Abstract
Despite the lower reactivity of natural phosphates compared to soluble fertilizers, their P bioavailability can increase over the cultivation years, due to the physicochemical processes and the activity of soil microbiota. Therefore, this work aimed to evaluate the α and β diversity of the rhizosphere microbiota of maize and sorghum genotypes grown under different sources and doses of phosphate fertilizers. Four commercial maize and four sorghum genotypes were grown under field conditions with three levels of triple superphosphate (TSP) and two types of rock phosphate sources: phosphorite (RockP) and bayóvar (RP) during two seasons. Maize and sorghum presented a significant difference on the genetic β diversity of both rhizosferic bacterial and arbuscular mycorrhizal fungi. Moreover, P doses within each phosphate source formed two distinct groups for maize and sorghum, and six bacterial phyla were identified in both crops with significant difference in the relative abundance of Firmicutes and Proteobacteria. It was observed that RockP fertilization increased Firmicutes population while Proteobacteria was the most abundant phylum after TSP fertilization in maize. In sorghum, a significant impact of fertilization was observed on the Acidobacteria and Proteobacteria phyla. TSP fertilization increased the Acidobacteria population compared to no fertilized (P0) and RockP while Proteobacteria abundance in RockP was reduced compared to P0 and TSP, indicating a shift toward a more copiotrophic community. Our results suggested that the reactivity of P source is the predominant factor in bacterial community’ structures in the maize and sorghum rhizosphere from the evaluated genotypes, followed by P source.




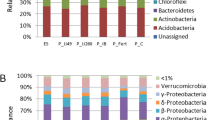
source formed two distinct groups for maize and sorghum: (I) 0 kg P2O5 ha−1 and (II) 50 and 100 kg P2O5 ha−1. Stress and ANOSIM p ≤ 0.05



Similar content being viewed by others
References
Bindraban PS, Dimkpa CO, Pandey R (2020) Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol Fertil Soils 56:299–317. https://doi.org/10.1007/s00374-019-01430-2
Cordell D, White S (2011) Peak phosphorus: clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 3:2027–2049. https://doi.org/10.3390/su3102027
Chowdhury RB, Moore GA, Weatherley AJ, Arora M (2017) Key sustainability challenges for the global phosphorus resource, their implications for global food security, and options for mitigation. J Clean Prod 140:945–963. https://doi.org/10.1016/j.jclepro.2016.07.012
Rashid M, Khalil S, Ayub N, Alam S, Latif F (2004) Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms (PSM) under in vitro conditions. Pak J Biol Sci 7:187–196. https://doi.org/10.3923/pjbs.2004.187.196
Mendes GO, De Freitas ALM, Pereira OL, Da Silva IR, Vassilev NB, Costa MD (2014) Mechanisms of phosphate solubilization by fungal isolates when exposed to different P sources. Ann Microbiol 64:239–249. https://doi.org/10.1007/s13213-013-0656-3
Timmusk S, Behers L, Muthoni J, Muraya A, Aronsson AC (2017) Perspectives and challenges of microbial application for crop improvement. Front Plant Sci 8:49. https://doi.org/10.3389/fpls.2017.00049
Kari A, Nagymáté Z, Romsics C, Vajna B, Kutasi J, Puspán I, Kárpáti E, Kovács R, Márialigeti K (2019) Monitoring of soil microbial inoculants and their impact on maize (Zea mays L.) rhizosphere using T-RFLP molecular fingerprint method. Appl Soil Ecol 138. https://doi.org/10.1016/j.apsoil.2019.03.010
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41. https://doi.org/10.1093/nar/gks808
Peiffer JA, Spor A, Koren O, Jin Z, Tringe SE, Dangl EE, Ley BR (2013) Diversity and heritability of the maize rhizosphere microbiome under field conditions. PNAS USA 110:6548–6553. https://doi.org/10.1073/pnas.1302837110
Yang Y, Wang N, Guo X, Zhang Y, Ye B (2017) Comparative analysis of bacterial community structure in the rhizosphere of maize by high-throughput pyrosequencing. Plos One 12:e0178425. https://doi.org/10.1371/journal.pone.0178425
De Vrieze J, Ijaz UZ, Saunders AM, Theuerl S (2018) Terminal restriction fragment length polymorphism is an “old school” reliable technique for swift microbial community screening in anaerobic digestion. Sci Rep 8:16818. https://doi.org/10.1038/s41598-018-34921-7
Bissett A, Brown MV, Siciliano SD, Thrall PH (2013) Microbial community responses to anthropogenically induced environmental change: towards a systems approach. Ecol Lett 1:128–139. https://doi.org/10.1111/ele.12109
Trabelsi D, Cherni A, Eddine BZA, Dhane-Fitouri S, Mhamdi R (2017) Fertilization of Phaseolus vulgaris with the Tunisian rock phosphate affects richness and structure of rhizosphere bacterial communities. Appl Soil Ecol 114. https://doi.org/10.1016/j.apsoil.2016.11.014
Macik M, Gryta A, Frac M (2020) Biofertilizers in agriculture: an overview on concepts, strategies and effects on soil microorganisms. Adv Agron 162:31–87. https://doi.org/10.1016/bs.agron.2020.02.001
Ji Y, Conrad R, Xu H (2020) Responses of archaeal, bacterial, and functional microbial communities to growth season and nitrogen fertilization in rice fields. Biol Fertil Soils 56. https://doi.org/10.1007/s00374-019-01404-4
Li P, Chen W, Han Y, Wang D, Zhang Y, Wu C (2020) Effects of straw and its biochar applications on the abundance and community structure of CO2-fixing bacteria in a sandy agricultural soil. Journal of Soils and Sediments. 20. https://doi.org/10.1007/s11368-020-02584-5
Bánfi R, Pohner Z, Szabó A, Herczeg G, Kovács GM, Nagy A, Márialigeti K, Vajna B (2021) Succession and potential role of bacterial communities during Pleurotus ostreatus production. FEMS Microbiol Ecol 97. https://doi.org/10.1093/femsec/fiab125
Karczewski K, Riss HW, Meyer EI (2017) Comparison of DNA-fingerprinting (T-RFLP) and high-throughput sequencing (HTS) to assess the diversity and composition of microbial communities in groundwater ecosystems. Limnologica 67:45–53. https://doi.org/10.1016/j.limno.2017.10.001
Johnston-Monje D, Mejia JL (2020) Botanical microbiomes on the cheap: Inexpensive molecular fingerprinting methods to study plant-associated communities of bacteria and fungi. Appl Plant Sci 8:e11334. https://doi.org/10.1002/aps3.11334
Novais RF, Smyth TJ (1999) Fósforo em solo e planta em condições tropicais. Universidade Federa de Viçosa, Viçosa
Marschner P, Solaiman Z, Rengel Z (2006) Rhizosphere properties of poaceae genotypes under P-limiting conditions. Plant Soil 283:11–24. https://doi.org/10.1007/s11104-005-8295-5
Tang XY, Placella SA, Dayde F, Bernard L, Robin A, Journet EP, Justes E, Hinsinger P (2016) Phosphorus availability and microbial community in the rhizosphere of intercropped cereal and legume along a P-fertilizer gradient. Plant Soil 407:119–134. https://doi.org/10.1007/s11104-016-2949-3
Silva UC, Medeiros JD, Leite LR, Morais DK, Cuadros-Orellana S, Oliveira CA, de Paula Lana UG, Gomes EA, Dos Santos VL (2017) Long-term rock phosphate fertilization impacts the microbial communities of maize rhizosphere. Front Microbiol 8:1266. https://doi.org/10.3389/fmicb.2017.01266
Gomes EA, Lana UGP, Quensen JF, de Sousa SM, Oliveira CA, Guo J, Guimarães LJM, Tiedje JM (2018) Root-associated microbiome of maize genotypes with contrasting phosphorus use efficiency. Phytobiomes 2. https://doi.org/10.1094/PBIOMES-03-18-0012-R
Santos HG, Jacomine PKT, Anjos LHC, Oliveira VA, Lumbreras JF, Coelho MR, Almeida JA, Cunha, TJF, Oliveira JB (2013) Sistema brasileiro de classificação de solos. Embrapa, Brasília
Embrapa, (1997) Manual de métodos e análise de solo. Brasil, Rio de Janeiro
La Montagne MG, Michel FC Jr, Holden PA, Reddy CA (2002) Evaluation of extraction and purification methods for obtaining PCR-amplifiable DNA from compost for microbial community analysis. J Microbiol Methods 49:255–264. https://doi.org/10.1016/s0167-7012(01)00377-3
Turner S, Pryer KM, Miao VPW, Palmer JD (1999) Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiology 46:327–338. https://doi.org/10.1111/j.1550-7408.1999.tb04612.x
Trouvelot S, van Tuinen D, Hijri M, Gianinazzi-Pearson V (1999) Visualization of ribosomal DNA loci in spore interphasic nuclei of glomalean fungi by fluorescence in situ hyhridization. Mycorrhiza 8:203–206. https://doi.org/10.1007/s005720050235
Tipaynoa S, Kimb C, Tongmin SAA (2012) T-RFLP analysis of structural changes in soil bacterial communities in response to metal and metalloid contamination and initial phytoremediation. Appl Soil Ecol 6:137–146. https://doi.org/10.1016/j.apsoil.2012.06.001
Verbruggen E, Van Der Heijden MGA, Weedon JT, Kowalchuk GA, Röling WF (2012) Community assembly, species richness and nestedness of arbuscular mycorrhizal fungi in agricultural soils. Mol Eco 21:2341–2353. https://doi.org/10.1111/j.1365-294X.2012.05534.x
Culman SW, Bukowski R, Gauch HG, Cadillo-Quiroz H, Buckley DH (2009) T-REX: software for the processing and analysis of T-RFLP data. BMC Bioinformatics 10:171. https://doi.org/10.1186/1471-2105-10-171
Fredriksson N, Hermansson M, Wilén BM (2014) Tools for T-RFLP data analysis using Excel. BMC Bioinformatics 15:361. https://doi.org/10.1186/s12859-014-0361-7
Shyu C, Soule T, Bent SJ, Foster JA, Forney LJ (2007) MiCA: a web- based tool for the analysis of microbial communities based on terminal-restriction fragment length polymorphisms of 16S and 18S rRNA genes. Microb Ecol 53:562–570. https://doi.org/10.1007/s00248-006-9106-0
Hammer O, Harper D, Ryan P (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen TL, Miller E, Bache SM, Müller K, Ooms J, Robinson D, Seidel DP, Spinu V, Takahashi K, Vaughan D, Wilke C, Woo K, Yutani H (2019) Welcome to the tidyverse. J Open Source Softw 4:1686. https://doi.org/10.21105/joss.01686
Epskamp S, Cramer A, Waldorp L, Schmittmann V, Borsboom D (2012) qgraph: network visualizations of relationships in psychometric data. J Stat Softw 48. https://doi.org/10.18637/jss.v048.i04
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, Roskot N, Heuer H, Berg G (2001) Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl Environ Microbiol 67:4742–4751. https://doi.org/10.1128/AEM.67.10.4742-4751.2001
Grayston SJ, Wang S, Campbell CD, Edwards AC (1998) Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem 30:369–378. https://doi.org/10.1016/S0038-0717(97)00124-7
Grayston S, Griffith G, Mawdsley JL, Campbell C, Bardgett RD (2001) Accounting for variability in soil microbial communities of temperate upland grassland ecosystems. Soil Biol Biochem 33:533–551. https://doi.org/10.1016/S0038-0717(00)00194-2
Bulgarelli D, Rott M, Schlaeppi K, Loren V, van Themaat E, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P (2012) Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488:91–95. https://doi.org/10.1038/nature11336
Bouffaud ML, Poirier MA, Muller D, Moënne-Loccoz Y (2014) Root microbiome relates to plant host evolution in maize and other Poaceae: Poaceae evolution and root bacteria. Environ Microbiol 16:2804–2814. https://doi.org/10.1111/1462-2920.12442
Wieland G, Neumann R, Backhaus H (2002) Variation of microbial communities in soil, rhizosphere, and rhizoplane in response to crop species, soil type, and crop development. Appl Environ Microbiol 67:5849–5854. https://doi.org/10.1128/AEM.67.12.5849-5854.2001
Inceoglu O, Al-Soud WA, Salles JF, Semenov AV, van Elsas JD (2011) Comparative analysis of bacterial communities in a potato field as determined by pyrosequencing. PLoS ONE 6:e23321. https://doi.org/10.1371/journal.pone.0023321
Pérez-Jaramillo JE, Mendes R, Raaijmakers JM (2016) Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol Biol 90:635–644. https://doi.org/10.1007/s11103-015-0337-7
Jansa J, Finlay R, Wallander H, Smith F, Smith S (2011). Role of mycorrhizal symbioses in phosphorus cycling. https://doi.org/10.1007/978-3-642-15271-9_6
Zhang Z-Z, Srivastava A, Wu Q-S, Li G-H (2015) Growth performance and rhizospheric traits of peach (Prunus persica) in response to mycorrhization on replant versus non-replant soil. Indian J Agric Sci 85:125–130
Teng W, Deng Y, Chen XP, Xu XF, Chen RY, Lv Y, Zhao YY, Zhao XQ, He X, Li B, Tong YP, Zhang FS, Li ZS (2013) Characterization of root response to phosphorus supply from morphology to gene analysis in field-grown wheat. J Exp Bot 64:1403–1411. https://doi.org/10.1093/jxb/ert023
Deng Y, Feng G, Chen X, Zou C (2017) Arbuscular mycorrhizal fungal colonization is considerable at optimal Olsen-P levels for maximized yields in an intensive wheat-maize cropping system. Field Crops Res 209:1–9. https://doi.org/10.1016/j.fcr.2017.04.004
Pantigoso HA, Manter DK, Vivanco JM (2020) Differential effects of phosphorus fertilization on plant uptake and rhizosphere microbiome of cultivated and non-cultivated potatoes. Microb Ecol 80. https://doi.org/10.1007/s00248-020-01486-w
Marques JM, da Silva TF, Vollu RE (2014) Plant age and genotype affect the bacterial community composition in the tuber rhizosphere of field-grown sweet potato plants. FEMS Microbiol Ecol 88:424–435. https://doi.org/10.1111/1574-6941.12313
Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, Del Rio TG, Edgar RC, Eickhorst T, Ley RE, Hugenholtz P, Tringe SG, Dangl JL (2012) Defining the core Arabidopsis thaliana root microbiome. Nature 488:86–90. https://doi.org/10.1038/nature11237
Aira M, Gómez-Brandón M, Lazcano C, Bååth E, Domínguez J (2010) Plant genotype strongly modifies the structure and growth of maize rhizosphere microbial communities. Soil Biol Biochem 42:2276–2281. https://doi.org/10.1016/j.soilbio.2010.08.029
Schlemper TR, Leite MFA, Lucheta AR, Shimels M, Bouwmeester HJ, van Veen JA, Kuramae EE (2017) Rhizobacterial community structure differences among sorghum cultivars in different growth stages and soils. FEMS Microbiol Ecol 93. https://doi.org/10.1093/femsec/fix096
Hunter PJ, Teakle, GR, Bending GD (2014) Root traits and microbial community interactions in relation to phosphorus availability and acquisition, with particular reference to Brassica. Front. Plant Sci 11. https://doi.org/10.3389/fpls.2014.00027
Zhang D, Cheng H, Geng L, Kan G, Cui S, Meng Q, Gai J, Yu D (2009) Detection of quantitative trait loci for phosphorus deficiency tolerance at soybean seedling stage. Euphytica 167:313–322. https://doi.org/10.1007/s10681-009-9880-0
Gosling P, Mead A, Proctor M, Hammond JP, Bending GD (2013) Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytol 198:546–556. https://doi.org/10.1111/nph.12169
Wang Y-F, Li Xiangzhen DR, Chen J, Du Y, Du D-L (2022) Key factors shaping prokaryotic communities in subtropical forest soils. Appl Soil Ecol 169:104162. https://doi.org/10.1016/j.apsoil.2021.104162
Osman J, DuBow M (2019) Bacterial communities on the surface of oligotrophic (nutrient-poor) soils. Curr Top Biotechnol 9:31–44
Neal AL, McLaren T, Campolino ML, Hughes D, Coelho AM, Lana UGP, Gomes EA, de Sousa SM (2021) Crop type exerts greater influence upon rhizosphere phosphohydrolase gene abundance and phylogenetic diversity than phosphorus fertilization. FEMS Microbiol Ecol 97:fiab033. https://doi.org/10.1093/femsec/fiab033
Leff JW, Jones SE, Prober SM, Barberán A, Borer ET, Firn JL, Harpole WS, Hobbie SE, Hofmockel KS, Knops JM, McCulley RL, La Pierre K, Risch AC, Seabloom EW, Schütz M, Steenbock C, Stevens CJ, Fierer N (2015) Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc Natl Acad Sci 112:10967–10972. https://doi.org/10.1073/pnas.1508382112
Tan H, Barret M, Mooij MJ, Rice O, Morrissey JP, Dobson A (2013) Long-term phosphorus fertilization increased the diversity of the total bacterial community and the phoD phosphorus mineralizer group in pasture soils. Biol Fertil Soils 49:661–672. https://doi.org/10.1007/s00374-012-0755-5
Robbins C, Thiergart T, Hacquard S, Garrido-Oter R, Gans W, Peiter E, Paul Schulze-Lefert Spaepen S (2018) Root-associated bacterial and fungal community profiles of Arabidopsis thaliana are robust across contrasting soil P levels. Phytobiomes 2. https://doi.org/10.1094/PBIOMES-09-17-0042-R
Acknowledgements
This work was supported by Empresa Brasileira de Pesquisa Agropecuaria, Embrapa (Grant number 12.14.10.003.00.00) and Conselho Nacional de Desenvolvimento Científico e Tecnologico, CNPq/INCT-Plant-Growth “Promoting Microorganisms for Agricultural Sustainability and Environmental Responsibility” (Grant number 465133/2014–2, Fundação Araucaria-STI, Capes). MLC was recipient of a research fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior, Capes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Luiz Henrique Rosa
Supplementary Information

ESM 1
Non-metric multidimensional scaling analysis (NMDS) using the Bray-Curtis distance matrix for the mycorrhizal arbuscular fungi community β diversity of different maize (a, b, c) and sorghum (d, e, f) genotypes grown under different fertilizers and P2O5 doses in 2016/17 season. Stress and ANOSIM p≤0.05.(PNG 527 kb)

ESM 2
Non-metric multidimensional scaling analysis (NMDS) using the Bray-Curtis distance matrix for the mycorrhizal arbuscular fungi community β diversity of different maize (a, b, c) and sorghum (d, e, f) genotypes grown under different fertilizers and P2O5 doses in 2017/18 season. Stress and ANOSIM p≤0.05. (TIF 139 kb)(PNG 532 kb)
Rights and permissions
About this article
Cite this article
Campolino, M.L., de Paula Lana, U.G., Gomes, E.A. et al. Phosphate fertilization affects rhizosphere microbiome of maize and sorghum genotypes. Braz J Microbiol 53, 1371–1383 (2022). https://doi.org/10.1007/s42770-022-00747-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00747-9