Abstract
Vermicomposting utilizes the synergistic effect of earthworms with microorganisms to accelerate the stabilization of organic matter in biowastes. Nevertheless, the exact mechanism behind the maturity of vermicompost and the growth of earthworms exposed to biochar of varying particle sizes remains unclear. This study presents an investigation of the effect of biochar particle size on earthworm (Eisenia fetida) survival, microbial diversity, and the quality of vermicompost products. To address these issues, pelletized dewatered sludge samples from a municipal sewage treatment plant were amended with pine-based biochar with particle sizes of 1–2 mm, 25–75 μm, 200 nm, and 60 nm as the substrate for vermicomposting. This study revealed that the addition of millimeter-scale biochar and micron-scale biochar significantly promoted the degradation of organic matter since the organic matter in the treatment with 1–2 mm biochar at the end of the vermicomposting experiment decreased by 12.6%, which was equivalent to a 1.9-fold increase compared with that of the control. Excessive nanopowdering of nanobiochar significantly affected the survival of earthworms and led to 24.4–33.3% cumulative mortality, while millimeter-scale (mm) biochar and micron-scale (μm) biochar achieved zero mortality. The findings of this study could be used for evaluating the potential impact of nanoscale biochar to earthworms and guiding biochar-augmented vermicomposting.
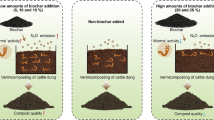
Graphical Abstract

Highlights
-
1.
The addition of 1–2 mm biochar resulted in a 1.9-fold greater reduction in organic matter compared to the control group.
-
2.
The introduction of 25–75 μm biochar led to a significant 94% increase in earthworm cocoons.
-
3.
The cumulative mortality rates of 200 nm and 60 nm biochar amendment reached peaks of 33.3% and 24.4% at first week, respectively.
-
4.
Earthworms excrete mucus that effectively removes attached nanobiochar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As a cost-effective and eco-friendly biotechnology, vermicomposting was first proposed by researchers in 1983 for sludge treatment (Frank et al. 1983) and has received increasing attention in recent years as a way to support the circular economy and UN sustainable development goals (Boruszko 2023; Vavouraki and Kornaros 2023). During vermicomposting, earthworms, which thrive solely in aerobic environments, assume the dual responsibility of aerating and maintaining the material, thus eliminating the requirement for costly turning equipment for conventional composting (Ndegwa and Thompson 2001). Unlike traditional thermophilic composting, vermicomposting must be performed at temperatures below 35 °C (Ndegwa and Thompson 2001). However, composting and vermicomposting can be integrated into a system by applying composting as the main processing unit and vermicomposting as the polishing unit (Hait and Tare 2011a). The types of organic waste for both vermicomposting and composting encompass a range of sources, including biowaste collected through source separation, municipal sewage sludge, and biowaste from the agri-food industry (Zhou et al. 2022). Primary sludge (Gupta and Garg 2008), digested sludge(Kızılkaya et al. 2014), industrial sludge (Yadav et al. 2011) and cattle manure-maize straw (Gong et al. 2023) have been used as raw substrates in vermicomposting. However, the long retention time of sludge during vermicomposting is one of the drawbacks of this technology (Lee et al. 2018). The addition of biochar can enhance the physicochemical properties of compost mixtures, stimulate microbial activities (Khan et al. 2020) and expedite the humification and biostabilization of sludge, leading to a shorter vermicomposting process (Xie et al. 2023; Zhang et al. 2014). The incorporation of biochar into composting brings additional advantages in terms of pesticide absorption and carbon sequestration when applied to soil (Siedt et al. 2021). Furthermore, biochar and earthworms have synergistic effects on the enhancement of soil structure and increase microbial population and activity (Zhang et al. 2021). In addition to its activating surface area and improving reactivity through physical and chemical means (Ahmad et al. 2013), biochar can also be activated by microorganisms and extracellular enzymes (Sizmur et al. 2017). Therefore, it is hypothesized that the addition of biochar is a promising solution for accelerating vermicomposting and achieving biologically stable compost products in a shorter duration.
The vermicomposting of sewage sludge with biochar amendment can be affected by the biochar type and biochar addition ratio (Liesch et al. 2010; Wu et al. 2023b). In the study by Liesch et al. (2010), two types of biochar (pine chip and poultry litter char) were tested at five application rates (0–90 mg ha−1) on Eisenia fetida growth and survival in artificial soils. The two highest application rates of poultry litter biochar resulted in 100% mortality and weight loss; this was mainly due to the high pH of the biochar (10.3) and the presence of toxic compounds such as As, Zn, Cu, Fe, and Al, as well as elevated Na and Mg content causing high salinity. During cattle dung vermicomposting, the influence of different biochar levels (ranging from 0 to 25% of the total feedstock) on earthworm (Eisenia fetida) activity and compost quality was assessed in the study by Wu et al. (2023b). They found that the addition of 5% biochar significantly enhanced earthworm total weight and cumulative burrowing activity. According to a study by Cao et al. (2021), the addition of biochar or nanocarbon impedes earthworm growth and cocoon production during vermicomposting of cow dung. Rashid et al. (2023) synthesized nanobiochar and analyzed its concentration-dependent influence on ammonia emission, nutrient mineralization, and nutrient uptake by maize from vermicompost and nanobiochar mixtures. However, the impact of biochar with different particle sizes on the survival of earthworms and their gut microbial communities during vermicomposting of sewage sludge remains unclear. The relationship among biochar size, surface area, and resulting biochar attachment size are crucial determinants of earthworm survival and compost quality.
The particle size of biochar utilized in the literature predominantly falls within the 2–3 mm range (Gong et al. 2023; Khan et al. 2019; Wu et al. 2023b). Some studies have used biochar with a particle size of less than 2 mm (Malińska et al. 2016) and as large as 0.5 cm (Gong et al. 2021). These studies on biochar addition to vermicomposting have overlooked the significance of biochar particle size at the micron-scale and nanoscale dimensions. The significance of biochar particle size is likely crucial, as these particles gradually decrease in size during vermicomposting and regulate the microenvironment within the compost pile by modifying the pore characteristics (Sanchez-Monedero et al. 2018). Larger biochar particles may undergo biofragmentation, analogous to the reduction in microplastic size during the vermicomposting process (Cui et al. 2022b). Furthermore, in vermicomposting products, biochar particles are expected to decrease in size due to both biochar weathering and the crushing action of earthworms following land application. We hypothesize that the biochar particle size plays a crucial role in earthworm reproduction and that adding biochar at an optimal particle size may enhance the conversion of sewage sludge in vermicomposting.
Therefore, the main purpose of this study was to evaluate the impact of biochar with different particle sizes on compost quality and earthworm activity in microbial communities. Furthermore, the effects of nanoscale biochar on earthworm abnormalities were investigated. These results are practical and instructional for the use of biochar in industrial-scale vermicomposting.
2 Materials and methods
2.1 Biochar and granulated sewage sludge
Biochar was produced from the pyrolysis of pine wood at 500 °C for 15 h. Prior to being added to the vermicomposting system, the biochar was ground to 1–2 mm, 25–75 μm, 200 nm and 60 nm, which were labeled as BC1-2, BC25-75, BC200, and BC60, respectively. To eliminate any potential toxic substances from the biochar, the biochar was immersed in deionized water, and shaken at a speed of 120 r min–1 for 72 h, passed through 0.45 μm membrane filters and then dried. The physicochemical properties of biochars of different sizes are listed in the Supplementary Materials (Table A1). In summary, as the biochar particle size decreased, the carbon content increased from 79.88 to 89.25%, while the hydrogen content decreased from 4.20 to 2.65%. Additionally, the surface area of the biochar samples, measured using the Brunauer‒Emmett‒Teller (BET) method, increased from 7.58 m2 g–1 to 327.19 m2 g–1. Dewatered sludge was collected from an anaerobic/anoxic/oxic (A2/O) wastewater treatment plant (WWTP) with a treatment capacity of 1.7 million m3 d–1 in Shanghai, China. Cationic polyacrylamide flocculant was added during sludge dewatering. Fresh dewatered sludge was granulated using a steel mesh with circular holes (diameter 5 mm) following the methods of Fu et al. (2015). After granulation, the sample was naturally air-dried in the shade for one week to remove ammonia, destroy the anaerobic environment, and make it suitable for earthworm survival. The physical and chemical properties of the raw materials used in the experiments are summarized in Table 1. In brief, the granulated sludge contained 78.7% moisture (21.3% total solid) with an organic fraction of 59.1% of dry solids. The pH of the sludge was 5.96. The pH of the pine-wood biochars (1–2 mm and 25–75 μm) was approximately 7.60, but it increased to 9.84 and 9.43 when the size was reduced to 200 nm and 60 nm, respectively. The electrical conductivity (EC) of the sludge was 3270 μS cm–1. The EC of the biochars (1–2 mm and 25–75 μm) varied from 334 to 365 μS cm–1, but it increased to 1333 μS cm–1 and 966 μS cm–1 when the size was reduced to 200 nm and 60 nm, respectively.
Earthworms (Eisenia fetida) were purchased from a vermicomposting plant in Shijiazhuang, Hebei, China. The earthworms were acclimated to dewatered and granulated sludge at room temperature (approximately 25 °C) for two months. The acclimated earthworms of uniform quality and size were selected for the next vermicomposting experiments.
2.2 Experimental design
To evaluate the effect of biochar particle size on sludge vermicomposting products, a total of six treatments were used. Each treatment was performed in triplicate with a total of 18 reactors. The vermicomposting reactor was a sealed transparent plastic square box with a volume of 1.8 L, which was 20.7 cm × 14.3 cm × 9.7 cm. Four circular holes with a diameter of 5 mm were drilled on top and bottom to facilitate water seepage and ventilation, respectively. At the same time, the cover was sealed to prevent the earthworms from escaping. The inoculation density of the earthworms was set at 33 earthworms/kg substrate, which suggests the addition of 30 earthworms (with approximately 0.8 g per earthworm) to each reactor. An inoculation density of earthworms (33 earthworms/kg wet sludge) is preferred based on previous experience with vermicomposting (Cui et al. 2022a). Table 2 summarizes the experimental setup. “SS” indicates the blank group, in which no earthworm or biochar was inoculated; “SSV0” indicates the control group, in which earthworms were inoculated while no biochar was added. The treatments containing biochar of 1–2 mm, 25–75 μm, 200 nm and 60 nm were referred to as “SSV1”, “SSV2”, “SSV3” and “SSV4”, respectively.
All reactors were incubated in the dark at 20 ± 2 °C for 35 days. The humidity was maintained at approximately 60-70% by spraying distilled water while aerobic conditions were maintained by turning the mixtures twice a week (Malińska et al. 2017). Intermittently, 30 g of mixture samples were taken at 0, 3, 7, 11, 14, 21, 28 and 35 days. For each sampling, adult worms and cocoons were manually selected to quantify the growth and reproduction of the earthworms. Meanwhile, the earthworm casts were collected for analysis of the diversity of the intestinal microbial community of the earthworms. The compost samples were collected in triplicate. One of these samples was used immediately as a fresh specimen for analyzing organic matter, water content, and enzyme activity. Another sample was stored, alongside the collected earthworm casts in a − 20 °C refrigerator for subsequent DNA extraction. The third part of the sample was dried at 40 °C until a constant weight was achieved and then ground and sieved through a 1 mm mesh. This sieved portion was then stored in a sealed desiccator for subsequent testing of physio-chemical parameters.
2.3 Analysis of vermicompost properties
The moisture content and organic matter (OM) content were determined by using a weight loss method (APHA 2005). Prior to determining the pH and electrical conductivity (EC), dry samples and deionized water were placed in a horizontal shaker at 200 r min–1 at a solid-to-liquid ratio of 1:10 (Zhang et al. 2015). After shaking for 4 h, the pH and EC of the supernatant were measured using a digital pH meter (pHs-2F, Shanghai Precision Scientific Instrument Co., Ltd., China) and a conductivity meter (DDS-307A, INESA Scientific Instrument Co., Ltd, China), respectively. The determination of ammonia nitrogen (AN) and total Kjeldahl nitrogen (TKN) was carried out using the sodium salicylate-sodium hypochlorite method on a continuous-flow autoanalyzer (AA3, Seal Analytical, Germany). Dissolved organic nitrogen (DON) was determined by subtracting the concentration of AN from the TKN value according to the ISO/DIS 15923-1 method. NO3−-N and NO2−-N were measured using the same continuous-flow autoanalyzer. At 16,000 r min–1, the water extract was centrifuged for 10 min and the supernatant was passed through a 0.45 μm polytetrafluoroethylene membrane filter. A total organic carbon analyzer (TOC-V CPH, Shimadzu, Japan) was used to measure the dissolved organic carbon (DOC) content and dissolved nitrogen (DN) of the filtrate. C/N ratio of the filtrate was calculated by dividing the DOC by DN. Spectrophotometric analysis was performed to measure the absorbance of liquid samples at 254 nm wavelength using a UV-1800 UV–Vis spectrophotometer (Shimadzu, Kyoto, Japan). The SUVA254 values were calculated by dividing the UV–Vis absorbance recorded at λ = 254 nm by the concentration of DOC.
2.4 Quantification analysis of the earthworms
During sampling, the adult earthworms, larvae and cocoons in the reactors were manually separated and counted. After all the adult earthworms were washed and dried, the total weight was determined. The abnormality and mortality rates of the earthworms were calculated according to Eq. 1 and Eq. 2, respectively.
where At and Mt are the number of abnormal earthworms in the reactor and the number of cumulative dead earthworms in the reactor on day t, respectively. The denominator “30” represents the initial number of earthworms inoculated in each reactor.
The mean individual biomass of the earthworms on day t (\(\overline{{W }_{t}}\), mg/worm) can be calculated according to Eq. 3:
where Wt (mg) and St are the total weight and the number of all surviving earthworms in the reactor on day t, respectively.
The reproduction rate can be calculated according to Eq. 4:
where N and S are the total cocoon number and the number of all surviving earthworms at the end of the vermicomposting experiment, respectively. The denominator “35” in the equation represents the duration of the vermicomposting experiments in days.
2.5 Dehydrogenase (DHA) and protease activity
The degradation of organic matter is enhanced through the assistance of diverse hydrolytic enzymes, such as DHA and proteases. Measurements of microbial enzyme activities, specifically those of dehydrogenases (DHAs) and proteases, were conducted on vermicompost samples collected at various sampling intervals. DHA was determined using the triphenyltetrazolium chloride (TTC) method (Huang et al. 2013). The foline phenol reagent method was used to determine the protease activity (Zhang et al. 2007). The absorbance was measured at a wavelength of 660 nm to characterize the protease activity in the sample. The instrument used was an ultraviolet spectrophotometer (UV-1800, Shimadzu, Japan).
2.6 Microbial analysis
The composition of the microbial communities in the vermicompost was significantly correlated with the intestinal microbes of the earthworms. Microbial analysis was conducted on vermicompost and intestinal microbial samples (earthworm casts). Before DNA extraction, frozen samples, which were stored at − 20 °C, were thawed at room temperature. DNA was extracted by using a PowerSoil® DNA isolation kit (MoBio Laboratories, Inc., California, USA). A spectrophotometer (NanoDrop 2000, Thermo Scientific, USA) was used to measure the A260/A280 and A260/A230 to assess the purity and concentration of DNA. The qualified DNA samples were stored at − 20 °C for subsequent analysis. Sequencing was performed on the Illumina PE300 sequencing platform (Illumina, USA). The primers used for amplification were ArBa515F_Arch806R for amplifying the V4 region of 16S rRNA to determine the composition of both archaea and bacteria. In addition, eukaryotic microbes in the compost samples were sequenced by using the primer SSU0817F_1196R. The raw sequencing reads were denoised to amplicon sequence variants (ASV) utilizing DADA2 version 1.10 with the default parameters (Callahan et al. 2016). Each ASV with 99% similarity threshold was taxonomically classified by QIIME 2 v.2020.2 (https://view.qiime2.org/) with SILVA 132 database.
2.7 Statistical analysis
Statistically significant differences in the means of parameters were tested based on one-way analysis of variance (ANOVA) with the mean separation by Tukey’s significant difference test at a 95% confidence level using R packages. Principle component analysis (PCA) and redundancy analysis (RDA) were performed using the R packages. The figures were drawn by the R software package ggplot2 or Origin Pro 8.5.1 (OriginLab Corporation, MA, USA).
3 Results and discussion
3.1 Effect of biochar on the quality of vermicomposting products
3.1.1 Changes in moisture content and OM
The moisture content and OM content in the sludge vermicomposting matrix changed with incubation time, as illustrated in Fig. 1a and b. In general, the changes in the trends of moisture content among the different treatments were maintained between 77% and 80%, which meets the normal growth requirements of earthworms. The OM in SSV1 and SSV2 at the end of the vermicomposting experiment decreased by 12.6% and 8.8%, respectively, which are 1.9-fold and 1.3-fold greater than the OM reduction in SSV0. This observation indicates that BC1-2 and BC25-75 combined with earthworm inoculation can effectively improve the degradation degree of sludge. Similarly, Xie et al. (2023) demonstrated that the addition of 100-μm biochar increased the organic carbon humification of vermicomposting products of dewatered sewage sludge. A previous study found that the synergy between earthworms, microorganisms, and biochar enhances the production of highly active enzymes, thereby accelerating organic matter degradation in vermicomposting (Sanchez-Hernandez et al. 2019). However, the degradation rate of OM in reactors with BC200 and BC60 addition was low, approximately 30% of that of the control group SSV0. One possible explanation is the initial inhibition of nanobiochar on earthworm growth and reproduction, which was also observed in the study of Cao et al. (2021).
3.1.2 pH and EC during biochar-augmented vermicomposting
The pH change for each reactor is shown in Fig. 1c. The decline in pH across all treatments during the initial stage can be ascribed to the generation of organic acids and the mineralization of nitrogen and phosphorus, which align with observations made in those studies on vermicomposting of sewage sludge (Hait and Tare 2011b; Villar et al. 2016). The subsequent pH increase can be explained by the utilization of acids by microorganisms and breakdown of proteins and amines (Beck-Friis et al. 2001).
EC serves as an useful indicator for evaluating the soluble salt concentration within the compost (McLachlan et al. 2004). As shown in Fig. 1d, the EC of the substrate water extracts in each treatment had a trend similar to that of the pH, first decreasing and then slowly increasing. At the end of the experiment, the changes in EC for SS, SSV0, SSV1, SSV2, SSV3, and SSV4 were − 1.02%, 12.68%, 21.73%, 0.95%, − 12.38% and − 14.13%, respectively. The rise in EC during the vermicomposting may be attributed to the organic matter loss through earthworm mineralization and release of different mineral salts in available forms (such as ammonium and potassium) (Garg et al. 2006; Suthar 2007). Nanoscale biochar exerts a negative impact on the growth of earthworms, thus affecting the mineralization of OM. Liesch et al. (2010) reported that high EC in poultry litter biochar is a cause of toxicity. Unlike the effects of biochar type, the impact of biochar particle size on the EC of compost is still unclear. In this research, none of the treatments significantly affected the compost EC, likely due to the washing pretreatment of the biochar and removal of minerals (Boakye et al. 2019). Table 1 shows that the raw 200 nm biochar had an EC of 1333 ± 29 μS cm–1, which was almost four times greater than that of biochar with particle sizes of 1–2 mm or 25–75 μm. Without washing pretreatment, the use of nanosized biochar in vermicomposting and subsequent land use could lead to the problem of high salinity as the maximum application rate of compost at 1000–2000 μS cm–1 is limited to 15 L m−2 for sensitive plants, and 60 L m−2 for tolerant plants (Hogg et al. 2002).
3.2 Effects of biochar on earthworm growth and reproduction during vermicomposting
3.2.1 Earthworm survival
During vermicomposting, the earthworms in the SSV0, SSV3 and SSV4 treatments died in different stages. Abnormal earthworms were also observed in SSV3 and SSV4. As shown in Fig. 2a, nanoscale biochar amendment in the early stages of vermicomposting increased the mortality and abnormality rates of earthworms. After 7 days, the cumulative mortality rates of SSV3 and SSV4 were as high as 33.3% and 24.4%, respectively. The high mortality rate of SSV3 and SSV4 can explain the abovementioned low degradation rate of OM in reactors with BC200 and BC60 addition. Nanoscale biochar adhered to the surface of the earthworms, which can be attributed to the escape and physical damage of the earthworms (Figure A1). In the middle and late stages of vermicomposting, the number of dead earthworms in the two nanoscale biochar treatments no longer increased because the earthworms continued to adapt to the environment. In these stages, earthworms secrete a large amount of mucus on the body surface and subsequently wash away the biochar particles attached on the body, as observed during the experiment and reported in the literature (Cui et al. 2023). Compared with the zero mortality in SSV1 and SSV2, earthworms escaped and died in the middle and late stages of SSV0 in the control group, which may be due to ammonia nitrogen accumulation caused by the ammonification process of sludge in the middle and late stages of vermicomposting, as shown in Figure A2b. Without the adsorption of ammonia nitrogen by biochar, a high level of ammonia is toxic to earthworms and affects their survival (Rollett et al. 2021).
a Changes in the mortality rate of earthworms and abnormality rate of living earthworms during vermicomposting; b changes in the mean individual biomass of earthworms; c number of cocoons during vermicomposting with different biochar particle sizes (Tukey HSD test, α = 0.05), which was manually counted from the initial 30 earthworms and 900 g pelletized sewage sludge; d redundancy analysis of the relationship among biomass, cocoons, damage rate and mortality rate of earthworms and environmental factors during the vermicomposting amended with different particle sizes of biochar. The presence of identical lowercase letters (a, b, c) above each bar indicates no significant difference (P > 0.05) between the various treatments
3.2.2 Growth and reproduction of earthworms
At the beginning of vermicomposting, the average earthworm growth rate of the control group SSV0 was greater than that of the SSV1 and SSV2 groups and then gradually decreased, as shown in Fig. 2b. At the end of vermicomposting, the average earthworm weights of SSV1 and SSV2 reached a maximum, which was not significantly different from that of the control group SSV0 (p > 0.05). As the biochar in SSV1 and SSV2 did not have any adverse effects on the growth of the earthworms, it can be inferred that the equal average earthworm weights among SSV1, SSV2 and SSV0 can be attributed to the limited availability of food for the earthworms in the batch vermicomposting experiment. In SSV3 and SSV4, the individual earthworm weight decline in the first week was due to individual death and physical damage (Figure A1). Subsequently, the individual weight of the earthworms began to increase and reached a maximum at the end of the experiment. There was no significant difference between the SSV3 and SSV4 (p > 0.05).
The reproductive capacity of the earthworms was measured by the number of cocoons laid, as shown in Fig. 2c. On Day 3, SSV0, SSV1 and SSV2 showed reproductive behavior and began to lay cocoons. Starting on Day 21, cocoon production in the SSV1 and SSV2 groups was significantly (p < 0.05) greater than that in the control group (SSV0). At the end of the experiment (Day 35), the mean spawning amount of SSV2 reached a maximum of 182.7 among the five treatments, which was 26% greater than that of SSV1. Compared with control group (SSV0), the number of earthworm cocoons increased by 94% and 53% for SSV2 and SSV1, respectively. The above results showed that the addition of biochar with 1–2 mm or 25–75 μm particles could effectively promote the reproduction of earthworms. Specifically, among all the treatments, biochar with a particle size ranging from 25 to 75 μm exhibited superiority. In other research studies, biochar with a size of 1–2 mm is commonly utilized (Gong et al. 2018; Malińska et al. 2016, 2017). As presented in Table A1, biochar particles with a coarser size of 1–2 mm exhibited a smaller surface area of 7.58 m2 g–1 than fine biochar particles with a coarser size of 25–75 μm, which had a significantly greater surface area of 120.96 m2 g–1. A greater surface area is responsible for bacterial attachment (Mohanty and Boehm 2014; Najm et al. 1990), which may facilitate bacterial reproduction and supply feed for earthworms.
In SSV3 and SSV4, the addition of nanoscale biochar caused the death and physical distortion of earthworm individuals at the early stage. Most of the abnormalities occurred near the ring zone of the earthworms, which seriously affected their reproductive ability. The earthworms in the two nanobiochar treatments did not start to reproduce until the middle and late stages of composting (after Day 21). At the end of the experiment, the mean numbers of cocoons laid by SSV3 and SSV4 were 44.0 and 46.7, respectively, which were significantly lower than those of the other three treatments (p < 0.05). These results indicated that nanobiochar impeded the reproduction of earthworms by damaging their clitellum. Only after the acclimation of the nanobiochar did the earthworms start to reproduce.
3.2.3 Correlation between vermicompost properties and earthworm growth and reproduction
Changes in growth and reproduction of earthworms can be attributed to changes in the physicochemical properties of the vermicompost. To test this hypothesis, the differences in the mean individual biomass, number of cocoons, cumulative mortality rate and damage rate of individual earthworms over time and the correlation between these earthworm biological parameters and environmental factors were analyzed by RDA for different treatments. The results are shown in Fig. 2d and it was found that RDA1 explained 42.43% of the variations and could distinguish the differences in earthworm growth and reproduction between the initial and final stages of vermicomposting, while RDA2 explained 20.28% of the variations through the differences in earthworm growth and reproduction among different treatments. On Day 0, the earthworm growth and reproduction of all five treatments were located in the upper left quadrant, which means that the initial conditions of earthworms were similar in different conditions. During the vermicomposting, almost both of the nanobiochar treatment groups SSV3 and SSV4 were in the lower half quadrant. At the end of the experiment, the earthworm growth and reproduction of control group SSV0, SSV1 and SSV2 were located in the upper right quadrant, while SSV3 and SSV4 were located in the lower right quadrant, which indicated that earthworm biomass and reproduction in each of the two groups showed similar trends. Indicators related to RDA1 and ranked in order of their influence on it were C/N, NO3−-N, NH4+-N, SUVA254, and DON, which were correlated with the degree of degradation and humification of the organic matter in the composting substrate, indicating that the availability of substrate significantly affects the growth and reproduction of earthworms. According to Dume et al. (2023), an 18:1 C/N ratio significantly increases the earthworm population. However, in our study, the C/N ratio, calculated by dividing DOC by DN, averaged approximately 4.15, primarily due to the composition of the sewage sludge feedstocks. This may explain why nitrogen-related parameters play a crucial role in earthworm biomass and reproduction. Meanwhile, changes in the physicochemical properties of the vermicompost substrate can influence the microbial community in the system, thus also affecting earthworm growth and reproduction. The average individual biomass of earthworms was positively correlated with the number of cocoons and all were positively correlated with DN, EC, NO3−-N, NH4+-N and SUVA254, while negatively correlated with pH, DON, DOC and C/N.
3.3 Effects of biochar on microbial activity and community structure during vermicomposting
3.3.1 Dehydrogenase and protease activity
The involvement of dehydrogenase in the initial breakdown of organics is well-established, as dehydrogenase catalyzes the extraction of hydrogen from organic compounds. As a result, dehydrogenase activity (DHA) serves as a measure for assessing the effectiveness of organic degradation. The DHA of compost substrates is used as a measure of overall microbial activity (Benitez et al. 1999). As depicted in Fig. 3a, the DHA of the initial compost of the six treatments varied in the range of 52.1–77.6 mg-TF h−1 g-dw−1, which suggested a high degradation rate in the early stage of vermicomposting. A subsequent decrease in the DHA concentration from Day 3 to Day 14 decelerated sludge degradation. This pattern is consistent with the findings of similar studies (Cui et al. 2018; Huang et al. 2013). However, in the study of Gong et al. (2018), the DHA concentration in the vermicomposting of plant waste began to increase, reached a maximum in the medium term and then decreased, which may be attributed to the different biowaste types.
In the experimental groups where biochar was added, the alteration in microbial activity can be attributed to the combined synergistic effects of earthworms and biochar. In general, the DHA of SSV1 and SSV2 consistently surpassed that of the control group (SSV0) and the blank group (SS), which suggests that biochar BC1-2 and BC25-75 can sustain a comparatively high level of microbial activity within the compost mixture. Upon completion of the vermicomposting process, the microbial activity observed in the SSV1 and SSV2 reactors surpassed that in both the control (SSV0) and blank (SS) groups. Subsequently, the SSV3 and SSV4 reactors demonstrated relatively lower microbial activity. At the end of vermicomposting, the relatively low DHA in SSV3 and SSV4 suggested the high biological stability of compost as this enzymatic activity is a reliable indicator of compost stability (Gómez et al. 2006; Tiquia 2005).
3.3.2 Changes in protease activity
The protease activity is strongly influenced by the availability of substrates, and several researchers have suggested that protease activity can serve as a dependable indicator for assessing the extent of substrate degradation (Lazcano et al. 2008). Hence, alterations in protease activity within compost can provide insights into the degradation of sludge and variations in microbial activity. Figure 3b shows the trend in protease activity within the compost among the different treatments. The initial protease activity of each treatment varied within the range of 28.7–36.9 mg-Tyr h−1 g-dw−1, and there were no significant differences (p > 0.05). Following the start of the experiment, the protease activity in the SSV1 exhibited an initial notable increase of 52%, followed by a subsequent decrease. This observation coincided with the accelerated degradation of OM (Fig. 1b) during the early stage of the experiment. The millimeter-scale biochar (BC1-2) did not adhere to the surface of the granular sludge but rather loosely filled the pores between the sludge particles. Consequently, this arrangement did not impact substrate availability or the oxygen content within the compost. However, the powdered micron and nanoscale biochar particles (BC25-75, BC200, BC60) adhered to and enveloped the outer surface of the granular sludge, which led to a significant reduction in substrate availability for microbial activity; moreover, it may create localized anaerobic conditions within the compost, consequently diminishing microbial activity. Consequently, during the initial 11-day period, the protease activities of SSV2, SSV3, and SSV4 exhibited a continuous decline. However, the subsequent biological activities of the earthworms facilitated the release of the enveloped sludge particles. The presence of biochar stimulated the secretion of mucus, serving as an attachment site for numerous microorganisms and enzymes. As a result, the protease activity rapidly increased, peaking on Day 14. Notably, the order of protease activity from highest to lowest was SSV4, SSV3, and SSV2. Nevertheless, all three treatments demonstrated lower protease activity than the maximum value achieved on the Day 7 in SSV1, which indicates that the 1–2 mm biochar greatly stimulated protease activity. As observed in similar studies (Awasthi et al. 2020; Gong et al. 2021), the presence of biochar increased the growth of protease-producing microorganisms and consequently elevated protease activities by increasing the porosity, surface area, and nutrient availability in compost. However, biochar particle size plays an important role in protease production. Among the tested particle sizes, 1–2 mm biochar greatly facilitated protease activity, which can be attributed to 1–2 mm biochar creating the optimal porosity and surface area for the growth of protease-producing bacteria.
3.3.3 Microbial composition of the vermicompost
Figure 4a shows the changes in the abundances of bacteria and archaea at the phylum level, with relative abundances greater than 2% in the vermicompost. Among all the samples, the relative abundance of Euryarchaeota accounted for approximately 0.04–3.22%, and Methanobacteria and Methanomicrobia (Class) were the main dominant species. Among all the samples, the common dominant bacterial phyla were Proteobacteria, Actinobacteria, Bacteroidetes and Chloroflexi, and the cumulative relative abundance reached 57–90%, which is consistent with the findings of previous reports (Domínguez et al. 2019; Li et al. 2020). This similarity may be attributed to the assimilation of indigenous microorganisms by earthworms. At the end of the vermicomposting, except for the two nanoscale biochar treatments (SSV3 and SSV4), there was no significant difference in the distribution of bacterial and archaeal species among the other treatments. The relative abundance of Proteobacteria in SSV3 and SSV4 decreased at the end of vermicomposting, but they were still the dominant bacteria with the highest proportion (22–44%). The highest values of the Shannon index and Observed ASVs were found in condition SSV0, followed by condition SSV1, as shown in Figure A3. At the end of the incubation, compared to the initial values, the Shannon index increased in all conditions except SSV3, with respective increases of 2.38%, 11.46%, 0.88%, 0.46%, -0.35%, and 1.78%. The Observed ASVs index also increased by 3.19%, 34.14%, 17.20%, 4.31%, 11.80%, and 0.73%, respectively. This indicates that, compared to normal composting, vermicomposting enhanced the abundance and diversity of bacteria and archaea in the vermicompost product. However, after adding biochar, the abundance and diversity of bacteria and archaea decreased, although they remained higher overall than those with normal composting. The RDA was performed to examine the relationship between the analyzed phyla and vermicompost properties, and the result showed that the first two components could explain 72.44% of the total variation (Fig. 4b). RDA showed significant correlations between bacteria and archaea abundance and the environmental variables: NO3−-N, moisture content, SUVA254, C/N and protease. NO3−-N and SUVA254 were positively correlated with Firmicutes, Actinobacteria, and Nitrospirae, while negatively correlated with Acidobacteria; in contrast, the C/N and protease showed the opposite pattern, being negatively correlated with Firmicutes, Actinobacteria, and Nitrospirae, and positively correlated with Acidobacteria.
Since we only identified two phylum levels of fungi, namely Opisthokonta (predominant) and SAR, we have opted not to incorporate the phylum level in the subsequent RDA analysis. Figure 4c shows the abundance changes at the genus level of the fungi whose relative abundance was higher than 2% in the compost under the different treatments. Overall, the majority of eukaryotes in all the samples were of the order Fungi accounting for 60–99% of the relative abundance. The eukaryotic community of this study differed from that of Fu et al. (2015), who reported that the eukaryotes in the vermicomposting products were mainly Protista and Fungi, among which Protista dominated the sewage sludge vermicomposting system without biochar amendment.
The RDA was performed to examine the relationship between the analyzed eukaryotic genera and vermicompost properties, and the result showed that the first two components could explain 72.69% of the total variation (Fig. 4d). NO2−-N and OM were the main factors influencing the composition of the eukaryotic community. OM was positively correlated with Rhinosporideacae, Laboulbeniomycetes and Uncultured eukaryote in Aspergillaceae while NO2−-N was negatively correlated with Cutaneotrichosporon. This observation suggests that the eukaryotic genera were significantly influenced by the degradation of organic matter during the vermicomposting of sewage sludge.
3.3.4 Microbial diversity of earthworm guts
The impact of earthworms on the degradation of organic waste within the vermicomposting process stems primarily from gut-associated activities (GAPs) (Domínguez et al. 2010). These activities encompass all the alterations that occur within the decaying organic material and microorganisms as they pass through the earthworm's digestive system (Domínguez et al. 2010). Figure 5a shows the changes in the abundances of bacteria and archaea at the phylum level, with relative abundances higher than 3% in the gut microbial samples of the earthworms in the treatments. Overall, the screened dominant strains were classified into Proteobacteria, Actinobacteria, Chloroflexi, Planctomycetes, Patescibacteria, Acidobacteria, Euryarchaeota, Bacteroidetes, Tenericutes, Firmicutes, Chlamydiae and Verrucomicrobia. In the intestinal microbial samples of earthworms, the relative abundance of Euryarchaeota ranged from 0.15 to 8.06%, which was greater than the content of Euryarchaeota in the compost samples. Most of the strains still belonged to the two classes Methanobacteria and Methanomicrobia. Among all the samples, the common dominant bacterial phyla were Proteobacteria, Actinobacteria, Bacteroidetes and Chloroflexi, and the cumulative relative abundance reached 47–91%.
Overall, after vermicomposting, the diversity of the intestinal bacteria and archaeal communities of the earthworms in each treatment decreased, and the proportions of the dominant bacterial species in the compost products were roughly similar. Specifically, from the perspective of changes in the abundance of the four dominant species of intestinal microorganisms, after the composting process, the relative abundance of Actinobacteria in all the treatments increased significantly. Except for SSV1, the changes in abundance were all between 12.13% and 14.99%. In addition, the abundance of Bacteroidetes decreased in the treatments, among which the abundance in SSV2 decreased from 19.52 to 3.42%, while the change range of the control groups (SSV0 and SSV1) was the smallest. The abundance of Proteobacteria in the control groups (SSV0, SSV1 and SSV4) increased significantly but decreased in the SSV2 and SSV3 groups. The changes in the abundance of Proteobacteria in SSV0, SSV2 and SSV4 were opposite to those in the compost. In addition, the changes in the abundance of Chloroflexi in the earthworm gut were opposite to those in the vermicompost. The variations in the abundances of Bacteroidetes and Chloroflexi in earthworm intestines are influenced by the composting substrate. Earthworms, through their feeding behavior, incorporate a portion of the substrate's microorganisms into their intestines. As a result, this process leads to alterations in the bacterial and archaeal communities within the earthworm intestines.
The findings were further validated by the findings of Knapp et al. (2009). The bacteria responsible for refractory OM, such as Actinobacteria, Bacteroidetes and Proteobacteria, in the gut of the earthworms still maintained a high abundance. However, due to the impact of nanoscale biochar on the life activity of earthworms, the proportions of Actinobacteria, Bacteroidetes and Proteobacteria in SSV3 and SSV4 were relatively low. RDA (Fig. 5b) showed the relationship between significant environmental variables and bacteria and archaea in vermicompost. RDA1 and RDA2 explained 50.98% and 23.67% of the variance, respectively, and NO3−-N, moisture, C/N were the main factors influencing the composition of the bacterial community in the earthworm gut. NO3−-N exhibited a positive correlation with Tenericutes, Actinobacteria, and Firmicutes, while showing a negative correlation with Acidobacteria. Moisture was positively correlated with Bacteroidetes and negatively correlated with Planctomycetes and Verrucomicrobia.
Figure 5c shows the changes in the abundance of fungi at the genus level (genera) with a relative abundance exceeding 2% in the gut microbial samples of earthworms. Notably, the predominant eukaryotic group across all samples was found to be order fungi, accounting for 79–99% of the relative abundance. Before the composting process commenced, the microbial diversity in the earthworm guts increased. However, as the earthworms ingested the sludge, the microorganisms from the substrate entered their guts, subsequently leading to alterations in the eukaryote community structure within the earthworm gut. Initially, Apiotrichum, Mortierella, Geotrichum and Cutaneotrichosoiron constituted the dominant species in the fungal community of the earthworm gut. During the later stage (after 14 days), Mortierella, a strain associated with chitin degradation (Gray and Baxby 1968), progressively emerged as the sole dominant strain, leading to reduced fungal diversity in all the treatments. However, the effect of adding biochar of different particle sizes on the composition of the fungal community in the gut of earthworms was not significant. As depicted in Fig. 5d, SUV254, N-related parameters, C/N and DHA, were the main vermicompost parameters affecting the eukaryotic genera in the earthworm gut.
3.4 Practical soil applications
Vermicompost and biochar are used as bio-conditioners in various types of soils, offering several benefits: enhancing the diversity of bacterial and fungal communities, as well as the activities of urease and alkaline phosphatase in greenhouse soils (Wu et al. 2023a); immobilizing heavy metals and improving soil fertility in contaminated soils (Wang et al. 2018); reducing ammonia and nitrous oxide emissions from a paddy soil (Wu et al. 2019). In particularly, nanosized biochar could serve as a beneficial soil amendment, enhancing soil fertility and boosting crop production (Shafiq et al. 2023). However, few studies have examined potential negative effects, such as increase of earthworm mortality and impacts of residual micro- and nano- biochar. The findings from this study demonstrate that the introduction of 25–75 μm biochar led to a significant 94% increase in earthworm cocoons, which suggested that the 25–75 μm biochar can be used for boosting the earworms reproduction when applied in soil. The rising use of engineered nanobiochar for water treatment and soil amendment has raised significant concerns about the mobility and toxicity of nanobiochar (Swaren et al. 2022). However, the final vermicompost product treated with nanobiochar in this study did not inhibit the growth and reproduction of earthworms, paving the way for the safe application of nanobiochar and vermicompost in soils without harming earthworms.
4 Conclusions
Biochar at the millimeter and micron scales can promote the degradation of organic matter in the process of vermicomposting sludge. The organic matter in 1-2 mm biochar treatment decreased by 12.6%, which was 1.9 times that observed in the control. Earthworm cocoons increased by 94% when 25–75 μm biochar was added. The cumulative mortality rates of 200 nm and 60 nm biochar amendments peaked at 33.3% and 24.4%, respectively, during the first week. At the millimeter scale, biochar can significantly increase the diversity and species richness of fungal communities in compost products. The changes in the gut microbiota of earthworms treated with biochar of different particle sizes showed similar trends. Therefore, when biochar is used in vermicomposting, powdered and nanoscale biochar should be avoided. Furthermore, the long-term vermicomposting effects of biochar amendment must be studied since the biochar particle size may gradually decrease over time.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Ok YS (2013) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99(3):19–33
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington
Awasthi MK, Duan Y, Awasthi SK, Liu T, Zhang Z (2020) Influence of bamboo biochar on mitigating greenhouse gas emissions and nitrogen loss during poultry manure composting. Bioresour Technol 303:122952
Beck-Friis B, Smårs S, Jönsson H, Kirchmann H (2001) SE—structures and environment: gaseous emissions of carbon dioxide, ammonia and nitrous oxide from organic household waste in a compost reactor under different temperature regimes. J Agric Eng Res 78(4):423–430
Benitez E, Nogales R, Elvira C, Masciandaro G, Ceccanti B (1999) Enzyme activities as indicators of the stabilization of sewage sludges composting with Eisenia foetida. Biores Technol 67(3):297–303
Boakye P, Tran HN, Lee DS, Woo SH (2019) Effect of water washing pretreatment on property and adsorption capacity of macroalgae-derived biochar. J Environ Manage 233:165–174
Boruszko D (2023) Impact of effective microorganisms on the vermicomposting of sewage sludge. Desalin Water Treat 288:273–282
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13(7):581–583
Cao YN, Tian YQ, Wu Q, Li JS, Zhu HY (2021) Vermicomposting of livestock manure as affected by carbon-rich additives (straw, biochar and nanocarbon): a comprehensive evaluation of earthworm performance, microbial activities, metabolic functions and vermicompost quality. Bioresour Technol 320:124404
Cui G, Li F, Li S, Bhat SA, Ishiguro Y, Wei Y, Yamada T, Fu X, Huang K (2018) Changes of quinolone resistance genes and their relations with microbial profiles during vermicomposting of municipal excess sludge. Sci Total Environ 644:494–502
Cui GY, Fu XY, Bhat SA, Tian WP, Lei XY, Wei YF, Li FS (2022) Temperature impacts fate of antibiotic resistance genes during vermicomposting of domestic excess activated sludge. Environ Res 207:112654
Cui GY, Lue F, Hu T, Zhang H, Shao LM, He PJ (2022) Vermicomposting leads to more abundant microplastics in the municipal excess sludge. Chemosphere 307:136042
Cui J, Jiang J, Chang E, Zhang F, Guo L, Fang D, Xu R, Wang Y (2023) Underlying reasons and factors associated with changes in earthworm activities in response to biochar amendment: a review. Biochar 5(1):79–79
Domínguez J, Aira M, Gómez-Brandón M (2010) Microbes at work: from wastes to resources. Springer, Berlin Heidelberg, pp 93–114
Domínguez J, Aira M, Kolbe AR, Gómez-Brandón M, Pérez-Losada M (2019) Changes in the composition and function of bacterial communities during vermicomposting may explain beneficial properties of vermicompost. Sci Rep 9(1):9657
Dume B, Hanc A, Svehla P, Michal P, Chane AD, Nigussie A (2023) Composting and vermicomposting of sewage sludge at various C/N ratios: technological feasibility and end-product quality. Ecotoxicol Environ Saf 263:115255
Frank R, Klauck C, Stonefield KI (1983) Metal transfer in vermicomposting of sewage-sludge and plant wastes. Bull Environ Contam Toxicol 31(6):673–679
Fu XY, Huang K, Cui GY, Chen XM, Li FS, Zhang XY, Li F (2015) Dynamics of bacterial and eukaryotic community associated with stability during vermicomposting of pelletized dewatered sludge. Int Biodeterior Biodegrad 104:452–459
Garg P, Gupta A, Satya S (2006) Vermicomposting of different types of waste using Eisenia foetida: a comparative study. Biores Technol 97(3):391–395
Gómez RB, Lima FV, Ferrer AS (2006) The use of respiration indices in the composting process: a review. Waste Manage Res 24(1):37–47
Gong XQ, Cai LL, Li SY, Chang SX, An ZF (2018) Bamboo biochar amendment improves the growth and reproduction of Eisenia fetida and the quality of green waste vermicompost. Ecotoxicol Environ Saf 156:197–204
Gong X, Zhang Z, Wang H (2021) Effects of Gleditsia sinensis pod powder, coconut shell biochar and rice husk biochar as additives on bacterial communities and compost quality during vermicomposting of pig manure and wheat straw. J Environ Manage 295:113136
Gong XQ, Zou L, Wang L, Zhang B, Jiang JX (2023) Biochar improves compost humification, maturity and mitigates nitrogen loss during the vermicomposting of cattle manure-maize straw. J Environ Manag 325:116432
Gray TRG, Baxby P (1968) Chitin decomposition in soil: II. The ecology of chitinoclastic micro-organisms in forest soil. Trans Br Mycol Soc 51(2):293–309
Gupta R, Garg VJ (2008) Stabilization of primary sewage sludge during vermicomposting. J Hazard Mater 153(3):1023–1030
Hait S, Tare V (2011a) Optimizing vermistabilization of waste activated sludge using vermicompost as bulking material. Waste Manage 31(3):502–511
Hait S, Tare V (2011b) Vermistabilization of primary sewage sludge. Biores Technol 102(3):2812–2820
Hogg D, Barth J, Favoino E, Centemero M, Caimi V, Amlinger F, Devliegher W, Brinton W, Antler S (2002) Comparison of compost standards within the EU, North America and Australasia: main report, WRAP. Programme
Huang K, Li F, Wei Y, Chen X, Fu X (2013) Changes of bacterial and fungal community compositions during vermicomposting of vegetable wastes by Eisenia foetida. Biores Technol 150:235–241
Khan MB, Cui XQ, Lazzat U, Zehra DA, Hamid Y, Hussain B, Tang L, Yang XE, He ZL (2019) Eisenia fetida and biochar synergistically alleviate the heavy metals content during valorization of biosolids via enhancing vermicompost quality. Sci Total Environ 684:597–609
Khan MB, Cui XQ, Jilani G, Tang L, Lu M, Cao XR, Sahito ZA, Hamid Y, Hussain B, Yang XE, He ZL (2020) New insight into the impact of biochar during vermi-stabilization of divergent biowastes: literature synthesis and research pursuits. Chemosphere 238:124679
Kızılkaya R, Türkay FŞH (2014) Vermicomposting of anaerobically digested sewage sludge with hazelnut husk and cow manure by earthworm Eisenia foetida. Compost Sci Util 22(2):68–82
Knapp BA, Podmirseg SM, Seeber J, Meyer E, Insam H (2009) Diet-related composition of the gut microbiota of Lumbricus rubellus as revealed by a molecular fingerprinting technique and cloning. Soil Biol Biochem 41(11):2299–2307
Lazcano C, Gómez-Brandón M, Domínguez J (2008) Comparison of the effectiveness of composting and vermicomposting for the biological stabilization of cattle manure. Chemosphere 72(7):1013–1019
Lee LH, Wu TY, Shak KPY, Lim SL, Ng KY, Nguyen MN, Teoh WH, Biotechnology, (2018) Sustainable approach to biotransform industrial sludge into organic fertilizer via vermicomposting: a mini-review. J Chem Technol Biotechnol 93(4):925–935
Li R, Liu Y, Sheng Y, Xiang Q, Zhou Y, Cizdziel JV (2020) Effect of prothioconazole on the degradation of microplastics derived from mulching plastic film: apparent change and interaction with heavy metals in soil. Environ Pollut 260:113988–113988
Liesch AM, Weyers SL, Gaskin J, Das KC (2010) Impact of two different biochars on earthworm growth and survival. Ann Environ Sci 4(1–9):1–9
Malińska K, Zabochnicka-Świątek M, Cáceres R, Marfà O (2016) The effect of precomposted sewage sludge mixture amended with biochar on the growth and reproduction of Eisenia fetida during laboratory vermicomposting. Ecol Eng 90:35–41
Malińska K, Golańska M, Caceres R, Rorat A, Weisser P, Ślęzak E (2017) Biochar amendment for integrated composting and vermicomposting of sewage sludge—the effect of biochar on the activity of Eisenia fetida and the obtained vermicompost. Biores Technol 225:206–214
McLachlan KL, Chong C, Voroney RP, Liu H-W, Holbein BE (2004) Variability of soluble salts using different extraction methods on composts and other substrates. Compost Sci Util 12(2):180–184
Mohanty SK, Boehm AB (2014) Escherichia coli removal in biochar-augmented biofilter: effect of infiltration rate, initial bacterial concentration, biochar particle size, and presence of compost. Environ Sci Technol 48(19):11535–11542
Najm IN, Snoeyink VL, Suidan MT, Lee CH, Richard Y (1990) Effect of particle size and background natural organics on the adsorption efficiency of PAC. J Am Water Works Ass 82(1):65–72
Ndegwa PM, Thompson SA (2001) Integrating composting and vermicomposting in the treatment and bioconversion of biosolids. Biores Technol 76(2):107–112
Rashid MI, Shah GA, Iqbal Z, Shahzad K, Ali N, Rehan M, Alhakamy NAA, Klemes JJ (2023) Nanobiochar reduces ammonia emission, increases nutrient mineralization from vermicompost, and improves maize productivity. J Clean Prod 414:137694
Rollett AJ, Bhogal A, Scullion J, Nicholson FA, Taylor MJ, Williams JR (2021) The effect of field application of food-based anaerobic digestate on earthworm populations. Soil Use Manag 37(3):648–657
Sanchez-Hernandez JC, Ro KS, Díaz FJ (2019) Biochar and earthworms working in tandem: research opportunities for soil bioremediation. Sci Total Environ 688:574–583
Sanchez-Monedero MA, Cayuela ML, Roig A, Jindo K, Mondini C, Bolan N (2018) Role of biochar as an additive in organic waste composting. Biores Technol 247:1155–1164
Shafiq F, Anwar S, Firdaus-e-Bareen, Zhang LX, Ashraf M (2023) Nano-biochar: Properties and prospects for sustainable agriculture. Land Degrad Dev 34(9):2445–2463
Siedt M, Schäffer A, Smith KEC, Nabel M, Ross-Nickoll M, van Dongen JT (2021) Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci Total Environ 751:141607
Sizmur T, Fresno T, Akgül G, Frost H, Moreno-Jiménez E (2017) Biochar modification to enhance sorption of inorganics from water. Biores Technol 246:34–47
Suthar S (2007) Vermicomposting potential of (Perrier) in different waste materials. Biores Technol 98(6):1231–1237
Swaren L, Safari S, Konhauser KO, Alessi DS (2022) Pyrolyzed biomass-derived nanoparticles: a review of surface chemistry, contaminant mobility, and future research avenues to fill the gaps. Biochar 4(1):33
Tiquia SM (2005) Microbiological parameters as indicators of compost maturity. J Appl Microbiol 99(4):816–828
Vavouraki AI, Kornaros M (2023) Vermi-conversion of anaerobic sludges by earthworms. Ferment Basel 9(6):512
Villar I, Alves D, Pérez-Díaz D, Mato S (2016) Changes in microbial dynamics during vermicomposting of fresh and composted sewage sludge. Waste Manage 48:409–417
Wang Y, Xu YA, Li D, Tang BC, Man SL, Jia YF, Xu H (2018) Vermicompost and biochar as bio-conditioners to immobilize heavy metal and improve soil fertility on cadmium contaminated soil under acid rain stress. Sci Total Environ 621:1057–1065
Wu D, Feng YF, Xue LH, Liu MQ, Yang B, Hu F, Yang LZ (2019) Biochar combined with vermicompost increases crop production while reducing ammonia and nitrous oxide emissions from a paddy soil. Pedosphere 29(1):82–94
Wu QQ, Zhang J, Liu XN, Chang TT, Wang Q, Shaghaleh H, Hamoud YA (2023a) Effects of biochar and vermicompost on microorganisms and enzymatic activities in greenhouse soil. Front Env Sci-Switz 10:1060277
Wu YP, Li QF, Zheng Y, Xiong XJ, Chen YF, Shaaban M, Hu RG (2023b) Optimizing biochar addition for vermicomposting: a comprehensive evaluation of earthworms’ activity, N2O emissions and compost quality. Biochar 5(1):4
Xie JC, Xia H, Guan MX, Huang K, Chen J (2023) Accelerating the humification mechanism of dissolved organic matter using biochar during vermicomposting of dewatered sludge. Waste Manage 159:102–113
Yadav A, Garg V (2011) Industrial wastes and sludges management by vermicomposting. Rev Environ Sci Bio/Technol 10(3):243–276
Zhang B, He P-J, Lü F, Shao L-M, Wang P (2007) Extracellular enzyme activities during regulated hydrolysis of high-solid organic wastes. Water Res 41(19):4468–4478
Zhang JN, Lü F, Shao LM, He PJ (2014) The use of biochar-amended composting to improve the humification and degradation of sewage sludge. Biores Technol 168:252–258
Zhang J, Lü F, Zhang H, Shao L, Chen D, He P (2015) Multiscale visualization of the structural and characteristic changes of sewage sludge biochar oriented towards potential agronomic and environmental implication. Sci Rep 5(1):9406–9406
Zhang QM, Li SZ, Saleem M, Ali MY, Xiang JH (2021) Biochar and earthworms synergistically improve soil structure, microbial abundance, activities and pyraclostrobin degradation. Appl Soil Ecol 168:104154
Zhou YW, Xiao R, Klammsteiner T, Kong XL, Yan BH, Mihai FC, Liu T, Zhang ZQ, Awasthi MK (2022) Recent trends and advances in composting and vermicomposting technologies: a review. Bioresour Technol 360:127591
Acknowledgements
The authors would like to thank Dr. Haowen Duan, Dr. Shasha Li, Miss. Wanying Liu and Miss. Yingtao Deng for the help with earthworm collection and sample pretreatment.
Funding
The study was financially supported by the National Natural Science Foundation of China (52000144), the Fundamental Research Funds for the Central Universities (22120220553, 2023-3-YB-11).
Author information
Authors and Affiliations
Contributions
Conceptualization: Wei Peng, Fan Lü; methodology: Yue Wang, Guangyu Cui, Qiyong Xu, Fan Lü, Wei Peng; software, formal analysis and investigation: Yue Wang; writing—original draft: Wei Peng; writing—review and editing: Wei Peng, Guangyu Cui, Fan Lü, Hua Zhang, Pinjing He; funding acquisition: Pinjing He, Fan Lü, Wei Peng; supervision: Pinjing He, Fan Lü. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interest.
Additional information
Handling editor: Bin Gao.
Supplementary Information
42773_2024_365_MOESM1_ESM.docx
Supplementary Material 1. Table A1 Characteristics of biochar of different particle sizes. Figure A1 Earthworm physical damage in the application of nanobiochar (after cleaning). Figure A2 Changes of (a) DOC, (b) NH4+-N, (c) DN, (d) NO3--N and (e) NO2--N in the water extracts of the different composts. Figure A3. Changes in (a) Shannon Index and (b) Observed ASVs of bacteria and archaea in the substrate during vermicomposting under different conditions. Figure A4. Changes in (a) Shannon Index and (b) Observed ASV of eukaryote in the substrate during vermicomposting under different conditions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peng, W., Wang, Y., Cui, G. et al. Compost quality, earthworm activities and microbial communities in biochar-augmented vermicomposting of dewatered activated sludge: the role of biochar particle size. Biochar 6, 73 (2024). https://doi.org/10.1007/s42773-024-00365-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-024-00365-8