Abstract
Biochar (BC) and nanoparticle-decorated biochar (NPs@BC) have emerged as potential high-performance function materials to facilitate simultaneous soil remediation and agricultural production. Therefore, there is an urgent need to incorporate environmental sustainability and human health targets into BC and NPs@BC selection and design processes. In contrast to extensive research on the preparation, modification, and environmental application of BC to soil ecosystems, reports about the adapted framework and material selection strategy of NPs@BC under environmental and human health considerations are still limited. Nevertheless, few studies systematically explored the impact of NPs@BC on soil ecosystems, including soil biota, geochemical properties, and nutrient cycles, which are critical for large-scale utilization as a multifunctional product. The main objective of this systematic literature review is to show the high degrees of contaminant removal for different heavy metals and organic pollutants, and to quantify the economic, environmental, and toxicological outcomes of NPs@BC in the context of sustainable agriculture. To address this need, in this review, we summarized synthesis techniques and characterization, and highlighted a linkage between the evolution of NPs@BC properties with the framework for sustainable NPs@BC selection and design based on environmental effects, hazards, and economic considerations. Then, research advances in contaminant remediation for heavy metals and organic pollutants of NPs@BC are minutely discussed. Eventually, NPs@BC positively acts on sustainable agriculture, which is declared. In the meantime, evaluating from the perspective of plant growth, soil characterizations as well as carbon and nitrogen cycle was conducted, which is critical for comprehending the NPs@BC environmental sustainability. Our work may develop a potential framework that can inform decision-making for the use of NPs@BC to facilitate promising environmental applications and prevent unintended consequences, and is expected to guide and boost the development of highly efficient NPs@BC for sustainable agriculture and environmental applications.
Graphical Abstract

Highlights
• Nanoparticle-decorated biochar as remediation materials fosters the attainment of soil remediation and sustainable agriculture.
• The synthesis, characterization, soil remediation, and environmental effects of nanoparticle-decorated biochar are described.
• A design and selection framework of nanoparticle-decorated biochar is proposed to evaluate the safety in all directions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A productive and sustainable agricultural system is essential to the development of human (Lowry et al. 2019). However, severe global soil contamination (i.e., heavy metal and organics pollutant) has greatly threatened human health and agricultural sustainability. Therefore, preventing soil degradation and contamination is urgent (Pineiro et al. 2020), especially with the increasing population and demand for agricultural land (Amundson et al. 2015; Mueller et al. 2012). Subsequently, economical and efficient environment function material as an emerging technology for soil remediation of pollutants comes into being (Brandl et al. 2015). Biochar (BC), a cheap, readily available, and environmentally friendly function material, has received increasing attention due to its unique physical and structural properties. For example, both China and the USA could annually generate 1.4 billion tons of agricultural biomass waste, which has the latent production of 4.2 × 108 tons of biochar per year (Turhollow et al. 2014). Nevertheless, some considerable disadvantages of raw BC (e.g., inferior mechanical strength, poor adsorption capacity for high-concentration contaminants, and high energy) restrict their proper utilization in the field of environmental remediation (Ma et al. 2014). Thus, overcoming the above limitations will be of great significance for enhancing the application of BC.
Nanotechnology and nanoparticles (NPs) pave the way for the agriculture industry, where they may be useful for environmental remediation purposes (Zhou and Hu 2017; Park et al. 2020; Petersen et al. 2021). NPs play crucial roles in the biogeochemical cycling of their constitutive elements due to the high surface energy and large specific surface area (Liu et al. 2020). Nonetheless, the high price, scarcity, and sustainability of NPs hinder them from the large-scale environmental applications (Falinski et al. 2018). Instead, nanoparticle-decorated biochar (NPs@BC) materials combine the merits of BC and NPs and significantly improve the performance of NPs/BC (Wang et al. 2019). Thus, NPs@BC materials show extraordinary talents, and these composite materials are rich in functional groups, which can counteract the drawbacks of pristine biochar and NPs in environmental remediation (Tu et al. 2020). NPs@BC can also be selectively designed for the target pollutants by loading nanoparticles. For example, MgO@BC, which is synthesized via straws and MgCl2·6H2O, reveals a much higher Cd2+ adsorption capacity (1058.8 mg g− 1) compared with the pristine biochar (Wang et al. 2021b). Magnetic biochar composites (Fe2O3/biochar) exhibit a huge potential for rapid and efficient adsorption of antibiotics (Liang et al. 2022a). Currently, a number of reviews have systematically summarized the synthesis, physicochemical properties of pure BC and NPs, and their agronomic and environmental applications, specifically including structure design and pyrolysis techniques, water and soil contaminant removal or stabilization, and health risk assessment (Liu et al. 2015; Zhang et al. 2022c). However, the existing literature on the above knowledge of NPs@BC remains limited, and few studies systematically examined the linkage between the evolution and properties of NPs@BC with the environmental function performance as well as the potential risk, which is critical for understanding the NPs@BC environmental application and utilization as a multifunctional product.
The number of papers regarding NPs@BC composites has increased significantly over the last decade, as shown in Additional file 1: Figure S1. However, only a few studies have been conducted on the standardization of NPs@BC, that is the characteristics, application, and evaluation of the NPs@BC. Although some studies have noticed the possible environmental risk related to NPs and BC (Kah et al. 2021; Pathy et al. 2020), the environmental sustainability assessment of NPs@BC is seldom addressed. Therefore, the objectives of the present review are to (1) systematically summarize the synthetic method and characterization of NPs@BC; (2) highlight an environmental sustainability assessment framework (including environmental effects, hazards, economic considerations, and crop growth) which is the standard for selection and design of NPs@BC; (3) elucidate the soil remediation (e.g., heavy metals and organics) application of NPs@BC; and (4) the salutary influence of NPs@BC on sustainable agriculture is described including crop growth, soil properties, and carbon and nitrogen cycling mediated by soil microorganism. This review will identify key knowledge gaps, provide new insights for selecting environmental applications of NPs@BC, and guide and advance the development of highly efficient NPs@BC for engineering applications.
2 Synthesis and characterization of NPs@BC
In the past years, NPs@BC components, as promising functional materials, have progressively increased in environmental remediation and agricultural application due to the unique properties. The detection and quantification of NPs@BC in environmental application systems require measurements that are specific to these unique properties of NPs@BC. These materials are manufactured under a range of conditions, including processes, and characterizations, some of which are summarized in Additional file 1: Table S1 and for which this information has been provided in the literature. Figure 1 provides an overview of selected techniques grouped by microscopic, spectroscopic, mass spectroscopy, and thermal categories for NPs@BC measurements after different synthesis processes. In addition, the interaction mechanisms between biochar and loading nanoparticles during the manufacturing processes are also discussed.
Summary of synthesis and characterization of NPs@BC. Schematic illustration shows the NPs@BC manufacturing routines, types, and functions of characterization at different decorated nanoparticles containing metal NPs, metal oxide NPs, and other NPs decorating biochar. XRD, X-ray diffraction; FT-IR, Fourier transform infrared spectroscopy; SEM, scanning electron microscopy; TEM, transmission electron microscopy; XANES, X-ray absorption near-edge structure; µ-XRF, micro-X-ray fluorescence; XPS, X-ray photoelectron spectroscopy; EDS, energy dispersive x-ray spectroscopy; EDX, energy dispersive x-Ray spectroscopy; TGA, thermo-gravimetric analysis; DTA, differential thermal analysis; BET, Brunauer Emmett teller
2.1 Synthesis methods
Metal/metal oxide nanoparticles (e.g., nZVI, Fe3O4, TiO2, and ZnO) and element-doped (e.g., N, P, B, S) BC combinations are the most commonly pursued nanohybrids, which exhibit appealing properties and multiple benefits for environmental applications. Moreover, the physicochemical properties of NPs@BC are strongly dependent on their size, shape, surface structure, and their bulk composition, which can be appropriately tuned during their synthesis under specific conditions; therefore, investigation and monitoring of the manufacturing of NPs@BC throughout are required. Consequently, based on the composition of the nanoparticles, this review classifies the NPs@BC materials into the following three categories: (1) metal nanoparticles coated biochar composites; (2) metal oxide nanoparticles decorated biochar composites; and (3) other nanoparticles loaded biochar. The synthesis of NPs@BC can be carried out by different methods such as co-precipitation, hydrothermal reaction, impregnation, ball milling, and sol-gel, among others. Manufacturing of NPs@BC composites generally involves two stages: feedstock (woods, rice husk, corn stalks, etc.) is pretreated with deionized water to remove impurities generally ahead of pyrolysis and the temperature would high than 400 °C and the pyrolysis process is conducted at nitrogen atmosphere; subsequently rinsing the obtained biochar with the hydrochloric acid solution is designed to eradicating inorganic components and coupled with hydrothermal, impregnation, co-precipitation, sol-gel, etc.
2.1.1 Metal nanoparticles coated biochar
The different modification approaches, such as nano-metallic particles (i.e., iron, copper, cobalt) decorating on biochar material, have been employed to increase the capacity of pristine biochar due to their small size, excellent specific surface area, higher reaction activity, and better adsorption performance. Moreover, BC could act as the catalyst supports to fix and prevent the aggregation of nanoparticles, and the loaded nanoparticles would provide the prominent active site, then boost the catalytic efficiency (Diao et al. 2020a; Huang et al. 2020). Thus, NPs@BC exhibits the characteristics of being efficient. For example, NaBH4 reduction combined with pyrolysis was used for preparing MgO@BC components and presented superior surface area (218.9 m2 g− 1) than biochar (27.9 m2 g− 1). BC presents deeply low affinity of adsorption (e.g., phosphate < 5 mg g− 1, humate < 10 mg g− 1). Instead, MgO@BC significantly accelerates the affinity for phosphate and humate, increasing by more than 660% and 280% after MgO impregnation, respectively (Li et al. 2017). Although NPs@BC composites exhibit a remarkable capacity for removing pollutants, metal ions of residue in the water and soil which derive from composite have been neglected. The traditional method for metal decorating BC is divided into the two steps, including biomass pyrolysis and metal nanoparticle loading (e.g., NaBH4 reduction and impregnation), and the detailed process is showed in Additional file 1: Table S1. Biomass pyrolysis can be broadly divided into two major categories (fast pyrolysis and slow pyrolysis) according to the operating conditions (Sharma et al. 2015). Fast pyrolysis is characterized by short vapor residence time (2 s) and fast heating rate (> 200 K min− 1) at 400–600 °C. On the contrary, the slow pyrolysis is characterized by a longer vapor residence time (mins to hours) and a slow heating rate (5–10 °C min− 1) at 300–800 °C (Lian and Xing 2017). The metal nanoparticles are loaded on the BC by putting them into a metal-salt solution aged for hours and are generally doped onto the BC surface containing enriched oxygen functional groups. Recent studies of nZVI@BC production by Zhang et al. (2022a) have confirmed the Fe-O bond (at 529.4 eV) exists in the XPS spectra of nZVI@BC. During the synthetic processes of NPs@BC, various factors such as the feedstock type, pyrolysis temperature, and ratio of biochar and metal nanoparticles influence the properties of NPs@BC. The surface area of nZVI@BC increases with increasing BC contents. For example, the surface area of nZVI@BC is 96.13 m2 g− 1, 107.51 m2 g− 1, 122.65 m2 g− 1, 119.11 m2 g− 1, when the mass ratio of nZVI/BC is 1:1, 1:2, 1:3 and 1:4, respectively. Since under higher BC contents, nZVI disperses well on the surface of BC. Whereas, owing to the increasing aggregation of BC sheets, the surface area is decreased with further increase of BC content (nZVI: BC = 1:4) (Hussain et al. 2017).
2.1.2 Metal oxide nanoparticles coated biochar
Nano-metal oxides, owing to diffusion activation energy and strong quantum effect, regularly have improved redox and adsorption capability and are environmentally friendly and inexpensive (Wang et al. 2019). Biochar modified with nano-metal oxides has a larger surface area, cation exchange capacity, and porosity than the pristine biochar and is enriched in surface functional groups (Bombuwala Dewage et al. 2018). Several nanocrystalline metal oxides have currently been applied to modifying biochar for hoisting adsorption capacity, as follows: (a) magnetic metal oxides such as CuFe2O4, MnFe2O4, Fe3O4, Co3O4 (Fu et al. 2019; Xu et al. 2020a; Zhao et al. 2020); (b) non-magnetic metal oxides such as MgO, ZnO, Fe2O3, TiO2 (Ibrahim et al. 2022). All in all, the nano-metal oxides with biochar can be prepared by either soaking feedstock into metal oxide salt solution as pre-pyrolysis treatment or soaking pyrolyzed material into metal-ion solution as post-pyrolysis treatment. The preparation methods generally need to mix BC with a metal salt solution to form a suspension. After that, aqueous alkali (e.g., NaOH, NH3·H2O) is added dropwise while stirring at room temperature ranging from 180 °C, and then precipitation occurs and is calcined at 600 °C. However, treating the feedstock with Fe, Mn, Mg, and Zn salts may result in the formation of their oxides on the biochar surface. Different kinds of nano-metal oxides are well distributed in the biochar matrix by generating covalent or noncovalent functionalization. Xing et al. (2021) successfully demonstrated that CuFeO2 is doped biochar the structural defects in the carbonaceous matrix that can catch the active redox-pairs/cycle, for instance, Fe0-Fe2+-Fe3+ using FTIR spectra of BC, and CuFeO2@BC. Similar results were obtained by Zhao et al. They identified that the absorption bands at 480 and 500 cm− 1 can be attributed to the stretching vibration of Cu-O bond in the octahedral structure and Fe-O bond formation. In addition, in the case of pure BC, CuFe2O4, these characteristic peaks could be in kept with the spectra of fresh CuFe2O4@BC composites (Zhao et al. 2020). A novel advanced oxidation technology based on sulfate radicals has been extensively used in the treatment of organic wastewater, which can be mainly fulfilled by the high oxidation potential and long half-life of sulfate radicals (Chen et al. 2018). •SO4− can be produced by activation of peroxy-mono-sulfate (PMS) or persulfate. Generally, numerous heterogeneous magnetic catalysts, for instance, CuFe2O4, MnFe2O4, etc., are served for PMS activation (Pang and Lei 2016; Xu et al. 2016). Consequently, a certain amount of nano-magnetic metal oxides coated biochar are allied with advanced oxidation technology, such as Fenton, to achieve the decomposition of refractory organic pollutants (Xin et al. 2021). The properties of NPs@BC greatly depend on the pyrolysis temperature. The increase in pyrolysis temperature results in the growth of aromatic structures and the loss of oxygen functional groups. For instance, in the FT-IR spectrum of Fe3O4@BC, the band at 1616 cm− 1 is assigned to C=C and C=O stretching increasing from 300 to 400 °C but gradually decreasing from 500 to 700 °C (Fang et al. 2015). The symmetric C–O stretching (1035 and 1120 cm− 1) is decreased from 300 to 400 °C. The C–H bending for aromatic out-of-plane deformation (873 cm− 1) becomes apparent for 500 and 700 °C (Ouyang et al. 2017).
2.1.3 Other nanoparticles coated biochar
The above two modification approaches, which acquire materials containing metal, would still lead to secondary pollution, giving rise to metal exudating (Zhao et al. 2021). Introducing functional nano-heteroatoms into biochar is a chemical activation method that can stimulate a synergistic effect a synergistic effect between heteroatoms and dopants, contributing to the exceptional enhancement of materials’ properties. More importantly, the doping and coating of nano-heteroatoms can reduce the leaching of metal ions and avoid secondary contamination. Both theoretical calculations and experimental investigations currently incorporate heteroatoms such as S into the carbon matrix, which expresses unique superior chemical stability (Ding et al. 2020; Zhou et al. 2018). On the contrary, a single bond is generated to combine biochar with metals or oxides (e.g., Cu-O, Fe-O bond). Heteroatoms doped biochar composites would fulfill electron-donor properties and enhanced π bonding in adjacent framework, resulting in the tailored electrocatalytic property (Ren et al. 2020). Thus, heteroatoms, involving N, S, or P, are regarded as an effective method for improving the stability and removal ability of contaminants for biochar (Liu et al. 2022). The pyrolysis, impregnation, ball milling, air pre-oxidation, and hydrothermal method can generally dope heteroatoms into biochar. The overall process would be broadly generalized in three phases: feedstocks are pyrolyzed to gain the biochar; dopants and biochar are aged, and the precursor based on biochar composites is carbonized under a nitrogen atmosphere. The feedstock is a factor impacting the composition of NPs@BC. The P-BC pyrolyzed by cornstalk and rice husk has a relatively higher silicon content than other P-BC that are pyrolyzed by wood and bamboo after modification (Zhang et al. 2020b). Heteroatoms-doped biochar generally exhibits a relatively larger specific surface area due to the released gases during the pyrolysis of precursor. After ammonium phosphate addition and air pre-oxidation treatment, during pyrolysis the release of CO from biomass results in the formation of micron-sized pores in P-biochar and large specific surface area (763 m2 g− 1) (Jin et al. 2020). However, BC prepared by sugarcane bagasse presents lower surface area (388 m2 g− 1) (Zhang et al. 2017). In a similar manner, Tang et al. reported that the N-P -BC is manufactured by pyrolysis from corn stalk and showed that N-P-BC is an effective adsorbent for the removal of sulfonamide because surface area and pore volume are 2.5–13.2 times higher than pristine biochar (Tang et al. 2021). Moreover, the surface area displays a positive relationship with the pyrolysis temperature, since the richer porous structure is generated at high pyrolysis temperatures (Dong et al. 2020). For example, the SBET is 43.9 m2 g− 1, 81.6 m2 g− 1, respectively, at 350 and 650 °C for N-P-BC (Tang et al. 2021). The different heteroatoms on BC contribute to different special functional groups of NPs@BC. The N-containing functional groups are formed by coupling N with oxygen and carbon atoms. The N1s spectrum is always assigned to pyridinic-N, pyrrolic-N, and quaternary-N at 398.9 eV, 399.9 eV, 400.8 eV, and 401.5 eV in high-resolution spectra of XPS (Liu et al. 2022). The S atom can form an -C-S-C- bond with C atoms in biochar. The P atoms doped into biochar primarily combine with C and O atoms generating C–P, P=O stretching at 1060 cm− 1, 1110 cm− 1, respectively (Zhang et al. 2022d; Zhou et al. 2022).
2.2 Characterization
Characterizations of NPs@BC are to investigate their structure and adhesion of nanoparticles on biochar, which are very helpful for their potential applications in industry. Consequently, structural analysis and functional group determination are necessary. Various methods have been developed to characterize the morphological structure, physicochemical property, stability, morphology, and element composition of NPs@BC components. In this section, we summarize the primary characterization techniques, including elemental analysis, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), the energy dispersive energy X-ray (EDX) spectroscopy, Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy, thermogravimetric analysis (TGA), magnetic hysteresis loops analysis by vibrating sample magnetometer (VSM), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Brunauer–Emmett–Teller (BET), energy dispersive X-ray spectroscopy (EDS), X-ray absorption near-edge structure (XANES), extended X-ray absorption fine structure (EXAFS), and confocal micro-X-ray fluorescence (µ-XRF) (see Fig. 1).
SEM is used to observe the microstructure and morphology of NPs@BC and the dispersion of nanoparticles. In addition, more detailed nano-level visual information can be provided by TEM, for example, thickness and size of the core–shell structure, as well as morphology. SEM was used by Mao et al. to confirm the MgO@BC composites’ surface morphology and the SEM images exhibit that MgO nanoparticles are effectively incorporated into the matrix and surface of the biochar. The images also clearly show the formation of flake-like structures of MgO nanoparticles in the surfaces and pores of the biochar (Ibrahim et al. 2022). This indicates that the pure metal nanoparticles, which are produced by metal compounds under the reducing atmosphere, are deposited on the surface of biochar and form the metal decorated biochar composites that present plentiful pore structure types, including abundant micropores and a few mesopores.
XRD, FT-IR, and Raman spectroscopy analysis are employed to explore the crystalline nanostructure, mineral contents, functional moieties, and phase composition of NPs@BC. For instance, XRD peaks of biochar at 27.44°, 28.62°, 36.77°, 40.06° and 45.53° are assigned to graphite phase, which indicates that corn stalks are successfully carbonized (Ibrahim et al. 2022; Li et al. 2020c). The XRD patterns of CuO@BC match the characteristic peaks of biochar and CuO without any detectable impurity peak, which implies the successful synthesis of BC-CuO composites. Furthermore, Li et al. utilized FT-IR to confirm that the surface of biochar possesses abundant functional groups and the existence of functional groups, including C–H, C–O, C=C, C=O and O–H, is found at 875, 1105, 1430, 1620, and 3419 cm− 1, respectively, and noted that the band at 1027 cm− 1 is assigned to C–O–Cu in the FTIR spectra of CuO@BC components (Li et al. 2020c).
Exploring the thermal stability of NPs@BC is crucial for realizing the resistance of NPs@BC composites to aging in nature (Liang et al. 2022b). The characterization about thermal stability contains three types such as TGA, DTA and DSC curves. Currently, the TGA curve is used to determine the variation trend of physicochemical properties of NPs@BC, which mainly describes the relationship of weight loss to elevated temperature. The surface area of NPs@BC is calculated via the BET method by means of calculating the quantity of N2 adsorbed on the NPs@BC surface at low temperatures (77 K) (Igalavithana et al. 2017). Besides, CO2 adsorption is a quite appropriate way and supplies a more accurate surface area than other techniques at high temperatures (273 K) (Kasozi et al. 2010). In general, large surface area is beneficial for the adsorption of heavy metals on NPs@BC. Furthermore, pore size and pore volume also play different roles, such as increasing or decreasing the adsorption capacity of NPs@BC for heavy-metal. The three metrics (see Additional file 1: Table S1) are the most related to the natures of NPs@BC about the remediation functions. The surface area and pore volume of NPs@BC composites are 1.03–3.61 and 1.13–2.30 times larger than those of pristine biochar (Ding et al. 2020; Fu et al. 2017; Rong et al. 2019; Xin et al. 2021). These results indicate that NPs dispersed into biochar increased the surface area by reducing the shrink of biochar matrix in the carbonization treatment. However, there is no definite relationship between NPs@BC and BC with respect to pore size. VSM technique is employed to analyze the saturated magnetization of NPs@BC. For example, the saturation magnetization is measured to be 6.71 emu g− 1 for magnetic functionalized biochar, manifesting the magnetic NPs@BC could be easily attracted by a magnet (Zhao and Lang 2018).
XANES, also referred to as NEXAFS (near-edge X-ray adsorption fine structure), is a photonic-based X-ray adsorption spectroscopy that provides a unique tool for studying chemical valence and electronic structure of NPs@BC which can indicate the presence of species that appear after modification. XPS is also performed to determine the valence states of elements and illustrate the elemental composition. What is more, the adjacent atomic structure can be drawn from EXAFs. µ-XRF, which is another novel approach, has been adopted to determine the elemental spatial distribution and chemical states in NPs@BC. For instance, Xu et al. prepared the Fe@BC composites, and µ-XRF was used to analyze the properties of the components. Fe nanoparticles are widely dispersed on the surface and inside of the biochar. Ca, which is a familiar inorganic element in biochar, is heterogeneously distributed, probably because of replacement by Fe. Furthermore, Fe is impregnated into the carbon structure and more enriched in the interior of the biochar as the pyrolysis temperature ranges from 300 to 900 °C (Xu et al. 2020c). XANES was used by Liu et al. proving that the S is embedded into the carbon structure of the biochar that possesses aromatic ring structures and these aromatic ring structures have delocalized electrons (π electrons). In the meanwhile, the thiadiazole in 2,5-dimercapto-1,3,4-thiadiazole (DMC) also has delocalized electrons. Thus, DMC is possibly bound to biochar by means of π–π interactions between the ring structures (Liu et al. 2018).
EDS, EDX, and XPS are adopted to determine the chemical composition and contents of elements of NPs@BC. According to the Fe 2p XPS spectrum of nZVI@BC, there were three different peaks, assigning to the Fe–O bond at 529.4 eV, C–O single bond in hydroxyl groups at 530.8 eV, and C=O at 532.1 eV, respectively (Zhang et al. 2022a). Therefore, nZVI is successfully coated onto the biochar surface, containing enriched oxygen functional groups with partial oxidation. The high-resolution N1s XPS spectrum analysis indicates that when N is doped in the pristine biochar, there are three kinds of nitrogen, graphitic nitrogen, pyrrolic nitrogen, and pyridinic nitrogen. They are higher electronegativity nitrogen moieties and can polarize the carbon charge, prompting the electron mobility and interaction of the NPs@BC with persulfate and pollutant (Zhao et al. 2021). Besides bonding to a 6-member C ring, the pyridinic N atom can also contribute its π-electron to the coupled C network, increasing the electron-donating properties (Cheng et al. 2017). Meanwhile, the pyrrolic nitrogen atom is bonded to a 5-member carbon ring. Both pyrrolic nitrogen and pyridinic nitrogen are equipped with unpaired electrons which are not delocalized into the π system and can accelerate the electron mediation mechanism (Chen et al. 2018). Moreover, severe electron deficiency around adjacent C is the result from graphitic nitrogen, which can promote persulfate adsorption and activation (Gao et al. 2020). For the phosphorus atom, there is an identical quantity of valence electrons comparing with N, and therefore phosphorus shares analogous features. However, the electronegativity is lower, and the covalent radius is larger than that of heteroatoms (N, S). Consequently, doping P could produce more defective sites around the biochar structure and simultaneously entice charge delocalization around the carbon lattice (Chen et al. 2017). In addition, the negative charge of adjacent carbon atoms is induced, creating an electron cloud and preventing the carbon lattice from a vigorous oxidation environment (Zhang et al. 2021a). The electronegativity of the sulfur atoms is lower, which can powerfully perturb the electron distribution in the biochar structure, and couple with the inherent graphitic nitrogen and pyridinic nitrogen to create more Lewis acid and basic sites (Zhao et al. 2021). In short, the direct electron transfer between nanometals and biochar takes place in the case of nanoparticles adhered on the surface of biochar rather than simply connected, and they are uniformly distributed over the surface or into the framework of the biochar existing in most nanoparticles and a few oxidation states.
3 Safety assessment of NPs@BC for design and selection
NPs@BC materials are regularly claimed to be more environmentally sustainable than traditional materials in view of their remarkable properties. And then, the conventional materials and chemicals are replaced with the NPs that have emerged with potentially high performance. Nevertheless, NPs@BC are entering air, water, and soil, where they could adversely affect organisms in ecosystems, and their manufacture could offset the intended benefits (Gilbertson et al. 2015). Moreover, actual environmental impacts of NPs@BC have not been documented, and there are uncertainties about the potential, and how to evaluate the impacts. Thus, after preparing NPs@BC but before applying a mass of NPs@BC in the soil remediation, it is essential to establish an integrated framework which can pre-screen nanoparticles and biochar from hazard properties, environmental effects, and economic considerations for adequate risk characterization and assessment. In this segment, the material selection and design framework for sustainable NPs@BC composites are developed and explored.
3.1 Environmental effects
The carbon footprint, referred to the direct and indirect CO2-eq emissions from an activity, is a widely used metric of climate change impacts (Xia et al. 2022). Nevertheless, carbon footprint cannot fully evaluate the environmental burden of products (Laurent et al. 2012). Therefore, given a comprehensive evaluation of the environmental performances of NPs@BC composites, the extended environmental effect metrics (e.g., cumulative energy demand, and greenhouse gas emission) are applied to understand the functional performance and environmental impacts of NPs@BC composites. Here, perspectives containing nanoparticles and biochar are served for clarifying the selection and design principle of NPs@BC. Cumulative energy demand (CED), an index for the embodied primary energy in a product, is calculated as the sum of primary energy that must be spent for the production process, use stage and disposal (Ntinas et al. 2017). Herein, CED has been used to assess life cycle environmental impacts of NPs and biochar materials. The CED of NPs and biochar in the Fig. 2a expresses the direct and indirect energy throughout the life cycle, including the fossil CED and the CED of nuclear, water, biomass, solar energy, and wind in the life cycle using in units of MJ kg− 1 (data in Additional file 1: Table S2) (Rahman et al. 2022). The energy consumption is based on three consumption stages, including the extraction, manufacturing, and application of the raw materials (Huijbregts et al. 2010). Selection of NPs@BC composites generally involves both the composition and geometry of the nanocomponents. The geometry of NPs can be a variety of shapes (sphere, rod, and ring, etc.) and is determined by the feedstock, and NPs@BC components are built up on the atomic or molecular level. These more novel techniques can include two steps, pyrolyzing biomass and decorating nanoparticles. All kinds of process options can each have influence on the overall environment; for example, preparation of NPs@BC involves chemical coprecipitation, which can generate extra waste streams that need to be treated prior to releasing. This increases the amount of required electricity consumption. In general, NPs@BC are typically used in environmental remediation, and transferring also requires energy. Preparation of NPs@BC material contributes the most to energy consumption. The results also show that Ni NPs tend to require 150 MJ kg− 1, and the nZVI is below the 1001.52 MJ kg− 1. However, the CED of metal oxide (TiO2) is higher than Fe3O4, which is ascribed to the synthesis methods of TiO2 named flame synthesis technique (Osterwalder et al. 2006). Flame spray synthesis involves the combustion of an organo-metallic precursor that originates from the reaction of titanium chloride (TiCl4) alone with an organic acid. The sulfate process requires 32–40 MJ kg− 1 and the chloride process only needs 19 MJ kg− 1. While Fe3O4 is manufactured by thermal decomposition, and the CED of the synthesis methods abides by the trend (descending order): biological, thermal decomposition, flame spray pyrolysis, sol-gel, co-precipitation, hydrothermal reaction, and electrochemistry. For all synthesis methods, the biological reaction, flame spray pyrolysis, and sol-gel methods, there are vast contributions to the CED from processing energy requirements (Rahman et al. 2022).
Proposed mechanisms of heavy metal and organic pollutant removal by NPs@BC. The heavy metal and organic pollutant removal by NPs@BC from soil environmental matrices considers the elemental composition, surface chemistry, and molecular structure. PAHs, polycyclic aromatic hydrocarbons; PAEs, phthalate esters; CPs, chlorophenols; ROS, reactive oxygen species
Greenhouse gas emission (GHG) is also selected to evaluate the impact of NPs@BC components on climate change. Biochar is carbon-rich charcoal as a consequence of pyrolysis of biomass materials. The majority of C in the biochar is in a highly stable state; however, the stability of the biochar does change with feedstock, processing, and environmental conditions. Thus, we assume that there is a conservative estimate about 80% of the carbon in the biochar is stable, and the remaining 20% of the C is labile, which is easy to release into the atmosphere as biogenic CO2 at first few years of applying NPs@BC to the soil (Roberts et al. 2010). From the climate change impacts, negative GHG implies more CO2 equivalent (CO2 eq) reductions than emissions. The GHG for biomass ranges from − 1193 to 262 t CO2 eq t− 1 dry biomass in Fig. 3b. The reason for the negative is that biomass pyrolyzes and returns to the soil for mitigating climate change (Roberts et al. 2010). In addition, land-use change plays a critical role in the life-cycle climate change impacts of NPs@BC components. For the switchgrass A scenario, in which the cropland is diverse from annual crops to perennial grass energy crops, the GHG is − 442 kg CO2 eq t− 1 dry switchgrass, while for the B scenario, which is land converse to cropland, it is 36 kg CO2 eq t− 1 dry switchgrass (Roberts et al. 2010).
Perspectives of the methodological framework for NPs@BC evaluation. a–d Environmental impact (cumulative energy demand as CED and greenhouse gas emission as GHG; a and b), environmental health impact (earthworm toxicity as LC50; c, price (d). Vertical dashed lines indicate single values taken or derived from literature. LC50, sublethal concentration
3.2 Hazards
Soil fauna is the consumers of bacteria, fungi, and other microorganisms. After NPs@BC release and distribution within the soil, organisms will be exposed, and unpredictable impacts may occur. Meanwhile, earthworms are regarded as ‘ecosystem engineers’ that play an important role in the biogeochemical cycling of elements in the soil environment and earthworms are the first soil organisms to have a standardized soil toxicity test developed (Sizmur and Richardson 2020). Therefore, the earthworm is selected as a dominant object for assessing the biological effect of NPs@BC on soil organisms. However, there is seldom literature referring to the influence of NPs@BC on soil fauna (Pathy et al. 2020). Thus, this segment evaluates the ecotoxicological effects of biochar and nanoparticles on earthworms, respectively. The influence of biochar/nanoparticles on fauna is normally manifested in four aspects: survival, growth, reproduction, and behavior. Sub-lethal effects of NPs/biochar consist of a decline in reproduction, the number of surviving offspring, and avoidance of polluted soil (Tatsi et al. 2018). It will be used to assess the sustainability of NPs@BC in the following section. The survival and growth of earthworms in biochar/nanoparticles amended soil are suppressed to a certain extent. For example, the mortality of earthworm in the Ni nanoparticle stress is influenced by Ni dosage and exposure time. The mortality of earthworms is 10% exposed to 800 mg Ni kg− 1 and then is 100% within 7 days in the 1500 mg Ni kg− 1 group, indicating a significant acute toxicity effect (Wang et al. 2020).
However, this study showed that the exposure to TiO2 (0, 5, 50, and 500 mg kg− 1) did not affect the growth, reproduction of earthworms in the long term (120 days) (Zhu et al. 2020). In addition, the weight loss of earthworms is observed when exposed to ZnO NPs alone in the early stage (14 days) (Zhang et al. 2022b). Simultaneously, there are marked reductions in fecundity and fertility of the earthworm, comparing with the control group, with exposure equal to or exceeding 250 mg Zn kg− 1 and 125 mg Zn kg− 1, respectively (García-Gómez et al. 2019). Avoidance behavior has been considered as a more sensitive endpoint than mortality, growth, etc. and has been extensively studied. Li et al. (2011) found the significant avoidance of earthworms (E. fetida) within 10% and 20% biochar. There is a synergistic effect existing between biochar and NPs. µ-XRF, and µ-XANES analysis are applied to evaluate Ce localization, speciation and persistence in CeO2/biochar-mixed exposed earthworms. Ce4+ and Ce3+ remain predominant, but the negatively charged functional groups on biochar interact with CeO2 that is pH-dependent positively charged, influencing hetero-aggregation, which is ultimately limiting CeO2 bioavailability (Servin et al. 2018). The former investigation is based on the direct action between NPs/biochar and soil fauna. Consequently, the following research needs to assess the impacts of NPs@BC in an environment where pollutants are present.
In addition, earthworms have large epidermis surfaces and can live in different types of soil (Lanno et al. 2004, Silvani et al. 2019). Pollutants and NPs that could dissolve in the pore water of soil are primarily diffused into the earthworm’s body via direct contact with the earthworm outer epidermis (Silvani et al. 2019). Thus, the sublethal concentration (LC50) of NPs and biochar in this work is applied to evaluate the impact of NPs@BC on soil fauna such as earthworm (e.g., growth, reproduction, enzyme activity, and survival rate), which indicates the toxicity of NPs@BC.
The toxicity of Ni, nZVI, Fe3O4, TiO2, and biochar is shown in Fig. 3c. TiO2 nanoparticles have little toxicity, and no prominent effect has been observed on survival, with impacts on Eisenia fetida metabolism, reproduction, and DNA becoming evident at concentrations of sub-lethal higher than 103 mg kg− 1 (Hu et al. 2010). Nonetheless, earthworms could avoid artificial soil supplemented with 1–5 g kg− 1 TiO2 NPs and distinguish particles from the nanometer to micrometer ranges (Schlich et al. 2012). The observed metabolomic response of earthworms is that alanine and other amino acids increased, and maltose decreased exposed to TiO2, which is not correlated with the sizes of TiO2 NPs (Whitfield Aslund et al. 2012). In regard to Fe3O4 nanoparticles, 67–200 mg kg− 1 NPs have an effect on the reproduction and survival of Eisenia fetida (Gomes et al. 2015). Earthworm acute toxicity test for biochar is conducted and earthworm mortality increases with the elevated application rate, manifesting significant dose-effect relationships. However, the biochar is rarely toxic when LC50 is higher than 3.8 × 104 mg kg− 1, and a portion of biochar (CC, SS biochar) has no influence on the mortality of Eisenia fetida at the tested doses (Shi et al. 2021). The biochar had an adverse impact on antioxidant enzyme activities and the lysosomal membrane stability of earthworms. The function of the organism’s antioxidant enzyme system is an important component of the innate defense system; thus, the earthworm antioxidant defensive index is another vital biomarker to explore the toxicity mechanisms of chemicals in soil ecotoxicology, which can provide early-warning signals for soil pollution (Shi et al. 2017). The antioxidant enzyme system of earthworm was damaged by biochar, and SOD could not catalyze the conversion of O2− to H2O2. CAT is difficult to convert H2O2 to water and oxygen, and POD is hard to eliminate excess H2O2. Thus, lipid peroxidation of the membrane system is induced, which contributes to disturbed lysosomal membrane stability (Markad et al. 2012). The coelomocyte lysosome of earthworms is a sensitive target organelle, of which membrane stability has served as a potential biomarker for the toxic impact of environmental pollutants (Shi et al. 2021). With respect to metal NPs, the toxicity of Ni is more severe than that of nZVI, and LC50 is 757–1202.44 mg kg− 1 and 200–500 mg kg− 1, respectively (El-Temsah and Joner 2012, Wang et al. 2020). Since metal nanoparticles could not provide the labile organic matter for earthworm growth, they also have adverse impacts on earthworms, for example, avoidance behaviors lead to the additional energy needs. More protein, glycogens, and lipids are metabolized, resulting in earthworm weight loss (Wang et al. 2021a).
3.3 Economic considerations
In this paper, to develop economically feasible and sustainable NPs@BC, an integrated framework is used to estimate the economic feasibility of NPs@BC, and Fig. 3d reflects the price of NPs components. The feedstock collection and pyrolysis of biochar are the primary cost; however, the feedstock and biochar transport and biochar application have negligible contributions to the whole expense (Meyer et al. 2011, Nematian et al. 2021). As we all know, biochar is a high-value-added product, and the value is not be neglected, which is divided into two components: (i) the P and K content of the biochar, which could improve fertilizer efficiency, and (ii) the GHG offsets, which are in the value assigned per ton of CO2eq emission reduction so that the expense of biochar is the cheapest. National development gap scenarios are considered, where values of $20 and $80 t− 1 CO2eq are used, according to the IPCC recommendations (Azzi et al. 2019). On the contrary, the cost of remaining NPs (i.e., nZVI, Ni, TiO2, and Fe3O4) is mainly put in the process of preparation, as a result of high energy-consuming enterprises to manufacture the metals; hence the price of them is higher than biochar. The price of metals and oxides is almost equal except for TiO2, which is 280–2908 times cheaper than other metal/oxide NPs. Here, which is more economical under the condition of the similar removal efficiency is the focus. For example, the immobilization is approximately 60% and the dosage of Fe3O4/nZVI is 10 g kg− 1, whereas the average price of Fe3O4 is 1.5 times higher than that of nZVI (Qiao et al. 2018; Wang et al. 2021a). Overall, the developed strategy can be integrated with conventional nanomaterials to develop sustainable and economic NPs@BC for various scenarios. The ranges reported here are the maximum and minimum values for each material. Therefore, we can find that TiO2@BC represents comprehensive competence, such as the lower CED, GHG, price, and non-poisonous. Whereas, it is interesting that nZVI/Fe3O4@BC is studied by lots of researchers, which may ascribe to the inferior removal efficiency of TiO2.
4 The remediation of NPs@BC for soil contamination
Relevant studies have explained the brilliant structure of NPs@BC with the aid of various characterizations, indicating that NPs@BC is considered a promising soil amendment in the removal of heavy metals and organic pollutants. The eradication ability of NPs@BC is vital to its soil application; therefore, we would elaborate on the NPs@BC composites’ environmental remediation and unravel the intrinsic mechanism to explain how heavy metals/organics are removed by NPs@BC and to improve engineering applications. The acquired details could shed new light on how NPs@BC can be used, regenerated, and even disposed.
4.1 Heavy metals
Pollution of heavy metals in the soil is a global environmental problem that threatens food safety and human health and has attracted considerable attention recently (Zhou et al. 2020). The atomic density of typical toxic heavy metal elements (Cd, Cr, Pb) and metalloids (i.e., As) is greater than 4.5 g cm− 3 (Tian et al. 2015). Toxic heavy metals are harmful to human health in terms of acute and chronic toxicity, such as endocrine disruption, reproduction impairment, neurotoxicity, and immunosuppression (Zheng et al. 2021). Thus, removing heavy metals from the soil, including stabilization, microbial remediation, and electrochemical processes, has become a hot topic in agriculture and environmental science (Li et al. 2020a; Wu et al. 2021; Zhang et al. 2021b). Herein, NPs@BC has been identified as a nontoxic soil amendment for stabilizing heavy metals now that they possess excellent performance at reducing the bioavailability, mobility, and toxicity of heavy metals in soil (Peng et al. 2022). Consequently, we evaluate the performance of NPs@BC for eradicating heavy metals in soil and then parameters are debated; in the end, the removal mechanism is discussed in this review.
Extensive research literatures have presented that NPs@BC can efficiently remove heavy metals from soil, and they have also evaluated the removal efficiency of NPs@BC for heavy metals in soil. The remediation of heavy metals in soil by NPs@BC is summarized in Table 1. NPs@BC has showed positive results for heavy metal immobilization. For example, using 8 g nZVI@BC per kg soil for 15 days, the immobilization efficiency of Cr(VI) and Crtotal is 100% and 91.94%, respectively (Su et al. 2016b). The soil fertility could be effectively improved by nZVI@BC, and the leachability of Fe is reduced. Bi@BC is widely applied in As remediation, and the immobilization rate of amorphous hydrous oxides of Fe- and Al-associated arsenic is 95%, which decreases over time (Zhu et al. 2019a). However, there is inconsistent finding in the literature about the effects of Fe@BC on the As dispatched. The As immobilization is only 17.98–35.18%, and there is no effect on the soil pH, which will not damage the soil properties (Wu et al. 2018a). In addition, biochar supported nanometals also present remarkable performance in various heavy metal mixed pollution such as As, Cd, and Pb. Immobilization rates of As, Cd, and Pb are 98.45%, 59.15%, and 82.14%, and leaching concentrations are 19.59, 1.15, and 1.52 µg L− 1, respectively (Yang et al. 2021). The encapsulation mechanisms of nZVI-BC involve complexation, precipitation, oxidation, reduction, and the formation of surface complexes. Furthermore, the NPs@BC composite materials produced by BC and metal-oxides (such as MgO and Fe3O4) can significantly improve the active sites, surface functional groups, and catalytic degradation capabilities. Thus, Fe3O4-modified BC can simultaneously accelerate the As, Cd, Pb removal from the farm and industrial lands, and the concentrations of As, Cd and Pb decrease by 28–32% (Wan et al. 2020). Pyrolysis at the end of magnetization stabilized Fe oxides on the biochar surface, resulting in a higher biochar recovery rate of approximately 65%, and a higher metal removal efficiency. MgO@BC also exhibits superior immobilization for Pb, Cd and Cu- contaminated soils, and the contents of available Pb, Cd, and Cu are reduced by 53.7%, 74.2%, and 84.2%, respectively, compared with the addition of biochar (Qi et al. 2022). To tempt better adsorption characteristics for the removal of heavy metals, polymers are one of the used substrates with BC due to their three-dimensional cross-linked structures and rich ionic functional groups. Consequently, heteroatom-modified biochar could promote removal efficiency for heavy metals. For instance, the immobilization rate of Cr in the soil is 99.99% for N-biochar (Rafique et al. 2021). Meri et al. (2018) revealed that the surface of functional material, with additional functional groups (–NH2), can have strong electrostatic interaction with Cr(VI) and expose a higher Cr(VI) removal efficiency.
The stability and the removal efficiency of heavy metals utilizing NPs@BC are influenced by multiple environmental conditions, such as soil minerals, biochar feedstock, pyrolysis temperature, mass ratios of nanoparticles and biochar, dosage, exogenous additive, and so on. Literatures convincingly have demonstrated the predominant function of soil physicochemical properties and feedstock sources of biochar in the mobility of Cd2+. There is approximately 93.9% of Cd2+ for wood chip biochar (Fe3O4@WCBC), which retains in the red soil column, while this value is 88.6% for Fe3O4 modified wheat straw biochar (Fe3O4@WSBC), decreased by about 5.64%. Furthermore, the values of maximum solid-phase retention capacity are 33.6 mg g− 1 and 26.1 mg g− 1 for Fe3O4@WCBC and Fe3O4@WSBC in paddy soil, respectively, which are two point five times higher than those of Fe3O4@WC (16.3 mg g− 1) and Fe3O4@WS (11.6 mg g− 1) in red soil (Chen et al. 2019). Paddy and red soils are dominated by iron oxides (bernalite, hematite and goethite), quartz, and clay minerals (kaolinite, montmorillonite, and albite). The reason for this difference is that the red soil has relatively lower contents of Fe, Al, and Mn than the paddy soil, which suggests that there is an availability of little surface deposition sites for Fe3O4@BC retention in red soil. The removal efficiency does increase as the dosage increases to a certain extent, and there is an optimal usage. The ferromanganese oxide impregnated biochar (FMBC) composites dosage increases range from 0.5% to 2%; in the meantime, the immobilization efficiency of As increases observably from 11% to 18.9%. 2% FMBC treatment displays the optimal promoting effect in controlling As(V) and As(III) concentrations in the polluted soils (Lin et al. 2017). There is an alike result about Cr(VI) immobilization mainly associated with amendment dosage. The immobilization efficiency of Cr(VI) is only 28.82%, while the dosage is 2 g kg− 1. On the contrary, the removal efficiency of Cr(VI) ranges from 79.54 to 100% as the dosage increases from 5 to 8 g kg− 1 (Su et al. 2016b). This situation may be a result of extended surface areas and reactive sites. Besides, the synergistic reaction occurs between nZVI and BC, and the reaction activity of nZVI is improved by BC. Additionally, the pyrolysis temperature is also a crucial parameter for the soil contaminant removal by NPs@BC. Pyrolysis conditions (such as temperature and gas flow) can affect the type of functional group and surface area of biochar composites. For instance, there is higher Cr immobilization at higher pyrolysis temperatures (700 ℃) (Rafique et al. 2021). The alcoholic (–OH), aliphatic and ester (–CO–) groups on the surface of biochar are removed as the pyrolysis temperature increases (Shakya et al. 2022). Besides, the decomposition of hemicellulose occurs at ˃ 350 °C contributing to fast release of volatile matters, which can shape pores (Wafiq et al. 2016). Thus, the immobilization capacity increases with an increase in temperature due to the improvement in the surface area. The exogenous additives also influence the removal efficiency of contaminants in soil. Naturally occurring humic acid (HA) and citric acid (CA) are common additives that may restrain the efficiency of nZVI by competing for reaction sites. The presence of HA abridges the stabilization efficiency of nZVI by occupying the adsorption sites of nZVI and forming soluble complexes with metals by means of complexation and electrostatic affinity (Xu et al. 2020b). The stabilization efficiency of As, Cd, and Pb significantly decreases by roughly 18%, 15%, and 25%, respectively, when the addition of CA exceeds 0.50% (Yang et al. 2021). For polymetallic nanoparticle loading BC, the immobilization of heavy metals also depends on the ratio of nanoparticles. The Fe–Mn–Ce oxide (FMCBC), in which the weight ratio of BC, Fe, Mn, and Ce is 24:2:3:4 (FMCBC1, w/w/w), 24:2:3:8 (FMCBC2, w/w/w), and 24:2:3:10 (FMCBC3, w/w/w), respectively, is applied to amend highly contaminated soil. Moreover, the application of FMCBC3 decreases As (specifically bound, a form of As in soil) concentration to the greatest extent among all treatments (Zhang et al. 2020a). The removal of As is predominantly manipulated by the surface of non-crystalline iron oxides in the soil, and the immobilization capacity of As is boosted due to increased Fe and other metal oxides (e.g., free Fe, Mn, and Ce oxides) in the soil (Smith et al. 2008).
4.2 Organic pollutants
NPs@BC acts as the adsorbent, reductant, and catalyst for the removal of organic pollutants (e.g., persistent organic pollutants, carcinogenic organic compounds, pesticides, and antibiotics) in environmental and agricultural applications due to their highly adsorptive, catalytic, and reductive ability. Table 1 summarizes the current knowledge regarding the removal and degradation ability of organic pollutants with different kinds of NPs@BC and pH or dose-dependent removal trends of all organic pollutants on NPs@BC. Recent studies have revealed that carcinogenic persistent organic pollutants (POPs), for example, polycyclic aromatic hydrocarbons (PAHs) and polybrominated diphenyl ethers (PBDEs), enter the food chain, and they will bring about a potential risk for human health and the environmentbecause of their polymer structure, carcinogenicity, low solubility, high molecular weight and persistence (Lian et al. 2022; Tian et al. 2021).
Till now, the modified biochar utilizing nano-metals, bimetals and metallic oxide has been employed for removing PBDEs efficiently. For instance, nZVI@BC is prepared by liquid reduction method, and decabromodiphenyl ether could be degraded within 4 h on the condition that the pH value is 3 and the molar ratio of persulfate to nZVI@BC is 3:1 (Li et al. 2019). The raw products, such as sawdust, urea, and FeSO4·7H2O, often act as a soil amendment (Fe/N co-doped biochar, Fe–N–BC) to disintegrate PAHs in crude oil-spiked soil. This soil amendment exhibits a substantial improvement in PAHs degradation rate: Fe/N@BC, and alkylbenzene Fe–N@BC are 13.48%, and 48.76%, respectively. Moreover, functionalized BC could increase the adsorption capacity of pristine BC towards total petroleum hydrocarbons (TPHs) due to the hydrophobic interactions (Gao et al. 2013; Li et al. 2022a). There are crucial factors (e.g., additives and dosage) to consider when NPs@BC materials disassemble POPs. H2O2 and Fe-N@BC mediated Fenton system mainly through enhancing the oxidation efficiency of C10–C23 (50-72%) and 2–4 rings PAHs (43–63%) (Li et al. 2022a). The NPs@BC composites of the material have a decisive influence on its performance. For example, the effect of the ratio of sodium dodecylbenzene sulfonate (SDBS) on its adsorption capacity and catalytic activity for TPHs was explored, and the results indicated that the high loading content of SDBS could contribute to more adsorption sites and upgrade the adsorption of TPHs (Zhang et al. 2019). Analogously, there is the impact of the molar ratio of persulfate to nZVI@BC on the degradation rate. The degradation rate is the fastest as the content of nZVI increases because of producing Fe2+ rapidly in a short time. When the molar ratio of persulfate/nZVI@BC is 1:2, it can activate persulfate to generate the •SO4− faster (Li et al. 2019). Furthermore, pH dramatically affects the response of NPs@BC to decomposed pollutants. The highest TPHs oxidation efficiency is 91.42 and 96.88%, respectively for different contents of soil organic matter (10.38% and 4.14%) at pH 7. Since the precipitation and agglomeration of soil particles can be generated in the acidic pH, the TPHs adsorption by Fe/N@BC would be impeded (Li et al. 2022a).
Chlorophenols (CPs) are widely used to manufacture fungicides, insecticides, and herbicides, and the United States Environmental Protection Agency has listed CPs as priority pollutants (Luo et al. 2022a). Removal of CPs from soil taking advantage of NPs@BC composite material is a promising approach; for example, CuFe2O4@BC can form by modified sol-gel method for o-nitro chlorobenzene degradation in soil. The CuFe2O4@BC catalyst possesses superior stability and activity under persulfate activation, which contributes to the highly efficient degradation of o-nitro chlorobenzene with 78.30% (Zhao et al. 2020). The study investigated the feasibility of the nZVI@BC, which was used for the degradation of 2,4-dichlorophenol in contaminated soil. The research findings revealed that persulfate could markedly improve the degradation of 2,4-dichlorophenol by nZVI@BC process and approximately 91% of 2,4-dichlorophenol degradation can realize within 4 h (Diao et al. 2020b). The eradication efficiency of CPs is related to pH value, additive, pollutant concentration, catalyst dosage, and dissolved oxygen. The degradation efficiencies of CPs decrease along as initial pH values increase because decreasing pH may inhibit the activation process and prohibit reductive dichlorination (Zhao et al. 2020). EDTA is a proverbial chelating agent that equips with six potential sites used for combining with metal cations, for instance, Fe2+ and Fe3+. The degradation efficiencies of 2,4-dichlorophenol with different concentrations of EDTA are compared, and the degradation increases by around 5% with 1.5 mM EDTA compared with no EDTA. Nevertheless, further increasing concentration of EDTA evidently suppresses the 2,4-dichlorophenol disintegration; thus, only 62.9% of 2,4-dichlorophenol is degraded under 2.0 mM EDTA (Diao et al. 2020b). The superfluous EDTA will compete with 2,4-dichlorophenol to consume the radical species. As we all know, humic acid (HA), as natural organic compound, usually exists in soil environments and is also a common additive. Various degrees of negative effects on the 2,4-dichlorophenol degradation with diverse concentrations of HA are discovered; to be furthermore, the suppressive effect decreases as the HA concentration decreases (Diao et al. 2020b). While the dosage of the catalyst increases, the degradation efficiency increases. It is rational that a mass of catalyst will increase the number of active sites, which contributes to much more efficient radical generation for the degradation process.
In the past years, considerable studies and efforts have been dedicated to researching the application of NPs@BC for the removal of organic pollutants, including MPs and atrazine. A novel nZVI@BC serves as a heterogeneous catalyst activating peroxymonosulfate (PMS) for the removal of atrazine. The synergistic effect has been successfully achieved during nZVI@BC/PMS process, and approximately 96% of atrazine disintegration is gained at the dosage of 2.0 g L− 1. This superior degradation efficiency mainly attributes to the synergistic effect of BC/PMS and nZVI/PMS processes. Dissipation of atrazine can be affected by dosage. When the dosage of nZVI@BC is less than 2.0 g L− 1, the positive correlation between nZVI@BC dosage and atrazine elimination is observed owing to the increased available reactive sites. Moreover, an analogous trend is also observed in the effect of PMS concentration on atrazine dissipation (Diao et al. 2021). Globally, phthalate esters (PAEs) are widely used in plastic products as typical plasticizers, which can present potential bioaccumulation of benzene carboxylic groups. Thus, it is imperative to eliminate PAEs from the ecological environment such as soil and sediment. Dibutyl phthalate (DBP) and di-(2-Ethylhexyl) phthalate (DEHP) are the two dominant plasticizers and residues that exist in soil, and have been enumerated as priority pollutants by the US Environmental Protection Agency (Zhu et al. 2019b). Fe–Mn oxide-modified-BC (FMBC) composites, synthesized by the impregnation method, are commonly used for the decomposition of PAEs in contaminated soil. Some Fe–Mn oxide particles are generally attached to the surface of the materials, and other particles are inserted into the surface of the materials. FMBC shows excellent performance in PAEs removal and satisfactory stability. The accumulation of DBP and DEHP is reduced in the grains after applying FMBC, for instance, the residual DBP decreases by 28.19–74.21% with increasing FMBC additions compared with the controls (Xu et al. 2021). The interaction between soil and FMBC impacts the connectivity, pore size, and volume shape of FMBC, which can furtherly impact the water-holding capacity and adsorption properties of FMBC (Chang et al. 2021). Gao et al. reported that DBP and DEHP accumulations in grains decrease by 38.2–74.3% and 25.9–65.6%, 27.6–69.8% and 22.9–67.9%, 19.9–65.6% and 33.2–73.9%, respectively with an increase in FMBC, when the initial concentration of PAEs is 10 mg kg− 1, 20 mg kg− 1, 40 mg kg− 1, respectively (Gao et al. 2021b). This shows that FMBC is more effective at decreasing DBP and DEHP uptake. Thus, BC has a good application prospect in soil remediation through modification.
Overall, NPs@BC is suitable for organic contaminants (e.g., PBDEs, CPs, MPs, and PAEs) in soil due to their economical cost, high surface area, small particle size, unique layered structure, abundant functional groups, and strong ion exchange capacity. Generally, adding NPs@BC composites in soil improves the removal of organic contaminants. However, the actual degradation performance depends on the properties of NPs@BC and organic pollutants, such as catalyst dosage, pH, and additives. Additionally, the utilization of NPs@BC, such as nZVI@BC, for in situ remediations of organic pollutant contaminated soil presents huge prospects due to their low toxicity, low cost, and excellent activity (Vidal et al. 2018). However, rare attention has been attracted to applying NPs@BC for soil amendment, especially in-situ remediation. Moreover, NPs@BC components are affected by the structural characteristics and physicochemical properties of NPs and BC. Therefore, the compound production of biochar and NPs can be regulated to obtain perfect properties and to promote its application efficiency in soil remediation.
4.3 Removal mechanisms
According to the above discussion, NPs@BC could eliminate target pollutants, including heavy metals and organic pollutants. The interaction mechanism between contaminants and NPs@BC identification is one of the basic fundamental principles that are essential to bridge the gap ranging from remediation investigations to engineering design of NPs@BC (Luo et al. 2021). The dissipation efficiency is highly affected by pH and dosage of materials, etc. The massive efforts have been conducted on the remediation characteristics of NPs@BC materials for heavy metals and organic compounds in the contaminated environments.
Numerous literatures apply essential characterization methods, for example, µ-XRF, XANES, and FTIR, etc. to elucidate the possible adsorption mechanisms, delivering mechanical information, detecting the NPs@BC, and pollutants interaction from the molecular level. Herein, the removal of heavy metals by NPs@BC is mainly attributed to physical adsorption, redox reaction, and precipitation. The contaminants are routinely adsorbed onto the surface of NPs@BC composites, and then the redox reaction of heavy metals such as Cr(VI), As(V), and Cd(II) occurs with the nanoparticles (Chen et al. 2021; Li et al. 2020b; Medha et al. 2021; Su et al. 2016a; Wen et al. 2021; Yang et al. 2021). Lin et al. applied ferromanganese oxide-biochar composites to remove As(V), which is the most major As species. The As(V) is reduced to As(III) in the meanwhile Fe and Mn plaques are formed on the rice root surface by ferromanganese oxide, which can strongly adsorb As(III) (Lin et al. 2017). Moreover, electrostatic attraction, inner-sphere/surface complexation, and cation–π interaction process have been considered as other mechanisms. They have been used to explain various removal processes of heavy metal ions with NPs@BC materials. FT-IR analysis was used to confirm that Cd2+ might be adsorbed on the surface of Fe3O4 by electrostatic attraction and form surface complexes between Cd2+, which is attributed to π-orbital metal bonding onto Cπ electrons (Chen et al. 2019). The NPs@BC, including semiquinone-type free radicals, phenolic-OH, and C=O, can be used as electron acceptors, and play an important part in oxidizing heavy metals (Wen et al. 2021). The redox reactions of heavy metals are performed on the biochar surface in the presence of strong oxidizing and reducing agents and result in strong inner-sphere complexation on the biochar surface (Wen et al. 2021; Yuan et al. 2017). Furthermore, chelation and ion exchange happen on the surface of NPs@BC materials, which improves their immobilization efficiency. Qi et al. verified the existence of an ion exchange process with the aid of measuring the released Ca2+ and Mg2+ before and after adsorption, and the released concentrations of Mg2+ and Ca2+ increase with increasing the adsorption capacity of MgO@BC for metal ions (Pb2+, Cu2+, Cd2+) (Qi et al. 2022). The calcium-based magnetic biochar (Ca-MBC) successfully decreases the concentration of As and Cd due to the formation of bi-dentate chelate and ternary surface complexes on the surface of Fe oxide. The XANES spectrum indicates that As(V) is the predomination on the surface of Ca-MBC. The µ-XRF results confirm that As is mainly distributed on Fe oxide, whereas Cd is bound to CaCO3, biochar, and Fe oxide (Wu et al. 2020). In summary, NPs@BC composites have high immobilization efficiency for heavy metals in soil. The potential mechanisms involve physical adsorption (e.g., intermolecular force, electrostatic attraction) and chemical adsorption. Moreover, chemical adsorption mainly includes surface/inner-sphere complexation, ion exchange, precipitation, cation/π–π interactions, and chelation, as well as other routes, such as redox reaction. Adsorption is the main removal mechanism for contaminants in the presence of biochar (Li and Shi 2022; Tang et al. 2022). Moreover, various adsorption kinetic (e.g., pseudo-first-order and pseudo-second-order) and isotherm models (e.g., Langmuir and Freundlich) are used to describe the adsorption process of contaminants onto the biochar or NPs@BC (Bianco et al. 2022; Xu et al. 2022). Here, the summary of reports about the adsorption characteristic of NPs@BC for contaminants is also listed in Additional file 1: Table S3. For example, Cheng et al. used crofton weed as the biomass to prepare MgO@BC to remove Pb2+ and Cd2+ (Cheng et al. 2022). The adsorption process of the MgO@BC is conformed with the pseudo-second-order and the adsorption capacities of MgO@BC that is calcined at 400–600 ℃ to Pb2+ and Cd2+ by the Hill model reach 384.08 mg g− 1 and 207.02 mg g− 1, respectively. The fitting coefficient of the Hill model (0.987/0.989) is much higher than that of the Langmuir model (0.981/0.921), indicating that the adsorption is monolayer adsorption and that the adsorption sites are relevant to the surface of the MgO@BC.
NPs@BC composites principally act as an adsorbent or reductant/oxidant for organic pollutant removal. For example, the degradation of decabromodiphenyl by Ni/Fe@BC is an integrated process, combining adsorption and reductive degradation. In addition, Ni/Fe@BC effectively adsorbs and immobilizes the degradation products and the Ni discharges into the soil (Wu et al. 2016). Fe3O4@BC exhibits excellent degradation efficiency of PAHs. Fe3O4@BC composites activate PS, which can produce Fe2+ species via an efficient electron transfer process that initiates a reaction to generate •SO4− and then PAHs are oxidized, including side-chain oxidation and ring oxidation. The process involves an addition reaction containing a double bond, and hydrogen substitution (Dong et al. 2017). Besides, the π–π interaction, hydrogen bond, electrostatic attraction and hydrophobic interaction are the common methods to eliminate organics in the natural environment. Li et al. found that the Fe-Ce@BC material shows great decomposition capacity to PAEs, and electrostatic attraction, hydrogen bond, and hydrophobic interaction between PAEs and oxygen-containing functional groups in biochar facilitate the degradation of PAEs, in view of the fact that PAEs are hydrophobic component fixing benzene rings and ortho-substituents. More importantly, π-electron donor and acceptor interactions with S2O82− activated by the Ce4+/Ce3+ and Fe3+/Fe2+ redox cycles on the Fe-Ce@BC surface are regarded as the dominant mechanism for PAEs degradation (Dong et al. 2020). Thus, NPs@BC composite materials could effectively remove organic pollutants through the physical adsorption (e.g., intermolecular force, electrostatic attraction) and various chemical adsorption (e.g., cation/π–π interactions, hydrogen binding, redox reaction, and hydrophobic interaction).
It is well known that the interaction of NPs@BC compounds with contaminants depends on the organic or inorganic chemicals, the structures of molecules, and the environmental conditions. The heavy metals naturally present the oxidation state, and organic composites are highly stable for urban and industrial sources and environmental behavior; meanwhile, positive/negative charges are on their surface. And large specific surface area, sufficient porous structure, and various surface functional groups of NPs@BC materials, for instance, carboxyl and hydroxyl are beneficial for environmental remediation. Consequently, pollutants (e.g., heavy metals and organics) can be removed in the soil with the help of physical adsorption (e.g., intermolecular force, electrostatic attraction) and redox reaction in Fig. 2, especially, the advanced oxidation processes (AOPs), mainly based on the generation of reactive species originating from the decomposition of oxidants, as an auxiliary approach in the course of the degradation of organic pollutants. Furthermore, NPs@BC composites are aromatic rings with strengthened π-polarity on their surface, which will allow the formation of strong π-electron donor and acceptor interactions with organics and metal ions; thus, the cation–π and π–π interactions occur between contaminants (e.g., heavy metals and organics) and NPs@BC. However, partial chemical adsorption processes, such as the ion exchange, precipitation, chelation, and metal-ligand (surface/inner-sphere) complexation are unique for heavy metal removal by NPs@BC. Hydrophobic interaction is an exclusive mechanism for organic contaminant removal due to their high hydrophobicity, octanol-water partition coefficients, and low volatility. In general, the removal mechanisms of contaminants in soil by NPs@BC involve physical and chemical adsorption as well as other routes of interaction (e.g., redox reaction). The kinetics in literatures about removal of pollutants are generally overviews, including various mechanisms. Thus, it is difficult to distinguish the kinetics between physical adsorption and chemical adsorption (Ding et al. 2022; Luo et al. 2022b). There are many studies that pay attention to the elimination of a class of pollutants in soil and wastewater. Whereas, the actual application filed is changeable and complex. Thus, follow-up research with respect to the simultaneous removal of multiple types of pollutants utilizing NPs@BC materials are of great significance for practical soil environment treatment.
5 The environment-promoting effects of NPs@BC on sustainable agriculture
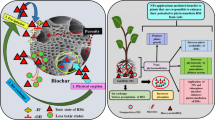
Focus on NPs@BC ecotoxicology invokes exposure scenarios relevant to NPs@BC applications such as agricultural soil remediation and scenarios that consider the effect of NPs@BC on sustainable agriculture. Achieving sustainable agricultural productivity is another enormous challenge of the new millennium aside from environmental remediation (Lowry et al. 2019). The general object of sustainable agriculture is for the sake of providing safe and nutritional food on existing agricultural land, taking advantage of resources and facilitating, or maintaining at least, farm productivity, and simultaneously preserving the function of the ecosystem (Pineiro et al. 2020; Wang et al. 2022). Nevertheless, sustainable agriculture is heavily subject to healthy soil with superior characteristics, for instance, divergent species, an abundant eco-friendly microbial population, as well as carbon/nitrogen cycling mediated by soil microorganisms. Currently, a certain of studies confirm that the release of pollutants (e.g., heavy metals, organic pollutants, and NPs) from NPs@BC in soil depends on NPs content in the raw materials and the transformation, morphology, mineral types, as well as dissolution of pollutants/NPs@BC under different soil environmental conditions, all of which deserve further investigation. The impacts of NPs@BC on the soil environment have received increasing attention taking sustainable development of agriculture into account. Therefore, based on the previous chapter, we illustrate the impacts of NPs@BC on the soil-plant system (see Fig. 4) from the perspective of three target objects, including crop growth, soil properties, and soil carbon and nitrogen cycling in agricultural soil.
Schematic diagram of the contribution of NPs@BC to sustainable agriculture. After being released into the soil, NPs@BC generally interacts with plant roots, soil biota, and contaminants. The schematic illustration of NPs@BC involves carbon and nitrogen cycle. Arrow widths roughly correspond to the sizes of the CO2 fluxes (Ogle 2018)
5.1 Crop growth
Responses of crop growth to NPs@BC application in soil include seed germination, plant growth, physiology, yield, and quality of the plant. The crop can take up NPs@BC and pollutants either through the roots or foliar contact; however, uptake, accumulation, and prohibition of them in crops may be different for plant cultivars, tissue growth conditions, and variety of NPs@BC and pollutants. For instance, the biochar along with TiO2 application substantially is lower 2–5 times than TiO2 NPs treatment on accumulating mass of Ti in wheat tissues, including shoots and roots. In the meantime, the root transfer factor of Ti decreases by threefold at a relatively higher exposure level (500 mg L− 1 TiO2@BC) compared with only TiO2 NPs treatment, as a result of the translocation of biochar within the plant system as well as the interaction of humic substances with NPs (Abbas et al. 2021). Simultaneously, nZVI@BC could also effectively prevent the upward translocation of pollutant Cr in the plant–soil system and decrease the accumulation of Cr in cabbage mustard compared with the nZVI (Su et al. 2016a). Owing to generating insoluble salts and plant resistance to the mobility of metal ions to their aerial parts, the plant roots are the easiest aggregation part by heavy metals. Thus, bioavailable metals generally concentrate on roots, and comparatively less amount is translocated in shoots (Rafique et al. 2021). In addition, iron plaques can be formed that retain certain ions and suppress heavy metal assimilation by plants for iron oxide nanoparticles (Lu et al. 2018).
The effect of NPs@BC on plants touches upon many aspects, including numerous growth parameters, such as fresh and dry biomass, seed germination rate, root and shoot lengths, and photosynthesis, etc. The length of the roots and the stems of the cabbage mustard grown in the Cr-contaminated soil treated by nZVI@BC is 21.33 and 41.34 mm larger, respectively, than that in the soil with no material amendment (Su et al. 2016a). MgO@BC treatment improves the root, shoot and leaf growth under severe salinity by about 54%, 21%, and 27%, respectively, compared to the non-biochar treatment (Farhangi et al. 2021). The metal oxide-loaded biochar nanocomposites have a greatly influence on water content and osmolytes. At a high level of salinity, the leaf water content of safflower is increased by approximately 3.6-7%, in response to MgO/MnO@BC composite treatment (Farhangi et al. 2021). The parameters of photosynthesis and gas exchange are valuable indicators of crops grown under stress conditions. The addition of biochar with TiO2 nanoparticles boosts the photosynthetic performance of wheat at every nanoparticle exposure level. The interactive effect of the TiO2@BC amendment is observed at correspondingly low nanoparticle concentration (200 mg L− 1) that induces the positive effect on crops’ photosynthetic relevant characteristics by triggering the net photosynthesis rate, maximum photosynthesis rate, dark respiration rate, and CO2 assimilation quantum yield (Abbas et al. 2021). The synergistic effect between biochar and NPs promotes plant growth by suppressing nanoparticle accumulation in plant tissues and increasing nutrient availability.
As we all know, plant abiotic and biological stresses generate an accumulation of reactive oxygen species (ROS), which can give rise to the oxidation of proteins, DNA, lipids, and carbohydrates, and a consequent abridgement in photosynthetic performance (Li et al. 2022b). In the meanwhile, the resultful antioxidant–enzyme systems can clear off ROS build-up in plants and prevent living organisms from oxidative stress. There is a study that assesses oxidative stress by quantitatively determining the activity of the antioxidant enzyme system in terms of superoxide dismutase (SOD), peroxidase activity (POD), and catalase activity (CAT). Comparing with the control group (only the soil), the activities of SOD, POD, and CAT in shoots of plants grown in the treatment of Ni-Fe@BC increase by 15.7%, 39.8%, and 14.9%, respectively. These results indicate that the oxidative stress on plants reduces in the treatment of Ni-Fe@BC (Wu et al. 2018b). The contents of chlorophyll are the effective index of crophealth and growth condition and are commonly used to observe the impact of NPs@BC on crop growth (Gao et al. 2022). The concentration of chlorophyll is affected by the dosage of NPs. For instance, relatively low exposure levels (100 mg L− 1) of CeO2 and biochar promote the production of chlorophyll a (12%), chlorophyll b (4.4%), and total chlorophyll (10%) in wheat tissues. On the contrary, the reduction rate of chlorophyll a, chlorophyll b, and total chlorophyll is 8–11%, 10–14%, and 10%, respectively, at 1000 and 2000 mg L− 1 of biochar amendment with CeO2 (Abbas et al. 2020). Consequently, NPs@BC composites play an important role in catalytic scavengers of ROS in isolated chloroplasts and further improve photosynthesis performance.
Plant growth requires relatively vast amounts of primary macronutrients (N, P, Ca, and K), and most usually are the limiting factor for crop growth and productivity. These macronutrients play an important role in metabolism and stop plants from biotic and abiotic stresses. In contrast, microelements Fe and Mn are conducive to human health. In the current study, NPs@BC amendments contribute to a tempestuous increase in the bioavailability and accumulation of N, P, K, Ca, Mn, and Fe in wheat tissues (Abbas et al. 2020; Gao et al. 2021b). Moreover, NPs@BC (such as Fe@BC) can increase the rice straw yield by 89.2% in As, Cd, Pb contaminated paddy soil, which may be the improvement of soil physicochemical properties and nutrient supply induced by the Fe@BC composites (Wen et al. 2021).
5.2 Soil geochemical properties
Biochar absorbs organic pollutants and microelements to prevent contaminants from stressing crops by means of its special characteristics, such as large specific surface area, porous structure as well as excellent adsorption capacity (Shen et al. 2020). Thus, biochar is proposed as a potential soil additive that could promote carbon storage by many researchers; furthermore, it could increase the value of agricultural products and improve plant growth (Lehmann et al. 2006). The NPs@BC composites combine the benefits of biochar with NPs. Meanwhile, the above discussion is that all the improvements of plants are mainly on account of applying NPs@BC. Due to the double characteristics of NPs@BC, their physicochemical properties play a major role in altering the soil geochemical properties.
Biomass that possesses higher ash content is deemed to have more excellent pH value. The operational temperature is higher during pyrolysis, and the alkalinity of biochar is greater. Consequently, the pH of NPs@BC plays a vital role in regulating soil alkalinity. Normally, the soil pH significantly increases when NPs@BC is applied to soil (Tang et al. 2020). The increased soil pH will give rise to the precipitants of metal oxides and hydroxides, caused by increased hydrolysis of heavy metals, which will reduce heavy metal bioavailability and immobilize heavy metals or metalloids (Jiang et al. 2012). Thus, the availability of the most metals, such as Ni, Cu, and Cd in soil, is able to reduce with NPs@BC applied. It mainly decreases the soluble and reducible fractions of Cd and As according to the BCR sequential extraction on soil samples (Wu et al. 2020). Available phosphorus and hydrolysable nitrogen are other indicators to evaluate the impact of different amendments on the soil traits. The result shows that Ni-Fe bimetallic nanoparticles would break up soil available phosphorus and attenuate soil fertility, which is harmful to plant growth. However, the addition of Ni-Fe@BC could observably increase (2.09 mg kg− 1) the content of available P in soil. The reason is that the NPs@BC involves excellent amounts of P and its availability is also high. At the same time, the determination result of available nitrogen indicates a similar trend to that of available phosphorus, increased by 187 mg kg− 1 (Wu et al. 2018b). Moreover, NPs@BC may transform the extent of organic carbon mineralization in the soil systems. The application of NPs@BC significantly enhances the soil total organic carbon (TOC) and dissolved organic carbon (DOC) content (Wen et al. 2021; Wu et al. 2020). Nevertheless, pristine biochar is more effective than Fe@BC in increasing the content of TOC in soil. For instance, the TOC content increases by 14 mg kg− 1 in the pristine BC-treated soil, whereas 11.7 mg kg− 1 for Fe@BC (Wen et al. 2021).
Soil enzyme activity is an important index of soil quality since it is directly related to the activity of microorganisms and the biogeochemical cycling of nutrients (Ahmad et al. 2012). The response of soil enzymes to the amendment is quick; thus, soil enzyme activity is generally used to estimate the soil functions with NPs@BC application. The β-glucosidase activity increases in the soil polluted with heavy metals or organic pollutants, which is connected with soil carbon and sulfur cycling and the improvement of soil pH after NPs@BC composites applying in the short or long-term (Chang et al. 2021). Meanwhile, enzymes have different degrees of sensitivity to NPs@BC. The activities of dehydrogenase, polyphenol oxidase, β-glucosidase, urease, and protease are significantly related with the doses of Fe–Mn oxide-modified biochar composites (FM@BC). On the contrary, the acid phosphatase activity presents the opposite trend (Chang et al. 2021). The most sensitive to the addition of FM@BC is polyphenol oxidase, followed by protease, β-glucosidase, dehydrogenase, urease, and acid phosphatase (Toscano et al. 2003). Moreover, the effect of NPs@BC addition on soil enzyme activity is often more impressive than that of biochar treatment. For example, FM@BC application increases polyphenol oxidase activity by 14.6–49.9% in polluted soil compared with biochar alone (Gao et al. 2021a).
5.3 Soil carbon and nitrogen cycle
Studies have demonstrated the impact of biochar on soil microbial activity and community structure (Palansooriya et al. 2019). Soil microbes are crucial components of the soil ecosystem and play a significant role in regulating nutrient cycling, maintaining soil health and resisting contaminants, which are important to sustainable agriculture. Consequently, they are indicators to explore the influence of NPs@BC on ecological functions and bio-geochemical processes (e.g., C and N cycles). On the one hand, changes in soil properties can drive soil microbial growth. Owing to the surface area, high pore volume, and negative surface charge, NPs@BC enriches the soil with nutrients, containing cations K, Na, N, and P, which are crucial for microbial growth (Kaur et al. 2022). The addition of NPs@BC in the soil declines the bulk density of soil and contributes to enhancing soil aeration. The improved bulk density of the soil improves the available water content and promotes the availability of essential nutrients to microbes. In addition, NPs@BC is rich in pore structure and volume, which provides accommodation for a large number of microbes and increases their abundance on its surface. On the other hand, there are negative effects and risk which is associated with the NPs@BC applied. The pyrolysis process of biomass would make it possible to generate a wide number of organic chemicals, including volatile organic compounds (VOCs), dioxins, and PAHs (Hale et al. 2012). Some studies illustrate that dioxin-like chemicals are generally deposited on biochar surfaces at low heat treatment temperatures (200–400 °C) and short residence times (Lyu et al. 2016). Although the content of hazardous substances is acceptable in the majority of biochar, NPs@BC may have a negative effect on soil microorganisms. For example, an apparent correlation between the content of PAHs in biochar and toxicity is claimed for Daphnia magna (Oleszczuk et al. 2013).
NPs@BC affects the physicochemical traits of soil and then impacts the soil microbiota directly or indirectly. The effect of NPs@BC composites on microorganisms is manifested in these aspects: microbial biomass, microbial activity, as well as microbial abundance and structure. The microbial diversity and community structure are intimately connected to the soil type to which Nps@BC is applied. For instance, biochar-supported Fe-Mn oxide (FM@BC) is applied for the dibutyl phthalate (DBP), and di-(2-ethylhexyl) phthalate (DEHP) contaminated soil that is collected from fluvo-aquic soils (FAS) and brown soils (BS) with a loam texture, respectively. Proteobacteria is dominant in FAS samples with different concentrations of contaminants except for 40 mg L− 1 DEHP-polluted soils repaired with FM@BC (Chang et al. 2021). There are ten most dominant bacterial phyla (i.e., Verrucomicrobia, Chloroflexi, Bacteroidetes, Gemmatimonadetes, etc.), and a proportion of them are more than 96.7% of the sequences assigned to soil bacteria. Actinobacteria, Firmicutes, and Proteobacteria are the prime taxa in all treatments, contributing the most significant relative abundance (> 65.5%) and are regarded as leading groups in BS samples (Gao et al. 2021a). Soil conditions, such as oxygen content, also influence the feedback of bacterial diversity and community structure. In the drier soil, the increased spatial isolation of microhabitats suppresses the competition among species, as a result, leading to easier inoculation of exogenous bacteria under aerobic conditions (Wang et al. 2021a). For example, Bacillus, Micromonospora, and Streptomyces are typical metal-reducing bacterial genera, are a primary bacterial community in all treatments, and the relative abundance ranges from 41.13–54.62%, 3.79–7.85%, and 5.90–8.51%, respectively, under aerobic conditions. Nevertheless, Ruminiclostridium, Clostridium, and Lentimicrobium are the major bacterial genera, which account for 22.61–35.45% in soil under anaerobic conditions. The relative abundance of Bacillus is from 2.4% to 7.6% under anaerobic conditions, which is much lower than under aerobic conditions. In conclusion, NPs@BC has the potential to alter the microbial community structure and diversity in various degrees related to the function of soil condition and variety.
Soil microbial biomass, containing soil microbial biomass carbon, nitrogen, and phosphorus, is a major regulator of essential ecosystem processes, such as nutrient cycling and organic matter decomposition (Crowther et al. 2019; Delgado et al. 2020). In soil, belowground fuel productivity and microbial biomass can be fueled by means of flows of carbon (Bastida et al. 2021). When the magnetic biochar is exerted on the soil, more suitable microbe habitats are supplied, and the rates of bacterial metabolism increase, resulting in an increase in biomass carbon (Wang et al. 2021a).
Soil microbes are the dominant drivers of nutrient cycling, where soil organic matter is mineralized and decomposed to release available forms of nutrients that can be adsorbed by plants and other microorganisms or remain in the soil–plant biogeochemical cycle. Any factors that influence the microbiota or nutrient cycling may compromise ecosystem functioning. The previous part has showed that NPs@BC might transform soil microbial composition and affect the soil microbial community’s metabolic capacity and processes referring to nutrient cycles, such as carbon and nitrogen cycling. The presence of NPs@BC could alter enzyme activity, functional genes, and microbial taxa linked with nitrogen/carbon cycling processes, and nitrogen-cycle-related genes are summarized in Fig. 4. Soil pH, bioavailable metals and soil electric conductivity (EC) have influence on bacterial community in addition to damaging the growth of phototrophs, resulting in the reduction of the carbon fixation genes (Zhang et al. 2021c). In addition, the dissolved black carbon (DOC) is discharged from biochar, which generates reactive oxygen species that could break the cellular components of phototrophs, giving rise to a reduction in the relative abundance of carbon fixation genes (Fu et al. 2016). The stimulation of soil carbon-degrading organisms by MgO@BC could be linked to the activity of cellulase, which induces more carbon in the form of glucose to increase the abundance of carbon-utilizing bacteria (Ibrahim et al. 2022). In part, the increase in some key orders, which is relevant to carbon-cycling bacteria in the modified biochar treatments, will regulate the C-cycle.
The cycle of biologically driven nitrogen transformations in natural environments involves the following pathway: nitrogen fixation (from ammonium, nitrate, or organic nitrogen) that is an process, nitrification, and denitrification, which are catabolic processes (Zhang et al. 2020c). The changes in nitrification and denitrification ascribe to the truth that biochar can change the the relative abundance of taxa and microbial diversity. The biochar does not mutate the total expression of archaeal and bacterial but reinforces the activity of nitrifiers and de-nitrifiers. Meanwhile, the expression of archaeal amoA abundance is sizably increased by biochar, but not amoA of bacteria (Xu et al. 2014). Thus, the bacteria may contribute lower than archaea to the improved nitrification by biochar. The enzyme activity that is linked to nitrogen transformation could also be interfered with by NPs, and proceed to affect the nitrogen cycling. For instance, ZnO NPs alter the transcriptional regulation of glycolysis and synthesis of poly-hydroxybutyrate, which causes the decrease in reducing the power available for the reduction of nitrate and N2O. Moreover, ZnO NPs substantially restrain the gene expression and the pivotal catalytic activities of denitrifying enzymes (i.e., glucokinase, phosphofructokinase, and pyruvate kinase) (Zheng et al. 2014).
In conclusion, the application of NPs and biochar materials could also modulate the growth and yield of crops, the survival, reproduction and avoidance behavior of soil fauna, and the physicochemical properties of soils, such as pH and organic matter that impact on the structure of soil microbial communities. In turn, rhizodeposition shapes soil microbial communities that conduct essential element cycling processes such as soil carbon and nitrogen metabolism (i.e., CO2, N2O emission). Hence, NPs@BC presents superior potential for maintaining the soil ecosystem function, such as soil health, crop productivity in agriculture, soil microbe and global warming, and nutrient cycles, which correspond to the aim of sustainable agriculture, and NPs@BC needs to be considered in future studies.
6 Conclusions and future perspectives
To date, there is rapidly growing interest in using NPs@BC composites for sustainable agriculture and soil amendment. Although extensive studies about contaminated soil are performed, including heavy metals and organics with the aid of NPs@BC, impacts of nano-biochar composites on the performance of crops, soil characters, fauna, and microbial communities are considered one of the most severe problems with respect to its effective application. Above all, an integrated framework of NPs@BC is proposed for sustainable selection and design to assess the possible trade-offs and risks comprehensively. As discussed in this review, our work offers a new thought that is related to undertake the safety assessment oriented environmental effects, hazard, and economic consideration. For the framework of sustainable NPs@BC selection and design, various indicators (e.g., CED, GHG, LC50, and price) need to be taken into account before the soil remediation, and future research needs to adjust the study objectives. To promote the possibility of large-scale operation of NPs@BC composites according to the promise exhibited in bench studies, the following research areas need to be enhanced:
-
1.
In the past few decades, rapid progress has been made in the production and application of biochar worldwide. However, industrial-scale pyrolysis of biomass for biochar production in general is still challenging. Producing biochar from biomass will produce promising co-benefits, such as the generation of gas biofuels, liquid biofuels, a great deal of heat steam that settle energy crisis from drastic fossil fuel depletion. In the future, newly improved technologies will be formulated, implemented and integrated together to take full advantage of biomass and its by-products. Besides, the in-situ characterization method is a standard tool for monitoring and is conducive to elucidating the complex growth dynamics usually presented during NPs@BC formation. For one, in-situ characterizations of NPs@BC have been important for exploiting reliable manufacturing processes. For another, in-situ monitoring of NPs@BC material formation and understanding how NPs@BC surfaces evolve are highly desirable as the unparalleled nanostructures of NPs@BC play a crucial role in soil remediation.
-
2.
NPs@BC material assessment framework is focused on performance, hazard, and material price, which are important to material engineers and scientists, and their environmental impacts will be increasingly urgent as the fabrication techniques of NPs@BC continue to develop and mature. In the subsequent study, the evaluation framework should collaborate intensively with machine learning, involving developing and defining approaches to model the unclear impacts as yet and co-developing better solution for effects already accounted. Furthermore, its future development should work out designing and manufacturing cheap specific NPs@BC for remediating certain polluted soils.
-
3.
In natural conditions, the contaminated sites generally contain multiple pollutants. For example, heavy metals always co-exist with other contaminants (i.e., chlorophenol, PAHs, etc.). Shaping co-contaminants in environments could reflect different environmental behavior and toxicity impacts compared with heavy metals alone. However, NPs@BC composites often only target a single contaminant in previous studies, and applying NPs@BC to dissipate organics and heavy metal co-contaminants in the environments is not clear. Therefore, brand new NPs@BC materials serving for various co-contaminants are extraordinarily imperative. Circumstances, for example, the effect of the PMS dose, need to be considered when NPs@BC composites are used in the actual engineering field, such as soil remediation. Since SO42- is produced after PMS decompose, the maximum concentration of SO42- is 250 mg L-1, in the standard for drinking water (GB 5749-2006) (Zhao et al. 2021).
-
4.
In the review, the scope of the impact of NPs@BC on sustainable agriculture is narrow, continuing to optimize the effect assessment of NPs@BC. The different environmental factors (i.e., P, S cycling, metabolism of soil biota) and different perspectives (i.e., human health) need to be considered in the NPs@BC evaluation, which is correlated to sustainable agriculture. Especially, it is obliged to take advantage of the multiple-omics techniques (e.g., transcriptomics, proteomics, and metabolomics) to explore the disparity of genes/metabolic pathways/ protein function related to crop growth and C/N/P/S cycling when NPs@BC is utilized in the soil.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbas Q, Liu G, Yousaf B et al (2020) Biochar-assisted transformation of engineered-cerium oxide nanoparticles: effect on wheat growth, photosynthetic traits and cerium accumulation. Ecotoxicol Environ Saf 187:109845. https://doi.org/10.1016/j.ecoenv.2019.109845
Abbas Q, Yousaf B, Mujtaba Munir MA et al (2021) Biochar-mediated transformation of titanium dioxide nanoparticles concerning TiO2NPs-biochar interactions, plant traits and tissue accumulation to cell translocation. Environ Pollut 270:116077. https://doi.org/10.1016/j.envpol.2020.116077
Ahmad M, Hashimoto Y, Moon DH et al (2012) Immobilization of lead in a korean military shooting range soil using eggshell waste: an integrated mechanistic approach. J Hazard Mater 209:392–401. https://doi.org/10.1016/j.jhazmat.2012.01.047
Amundson R, Berhe AA, Hopmans JW et al (2015) Soil and human security in the 21st century. Science 348(6235):1261071. https://doi.org/10.1126/science.1261071
Azzi ES, Karltun E, Sundberg C (2019) Prospective life cycle assessment of large-scale biochar production and use for negative emissions in Stockholm. Environ Sci Technol 53(14):8466–8476. https://doi.org/10.1021/acs.est.9b01615
Bastida F, Eldridge DJ, Garcia C et al (2021) Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J 15(7):2081–2091. https://doi.org/10.1038/s41396-021-00906-0
Bianco F, Marcińczyk M, Race M et al (2022) Low temperature-produced and VFA-coated biochar enhances phenanthrene adsorption and mitigates toxicity in marine sediments. Sep Purif Technol 296:121414. https://doi.org/10.1016/j.seppur.2022.121414
Bombuwala Dewage N, Liyanage AS, Pittman CU et al (2018) Fast nitrate and fluoride adsorption and magnetic separation from water on α-Fe2O3 and Fe3O4 dispersed on Douglas fir biochar. Bioresour Technol 263:258–265. https://doi.org/10.1016/j.biortech.2018.05.001
Brandl F, Bertrand N, Lima EM et al (2015) Nanoparticles with photoinduced precipitation for the extraction of pollutants from water and soil. Nat Commun 6(1):7765. https://doi.org/10.1038/ncomms8765
Chang X, Song Z, Xu Y et al (2021) Response of soil characteristics to biochar and Fe-Mn oxide-modified biochar application in phthalate-contaminated fluvo-aquic soils. Ecotoxicol Environ Saf 225:112755. https://doi.org/10.1016/j.ecoenv.2021.112755
Chen Y, Zhang X, Chen W et al (2017) The structure evolution of biochar from biomass pyrolysis and its correlation with gas pollutant adsorption performance. Bioresour Technol 246:101–109. https://doi.org/10.1016/j.biortech.2017.08.138
Chen X, Oh W, Da, Hu Z, Ting et al (2018) Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes. Appl Catal B 225:243–257. https://doi.org/10.1016/j.apcatb.2017.11.071
Chen M, Tao X, Yi, Wang D, Jun et al (2019) Facilitated transport of cadmium by biochar-Fe3O4 nanocomposites in water-saturated natural soils. Sci Total Environ 684:265–275. https://doi.org/10.1016/j.scitotenv.2019.05.326
Chen X, Dai Y, Fan J et al (2021) Application of iron-biochar composite in topsoil for simultaneous remediation of chromium-contaminated soil and groundwater: immobilization mechanism and long-term stability. J Hazard Mater 405:124226. https://doi.org/10.1016/j.jhazmat.2020.124226
Cheng G, Chun, Li G, Lan Di, Liu C et al (2017) Efficient synthesis of nitrogen- and S-Co-doped ketjenblack with a single-source precursor for enhancing oxygen reduction reaction activity. Chem Eur J 23(15):3674–3682. https://doi.org/10.1002/chem.201604930
Cheng S, Zhao S, Guo H et al (2022) High-efficiency removal of lead/cadmium from wastewater by MgO modified biochar derived from crofton weed. Bioresour Technol 343:126081. https://doi.org/10.1016/j.biortech.2021.126081
Crowther T, van den Hoogen J, Wan J et al (2019) The global soil community and its influence on biogeochemistry. Science 365(6455):550. https://doi.org/10.1126/science.aav0550
Delgado B, Manuel, Reich PB, Trivedi C et al (2020) Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat Ecol Evol 4(2):210–220. https://doi.org/10.1038/s41559-019-1084-y
Diao Z, Hui, Dong F, Xin, Yan L et al (2020a) Synergistic oxidation of Bisphenol A in a heterogeneous ultrasound-enhanced sludge biochar catalyst/persulfate process: reactivity and mechanism. J Hazard Mater 384:121385. https://doi.org/10.1016/j.jhazmat.2019.121385
Diao Z, Hui, Yan L, Dong F, Xin et al (2020b) Degradation of 2,4-dichlorophenol by a novel iron based system and its synergism with cd(II) immobilization in a contaminated soil. Chem Eng J 379:122313. https://doi.org/10.1016/j.cej.2019.122313
Diao Z, Hui, Zhang W, Xuan, Liang J, Yi et al (2021) Removal of herbicide atrazine by a novel biochar based iron composite coupling with peroxymonosulfate process from soil: synergistic effect and mechanism. Chem Eng J 409:127684. https://doi.org/10.1016/j.cej.2020.127684
Ding D, Yang S, Qian X et al (2020) Nitrogen-doping positively whilst sulfur-doping negatively affect the catalytic activity of biochar for the degradation of organic contaminant. Appl Catal B 263:118348. https://doi.org/10.1016/j.apcatb.2019.118348
Ding L, Wang Y, Tong L et al (2022) N-doped biochar-catalyzed dechlorination of carbon tetrachloride in sulfide-containing aqueous solutions: performances, mechanisms and pathways. Water Res 223:119006. https://doi.org/10.1016/j.watres.2022.119006
Dong C, Di, Chen C, Wen, Hung C, Mao (2017) Synthesis of magnetic biochar from bamboo biomass to activate persulfate for the removal of polycyclic aromatic hydrocarbons in marine sediments. Bioresour Technol 245:188–195. https://doi.org/10.1016/j.biortech.2017.08.204
Dong C, Di, Chen C, Wen, Nguyen T, Binh et al (2020) Degradation of phthalate esters in marine sediments by persulfate over Fe-Ce/biochar composites. Chem Eng J 384:123301. https://doi.org/10.1016/j.cej.2019.123301
El-Temsah YS, Joner EJ (2012) Ecotoxicological effects on earthworms of fresh and aged nano-sized zero-valent iron (nZVI) in soil. Chemosphere 89(1):76–82. https://doi.org/10.1016/j.chemosphere.2012.04.020
Falinski MM, Plata DL, Chopra SS et al (2018) A framework for sustainable nanomaterial selection and design based on performance, hazard, and economic considerations. Nat Nanotechnol 13(8):708–714. https://doi.org/10.1038/s41565-018-0120-4
Fang G, Zhu C, Dionysiou DD et al (2015) Mechanism of hydroxyl radical generation from biochar suspensions: implications to diethyl phthalate degradation. Bioresour Technol 176:210–217. https://doi.org/10.1016/j.biortech.2014.11.032
Farhangi A, Salar, Ghassemi G, Kazem (2021) Changes in soil properties and salt tolerance of safflower in response to biochar-based metal oxide nanocomposites of magnesium and manganese. Ecotoxicol Environ Saf 211:111904. https://doi.org/10.1016/j.ecoenv.2021.111904
Fu H, Liu H, Mao J et al (2016) Photochemistry of dissolved black carbon released from biochar: reactive oxygen species generation and phototransformation. Environ Sci Technol 50(3):1218–1226. https://doi.org/10.1021/acs.est.5b04314
Fu D, Chen Z, Xia D et al (2017) A novel solid digestate-derived biochar-Cu NP composite activating H2O2 system for simultaneous adsorption and degradation of tetracycline. Environ Pollut 221:301–310. https://doi.org/10.1016/j.envpol.2016.11.078
Fu H, Zhao P, Xu S et al (2019) Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: insights on the mechanism. Chem Eng J 375:121980. https://doi.org/10.1016/j.cej.2019.121980
Gao B, Wang P, Zhou H et al (2013) Sorption of phthalic acid esters in two kinds of landfill leachates by the carbonaceous sorbents. Bioresour Technol 136:295–301. https://doi.org/10.1016/j.biortech.2013.03.026
Gao Y, Li T, Zhu Y et al (2020) Highly nitrogen-doped porous carbon transformed from graphitic carbon nitride for efficient metal-free catalysis. J Hazard Mater 393:121280. https://doi.org/10.1016/j.jhazmat.2019.121280
Gao M, Chang X, Xu Y et al (2021a) Effects of Fe-Mn impregnated biochar on enzymatic activity and bacterial community in phthalate-polluted brown soil planted with wheat. Environ Pollut 284:117179. https://doi.org/10.1016/j.envpol.2021.117179
Gao M, Xu Y, Chang X et al (2021b) Fe-Mn oxide modified biochar decreases phthalate uptake and improves grain quality of wheat grown in phthalate-contaminated fluvo-aquic soil. Chemosphere 270:129428. https://doi.org/10.1016/j.chemosphere.2020.129428
Gao ZY, Wang SC, Zhang YX et al (2022) Single and combined toxicity of polystyrene nanoplastics and copper on Platymonas helgolandica var. tsingtaoensis: perspectives from growth inhibition, chlorophyll content and oxidative stress. Sci Total Environ 829:154571. https://doi.org/10.1016/j.scitotenv.2022.154571
García-Gómez C, Babín M, García S et al (2019) Joint effects of zinc oxide nanoparticles and chlorpyrifos on the reproduction and cellular stress responses of the earthworm Eisenia andrei. Sci Total Environ 688:199–207. https://doi.org/10.1016/j.scitotenv.2019.06.083
Gilbertson LM, Zimmerman JB, Plata DL et al (2015) Designing nanomaterials to maximize performance and minimize undesirable implications guided by the principles of Green Chemistry. Chem Soc Rev 44(16):5758–5777. https://doi.org/10.1039/C4CS00445K
Gomes SI, Caputo G, Pinna N et al (2015) Effect of 10 different TiO2 and ZrO2 (nano)materials on the soil invertebrate Enchytraeus crypticus. Environ Toxicol Chem 34(10):2409–2416. https://doi.org/10.1002/etc.3080
Hale SE, Lehmann J, Rutherford D et al (2012) Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ Sci Technol 46(5):2830–2838. https://doi.org/10.1021/es203984k
Hu CW, Li M, Cui YB et al (2010) Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol Biochem 42(4):586–591. https://doi.org/10.1016/j.soilbio.2009.12.007
Huang Y, Fong, Huang Y, Yang, Chiueh P, Te et al (2020) Heterogeneous Fenton oxidation of trichloroethylene catalyzed by sewage sludge biochar: experimental study and life cycle assessment. Chemosphere 249:126139. https://doi.org/10.1016/j.chemosphere.2020.126139
Huijbregts MAJ, Hellweg S, Frischknecht R et al (2010) Cumulative energy demand as predictor for the environmental burden of commodity production. Environ Sci Technol 44(6):2189–2196. https://doi.org/10.1021/es902870s
Hussain I, Li M, Zhang Y et al (2017) Insights into the mechanism of persulfate activation with nZVI/BC nanocomposite for the degradation of nonylphenol. Chem Eng J 311:163–172. https://doi.org/10.1016/j.cej.2016.11.085
Ibrahim MM, Guo L, Wu F et al (2022) Field-applied biochar-based MgO and sepiolite composites possess CO2 capture potential and alter organic C mineralization and C-cycling bacterial structure in fertilized soils. Sci Total Environ 813:152495. https://doi.org/10.1016/j.scitotenv.2021.152495
Igalavithana AD, Mandal S, Niazi NK et al (2017) Advances and future directions of biochar characterization methods and applications. Crit Rev Environ Sci Technol 47(23):2275–2330. https://doi.org/10.1080/10643389.2017.1421844
Irshad MK, Noman A, Alhaithloul HAS et al (2020) Goethite-modified biochar ameliorates the growth of rice (Oryza sativa L.) plants by suppressing cd and As-induced oxidative stress in cd and as co-contaminated paddy soil. Sci Total Environ 717:137086. https://doi.org/10.1016/j.scitotenv.2020.137086
Jiang T, Yu, Jiang J, Xu R, Kou et al (2012) Adsorption of pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 89(3):249–256. https://doi.org/10.1016/j.chemosphere.2012.04.028
Jin Z, Wang B, Ma L et al (2020) Air pre-oxidation induced high yield N-doped porous biochar for improving toluene adsorption. Chem Eng J 385:123843. https://doi.org/10.1016/j.cej.2019.123843
Kah M, Johnston LJ, Kookana RS et al (2021) Comprehensive framework for human health risk assessment of nanopesticides. Nat Nanotechnol 16(9):955–964. https://doi.org/10.1038/s41565-021-00964-7
Kasozi GN, Zimmerman AR, Nkedi-Kizza P et al (2010) Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ Sci Technol 44(16):6189–6195. https://doi.org/10.1021/es1014423
Kaur T, Devi R, Kumar S et al (2022) Microbial consortium with nitrogen fixing and mineral solubilizing attributes for growth of barley (Hordeum vulgare L.). Heliyon 8(4):326. https://doi.org/10.1016/j.heliyon.2022.e09326
Lanno R, Wells J, Conder J et al (2004) The bioavailability of chemicals in soil for earthworms. Ecotoxicol Environ Saf 57(1):39–47. https://doi.org/10.1016/j.ecoenv.2003.08.014
Laurent A, Olsen SI, Hauschild MZ (2012) Limitations of carbon footprint as indicator of environmental sustainability. Environ Sci Technol 46(7):4100–4108. https://doi.org/10.1021/es204163f
Lehmann J, Gaunt J, Rondon M (2006) Biochar sequestration in terrestrial ecosystems—a review. Mitig Adapt Strat Glob Change 11(2):403–427. https://doi.org/10.1007/s11027-005-9006-5
Li X, Shi J (2022) Simultaneous adsorption of tetracycline, ammonium and phosphate from wastewater by iron and nitrogen modified biochar: kinetics, isotherm, thermodynamic and mechanism. Chemosphere 293:133574. https://doi.org/10.1016/j.chemosphere.2022.133574
Li D, Hockaday WC, Masiello CA et al (2011) Earthworm avoidance of biochar can be mitigated by wetting. Soil Biol Biochem 43(8):1732–1737. https://doi.org/10.1016/j.soilbio.2011.04.019
Li R, Wang JJ, Zhou B et al (2017) Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J Clean Prod 147:96–107. https://doi.org/10.1016/j.jclepro.2017.01.069
Li H, Zhu F, He S (2019) The degradation of decabromodiphenyl ether in the e-waste site by biochar supported nanoscale zero-valent iron /persulfate. Ecotoxicol Environ Saf 183:109540. https://doi.org/10.1016/j.ecoenv.2019.109540
Li H, Tian Y, Liu W et al (2020a) Impact of electrokinetic remediation of heavy metal contamination on antibiotic resistance in soil. Chem Eng J 400:125866. https://doi.org/10.1016/j.cej.2020.125866
Li Y, Tian X, Liang J et al (2020) Remediation of hexavalent chromium in contaminated soil using amorphous iron pyrite: effect on leachability, bioaccessibility, phytotoxicity and long-term stability. Environ Pollut 264:114804. https://doi.org/10.1016/j.envpol.2020.114804
Li X, Xu J, Yang Z (2022a) Efficient oriented interfacial oxidation of petroleum hydrocarbons by functionalized Fe/N co-doped biochar-mediated heterogeneous Fenton for heavily contaminated soil remediation. Chem Eng J 450:138466. https://doi.org/10.1016/j.cej.2022.138466
Li Y, Tang Z, Pan Z et al (2022b) Calcium-mbilizing properties of salvia miltiorrhiza-derived carbon dots confer enhanced environmental adaptability in plants. ACS Nano 16(3):4357–4370. https://doi.org/10.1021/acsnano.1c10556
Li Z, Liu D, Huang W et al (2020c) Biochar supported CuO composites used as an efficient peroxymonosulfate activator for highly saline organic wastewater treatment. Sci Total Environ 721:137764. https://doi.org/10.1016/j.scitotenv.2020.137764
Lian F, Xing B (2017) Black carbon (biochar) in water/soil environments: molecular structure, sorption, stability, and potential risk. Environ Sci Technol 51(23):13517–13532. https://doi.org/10.1021/acs.est.7b02528
Lian L, Huang T, Ke X et al (2022) Globalization-driven industry relocation significantly reduces arctic PAH contamination. Environ Sci Technol 56(1):145–154. https://doi.org/10.1021/acs.est.1c05198
Liang H, Zhu C, Ji S et al (2022a) Magnetic Fe2O3/biochar composite prepared in a molten salt medium for antibiotic removal in water. Biochar 4(1):3. https://doi.org/10.1007/s42773-021-00130-1
Liang W, Wang G, Peng C et al (2022b) Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: a critical review. J Hazard Mater 426:127993. https://doi.org/10.1016/j.jhazmat.2021.127993
Lin L, Gao M, Qiu W et al (2017) Reduced arsenic accumulation in indica rice (Oryza sativa L.) cultivar with ferromanganese oxide impregnated biochar composites amendments. Environ Pollut 231:479–486. https://doi.org/10.1016/j.envpol.2017.08.001
Liu W-J, Jiang H, Yu H-Q (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115(22):12251–12285. https://doi.org/10.1021/acs.chemrev.5b00195
Liu P, Ptacek CJ, Elena KMA et al (2018) Evaluation of mercury stabilization mechanisms by sulfurized biochars determined using X-ray absorption spectroscopy. J Hazard Mater 347:114–122. https://doi.org/10.1016/j.jhazmat.2017.12.051
Liu L, Yin Y, Hu L et al (2020) Revisiting the forms of trace elements in biogeochemical cycling: analytical needs and challenges. Trends Anal Chem 129:115953. https://doi.org/10.1016/j.trac.2020.115953
Liu Y, Chen Y, Li Y et al (2022) Fabrication, application, and mechanism of metal and heteroatom co-doped biochar composites (MHBCs) for the removal of contaminants in water: a review. J Hazard Mater 431:128584. https://doi.org/10.1016/j.jhazmat.2022.128584
Lowry GV, Avellan A, Gilbertson LM (2019) Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat Nanotechnol 14(6):517–522. https://doi.org/10.1038/s41565-019-0461-7
Lu HP, Li ZA, Gasco G et al (2018) Use of magnetic biochars for the immobilization of heavy metals in a multi-contaminated soil. Sci Total Environ 622–623:892–899. https://doi.org/10.1016/j.scitotenv.2017.12.056
Luo J, Yu D, Hristovski KD et al (2021) Critical review of advances in engineering nanomaterial adsorbents for metal removal and recovery from water: mechanism identification and engineering design. Environ Sci Technol 55(8):4287–4304. https://doi.org/10.1021/acs.est.0c07936
Luo Y, Hao, Cai Y, Long X et al (2022a) Palladium pd(0) loading-controlled catalytic activity and selectivity for chlorophenol hydrodechlorination and hydrosaturation. Environ Sci Technol 56(7):4447–4456. https://doi.org/10.1021/acs.est.1c08347
Luo Z, Liu M, Tang D et al (2022b) High H2O2 selectivity and enhanced Fe2+ regeneration toward an effective electro-Fenton process based on a self-doped porous biochar cathode. Appl Catal B 315:121523. https://doi.org/10.1016/j.apcatb.2022.121523
Lyu H, He Y, Tang J et al (2016) Effect of pyrolysis temperature on potential toxicity of biochar if applied to the environment. Environ Pollut 218:1–7. https://doi.org/10.1016/j.envpol.2016.08.014
Ma Y, Liu W, Jun, Zhang N et al (2014) Polyethylenimine modified biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresour Technol 169:403–408. https://doi.org/10.1016/j.biortech.2014.07.014
Markad VL, Kodam KM, Ghole VS (2012) Effect of fly ash on biochemical responses and DNA damage in earthworm, Dichogaster curgensis. J Hazard Mater 215:191–198. https://doi.org/10.1016/j.jhazmat.2012.02.053
Medha I, Chandra S, Vanapalli KR et al (2021) (3-Aminopropyl)triethoxysilane and iron rice straw biochar composites for the sorption of cr (VI) and zn (II) using the extract of heavy metals contaminated soil. Sci Total Environ 771:144764. https://doi.org/10.1016/j.scitotenv.2020.144764
Meri NH, Alias AB, Talib N et al (2018) Effect of chemical washing pre-treatment of empty fruit bunch (EFB) biochar on characterization of hydrogel biochar composite as bioadsorbent. In: 3rd international conference on global sustainability and chemical engineering (ICGSCE) 358. https://doi.org/10.1088/1757-899X/358/1/012018
Meyer S, Glaser B, Quicker P (2011) Technical, economical, and climate-related aspects of biochar production technologies: a literature review. Environ Sci Technol 45(22):9473–9483. https://doi.org/10.1021/es201792c
Mueller ND, Gerber JS, Johnston M et al (2012) Closing yield gaps through nutrient and water management. Nature 490(7419):254–257. https://doi.org/10.1038/nature11420
Nematian M, Keske C, Ng’ombe JN (2021) A techno-economic analysis of biochar production and the bioeconomy for orchard biomass. Waste Manage (Oxford) 135:467–477. https://doi.org/10.1016/j.wasman.2021.09.014
Ntinas GK, Neumair M, Tsadilas CD et al (2017) Carbon footprint and cumulative energy demand of greenhouse and open-field tomato cultivation systems under Southern and Central European climatic conditions. J Clean Prod 142:3617–3626. https://doi.org/10.1016/j.jclepro.2016.10.106
Ogle K (2018) Hyperactive soil microbes might weaken the terrestrial carbon sink. Nature 560(7716):32–33. https://doi.org/10.1038/d41586-018-05842-2
Oleszczuk P, Josko I, Kusmierz M (2013) Biochar properties regarding to contaminants content and ecotoxicological assessment. J Hazard Mater 260:375–382. https://doi.org/10.1016/j.jhazmat.2013.05.044
Osterwalder N, Capello C, Hungerbühler K et al (2006) Energy consumption during nanoparticle production: how economic is dry synthesis? J Nanopart Res 8(1):1. https://doi.org/10.1007/s11051-005-8384-7
Ouyang D, Yan J, Qian L et al (2017) Degradation of 1,4-dioxane by biochar supported nano magnetite particles activating persulfate. Chemosphere 184:609–617. https://doi.org/10.1016/j.chemosphere.2017.05.156
Palansooriya KN, Wong JTF, Hashimoto Y et al (2019) Response of microbial communities to biochar-amended soils: a critical review. Biochar 1(1):3–22. https://doi.org/10.1007/s42773-019-00009-2
Pang Y, Xiong, Lei H, Yi (2016) Degradation of p-nitrophenol through microwave-assisted heterogeneous activation of peroxymonosulfate by manganese ferrite. Chem Eng J 287:585–592. https://doi.org/10.1016/j.cej.2015.11.076
Park H, May A, Portilla L et al (2020) Magnetite nanoparticles as efficient materials for removal of glyphosate from water. Nat Sustain 3(2):129–135. https://doi.org/10.1038/s41893-019-0452-6
Pathy A, Ray J, Paramasivan B (2020) Biochar amendments and its impact on soil biota for sustainable agriculture. Biochar 2(3):287–305. https://doi.org/10.1007/s42773-020-00063-1
Peng Y, Yan Y, Wang J et al (2022) CdSe cluster-modified biogenic α-FeOOH based on macroporous biochar for Fenton-like reaction of as(III). Appl Surf Sci 589:152872. https://doi.org/10.1016/j.apsusc.2022.152872
Petersen EJ, Goss GG, von der Kammer F et al (2021) New guidance brings clarity to environmental hazard and behaviour testing of nanomaterials. Nat Nanotechnol 16(5):482–483. https://doi.org/10.1038/s41565-021-00889-1
Pineiro V, Arias J, Dürr J et al (2020) A scoping review on incentives for adoption of sustainable agricultural practices and their outcomes. Nat Sustain 3(10):809–820. https://doi.org/10.1038/s41893-020-00617-y
Qi X, Yin H, Zhu M, Han et al (2022) MgO-loaded nitrogen and phosphorus self-doped biochar: high-efficient adsorption of aquatic Cu2+, Cd2+, and Pb2+ and its remediation efficiency on heavy metal contaminated soil. Chemosphere 294:133733. https://doi.org/10.1016/j.chemosphere.2022.133733
Qiao JT, Liu TX, Wang XQ et al (2018) Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 195:260–271. https://doi.org/10.1016/j.chemosphere.2017.12.081
Rafique MI, Usman ARA, Ahmad M et al (2021) Immobilization and mitigation of chromium toxicity in aqueous solutions and tannery waste-contaminated soil using biochar and polymer-modified biochar. Chemosphere 266:129198. https://doi.org/10.1016/j.chemosphere.2020.129198
Rahman A, Kang S, McGinnis S et al (2022) Life cycle impact assessment of iron oxide (Fe3O4/γ-Fe2O3) nanoparticle synthesis routes. ACS Sustainable Chem Eng 10(10):3155–3165. https://doi.org/10.1021/acssuschemeng.1c05763
Ren J, Tao, Wan C, Ying, Pei T, Yao et al (2020) Promotion of electrocatalytic nitrogen reduction reaction on N-doped porous carbon with secondary heteroatoms. Appl Catal B 266:118633. https://doi.org/10.1016/j.apcatb.2020.118633
Roberts KG, Gloy BA, Joseph S et al (2010) Life cycle assessment of biochar systems: estimating the energetic, economic, and climate change potential. Environ Sci Technol 44(2):827–833. https://doi.org/10.1021/es902266r
Rong X, Xie M, Kong L et al (2019) The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation. Chem Eng J 372:294–303. https://doi.org/10.1016/j.cej.2019.04.135
Schlich K, Terytze K, Hund R, Kerstin (2012) Effect of TiO2 nanoparticles in the earthworm reproduction test. Environ Sci Europe 24(1):5. https://doi.org/10.1186/2190-4715-24-5
Servin AD, Castillo-Michel H, Hernandez-Viezcas JA et al (2018) Bioaccumulation of CeO2 nanoparticles by earthworms in biochar-amended soil: a synchrotron microspectroscopy study. J Agric Food Chem 66(26):6609–6618. https://doi.org/10.1021/acs.jafc.7b04612
Shakya A, Vithanage M, Agarwal T (2022) Influence of pyrolysis temperature on biochar properties and cr(VI) adsorption from water with groundnut shell biochars: mechanistic approach. Environ Res 215:114243. https://doi.org/10.1016/j.envres.2022.114243
Sharma A, Pareek V, Zhang D (2015) Biomass pyrolysis-a review of modelling, process parameters and catalytic studies. Renew Sustain Energy Rev 50:1081–1096. https://doi.org/10.1016/j.rser.2015.04.193
Shen X, Zeng J, Zhang D et al (2020) Effect of pyrolysis temperature on characteristics, chemical speciation and environmental risk of Cr, Mn, Cu, and Zn in biochars derived from pig manure. Sci Total Environ 704:135283. https://doi.org/10.1016/j.scitotenv.2019.135283
Shi Z, Tang Z, Wang C (2017) A brief review and evaluation of earthworm biomarkers in soil pollution assessment. Environ Sci Pollut Res 24(15):13284–13294. https://doi.org/10.1007/s11356-017-8784-0
Shi Z, Yan J, Ren X et al (2021) Effects of biochar and thermally treated biochar on Eisenia fetida survival, growth, lysosomal membrane stability and oxidative stress. Sci Total Environ 770:144778. https://doi.org/10.1016/j.scitotenv.2020.144778
Silvani L, Hjartardottir S, Bielska L et al (2019) Can polyethylene passive samplers predict polychlorinated biphenyls (PCBs) uptake by earthworms and turnips in a biochar amended soil? Sci. Total Environ 662:873–880. https://doi.org/10.1016/j.scitotenv.2019.01.202
Sizmur T, Richardson J (2020) Earthworms accelerate the biogeochemical cycling of potentially toxic elements: results of a meta-analysis. Soil Biol Biochem 148:107865. https://doi.org/10.1016/j.soilbio.2020.107865
Smith E, Naidu R, Weber J et al (2008) The impact of sequestration on the bioaccessibility of arsenic in long-term contaminated soils. Chemosphere 71(4):773–780. https://doi.org/10.1016/j.chemosphere.2007.10.012
Su H, Fang Z, Tsang PE et al (2016a) Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environ Pollut 214:94–100. https://doi.org/10.1016/j.envpol.2016.03.072
Su H, Fang Z, Tsang PE et al (2016b) Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J Hazard Mater 318:533–540. https://doi.org/10.1016/j.jhazmat.2016.07.039
Tang X, Shen H, Chen M et al (2020) Achieving the safe use of Cd- and As-contaminated agricultural land with an Fe-based biochar: a field study. Sci Total Environ 706:135898. https://doi.org/10.1016/j.scitotenv.2019.135898
Tang W, Zanli BLGL, Chen J (2021) O/N/P-doped biochar induced to enhance adsorption of sulfonamide with coexisting Cu2+/ cr (VI) by air pre-oxidation. Bioresour Technol 341:125794. https://doi.org/10.1016/j.biortech.2021.125794
Tang Y, Li Y, Zhan L et al (2022) Removal of emerging contaminants (bisphenol A and antibiotics) from kitchen wastewater by alkali-modified biochar. Sci Total Environ 805:150158. https://doi.org/10.1016/j.scitotenv.2021.150158
Tatsi K, Shaw BJ, Hutchinson TH et al (2018) Copper accumulation and toxicity in earthworms exposed to CuO nanomaterials: effects of particle coating and soil ageing. Ecotoxicol Environ Saf 166:462–473. https://doi.org/10.1016/j.ecoenv.2018.09.054
Tian HZ, Zhu C, Gao J et al (2015) Quantitative assessment of atmospheric emissions of toxic heavy metals from anthropogenic sources in China: historical trend, spatial distribution, uncertainties, and control policies. Atmos Chem Phys 15:12107–12166. https://doi.org/10.5194/acpd-15-12107-2015
Tian H, Wang Z, Zhu T et al (2021) Degradation prediction and products of polycyclic aromatic hydrocarbons in soils by highly active bimetals/AC-activated persulfate. ACS ES&T Engg 1(8):1183–1192. https://doi.org/10.1021/acsestengg.1c00063
Toscano G, Colarieti ML, Greco G (2003) Oxidative polymerisation of phenols by a phenol oxidase from green olives. Enzyme Microb Technol 33(1):47–54. https://doi.org/10.1016/S0141-0229(03)00080-2
Tu Y, Peng Z, Huang J et al (2020) Preparation and characterization of magnetic biochar nanocomposites via a modified solvothermal method and their use as efficient heterogeneous Fenton-like catalysts. Ind Eng Chem Res 59(5):1809–1821. https://doi.org/10.1021/acs.iecr.9b04590
Turhollow A, Perlack R, Eaton L et al (2014) The updated billion-ton resource assessment. Biomass Bioenergy 70:149–164. https://doi.org/10.1016/j.biombioe.2014.09.007
Vidal J, Saez C, Cañizares P et al (2018) ZVI—reactive barriers for the remediation of soils polluted with clopyralid: are they really worth? Chem Eng J 350:100–107. https://doi.org/10.1016/j.cej.2018.05.142
Wafiq A, Reichel D, Hanafy M (2016) Pressure influence on pyrolysis product properties of raw and torrefied Miscanthus: role of particle structure. Fuel 179:156–167. https://doi.org/10.1016/j.fuel.2016.03.092
Wan X, Li C, Parikh SJ (2020) Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. Environ Pollut 261:114157. https://doi.org/10.1016/j.envpol.2020.114157
Wang S, Zhao M, Zhou M et al (2019) Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: a critical review. J Hazard Mater 373:820–834. https://doi.org/10.1016/j.jhazmat.2019.03.080
Wang G, Xia X, Yang J et al (2020) Exploring the bioavailability of nickel in a soil system: physiological and histopathological toxicity study to the earthworms (Eisenia fetida). J Hazard Mater 383:121169. https://doi.org/10.1016/j.jhazmat.2019.121169
Wang L, Chen H, Wu J et al (2021a) Effects of magnetic biochar-microbe composite on cd remediation and microbial responses in paddy soil. J Hazard Mater 414:125494. https://doi.org/10.1016/j.jhazmat.2021.125494
Wang Y, Wang L, Li Z et al (2021b) MgO-laden biochar enhances the immobilization of Cd/Pb in aqueous solution and contaminated soil. Biochar 3(2):175–188. https://doi.org/10.1007/s42773-020-00080-0
Wang Y, Zheng K, Zhan W et al (2021c) Highly effective stabilization of cd and Cu in two different soils and improvement of soil properties by multiple-modified biochar. Ecotoxicol Environ Saf 207:111294. https://doi.org/10.1016/j.ecoenv.2020.111294
Wang D, Saleh NB, Byro A et al (2022) Nano-enabled pesticides for sustainable agriculture and global food security. Nat Nanotechnol 17(4):347–360. https://doi.org/10.1038/s41565-022-01082-8
Wen E, Yang X, Chen H et al (2021) Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil. J Hazard Mater 407:124344. https://doi.org/10.1016/j.jhazmat.2020.124344
Whitfield Aslund ML, Mcshane H, Simpson MJ et al (2012) Earthworm sublethal responses to Titanium dioxide nanomaterial in soil detected by 1H NMR metabolomics. Environ Sci Technol 46(2):1111–1118. https://doi.org/10.1021/es202327k
Wu J, Yi Y, Li Y et al (2016) Excellently reactive Ni/Fe bimetallic catalyst supported by biochar for the remediation of decabromodiphenyl contaminated soil: reactivity, mechanism, pathways and reducing secondary risks. J Hazard Mater 320:341–349. https://doi.org/10.1016/j.jhazmat.2016.08.049
Wu C, Cui M, Xue S et al (2018a) Remediation of arsenic-contaminated paddy soil by iron-modified biochar. Environ Sci Pollut Res 25(21):20792–20801. https://doi.org/10.1007/s11356-018-2268-8
Wu J, Yi Y, Fang Z et al (2018b) Effects of biochar on phytotoxicity and translocation of polybrominated diphenyl ethers in Ni/Fe bimetallic nanoparticle-treated soil. Environ Sci Pollut Res 25(3):2570–2579. https://doi.org/10.1007/s11356-017-0627-5
Wu J, Li Z, Huang D et al (2020) A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J Hazard Mater 387:122010. https://doi.org/10.1016/j.jhazmat.2019.122010
Wu C, Li F, Yi S et al (2021) Genetically engineered microbial remediation of soils co-contaminated by heavy metals and polycyclic aromatic hydrocarbons: advances and ecological risk assessment. J Environ Manage 296:113185. https://doi.org/10.1016/j.jenvman.2021.113185
Xia X, Li P, Xia Z et al (2022) Life cycle carbon footprint of electric vehicles in different countries: a review. Sep Purif Technol 301:122063. https://doi.org/10.1016/j.seppur.2022.122063
Xin S, Liu G, Ma X et al (2021) High efficiency heterogeneous Fenton-like catalyst biochar modified CuFeO2 for the degradation of tetracycline: economical synthesis, catalytic performance and mechanism. Appl Catal B 280:119386. https://doi.org/10.1016/j.apcatb.2020.119386
Xu H, Juan, Wang X, Hui, Li H et al (2014) Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ Sci Technol 48(16):9391–9399. https://doi.org/10.1021/es5021058
Xu Y, Ai J, Zhang H (2016) The mechanism of degradation of bisphenol a using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process. J Hazard Mater 309:87–96. https://doi.org/10.1016/j.jhazmat.2016.01.023
Xu H, Zhang Y, Li J et al (2020a) Heterogeneous activation of peroxymonosulfate by a biochar-supported Co3O4 composite for efficient degradation of chloramphenicols. Environ Pollut 257:113610. https://doi.org/10.1016/j.envpol.2019.113610
Xu W, Hu X, Lou Y et al (2020b) Effects of environmental factors on the removal of heavy metals by sulfide-modified nanoscale zerovalent iron. Environ Res 187:109662. https://doi.org/10.1016/j.envres.2020.109662
Xu Y, Xie X, Feng Y et al (2020c) As(III) and as(V) removal mechanisms by Fe-modified biochar characterized using synchrotron-based X-ray absorption spectroscopy and confocal micro-X-ray fluorescence imaging. Bioresour Technol 304:122978. https://doi.org/10.1016/j.biortech.2020.122978
Xu Y, Song Z, Chang X et al (2021) Effects of Fe-Mn oxide-modified biochar composite applications on phthalate esters (PAEs) accumulation in wheat grains and grain quality under PAEs-polluted brown soil. Ecotoxicol Environ Saf 208:111624. https://doi.org/10.1016/j.ecoenv.2020.111624
Xu Y, Xia H, Zhang Q et al (2022) Adsorption of cadmium(II) in wastewater by magnesium oxide modified biochar. Arab J Chem 15(9):104059. https://doi.org/10.1016/j.arabjc.2022.104059
Yang D, Yang S, Yuan H et al (2021) Co-benefits of biochar-supported nanoscale zero-valent iron in simultaneously stabilizing soil heavy metals and reducing their bioaccessibility. J Hazard Mater 418:126292. https://doi.org/10.1016/j.jhazmat.2021.126292
Yin D, Wang X, Peng B et al (2017) Effect of biochar and Fe-biochar on Cd and as mobility and transfer in soil-rice system. Chemosphere 186:928–937. https://doi.org/10.1016/j.chemosphere.2017.07.126
Yu Z, Qiu W, Wang F et al (2017) Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in an indica rice (Oryza sativa L.) cultivar. Chemosphere 168:341–349. https://doi.org/10.1016/j.chemosphere.2016.10.069
Yuan Y, Bolan N, Prévoteau A et al (2017) Applications of biochar in redox-mediated reactions. Bioresour Technol 246:271–281. https://doi.org/10.1016/j.biortech.2017.06.154
Zhang X, Gao B, Zheng Y et al (2017) Biochar for volatile organic compound (VOC) removal: sorption performance and governing mechanisms. Bioresour Technol 245:606–614. https://doi.org/10.1016/j.biortech.2017.09.025
Zhang M, Zhao C, Li J et al (2019) Organo-layered double hydroxides for the removal of polycyclic aromatic hydrocarbons from soil washing effluents containing high concentrations of surfactants. J Hazard Mater 373:678–686. https://doi.org/10.1016/j.jhazmat.2019.03.126
Zhang G, Liu X, Gao M et al (2020a) Effect of Fe-Mn-Ce modified biochar composite on microbial diversity and properties of arsenic-contaminated paddy soils. Chemosphere 250:126249. https://doi.org/10.1016/j.chemosphere.2020.126249
Zhang H, Shao J, Zhang S et al (2020b) Effect of phosphorus-modified biochars on immobilization of Cu (II), cd (II), and as (V) in paddy soil. J Hazard Mater 390:121349. https://doi.org/10.1016/j.jhazmat.2019.121349
Zhang X, Ward BB, Sigman DM (2020c) Global nitrogen cycle: critical enzymes, organisms, and processes for nitrogen budgets and dynamics. Chem Rev 120(12):5308–5351. https://doi.org/10.1021/acs.chemrev.9b00613
Zhang K, Min X, Zhang T et al (2021a) Selenium and nitrogen co-doped biochar as a new metal-free catalyst for adsorption of phenol and activation of peroxymonosulfate: elucidating the enhanced catalytic performance and stability. J Hazard Mater 413:125294. https://doi.org/10.1016/j.jhazmat.2021.125294
Zhang Q, Guo, Zou D, Sheng, Zeng X, Yi et al (2021b) Effect of the direct use of biomass in agricultural soil on heavy metals—activation or immobilization? Environ Pollut 272:115989. https://doi.org/10.1016/j.envpol.2020.115989
Zhang Y, Yan C, Liu H et al (2021c) Bacterial response to soil property changes caused by wood ash from wildfire in forest soils around mining areas: relevance of bacterial community composition, carbon and nitrogen cycling. J Hazard Mater 412:125264. https://doi.org/10.1016/j.jhazmat.2021.125264
Zhang S, Kong Z, Wang H et al (2022a) Enhanced nitrate removal by biochar supported nano zero-valent iron (nZVI) at biocathode in bioelectrochemical system (BES). Chem Eng J 433:133535. https://doi.org/10.1016/j.cej.2021.133535
Zhang S, Ren S, Pei L et al (2022b) Ecotoxicological effects of polyethylene microplastics and ZnO nanoparticles on earthworm Eisenia fetida. Appl Soil Ecol 176:104469. https://doi.org/10.1016/j.apsoil.2022.104469
Zhang Y-J, Huang G-X, Winter LR et al (2022c) Simultaneous nanocatalytic surface activation of pollutants and oxidants for highly efficient water decontamination. Nat Commun 13(1):3005. https://doi.org/10.1038/s41467-022-30560-9
Zhang Y, Xu M, He R et al (2022d) Effect of pyrolysis temperature on the activated permonosulfate degradation of antibiotics in nitrogen and sulfur-doping biochar: key role of environmentally persistent free radicals. Chemosphere 294:133737. https://doi.org/10.1016/j.chemosphere.2022.133737
Zhao H, Lang Y (2018) Adsorption behaviors and mechanisms of florfenicol by magnetic functionalized biochar and reed biochar. J Taiwan Inst Chem Eng 88:152–160. https://doi.org/10.1016/j.jtice.2018.03.049
Zhao Y, Song M, Cao Q et al (2020) The superoxide radicals’ production via persulfate activated with CuFe2O4@Biochar composites to promote the redox pairs cycling for efficient degradation of o-nitrochlorobenzene in soil. J Hazard Mater 400:122887. https://doi.org/10.1016/j.jhazmat.2020.122887
Zhao C, Shao B, Yan M et al (2021) Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: a review. Chem Eng J 416:128829. https://doi.org/10.1016/j.cej.2021.128829
Zheng X, Su Y, Chen Y et al (2014) Zinc oxide nanoparticles cause inhibition of microbial denitrification by affecting transcriptional regulation and enzyme activity. Environ Sci Technol 48(23):13800–13807. https://doi.org/10.1021/es504251v
Zheng J, Li M, Tang B et al (2021) Levels, spatial distribution, and impact factors of heavy metals in the hair of metropolitan residents in China and human health implications. Environ Sci Technol 55(15):10578–10588. https://doi.org/10.1021/acs.est.1c02001
Zhou Q, Hu X (2017) Systemic stress and recovery patterns of rice roots in response to graphene oxide nanosheets. Environ Sci Technol 51:2022−2030. https://doi.org/10.1021/acs.est.6b05591
Zhou Q, Jiang X, Li X et al (2018) Preparation of high-yield N-doped biochar from nitrogen-containing phosphate and its effective adsorption for toluene. RSC Adv 8(53):30171–30179. https://doi.org/10.1039/c8ra05714a
Zhou Q, Liu Y, Li T et al (2020) Cadmium adsorption to clay-microbe aggregates: implications for marine heavy metals cycling. Geochim Cosmochim Acta 290:124−136. https://doi.org/10.1016/j.gca.2020.09.002
Zhou M, Xu Y, Luo G et al (2022) Facile synthesis of phosphorus-doped porous biochars for efficient removal of elemental mercury from coal combustion flue gas. Chem Eng J 432:134440. https://doi.org/10.1016/j.cej.2021.134440
Zhu N, Qiao J, Yan T (2019a) Arsenic immobilization through regulated ferrolysis in paddy field amendment with bismuth impregnated biochar. Sci Total Environ 648:993–1001. https://doi.org/10.1016/j.scitotenv.2018.08.200
Zhu TK, Du PP, Zeng LJ et al (2019) Variation in metabolism and degradation of di-n-butyl phthalate (DBP) by high- and low-DBP accumulating cultivars of rice (Oryza sativa L.) and crude enzyme extracts. Sci Total Environ 668:1117–1127. https://doi.org/10.1016/j.scitotenv.2019.03.047
Zhu Y, Wu X, Liu Y et al (2020) Integration of transcriptomics and metabolomics reveals the responses of earthworms to the long-term exposure of TiO2 nanoparticles in soil. Sci Total Environ 719:137492. https://doi.org/10.1016/j.scitotenv.2020.137492
Acknowledgements
Thanks for the editor professor Wenfu Chen and the anonymous reviewers’ comments. The authors also appreciate the financial support from the National Natural Science Foundation of China (NO. 42107306, U1906222), the Fellowship of China Postdoctoral Science Foundation (NO. 2020M680867), the National Key Research and Development Project (NO. 2019YFC1804104), and Ministry of Education, People’s Republic of China as a 111 program (NO. T2017002).
Funding
This work was financially supported by the National Natural Science Foundation of China (NO. 42107306, U1906222), the Fellowship of China Postdoctoral Science Foundation (NO. 2020M680867), the National Key Research and Development Project (NO. 2019YFC1804104), and Ministry of Education, People’s Republic of China as a 111 program (NO. T2017002).
Author information
Authors and Affiliations
Contributions
TZ: literature collection and analysis, data curation, investigation, writing—original draft. SO: literature investigation, data curation, formal analysis, writing—review and editing. QZ: conceptualization, writing—review and editing, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Supplementary Information
Additional file 1: Fig. S1.
Scientific production on NPs@BC composites in the Web of Science database. keywords used for the search “biochar-supported nanoparticles.” NPs@BC, nanoparticles decorated biochar. Table S1. Recent researches on synthesis and characterization of NPs@BC composites. Table S2. Data of CED and GHG for NPs@BC composites. Table S3. Summary of reports about the adsorption kinetic and isotherm models of contaminants onto the NPs@BC composites.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, T., Ouyang, S. & Zhou, Q. Synthesis, characterization, safety design, and application of NPs@BC for contaminated soil remediation and sustainable agriculture. Biochar 5, 5 (2023). https://doi.org/10.1007/s42773-022-00198-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00198-3