Abstract
Numerous studies have demonstrated that soil applications of biochar contribute to plant disease suppression and growth promotion. Here, we quantitatively evaluated the performance of biochars on plant disease suppression and production using meta-analysis of literature data. The results indicated that biochar amendment dramatically reduced disease severity (DS) by 47.46% while increasing plant biomass by 44.05%. The highest disease suppression was achieved with soil application of straw-derived biochar compared to biochar from other feedstocks, while no significant increase in yield was found with straw-derived biochar. Biochars pyrolyzed at medium temperatures (350–600 °C) facilitate both disease controlling and growth promotion. Soil application of biochars between 3 and 5% significantly decreased plant DS by 59.11%, and inverted U-shaped biochar dose/DS suppression curve and biochar dose/growth curve were observed. In cash crop fields, the DS of plants amended with biochar was reduced over 50%, which was significantly higher than that of grain crops and perennial trees. Furthermore, biochar performance on plant disease suppression was higher for airborne pathogens than for soilborne pathogens, possibly due to the systemic activation of plant defences by biochar amendment. Additionally, a reduction of DS by biochar was observed on plants grown in agricultural soils. Our work contributes to the standardization of biochar production and provides a reference for improving the function of biochar in disease control.
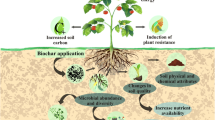
Graphical Abstract

Article highlights
-
A meta-analysis of biochar preparation and application methods for disease severity and plant growth is needed.
-
Soil application of biochar from straw at 350–600 °C and at 3–5% rate was effective in reducing disease severity.
-
Biochar application has better suppression effect on cash crop diseases caused by airborne pathogens.
-
The results of the meta-analysis are particularly useful for maximizing the effect of biochar on plant disease control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plant diseases have been a persistently great challenge to global food security (Strange and Scott 2005; Ristaino et al. 2021). The average yield losses associated with pathogens and pests globally was estimated at 21.5%, 30.0%, 22.5%, 17.2%, and 21.4% for wheat, rice, maize, potato, and soybean, respectively (Savary et al. 2019). In addition, the occurrence of plant diseases leads to famines and threatens human health due to the shortage of food (Ristaino et al. 2021). This situation became even worse as growing evidence showed that the rising global temperature intensified both the range and severity of plant diseases (Evans et al. 2008; Barford 2013; Chaloner et al. 2021). Chemical control has always been the major and effective method for plant disease control (Hirooka and Ishii 2013; Elmer et al. 2018). However, the excessive application of such agrochemicals is detrimental to the ecosystems and even toxic to human beings (Beketov et al. 2013; Nicolopoulou-Stamati et al. 2016; Yang et al. 2022b). With the growing strict regulation of chemical pesticides, growing studies were focused on plant disease control using biocontrol, which refers to plant disease control using a population of beneficial organisms to reduce or prevent plant infection by pathogens (Abbas et al. 2019; He et al. 2021; Yang and Zhang 2019).
Increasing studies have shown that plant health and productivity are reliant on the ecosystem services provided by indigenous soil and plant-associated microbiomes (Dignam et al. 2019; Huang et al. 2020). Soil with healthy rhizosphere microbial communities can greatly reduce the incidence of plant diseases by preying on, inhibiting, and metabolically inactivating competing pathogens (De Corato 2020; Nwokolo et al. 2021). For example, the diversity and richness of Pseudomonas species were primary parameters that explained the variation of disease suppressive capacity of soils among different treatment, and a more frequent organic residue returning enhanced the diversity and activities of plant-beneficial bacterial (Dignam et al. 2019). Therefore, healthy soil management is considered as an effective method to plant disease control (Nwokolo et al. 2021).
Biochar, the carbon-rich solid material derived from thermal conservation of biomass under oxygen-deficient environment, has great potential as a soil amendment, pollution remediation, and carbon sequestration (Joseph and Lehmann 2015; Chen et al. 2019). Land application of biochar helps to improve soil physicochemical properties as well as nutrient biogeochemical cycling by impacting soil microorganism abundance and richness (Gul and Whalen 2016; Yu et al. 2019). Due to their specific chemical properties, abundant nutrients, and porous structure, biochar is capable of recruiting microbes and its land application is capable of reshaping the microbial community structure in soils (Lehmann et al. 2011; Zhu et al. 2017).
The first report by Elad et al. (2010) on biochar application for plant disease suppression indicated that soil application of biochar derived from citrus wood induced systemic resistance to Botrytis cinerea and Leveillula taurica on both pepper and tomato. They further attributed the induced response of biochar on plant disease suppression to the phytotoxic compounds or chemical elicitors (Elad et al. 2010). Subsequently, continuous research has been conducted to explore the influence of biochar on plant pathogens, and the knowledge on biochar’s influence on plant disease control has expanded. For example, increased microbial diversity and changes in metabolic potential in the rhizosphere microbiome enhanced plant growth and disease suppression (Kolton et al. 2017). However, the efficiency of biochar on plant disease suppression was dose-dependent and greatly varied among biochars, plant species, and cultivating systems. Therefore, a comprehensive and quantitative response synthesis of the preparation methods and application concentration of biochar affecting disease severity and plant growth is greatly needed (Poveda et al. 2021; de Medeiros et al. 2021). Although a few recently published articles detailed the influence of biochar on plant pathogen control, for example, Frenkel et al. (2017) and Poveda et al. (2021), a quantitative evaluation of the biochar’s influence on plant disease suppression is needed. Here, a meta-analysis was conducted to make a comprehensive evaluation of biochar performance on plant disease suppression and explore the impacts of different agricultural and environmental conditions. The results of this study could provide the basis for clean and safe plant disease control and greatly expand the scope of biochar application in agriculture.
2 Materials and methods
2.1 Literature collection
To establish a comprehensive database concerning the influence of biochar on plant disease suppression, peer-reviewed articles published between 2010 and April 2022 were systematic searched from the online database of Web of Science (http://apps.webofknowledge.com), Elsevier Science Direct (https://www.sciencedirect.com), and Google Scholar (https://scholar.google.com) with keywords “biochar” and “plant disease” or “plant-pathogen”. The following criteria were used to select articles: (1) at least three replications per treatment; (2) both biochar and control treatment in the same experimental site or under the same experimental conditions; (3) reported biochar application rates; and (4) contained statistics data of disease severity and plant growth (i.e., root or shoot dry weight).
A total of 49 research articles were included in this meta-analysis after passing the acceptance criteria (Additional file 4: Table S1). The spatial distribution of targeted sites was shown in Additional file 1: Fig. S1. As indicated, the research on biochar influence on plant disease attracted worldwide interest, while the majority of studies were conducted in China and the USA.
2.2 Data categorization and treatment
The following categorical groups were established to assess the influence of biochar on plant disease suppression: biochar feedstock types (grouped into food waste, greenhouse waste, straw, and wood), production temperatures (grouped into ≤ 350 °C, 350–600 °C, and ≥ 600 °C), biochar application rates (grouped into ≤ 1%, 1–3%, 3–5%, 5–10% and ≥ 10%), plant types (grouped into cash crops, grain crops, and trees), plant pathogens (grouped into airborne and soilborne), and cultivation systems (grouped into soil and commercial potting mixture) (Table 1).
As various resistance assessment indexes were used in each study, we chose a more centralized disease severity (%) for assessment. Depending on plant species, plant growth was measured as plant dry weight, shoot dry weight, root dry weight, grain yield, and fruit per plant in each study. If more than one growth data was provided (e.g., both shoot and root biomass) in one study, the data point that is related to the diseased part of the plant was used in the meta-analysis (Additional file 5: Table S2). For data presented in graphs and figures, values of the mean and standard deviation were extracted using GetData Graph Digitizer 2.26 (http://getdata-graph-digitizer.com).
2.3 Meta-analysis
In the presented study, the response ratio (RR, thereafter response ratios) was measured to compare the impact of biochar application on the occurrence of plant disease and the influence of pathogens on plant growth. To facilitate statistical tests, a natural logarithm of RR was calculated as the effect size (Hedges et al. 1999).
where Xt represents the mean under biochar application treatment, and Xc represents the mean of the control group for a given experiment. The log-transformed values were used for meta-analysis in the calculation of summary biochar effects and confidence limits. In the tested variable, the values of RR greater than zero indicated that the application of biochar has a positive effect, while those less than zero indicated a negative effect. The values of RR close to zero indicate little or no effect of biochar addition.
In all meta-analyses and meta-regressions, cumulative effect sizes (RR+) were weighed by the inverse of the sampling variances, and the calculation formula is as follows:
where St and Sc represent the standard deviations of Xc and Xt, respectively. Nt and NC represent the sample sizes of the treatment group and the control group, respectively. w represents the weighting factor. τ2 represents the between-study variance component (Veroniki et al. 2016).
The percentage changes in disease suppression and plant productivity (Additional file 6: Table S3) were calculated from the weighted effect size (RR+) and confidence interval (CI) through exponential transformation (Wang et al. 2019b):
2.4 Statistical analysis
Random-effects meta-analysis was performed in R 3.2.2 software with the metafor package to assess overall heterogeneity in the data due to multiple effect sizes per study and species (Viechtbauer 2010; R Core Team 2013). All analyses were run separately for plant disease severity and biomass. Once the significant heterogeneity was observed in the random-effects meta-analysis, meta-subgroup analyses were conducted to explain with different categorical groups. Publication bias was tested using Funnel plots and Egger tests by including precision (1/SE) as a covariate in rma.mv function, and using meta-analytic residuals (Egger et al. 1997).
3 Results and discussion
3.1 Biochar application on disease suppression and plant growth
Severe plant diseases dramatically reduced plant growth (Fig. 1a, p < 0.001). By comparison, biochar application leads to an average reduction in plant disease severity by 47.46% (CI = 41.42%, 52.88%) and an elevation of plant biomass by 44.05% (CI = 22.41%, 69.52%) (Fig. 1b). Additionally, the broad interpretation range of effect sizes indicated a high variance of biochar performance on plant disease suppression and growth enhancement. Accordingly, a series of in-depth analyses were carried out for each categorical biochar group.
3.2 Influence of biochar properties
Studies indicated that feedstock varieties and production conditions had a great influence on biochar physicochemical properties, and hence impacted their performance in agricultural and environmental applications (Kambo and Dutta 2015; Haris et al. 2021; Zhou et al. 2021). Likely, in this study, biochar feedstock, production temperature, as well as application rates had a great influence on biochar performance on plant disease control and plant growth enhancement (Fig. 2).
3.2.1 Biochar feedstock
As illustrated in Fig. 2a, biochars, regardless of the feedstock species, effectively reduced plant disease severity. More specifically, biochars produced from straw residue (− 0.81, p < 0.001) showed greatest suppression on plant disease (up to 55.29%). Followed by biochars produced from wood (− 0.62, p < 0.001), which inhibit plant diseases by 46.1%. The influence of biochars derived from greenhouse waste had the least effect on plant disease inhibition (− 0.55, p < 0.001), but still reached 42.4%. In terms of plant biomass enhancement, the application of greenhouse waste-derived biochars dramatically promoted plant growth, with the mean effect size at 1.11 (p < 0.001). However, the influence on plant biomass is limited for biochars derived from straw and wood (Fig. 2b).
Feedstock choice has the largest influence on biochar physicochemical properties compared to pyrolysis temperatureand pyrolysis type (Ippolito et al. 2020). As biochars derived from different feedstocks are distinct in the elemental composition and ash constitution (Zhou et al. 2021; Weber and Quicker 2018), dissimilarities in the levels of active substances in biochars, such as soluble organic compounds, silicon, and calcium, etc., would be the main reason for the distinct performance of biochar on plant disease suppression (Dordas 2008). For example, Wang et al. (2019c) attributed the greater plant disease suppression to the higher silicon (Si) content in straw residue derived biochars. As a beneficial element, Si is beneficial for plant growth and efficient Si in plants can inhibit the penetration of pathogenic fungi by strengthening cell walls (Shabbir et al. 2020). For this reason, the application of high-Si biochar in soils reduced gray leaf spots by over 50% (Wang et al. 2019a). Probably, calcium (Ca) and potassium (K), which were also abundant elements in biochars, are essential for maintaining plant cell walls and cell membranes, as well as enhancing growth and metabolism, respectively (Wang et al. 2013; Sugimoto et al. 2010).
Furthermore, studies also found biochar derived from lignocellulosic material has a high surface area and well-developed porous structure, which helps the adsorption of cell wall-degrading enzyme and toxic metabolites, thus effectively reducing the infection by pathogens (Daoud et al. 2010; Jaiswal et al. 2018).
In contrast, the enhanced plant growth was mainly attributed to the abundant nutrients (mainly nitrogen and phosphorus) in biochars derived from greenhouse waste. Biochar land application elevates crop production and the increases in crop yield was greater among biochars produced from animal waste compared with those from crop residue and wood waste (Farhangi-Abriz et al. 2021), which is likely a function of greater amino acids and proteins present in these materials.
3.2.2 Pyrolysis temperature
Figure 2c shows the influence of pyrolysis temperature on the performance of biochar on plant disease suppression. The application of biochar produced between 350 and 600 °C dramatically reduced plant disease severity by 44.8% (− 0.59, p < 0.001). However, no significant differences were observed for biochars produced ≤ 350 °C and ≥ 600 °C. Additionally, the application of biochar that is produced at lower temperatures effectively elevated plant biomass (1.16, p < 0.001). By comparison, the influence of biochar produced in the medium temperature range (350–600 °C) was lower than that of biochar produced at lower temperatures, and the application of biochar produced at temperatures ≥ 600 °C had no significant effect on crop growth (Fig. 2d).
Numerous studies have indicated that pyrolysis temperature is the most important factor that determined biochar physicochemical properties (Hassan et al. 2020; Anand et al. 2022). Apart from the surface area, pH, and cation exchange capacity, which help to mediate soil physiochemical properties, the formation of organic compounds, especially those dissolved compounds, would also affect biochar disease suppression efficiency as well as plant growth. For example, the formation of volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs), polychlorinated dioxins and furans (PCDD/DFs) with the carbonization of organic compounds makes biochar toxic to both soil microorganisms and plants (Hale et al. 2012; Buss and Mašek 2014). Lyu et al. (2016) found that PCDD/DFs and PAHs were mainly produced at 300–400 °C, while biochars produced at higher temperatures became less toxic and lower potencies of AhR-mediated effects. Accordingly, we suggested that the reduction in plant disease occurrence was mainly attributed to toxin formation, while the comprehensive influence of toxicity and fertility determined the overall plant growth under biochar-involved treatments. Furthermore, studies found organic compounds in biochars like benzoic acid, glycol, and phenols, function as plant immunity inducers to enable plants to perform immune response by stimulating systemic resistance (Mehari et al. 2015; Jaiswal et al. 2020; Yang et al. 2022).
3.2.3 Application rate
As indicated, biochar applied at rates of < 10% had positive effects on plant disease suppression. Compared with the control, biochar application at a rate of ≤ 1%, 1–3%, 3–5%, and 50–10% significantly reduced plant disease severity by 37.0% (p < 0.01), 50.1% (p < 0.001), 59.1% (p < 0.001) and 41.5% (p < 0.05), respectively. Only a higher application rate of 10% had no significant effect on plant disease severity (Fig. 2e). Likewise, the greatest plant growth enhancement was also observed under an application rate of 3–5% (Fig. 2f).
As a multifunctional soil amendment, soil application of biochar helps to improve soil physicochemical properties including pH, porosity, bulk density, water retention, organic compounds, and nutrient contents (Herath et al. 2013; Zhang et al. 2021). All these changes in the soil microenvironment regulate the abundance and distribution of soil microorganisms and ultimately affect the occurrence of plant diseases. Since the optimal growth conditions for soil-borne plant pathogens are narrow and greatly impacted by soil Eh–pH, biochar induced pH increase in the rhizosphere dramatically reduced the viability of pathogens. For example, the difference in the soil pH, Ca, Mg, and CEC greatly impacted Pythium species diversity, community composition, and disease incidence (Broders et al. 2009). Likely, the lesion, lance, spiral, and pin nematode population densities are at moderate-high risk levels based on soil region, cropping sequence, tillage, soil pH, silt content, and electrical conductivity (Simon et al. 2018).
It is worth noting that the application rate of biochar is not the only factor that affects soil properties, which are also dependent on biochar properties, such as particle size, elemental composition, and ash content (Fu et al. 2021). For example, the particle size distribution of biochar is an important factor that determines the diminishing of pathogenic bacterial (Sasidharan et al. 2016). Furthermore, although various application rates were used in research from < 5 t ha−1 to over 100 t ha−1, an application rate less than 2.5% was recommended by International Biochar Initiative (IBI 2015).
3.3 Effects of plant type, plant pathogen, and planting system
3.3.1 Plant type
Biochar application can dramatically suppress disease severity of cash crops (including vegetables, berries and tobacco) by 51.5% (p < 0.001), and enhanced plant growth by 52.6% (p < 0.001) (Fig. 3a). However, the biochar amendment had limited influence on plant disease and plant growth for cereal grains and perennial trees (Fig. 3b). Accordingly, biochar application on cash crops would be the most optimal in terms of disease suppression and plant growth enhancement.
Our findings were similar to those from a previous study, in which Ojiambo and Scherm (2006) declared that biocontrol is more effective on annual crops than perennials plants. One possible reason for the distinct efficiencies of biochar on plant disease suppression among different plant types is the agricultural practice, including planting, fertilization, and management. Generally, a long-term intensive planting leads to more severe plant disease in the vegetable field than the cropland and perennial ecosystems (Huang et al. 2013; Kerdraon et al. 2019; Fareed Mohamed Wahdan et al. 2020). For example, the cropping history, litter saprotrophic fungi and spatial patterns greatly impacted the composition of soil-borne pathogens and the outbreak of plant disease (Van Agtmaal et al. 2017). For these reasons, biochar influence on plant disease suppression through modifying soil microbe structure was magnified in those less-intensive crop systems (Zhang et al. 2021; Rasul et al. 2022). However, current studies related to biochar performance on disease suppression was mainly focused on vegetables, and limited studies were conducted for crops and trees. Additionally, the forest plot should be updated to be more objective when more data become available.
3.3.2 Plant pathogens
Plant diseases evaluated in this meta-analysis were caused by a wide variety of plant pathogens, and wecategorized them into the plant diseases caused by airborne pathogens and plant disease caused by soilborne pathogens according to the site of infection that occurred. Contradicted to our assumption, biochar application dramatically reduced the occurrence of plant diseases caused by airborne pathogens (60.0%, p < 0.001) than that caused by soilborne pathogens (34.0%, p < 0.001) (Fig. 3c). Simultaneously, biochar application greatly elevated the biomass of plants infected by airborne pathogens (50.7%, p < 0.001). However, no obvious growth-enhancing effects were observed among plants infected by soilborne pathogens (Additional file 7: Table S4).
Further analysis indicated that plant foliar diseases caused by four airborne-pathogen species were effectively inhibited by biochar amendment, and the efficiencies decreased in the order of L. taurica (powdery mildew) > A. solani (early blight) > B. cinerea (gray mould) > P. aphanis (powdery mildew) (Additional file 2: Fig. S2). By comparison, only half of the soilborne pathogen types were effectively controlled by the land application of biochars, which were Phytophthora species, Ralstonia solanacearum, Fusarium species, and replant disease (Fig. 3e). Moreover, biochar application greatly enhanced the growth of plants infected by A. solani (92.0%, p < 0.001) and replant disease (69.2%, p < 0.05). However, soil application of biochar significantly decreased the growth of plants infected by Rhizoctonia solani (p < 0.01). This result is consistence with the report by Copley et al. (2015), who found that an increasing biochar application rate increased Rhizoctonia damping-off of all 11 plant species. The authors attributed the increased damping-off severity under biochar amendments to the potential metabolism of organic compounds in biochars. Further investigation found biochar application increased the susceptibility of soybeans to a foliar disease by modifying the expression of soybean genes and changes in salicylic acid hormonal balance (Copley et al. 2017).
Intriguingly, biochar additions to soil significantly reduced the incidence of replant disease (Fig. 3e), which is a soilborne disease that negatively affects tree growth and reduces the yields in replanted orchards (Wang et al. 2019d). Numerous nematodes, fungi, oomycete and bacteria can cause replant disease, of which Fusarium, Rhizoctonia, Cylindrocarpon, Phytophthora, and Pythium are the main pathogenic genera associated with this soilborne disease (Tewoldemedhin et al. 2011). Although the mechanisms are still unknown, biochar effectively alleviated replant disease on peach and apple trees, respectively, compared with the control (Wang et al. 2019d; Atucha and Litus 2015).
3.3.3 Cultivation system
As shown in Additional file 4: Table S1, 32 of 49 collected reports (65.3%) selected pot experiments to study the influence of biochar amendment on plant disease suppression. It should be noted that most of the plant growing medium used in these studies belonged to potting mixture with soil and commercial horticultural substrates, in which the organic matter content was much higher than that in agricultural soils. Therefore, we categorized the cultivation systems into two groups: agricultural soils and potting mixture. Biochar application has significant disease suppression effects on plants grown in agricultural soils (54.9%, p < 0.001), whereas biochar addition did not obviously reduce the disease severity on plants grown in potting mixture (Fig. 3e, f). Correspondingly, biochar involvement dramatically elevated the plant production by 57.8% (p < 0.001) in the pot system (Fig. 3f), whereas no obvious growth-promoting effect was observed in potting mixture system (Additional file 7: Table S4).
Soil organic matter (SOM) is the foundation for agricultural soil. SOM content is a critical indicator of soil health through positive impact on soil properties and processes (Doran and Zeiss 2000; Lal 2020). The further analysis illustrated a 68.7% reduction in disease severity (p < 0.001), and a 49.6% increase in plant production (p < 0.001) when SOM content < 10% (Additional file 3: Fig. S3). In different land use types and history, the actual SOM content in soils ranged between 1.7% and 8.8% (Pulleman et al. 2000). Biochar amendment has a positive effect on increasing SOM content, thereby promoting crop health and crop yields. By comparison, biochar involvement has little effect on promoting plant growth in the potting mixture, due to the fact that horticultural substrates contain a higher proportion of organic matter content.
3.4 Potential mechanisms of biochar on plant disease suppression and future implications
As a whole, the mechanisms of biochar application for plant disease suppression and growth enhancement were generally the enhanced plant nutrient supplication, improved host systemic resistance, modified soil-microbial communities and functioning, reduced allopathic and phytotoxic compounds, and established complex rhizosphere-root-soil-pathogen system (Graber et al. 2014; Bonanomi et al. 2015) (Fig. 4). Of note, soil type, soil physicochemical properties and nutrient/pathogen content in soil varied in each study, which had a significant impact on the disease suppression under biochar amendment (de Medeiros et al. 2021). We acknowledge that there are additional factors potentially affecting the efficiencies of biochar land application on plant disease suppression and plant growth that were not included in our meta-analysis. There was still much uncertainty on the relationship between the underlying biochar physicochemical properties and plant disease suppression performance of biochar, which is useful to guide engineering biochar production for achieving better disease suppression efficiencies.
Scheme of biochar application for plant disease suppression in the root and shoot parts. (part of the figure is adapt from https://www.onhydroponics.com/index.php/en/74-news-nutrients-en/99-silicon-supplementation-en)
The results of our meta-analysis also showed that the addition of biochar to soil exposes a significantly greater effect on the progress of diseases caused by airborne plant pathogens than that caused by soilborne plant pathogens, especially for vegetables and fruits (Fig. 3). These observations suggested that the effect of biochar on the priming of plant systemic resistance is stronger than the direct toxicity to plant pathogens, although biochar amendment has been reported to affect the progress of diseases caused by soilborne plant pathogens. Biochar has a porous structure with high surface area, which makes it an effective soil amendment for increasing for microflora settlement (Yang et al. 2020; Quilliam et al. 2013). When biochar is added to soils, it can profoundly affect the complex rhizosphere microbiome, resulting in direct and indirect domino effects on plant development and disease progress (Graber et al. 2014; Bonanomi et al. 2015). In turn, high surface area and porous structure of biochar may provide a suitable habitat for soilborne plant pathogens simultaneously, thereby compromising the disease-suppressive effect of biochar application (Fig. 4).
Additionally, emerging evidence indicated that biochar can alter the rhizosphere microbial community for plant disease management (Silva et al. 2020; Zheng et al. 2020). For instance, the 80 g/kg biochar treatment promoted plant growth by improving fungal communities in the rhizosphere of apple trees and decreasing the abundance of the soilborne pathogen F. solani (Wang et al. 2019d). Rice hull biochar amendment significantly suppressed the disease incidence and index of bacterial wilt through increasing soil bacterial composition and decreasing pathogen Ralstonia abundance (Chen et al. 2020). Likewise, Biochar addition increased the richness and diversity of the bacterial community in the tobacco rhizosphere to protect plants against bacterial wilt (Zhang et al. 2017). These results imply that the analysis of biochar-soil-microbiota interactions is extremely important for assessing the effect of biochar on plant disease suppression. Nevertheless, to date, there is little reports regarding the relationship between plant disease suppression with biochar addition and rhizosphere microbial abundance. Moreover, the microbial community research methods and index statistics are varied, which makes the data difficult to extract. The meta-analysis of biochar amendment on controlling plant diseases by altering soil microbial communities can be continued only after numerous related reports emerge in the future.
4 Conclusion
Biochar soil application can be effective in decreasing plant disease severity while increasing plant biomass simultaneously. However, feedstock type, pyrolysis temperature and application rate all impacted biochar performance on plant disease suppression. In general, soil application of biochar from grain residues at a medium temperature and at 3%-5% was effective in reducing disease severity. Simultaneously, plant type, plant pathosystem and cultivation system also influenced the effect of biochar application on disease suppression and plant growth promotion. In the future, it is necessary to standardize the production and application of biochar to maximize its effect on plant disease control.
Availability of data and materials
E-supplementary data for this work can be found in the e-version of this paper online.
References
Abbas A, Khan SU, Khan WU, Saleh TA, Khan MHU, Ullah S, Ali A, Ikram M (2019) Antagonist effects of strains of Bacillus spp. against Rhizoctonia solani for their protection against several plant diseases: alternatives to chemical pesticides. CR Biol 342(5):124–135
Anand A, Kumar V, Kaushal P (2022) Biochar and its twin benefits: crop residue management and climate change mitigation in India. Renew Sustain Energy Rev 156:111959
Atucha A, Litus G (2015) Effect of biochar amendments on peach replant disease. HortScience 50(6):863–868
Barford E (2013) Crop pests advancing with global warming. Nature 10:13644
Beketov MA, Kefford BJ, Schäfer RB, Liess M (2013) Pesticides reduce regional biodiversity of stream invertebrates. Proc Natl Acad Sci 110(27):11039–11043
Bonanomi G, Ippolito F, Scala F (2015) A “black” future for plant pathology? Biochar as a new soil amendment for controlling plant diseases. J Plant Pathol 97(2):223–234
Broders K, Wallhead M, Austin G, Lipps P, Paul P, Mullen R, Dorrance A (2009) Association of soil chemical and physical properties with Pythium species diversity, community composition, and disease incidence. Phytopathology 99(8):957–967
Buss W, Mašek O (2014) Mobile organic compounds in biochar—a potential source of contamination–phytotoxic effects on cress seed (Lepidium sativum) germination. J Environ Manage 137:111–119
Chaloner TM, Gurr SJ, Bebber DP (2021) Plant pathogen infection risk tracks global crop yields under climate change. Nat Clim Chang 11(8):710–715
Chen WF, Meng J, Han XR, Lan Y, Zhang WM (2019) Past, present, and future of biochar. Biochar 1(1):75–87. https://doi.org/10.1007/s42773-019-00008-3
Chen S, Qi GF, Ma GQ, Zhao XY (2020) Biochar amendment controlled bacterial wilt through changing soil chemical properties and microbial community. Microbiol Res 231:126373. https://doi.org/10.1016/j.micres.2019.126373
Copley TR, Aliferis KA, Jabaji S (2015) Maple bark biochar affects Rhizoctonia solani metabolism and increases damping-off severity. Phytopathology 105(10):1334–1346
Copley T, Bayen S, Jabaji S (2017) Biochar amendment modifies expression of soybean and Rhizoctonia solani genes leading to increased severity of Rhizoctonia Foliar Blight. Front Plant Sci 8:221
Daoud FB-O, Kaddour S, Sadoun T (2010) Adsorption of cellulase Aspergillus niger on a commercial activated carbon: kinetics and equilibrium studies. Colloids Surf, B 75(1):93–99
De Corato U (2020) Disease-suppressive compost enhances natural soil suppressiveness against soil-borne plant pathogens: a critical review. Rhizosphere 13:100192
de Medeiros EV, Lima NT, de Sousa Lima JR, Pinto KMS, da Costa DP, Franco Junior CL, Souza RMS, Hammecker C (2021) Biochar as a strategy to manage plant diseases caused by pathogens inhabiting the soil: a critical review. Phytoparasitica 49(4):713–726
Dignam BE, O’Callaghan M, Condron LM, Raaijmakers JM, Kowalchuk GA, Wakelin SA (2019) Impacts of long-term plant residue management on soil organic matter quality, Pseudomonas community structure and disease suppressiveness. Soil Biol Biochem 135:396–406
Doran JW, Zeiss MR (2000) Soil health and sustainability: managing the biotic component of soil quality. Appl Soil Ecol 15(1):3–11. https://doi.org/10.1016/S0929-1393(00)00067-6
Dordas C (2008) Role of nutrients in controlling plant diseases in sustainable agriculture a review. Agron Sustain Dev 28(1):33–46
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ Brit Med J 315(7109):629–634
Elad Y, David DR, Harel YM, Borenshtein M, Kalifa HB, Silber A, Graber ER (2010) Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology 100(9):913–921
Elmer W, Ma C, White J (2018) Nanoparticles for plant disease management. Curr Opin Environ Sci Health 6:66–70
Evans N, Baierl A, Semenov MA, Gladders P, Fitt BD (2008) Range and severity of a plant disease increased by global warming. J R Soc Interface 5(22):525–531
Fareed Mohamed Wahdan S, Hossen S, Tanunchai B, Schädler M, Buscot F, Purahong W (2020) Future climate significantly alters fungal plant pathogen dynamics during the early phase of wheat litter decomposition. Microorganisms 8(6):908
Farhangi-Abriz S, Torabian S, Qin R, Noulas C, Lu Y, Gao S (2021) Biochar effects on yield of cereal and legume crops using meta-analysis. Sci Total Environ 775:145869
Frenkel O, Jaiswal AK, Elad Y, Lew B, Kammann C, Graber ER (2017) The effect of biochar on plant diseases: what should we learn while designing biochar substrates? J Environ Eng Landsc 25(2):105–113
Fu G, Qiu X, Xu X, Zhang W, Zang F, Zhao C (2021) The role of biochar particle size and application rate in promoting the hydraulic and physical properties of sandy desert soil. CATENA 207:105607
Graber E, Frenkel O, Jaiswal A, Elad Y (2014) How may biochar influence severity of diseases caused by soilborne pathogens? Carbon Manag 5(2):169–183
Gul S, Whalen JK (2016) Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol Biochem 103:1–15
Hale SE, Lehmann J, Rutherford D, Zimmerman AR, Bachmann RT, Shitumbanuma V, O’Toole A, Sundqvist KL, Arp HPH, Cornelissen G (2012) Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ Sci Technol 46(5):2830–2838
Haris M, Hamid Y, Usman M, Wang L, Saleem A, Su F, Guo J, Li Y (2021) Crop-residues derived biochar: synthesis, properties, characterization and application for the removal of trace elements in soils. J Hazard Mater 416:126212
Hassan M, Liu Y, Naidu R, Parikh SJ, Du J, Qi F, Willett IR (2020) Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: a meta-analysis. Sci Total Environ 744:140714
He D-C, He M-H, Amalin DM, Liu W, Alvindia DG, Zhan J (2021) Biological control of plant diseases: an evolutionary and eco-economic consideration. Pathogens 10(10):1311
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80(4):1150–1156
Herath H, Camps-Arbestain M, Hedley M (2013) Effect of biochar on soil physical properties in two contrasting soils: an Alfisol and an Andisol. Geoderma 209:188–197
Hirooka T, Ishii H (2013) Chemical control of plant diseases. J Gen Plant Pathol 79(6):390–401
Huang L-F, Song L-X, Xia X-J, Mao W-H, Shi K, Zhou Y-H, Yu J-Q (2013) Plant-soil feedbacks and soil sickness: from mechanisms to application in agriculture. J Chem Ecol 39(2):232–242
Huang X, Liu S, Liu X, Zhang S, Li L, Zhao H, Zhao J, Zhang J, Cai Z (2020) Plant pathological condition is associated with fungal community succession triggered by root exudates in the plant-soil system. Soil Biol Biochem 151:108046
IBI (2015) Standardized product definition and product testing guidelines for biochar that is used in soil. Int Biochar Initiative 23
Ippolito JA, Cui LQ, Kammann C, Wrage-Monnig N, Estavillo JM, Fuertes-Mendizabal T, Cayuela ML, Sigua G, Novak J, Spokas K, Borchard N (2020) Feedstock choice, pyrolysis temperature and type influence biochar characteristics: a comprehensive meta-data analysis review. Biochar 2(4):421–438. https://doi.org/10.1007/s42773-020-00067-x
Jaiswal AK, Frenkel O, Tsechansky L, Elad Y, Graber ER (2018) Immobilization and deactivation of pathogenic enzymes and toxic metabolites by biochar: a possible mechanism involved in soilborne disease suppression. Soil Biol Biochem 121:59–66
Jaiswal AK, Alkan N, Elad Y, Sela N, Philosoph AM, Graber ER, Frenkel O (2020) Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato against Fusarium crown and root rot disease. Sci Rep 10(1):13934
Joseph S, Lehmann J (2015) Biochar for environmental management: an introduction. In: Biochar for environmental management: science and technology.
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew Sustain Energy Rev 45:359–378
Kerdraon L, Laval V, Suffert F (2019) Microbiomes and pathogen survival in crop residues, an ecotone between plant and soil. Phytobiomes J 3(4):246–255
Kolton M, Graber ER, Tsehansky L, Elad Y, Cytryn E (2017) Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol 213(3):1393–1404
Lal R (2020) Soil organic matter content and crop yield. J Soil Water Conserv 75(2):27a–32a. https://doi.org/10.2489/jswc.75.2.27A
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43(9):1812–1836
Lyu H, He Y, Tang J, Hecker M, Liu Q, Jones PD, Codling G, Giesy JP (2016) Effect of pyrolysis temperature on potential toxicity of biochar if applied to the environment. Environ Pollut 218:1–7
Mehari ZH, Elad Y, Rav-David D, Graber ER, Meller Harel Y (2015) Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil 395(1):31–44
Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamati P, Hens L (2016) Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health 4:148
Nwokolo NL, Enebe MC, Chigor CB, Chigor VN, Dada OA (2021) The contributions of biotic lines of defence to improving plant disease suppression in soils: a review. Rhizosphere 19:100372
Ojiambo P, Scherm H (2006) Biological and application-oriented factors influencing plant disease suppression by biological control: a meta-analytical review. Phytopathology 96(11):1168–1174
Poveda J, Martínez-Gómez Á, Fenoll C, Escobar C (2021) The use of biochar for plant pathogen control. Phytopathology 111(9):1490–1499
Pulleman MM, Bouma J, van Essen EA, Meijles EW (2000) Soil organic matter content as a function of different land use history. Soil Sci Soc Am J 64(2):689–693. https://doi.org/10.2136/sssaj2000.642689x
Quilliam RS, Glanville HC, Wade SC, Jones DL (2013) Life in the ’charosphere’—does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol Biochem 65:287–293. https://doi.org/10.1016/j.soilbio.2013.06.004
R Core Team (2013) R: a language and environment for statistical computing
Rasul M, Cho J, Shin H-S, Hur J (2022) Biochar-induced priming effects in soil via modifying the status of soil organic matter and microflora: a review. Sci Total Environ 805:150304
Ristaino JB, Anderson PK, Bebber DP, Brauman KA, Cunniffe NJ, Fedoroff NV, Finegold C, Garrett KA, Gilligan CA, Jones CM (2021) The persistent threat of emerging plant disease pandemics to global food security. Proc Natl Acad Sci 118(23):e2022239118
Sasidharan S, Torkzaban S, Bradford SA, Kookana R, Page D, Cook PG (2016) Transport and retention of bacteria and viruses in biochar-amended sand. Sci Total Environ 548:100–109
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A (2019) The global burden of pathogens and pests on major food crops. Nat Ecol Evol 3(3):430–439
Shabbir I, Abd Samad MY, Othman R, Wong M-Y, Sulaiman Z, Jaafar NM, Bukhari SAH (2020) White root rot disease suppression in rubber plant with microbial co-inoculants and silicon addition. Rhizosphere 15:100221
Sileshi GW, Gebeyehu S (2021) Emerging infectious diseases threatening food security and economies in Africa. Glob Food Sec 28:100479
Silva LG, de Andrade CA, Bettiol W (2020) Biochar amendment increases soil microbial biomass and plant growth and suppresses Fusarium wilt in tomato. Tropical Plant Pathol 45(1):73–83
Simon A, Lopez-Nicora HD, Niblack TL, Dayton EA, Tomashefski D, Paul PA (2018) Cropping practices and soil properties associated with plant-parasitic nematodes in corn fields in Ohio. Plant Dis 102(12):2519–2530
Strange RN, Scott PR (2005) Plant disease: a threat to global food security. Annu Rev Phytopathol 43:83–116
Sugimoto T, Watanabe K, Yoshida S, Aino M, Furiki M, Shiono M, Matoh T, Biggs A (2010) Field application of calcium to reduce Phytophthora stem rot of soybean, and calcium distribution in plants. Plant Dis 94(7):812–819
Tewoldemedhin YT, Mazzola M, Botha WJ, Spies CFJ, McLeod A (2011) Characterization of fungi (Fusarium and Rhizoctonia) and oomycetes (Phytophthora and Pythium) associated with apple orchards in South Africa. Eur J Plant Pathol 130(2):215–229. https://doi.org/10.1007/s10658-011-9747-9
Van Agtmaal M, Straathof A, Termorshuizen A, Teurlincx S, Hundscheid M, Ruyters S, Busschaert P, Lievens B, de Boer W (2017) Exploring the reservoir of potential fungal plant pathogens in agricultural soil. Appl Soil Ecol 121:152–160
Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, Kuss O, Higgins JPT, Langan D, Salanti G (2016) Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 7(1):55–79
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor Package. J Stat Softw 36(3):1–48
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14(4):7370–7390
Wang M, Wang JJ, Tafti ND, Hollier CA, Myers G, Wang X (2019a) Effect of alkali-enhanced biochar on silicon uptake and suppression of gray leaf spot development in perennial ryegrass. Crop Prot 119:9–16
Wang Y, Villamil MB, Davidson PC, Akdeniz N (2019b) A quantitative understanding of the role of co-composted biochar in plant growth using meta-analysis. Sci Total Environ 685:741–752
Wang Y, Xiao X, Zhang K, Chen B (2019c) Effects of biochar amendment on the soil silicon cycle in a soil-rice ecosystem. Environ Pollut 248:823–833
Wang YF, Ma ZT, Wang XW, Sun QR, Dong HQ, Wang GS, Chen XS, Yin CM, Han ZH, Mao ZQ (2019d) Effects of biochar on the growth of apple seedlings, soil enzyme activities and fungal communities in replant disease soil. Sci Hortic-Amsterdam 256:108641
Weber K, Quicker P (2018) Properties of biochar. Fuel 217:240–261
Yang YH, Zhang T (2019) Antimicrobial activities of tea polyphenol on phytopathogens: a review. Molecules 24(4):816
Yang W, Shang JY, Li BG, Flury M (2020) Surface and colloid properties of biochar and implications for transport in porous media. Crit Rev Env Sci Tec 50(23):2484–2522. https://doi.org/10.1080/10643389.2019.1699381
Yang B, Yang S, Zheng W, Wang Y (2022) Plant immunity inducers: from discovery to agricultural application. Stress Biol 2(1):5
Yang Y, Chen T, Liu X, Wang S, Wang K, Xiao R, Chen X, Zhang T (2022b) Ecological risk assessment and environment carrying capacity of soil pesticide residues in vegetable ecosystem in the Three Gorges Reservoir Area. J Hazardous Mater. 435:128987
Yu H, Zou W, Chen J, Chen H, Yu Z, Huang J, Tang H, Wei X, Gao B (2019) Biochar amendment improves crop production in problem soils: a review. J Environ Manage 232:8–21
Zhang CS, Lin Y, Tian XY, Xu Q, Chen ZH, Lin W (2017) Tobacco bacterial wilt suppression with biochar soil addition associates to improved soil physiochemical properties and increased rhizosphere bacteria abundance. Appl Soil Ecol 112:90–96. https://doi.org/10.1016/j.apsoil.2016.12.005
Zhang Y, Wang J, Feng Y (2021) The effects of biochar addition on soil physicochemical properties: a review. CATENA 202:105284
Zheng X, Wang Z, Zhu Y, Wang J, Liu B (2020) Effects of a microbial restoration substrate on plant growth and rhizosphere bacterial community in a continuous tomato cropping greenhouse. Sci Rep 10(1):13729
Zhou Y, Qin S, Verma S, Sar T, Sarsaiya S, Ravindran B, Liu T, Sindhu R, Patel AK, Binod P (2021) Production and beneficial impact of biochar for environmental application: a comprehensive review. Biores Technol 337:125451
Zhu X, Chen B, Zhu L, Xing B (2017) Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pollut 227:98–115
Acknowledgements
Not applicable.
Funding
This research was financially supported by the National Natural Science Foundation of China (No. U20A2047, 41907062), Changjiang Scholarship, Ministry of Education, China; and State Cultivation Base of Eco-agriculture for Southwest Mountainous Land, Southwest University.
Author information
Authors and Affiliations
Contributions
YY: Methodology, Investigation, Data Curation, Writing—Original Draft; TC: Formal analysis, Data Curation, Software, Visualization; RX: Writing—Review and Editing; XC: Supervision, Project administration, Funding acquisition; TZ: Conceptualization, Project administration, Funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Supplementary Information
Additional file 1: Fig. S1.
Geographical location of the 49 studies included in the meta-analysis. Locations as orange dots.
Additional file 2: Fig. S2.
Influence of biochar application on on plant disease suppression (a) and plant growth (b) under different pathogen-infections, respectively.
Additional file 3: Fig. S3.
Influence of biochar application on on plant disease suppression (a) and plant growth (b) under different organic matters in growing substrates, respectively.
Additional file 4: Table S1.
List of the 49 studies analyzed in the meta-analysis.
Additional file 5: Table S2.
List of data sources from the 49 studies analyzed in the meta-analysis.
Additional file 6: Table S3.
The percentage changes in disease suppression and plant productivity.
Additional file 7: Table S4.
Effect sizes and regression coefficients for disease suppression and plant productivity. Average random-model effect sizes (“Mean Effect size”) and 95% Confidence Intervals (“LCI” and “UCI”) were calculated by the categorical groups of biochar and plants separately.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Chen, T., Xiao, R. et al. A quantitative evaluation of the biochar’s influence on plant disease suppress: a global meta-analysis. Biochar 4, 43 (2022). https://doi.org/10.1007/s42773-022-00164-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00164-z