Abstract
Despite great efforts and achievement of nanomaterials in immune-associated diseases, the selection of appropriate nanomaterials and preparation technology remain some challenges and vast room for improvement. Immunotherapy has received tremendous attention throughout the medical process due to its clinical successes with the pathways of immunoactivation or immunosuppression. Recently, fibrous nanomaterials have facilitated advances in tissue repair and cancer treatments owing to the superiority of multi-channel structure, biocompatibility, tunable size and controlled surface modification. The immunoactivation-based nanofibers can potentially deliver functional agents to lesions and further actively promote immunologic intervention. On the contrary, the immunosuppression-based nanofibers prevent the immune system from overreacting through the blockage of critical pathways in vivo. This review summarizes the current application of nanofiber materials in diverse diseases, including cancer therapy, tissue regeneration (cartilage/bone, skin, tendon, nerves), myocardial infarction, psoriasis and organ defects. Some common fabrication technologies of biomedical nanofibers are also introduced. Meanwhile, the existing technical barriers and perspectives are rationally discussed, providing a constructive inspiration for the follow-up basic research and clinical transformation of nanofibers in the vibrant biomedical fields.
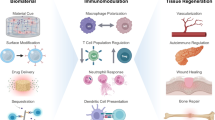
Graphical Abstract

Schematic illustration of nanofibers applied in immunoregulation-based therapy. A variety of diseases can be treated via regulating immune microenvironment including tendon regeneration, bone/cartilage repair, nerve regeneration, skin regeneration and cancer therapy. Therefore, multifunctional nanofibers can provide opportunities for future construction of efficient immune therapy for cancer immunotherapy, tissue regeneration




Reproduced with permission from Ref. [86], Copyright 2021, John Wiley and Sons

Reproduced with permission from Ref. [105], Copyright 2022, Elsevier

Reproduced with permission from Ref. [113], Copyright 2021, American Chemical Society

Reproduced with permission from Ref. [120], Copyright 2020, Elsevier

Reproduced with permission from Ref. [127], Copyright 2021, John Wiley and Sons

Reproduced with permission from Ref. [132], Copyright 2021, Elsevier
Similar content being viewed by others
References
Chen SX, Boda SK, Batra SK, Li XR, Xie JW. Emerging roles of electrospun nanofibers in cancer research. Adv Healthc Mater 2018;7:1701024.
Hu XL, Liu S, Zhou GY, Huang YB, Xie ZG, Jing XB. Electrospinning of polymeric nanofibers for drug delivery applications. J Controlled Release 2014;185:12.
Xue JJ, Wu T, Dai YQ, Xia YN. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev 2019;119:5298.
Wu T, Xue JJ, Li HX, Zhu CL, Mo XM, Xia YN. General method for generating circular gradients of active proteins on nanofiber scaffolds sought for wound closure and related applications. ACS Appl Mater Interfaces 2018;10:8536.
Shafiq M, Chen YJ, Hashim R, He CL, Mo XM, Zhou XJ. Reactive oxygen species-based biomaterials for regenerative medicine and tissue engineering applications. Front Bioeng Biotechnol 2021;9:821288.
Makey G, Galioglu S, Ghaffari R, Engin ED, Yıldırım G, Yavuz Ö, Bektaş O, Nizam ÜS, Akbulut Ö, Şahin Ö, Güngör K, Dede DK, Demir HV, Ilday FÖ, Ilday S. Universality of dissipative self-assembly from quantum dots to human cells. Nat Phys 2020;16:795.
Korevaar PA, Newcomb CJ, Meijer SIEW, Stupp SI. Pathway selection in peptide amphiphile assembly. J Am Chem Soc 2014;136:8540.
Cosgrove B, Mui KL, Driscoll TP, Caliari SR, Mehta KD, Assoian RK, Mauck BJA, RL,. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat Mater 2016;15:1297.
Wang BX, Lv XG, Li Z, Yao YB, Yan ZY, Sheng JL, Chen SY. A simple method for controlling the bacterial cellulose nanofiber density in 3D scaffolds and its effect on the cell behavior. Cellulose 2019;26:7411.
Xiong RH, Hua DW, Van Hoeck J, Berdecka D, Léger L, De Munter S, Fraire JC, Raes L, Harizaj A, Sauvage F, Goetgeluk G, Pille M, Aalders J, Belza J, Van Acker T, Bolea-Fernandez E, Si T, Vanhaecke F, De Vos WH, Vandekerckhove B, van Hengel J, Raemdonck K, Huang CB, De Smedt SC, Braeckmans K. Photothermal nanofibres enable safe engineering of therapeutic cells. Nat Nanotechnol 2021;16:1281.
Fu LW, Zhou XJ, He CL. Polymeric Nanosystems for immunogenic cell death-based cancer immunotherapy. Macromol Biosci 2021;21:e2100075.
Fu LW, Zhang WY, Zhou XJ, Fu JZ, He CL. Tumor cell membrane-camouflaged responsive nanoparticles enable MRI-guided immuno-chemodynamic therapy of orthotopic osteosarcoma. Bioact Mater 2022;17:221.
McDaniel MX, Chawla AS, Jain A, Meibers H, Saha I, Gao Y, Jain V, Roskin K, Way S, Pasare S. Effector memory CD4(+) T cells induce damaging innate inflammation and autoimmune pathology by engaging CD40 and TNFR on myeloid cells. Sci Immunol 2022;7:eabk0182.
Zhang XD, Kang Y, Wang JQ, Yan JJ, Chen Q, Cheng H, Huang P, Gu Z. Engineered PD-L1-expressing platelets reverse new-onset Type 1 diabetes. Adv Mater 2020;32:e1907692.
Hou MY, Wei YS, Zhao ZY, Han WQ, Zhou RX, Zhou Y, Zheng YR, Yin LC. Immuno-engineered nanodecoys for the multi-target anti-inflammatory treatment of autoimmune diseases. Adv Mater 2022;34:e2108817.
Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Del Rev 2016;97:4.
Barnes CP, Sell SA, Boland ED, Simpson DG, Barnes BGL, CP, Sell SA, Boland ED, Simpson DG, Bowlin GL,. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev 2007;59:1413.
Basu P, Repanas A, Chatterjee A, Glasmacher B, NarendraKumar U, Manjubala I. PEO-CMC blend nanofibers fabrication by electrospinning for soft tissue engineering applications. Mater Lett 2017;195:10.
Ogueri KS, Laurencin CT. Nanofiber technology for regenerative engineering. ACS Nano 2020;14:9347.
Sun YQ, Li W, Wu XL, Zhang N, Zhang YN, Ouyang SY, Song XY, Fang XY, Seeram R, Xue W, He LM, Wu WT. Functional self-assembling peptide nanofiber hydrogels designed for nerve degeneration. ACS Appl Mater Interfaces 2016;8:2348.
Rufaihah AJ, Yasa IC, Ramanujam VS, Arularasu SC, Kofidis T, Guler MO, Tekinay AB. Angiogenic peptide nanofibers repair cardiac tissue defect after myocardial infarction. Acta Biomater 2017;58:102.
Hajiali F, Tajbakhsh S, Shojaei A. Fabrication and properties of polycaprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: a review. Polym Rev 2018;58:164.
Wang X, Ali M, Lacerda C. A three-dimensional collagen-elastin scaffold for heart valve tissue engineering. Bioengineering 2018;5:69.
He CL, Nie W, Feng W. Engineering of biomimetic nanofibrous matrices for drug delivery and tissue engineering. J Mater Chem B 2014;2:7828.
Gui LQ, Muto A, Chan SA, Breuer CK, Niklason LE. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng Part A 2009;15:2665.
Pashneh-Tala S, MacNeil S, Claeyssens F. The tissue-engineered vascular graft-past, present, and future. Tissue Eng Part B Rev 2016;22:68.
Ha YJ, Ma XJ, Li SK, Li T, Li ZH, Qian YH, Shafiq M, Wang JW, Zhou XJ, He CL. Bone microenvironment-mimetic scaffolds with hierarchical microstructure for enhanced vascularization and bone regeneration. Adv Funct Mater 2022;32:2200011.
Picchetti P, Moreno-Alcantar G, Talamini L, Mourgout A, Aliprandi A, Cola LD. Smart nanocages as a tool for controlling supramolecular aggregation. J Am Chem Soc 2021;143:7681–7.
Eskandari S, Guerin T, Toth I, Stephenson RJ. Recent advances in self-assembled peptides: Implications for targeted drug delivery and vaccine engineering. Adv Drug Deliv Rev 2017;110:169.
Wen ZP, Zhan J, Li HK, Xu GH, Ma SD, Zhang JW, Li ZH, Ou CW, Yang ZM, Cai YB, Chen MS. Dual-ligand supramolecular nanofibers inspired by the renin-angiotensin system for the targeting and synergistic therapy of myocardial infarction. Theranostics 2021;11:3725.
Zha KK, Li X, Yang Z, Tian GZ, Sun ZQ, Sui X, Dai YJ, Liu SY, Guo QY. Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application. NPJ Regen Med 2021;6:14.
Chen S, Fu PL, Wu HS, Pei M. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res 2017;370:53.
Lu Y, Zhou L, Wang LJ, He S, Ren HL, Zhou N, Hu ZM. The role of SIRT1 in BMP2-induced chondrogenic differentiation and cartilage maintenance under oxidative stress. Aging (Albany NY) 2020;12:9000.
Fibel KH, Hillstrom HJ, Halpern BC. State-of-the-art management of knee osteoarthritis. World J Clin Cases 2015;3:89.
Pereira D, Ramos E, Branco J. Osteoarthritis. Acta Med Port 2015;28:99.
Wang YY, Yao J, Yuan MT, Zhang ZW, Hu WP. Osteoblasts can induce dental pulp stem cells to undergo osteogenic differentiation. Cytotechnology 2013;65:223.
Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2017;13:302.
Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 2010;6:625.
Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 2017;19:18.
Chen DG, Xie J, Fiskesund R, Dong WQ, Liang XY, Lv JD, Jin X, Liu JY, Mo SQ, Zhang TZ, Cheng FR, Zhou YB, Zhang HF, Tang K, Ma JW, Liu YY, Huang B. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat Commun 2018;9:873.
De Lange-Brokaar BJE, Ioan-Facsinay A, Van Osch GJVM, Zuurmond AM, Schoones J, Toes REM, Huizinga TWJ, Kloppenburg M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthr Cartil 2012;20:1484.
Li MZ, Yin H, Yan ZN, Li HY, Wu J, Wang Y, Wei F, Tian GZ, Ning C, Li H, Gao CJ, Fu LW, Jiang SP, Chen MX, Sui X, Liu SY, Chen ZW, Guo QY. The immune microenvironment in cartilage injury and repair. Acta Biomater 2020;140:23.
Wynn TA, Vannella KM. Macrophages in tissue repair. Regeneration, and fibrosis. Immunity 2016;44:450.
Chen YJ, Shafiq M, Liu MY, Morsi Y, Mo XM. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact mater 2020;5:963.
Chen YJ, Jia ZH, Shafiq M, Xie XR, Xiao XH, Castro R, Rodrigues J, Wu JL, Zhou GD, Mo XM. Gas foaming of electrospun poly(L-lactide-co-caprolactone)/silk fibroin nanofiber scaffolds to promote cellular infiltration and tissue regeneration. Colloids Surf B 2021;201:111637.
Chen YJ, Xu W, Shafiq M, Song DY, Xie XR, Yuan ZC, El-Newehy M, El-Hamshary H, Morsi Y, Liu Y, Mo XM. Chondroitin sulfate cross-linked three-dimensional tailored electrospun scaffolds for cartilage regeneration. Mater Sci Eng C 2022;134:112643.
Chen YJ, Xu W, Shafiq M, Tang JC, Hao JX, Xie XR, Yuan ZC, Xiao XH, Liu Y, Mo XM. Three-dimensional porous gas-foamed electrospun nanofiber scaffold for cartilage regeneration. J Colloid Interface Sci 2021;603:94.
Zhou FF, Zhang XZ, Cai DD, Li J, Mu Q, Zhang W, Zhu SA, Jiang YZ, Shen WL, Zhang SF, Ouyang HW. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater 2017;63:64.
Chen S, Chen WM, Chen YN, Mo XM, Fan CY. Chondroitin sulfate modified 3D porous electrospun nanofiber scaffolds promote cartilage regeneration. Mater Sci Eng C 2021;118:111312.
Liang RM, Zhao JM, Li B, Cai PA, Loh XJ, Xu CH, Chen P, Kai D, Zheng L. Implantable and degradable antioxidant poly(ε-caprolactone)-lignin nanofiber membrane for effective osteoarthritis treatment. Biomaterials 2020;230:119601.
Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim WS, Alim K, Mammoto A, Ingber DE, Duda GN, Mooney DJ. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater 2015;14:1269.
Ye KQ, Liu DH, Kuang HZ, Cai JY, Chen WM, Sun BB, Xia LG, Fang B, Morsi Y, Mo XM. Three-dimensional electrospun nanofibrous scaffolds displaying bone morphogenetic protein-2-derived peptides for the promotion of osteogenic differentiation of stem cells and bone regeneration. J Colloid Interface Sci 2019;543:625.
He JH, Chen GB, Liu MY, Xu ZL, Chen H, Yang L, Lv YG. Scaffold strategies for modulating immune microenvironment during bone regeneration. Mater Sci Eng C 2020;108:110411.
Pedrosa CR, Arl D, Grysan P, Khan I, Durrieu S, Krishnamoorthy S, Durrieu MC. Controlled nanoscale topographies for osteogenic differentiation of Mesenchymal stem cells. ACS Appl Mater Interfaces 2019;11:8858.
Patel KD, Kim TH, Mandakhbayar N, Singh RK, Jang JH, Lee JH, Kim HW. Coating biopolymer nanofibers with carbon nanotubes accelerates tissue healing and bone regeneration through orchestrated cell- and tissue-regulatory responses. Acta Biomater 2020;108:97.
Huang JY, Li RQ, Yang JH, Cai M, Lee YC, Wang AX, Cheng B, Wang Y. Bioadaptation of implants to in vitro and in vivo oxidative stress pathological conditions via nanotopography-induced FoxO1 signaling pathways to enhance Osteoimmunal regeneration. Bioact Mater 2021;6:3164.
Saino E, Focarete MLC, Gualandi CE, Emanuele E, Cornaglia AI, Cornaglia AIM, Imbriani ML, Visai L. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromol 2011;12:1900.
Liu XZ, Chen MM, Luo JC, Zhao H, Zhou XC, Gu QL, Yang HL, Zhu XS, Cui WG, Shi Q. Immunopolarization-regulated 3D printed-electrospun fibrous scaffolds for bone regeneration. Biomaterials 2021;276:121037.
Jin S, Gao J, Yang RL, Yuan C, Wang RL, Zou Q, Zuo Y, Zhu MF, Li YB, Man Y, Li JD. A baicalin-loaded coaxial nanofiber scaffold regulated inflammation and osteoclast differentiation for vascularized bone regeneration. Bioact Mater 2021;8:559.
Wei YJ, Liu ZQ, Zhu X, Jiang L, Shi WD, Wang YJ, Xu N, Gang FL, Wang XM, Zhao LY, Lin J, Sun XD. Dual directions to address the problem of aseptic loosening via electrospun PLGA @ aspirin nanofiber coatings on titanium. Biomaterials 2020;257:120237.
Lee J, Lee S, Ahmad T, Madhurakkat Perikamana SK, Kim EM, Lee SW, Shin H. Bioactive membrane immobilized with lactoferrin for modulation of bone regeneration and inflammation. Tissue Eng Part A 2020;26:1243.
Chai NW, Zhang JT, Zhang QQ, Du HB, He X, Yang J, Zhou XJ, He J, He CL. Construction of 3D printed constructs based on microfluidic microgel for bone regeneration. Compos B 2021;223:109100.
Gu J, Zhang Q, Geng M, Wang W, Yang J, Khan AUR, Du H, Sha Z, Zhou X, He C. Construction of nanofibrous scaffolds with interconnected perfusable microchannel networks for engineering of vascularized bone tissue. Bioact Mater 2021;6:3254.
Adamu BF, Gao J, Jhatial AK, Kumelachew DM. A review of medicinal plant-based bioactive electrospun nano fibrous wound dressings. Mater Des 2021;209:109942.
Merrell JG, McLaughlin SW, Tie L, Laurencin CT, Chen AF, Nair LS. Curcumin-loaded poly(epsilon-caprolactone) nanofibres: diabetic wound dressing with anti-oxidant and anti-inflammatory properties. Clin Exp Pharmacol Physiol 2009;36:1149.
Gizaw M, Thompson J, Faglie A, Lee SY, Neuenschwander P, Chou SF. Electrospun fibers as a dressing material for drug and biological agent delivery in wound healing applications. Bioengineering (Basel) 2018;5:9.
Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science 2017;356:1026.
Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res 2017;58:81.
Delavary MB, Van der Veer WM, Van Egmond M, Niessen FB, Beelen RHJ. Macrophages in skin injury and repair. Immunobiology 2011;216:753.
Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 2009;37:1528.
Chen L, Zhang LC, Zhang HB, Sun XM, Liu D, Zhang JM, Zhang YG, Cheng LY, Santos HA, Cui WG. Programmable immune activating electrospun fibers for skin regeneration. Bioact Mater 2021;6:3218.
Wang CB, Chu CY, Zhao XW, Yang Y, Hu C, Liu L, Li J, Qu YL, Man Y. The diameter factor of aligned membranes facilitates wound healing by promoting epithelialization in an immune way. Bioact Mater 2021;11:206.
Liu S, Zhang QF, Yu J, Shao NN, Lu HT, Guo JS, Qiu XP, Zhou DF, Huang YB. Absorbable thioether grafted hyaluronic acid nanofibrous hydrogel for synergistic modulation of inflammation microenvironment to accelerate vhronic diabetic wound healing. Adv Healthc Mater 2020;9:e2000198.
Nakkala JR, Duan YY, Ding J, Muhammad W, Zhang DT, Mao ZW, Ouyang HW, Gao CY. Macrophage membrane-functionalized nanofibrous mats and their immunomodulatory effects on macrophage polarization. Acta Biomater 2022;141:24.
Ogle ME, Segar CE, Sridhar S, Botchwey EA. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp Biol Med (Maywood) 2016;241:1084.
Mauro A, Russo V, Marcantonio LD, Berardinelli P, Martelli A, Muttini A, Mattioli M, Barboni B. M1 and M2 macrophage recruitment during tendon regeneration induced by amniotic epithelial cell allotransplantation in ovine. Res Vet Sci 2016;105:92.
Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol 2016;17:9.
Nourissat G, Berenbaum F, Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol 2015;11:223.
McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med 2014;43:491.
Hays PL, Kawamura S, Deng XH, Dagher E, Mithoefer K, Ying L, Rodeo SA. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am 2008;90:565.
Noël W, Raes G, Hassanzadeh Ghassabeh G, De Baetselier P, Beschin A. Alternatively activated macrophages during parasite infections. Trends Parasitol 2004;20:126.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122:787.
Sunwoo JY, Eliasberg CD, Carballo CB, Rodeo SA. The role of the macrophage in tendinopathy and tendon healing. J Orthop Res 2020;38:1666.
Saveh-Shemshaki N, Nair LS, Laurencin CT. Nanofiber-based matrices for rotator cuff regenerative engineering. Acta Biomater 2019;94:64.
Li WJ, Mauck RL, Cooper JA, Yuan X, Tuan RS. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J Biomech 2007;40:1686.
Cai CD, Zhang XS, Li YG, Liu XZ, Wang S, Lu MK, Yan X, Deng LF, Liu S, Wang F, Fan CY. Self-healing hydrogel embodied with macrophage-regulation and responsive-gene-silencing properties for synergistic prevention of peritendinous adhesion. Adv Mater 2021;34:e2106564.
Schoenenberger AD, Tempfer H, Lehner C, Egloff J, Mauracher M, Bird A, Widmer J, Maniura-Weber K, Fucentese SF, Traweger A, Silvan U, Snedeker JG. Macromechanics and polycaprolactone fiber organization drive macrophage polarization and regulate inflammatory activation of tendon in vitro and in vivo. Biomaterials 2020;249:120034.
Liu S, Pan GQ, Liu GW, Neves JD, Song S, Chen S, Cheng BJ, Sun ZY, Sarmento B, Cui WG, Fan CY. Electrospun fibrous membranes featuring sustained release of ibuprofen reduce adhesion and improve neurological function following lumbar laminectomy. J Control Release 2017;264:1.
Shalumon KT, Sheu C, Chen C-H, Chen S-H, Jose G, Kuo C-Y, Chen J-P. Multi-functional electrospun antibacterial core-shell nanofibrous membranes for prolonged prevention of post-surgical tendon adhesion and inflammation. Acta Biomater 2018;72:121.
Lucke S, Walschus U, Hoene A, Schnabelrauch M, Nebe JB, Finke B, Schlosser M. The in vivo inflammatory and foreign body giant cell response against different poly(l-lactide-co-d/l-lactide) implants is primarily determined by material morphology rather than surface chemistry. J Biomed Mater Res A 2018;106:2726.
Grafahrend D, Heffels KH, Beer MV, Gasteier P, Möller M, Boehm G, Dalton PD, Groll J. Degradable polyester scaffolds with controlled surface chemistry combining minimal protein adsorption with specific bioactivation. Nat Mater 2011;10:67.
Zhang BY, Luo Q, Chen Z, Shi YS, Ju Y, Yang L, Song GB. Increased nuclear stiffness via FAK-ERK1/2 signaling is necessary for synthetic mechano-growth factor E peptide-induced tenocyte migration. Sci Rep 2016;6:18809.
Song Y, Xu K, Yu C, Dong LL, Chen PX, Lv YG, Chiang MYM, Li LH, Liu WQ, Yang L. The use of mechano growth factor to prevent cartilage degeneration in knee osteoarthritis. J Tissue Eng Regen Med 2018;12:738.
Song Y, Li LH, Zhao WK, Qian YN, Dong LL, Fang YN, Yang L, Fan YB. Surface modification of electrospun fibers with mechano-growth factor for mitigating the foreign-body reaction. Bioact Mater 2021;6:2983.
Wolf MT, Dearth CL, Ranallo CA, LoPresti ST, Carey LE, Daly KA, Brown BN, Badylak SF. Macrophage polarization in response to ECM coated polypropylene mesh. Biomaterials 2014;35:6838.
Pajarinen J, Kouri VP, Jämsen E, Li TF, Mandelin J, Konttinen YT. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomater 2013;9:9229.
Wang S, Lu MK, Wang W, Yu SY, Yu RY, Cai CD, Li YG, Shi ZM, Zou J, He M, Xie WQ, Yu DJ, Jin HF, Li HZ, Xiao WF, Fan CY, Wu F, Li YS, Liu S. Macrophage polarization modulated by NF-κB in polylactide membranes-treated peritendinous adhesion. Small. 2022;18:e2104112.
Gu XS, Ding F, Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014;35:6143.
Qian Y, Yao ZX, Wang X, Cheng Y, Fang ZW, Yuan W-E, Fan CY, Ouyang YM. (-)-Epigallocatechin gallate-loaded polycaprolactone scaffolds fabricated using a 3D integrated moulding method alleviate immune stress and induce neurogenesis. Cell Prolif 2020;53:e12730.
Qian Y, Lin H, Yan ZW, Shi JL, Fan CY. Functional nanomaterials in peripheral nerve regeneration: scaffold design, chemical principles and microenvironmental remodeling. Mater Today 2021;51:165.
Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol 2011;178:19.
Pateman CJ, Harding AJ, Glen A, Taylor CS, Christmas CR, Robinson PP, Rimmer S, Boissonade FM, Claeyssens F, Haycock JW. Nerve guides manufactured from photocurable polymers to aid peripheral nerve repair. Biomaterials 2015;49:77.
Jia YC, Yang WC, Zhang KH, Qiu S, Xu J, Wang CY, Chai YM. Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta Biomater 2019;83:291.
Dong XH, Liu SY, Yang YY, Gao S, Li WL, Cao JS, Wan Y, Huang ZQ, Fan GW, Chen Q, Wang HJ, Zhu MF, Kong DL. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials 2021;272:120767.
Dong XZ, Wu P, Yan LS, Liu K, Wei WY, Cheng Q, Liang XY, Chen Y, Dai HL. Oriented nanofibrous P(MMD-co-LA)/Deferoxamine nerve scaffold facilitates peripheral nerve regeneration by regulating macrophage phenotype and revascularization. Biomaterials 2022;280:121288.
Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology 2004;101:468.
David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 2011;12:388.
Xi K, Gu Y, Tang JC, Chen H, Xu Y, Wu L, Cai F, Deng LF, Yang HL, Shi Q, Cui WG, Chen L. Microenvironment-responsive immunoregulatory electrospun fibers for promoting nerve function recovery. Nat Commun 2020;11:4504.
Müller K, Fedosov DA, Gompper G. Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci Rep 2014;4:4871.
Huang H, Dong CH, Chang MQ, Ding L, Chen L, Feng W, Chen Y. Mitochondria-specific nanocatalysts for chemotherapy-augmented sequential chemoreactive tumor therapy. Exploration 2021;1:50.
Zheng YH, Han YB, Sun Q, Zhen L. Harnessing anti-tumor and tumor-tropism functions of macrophages via nanotechnology for tumor immunotherapy. Exploration 2021. https://doi.org/10.1002/EXP.20210166 .
Rackley L, Stewart J, Salotti J, Krokhotin A, Shah A, Halman JR, Juneja R, Smollett J, Lee L, Roark K, Viard M, Tarannum M, Vivero-Escoto J, Johnson FP, Dobrovolskaia MA, Dokholyan NV, Franco E, Afonin KA. RNA fibers as optimized nanoscaffolds for siRNA coordination and reduced immunological recognition. Adv Funct Mater 2018;28:1805959.
Guo ZP, Hu YY, Zhao MY, Hao K, He P, Tian HY, Chen XS, Chen MW. Prodrug-based versatile nanomedicine with simultaneous physical and physiological tumor penetration for enhanced cancer chemo-immunotherapy. Nano Lett 2021;21:3721.
Teng FF, Meng XJ, Kong L, Yu JM. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: a systematic review. Cancer Lett 2018;414:166.
Chen Q, Chen GJ, Chen JW, Shen JJ, Zhang XD, Wang JQ, Chan A, Gu Z. Bioresponsive protein complex of aPD1 and aCD47 antibodies for enhanced immunotherapy. Nano Lett 2019;19:4879.
Park MH, Hong JT. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cell 2016;5:15.
Goswami KK, Ghosh T, Ghosh S, Sarkar M, Bose A, Baral R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol 2017;316:1.
Oude Munnink TH, Henstra MJ, Segerink LI, Movig KLL, Brummelhuis-Visser P. Therapeutic drug monitoring of monoclonal antibodies in inflammatory and malignant disease: Translating TNF-α experience to oncology. Clin Pharmacol Ther 2016;99:419.
Chen MC, Tan YJ, Dong ZL, Lu JQ, Han X, Jin QT, Zhu WJ, Shen JJ, Cheng L, Liu Z, Chen Q. Injectable anti-inflammatory nanofiber hydrogel to achieve systemic immunotherapy post local administration. Nano Lett 2020;20:6763.
Liu R, An Y, Jia WF, Wang YS, Wu Y, Zhen YH, Cao J, Gao HL. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J Control Release 2020;321:589.
Seo H, Jeon I, Kim BS, Park M, Bae E, Song B, Koh CH, Shin KS, Kim I, Choi K, Oh T, Min J, Min BS, Han YD, Kang SJ, Shin SJ, Chung Y, Kang C. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat Commun 2017;8:15776.
Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015;35:S185.
Shin HM, Ju Y, Kim G, Lee JW, Seo MW, Sim JH, Yang J, Noh S, Kim J, Kim H-R. Recyclable cytokines on short and injectable polylactic acid fibers for enhancing T-cell function. Adv Funct Mater 2019;29:1808361.
Maman S, Witz IP. A history of exploring cancer in context. Nat Rev Cancer 2018;18:359.
Finny AS, Jiang C, Andreescu S. 3D printed hydrogel-based sensors for quantifying UV exposure. ACS Appl Mater Interfaces 2020;12:43341.
Kwon B, Han E, Yang W, Cho W, Yoo W, Hwang J, Kwon BM, Lee D. Nano-fenton reactors as a new class of oxidative stress amplifying anticancer therapeutic agents. ACS Appl Mater Interfaces 2016;8:5887.
Qin Y, Tong F, Zhang W, Zhou Y, He SQ, Xie R, Lei T, Wang YS, Peng SJ, Li ZF, Leong J, Gao HL, Lu LG. Self-Delivered supramolecular nanomedicine with transformable shape for ferrocene-amplified photodynamic therapy of breast cancer and bone metastases. Adv Funct Mater 2021;31:2104645.
Liu CF, Li CP, Pang C, Li MQ, Li HX, Li PX, Fan L, Liu H, Tian W. Correction to supramolecular drug-drug complex vesicles enable sequential drug release for enhanced combination therapy. ACS Appl Mater Interfaces 2020;12:44382.
Klopfleisch R, Jung F. The pathology of the foreign body reaction against biomaterials. J Biomed Mater Res A 2017;105:927.
Chen R, Canales A, Anikeeva P. Neural recording and modulation technologies. Nat Rev Mater 2016;2:16093.
Mariani E, Lisignoli G, Borzì RM, Pulsatelli L. Biomaterials: foreign bodies or tuners for the immune response? Int J Mol Sci 2019;20:636.
Hernandez JL, Park J, Yao S, Blakney AK, Nguyen HV, Katz BH, Jensen JT, Woodrow KA. Effect of tissue microenvironment on fibrous capsule formation to biomaterial-coated implants. Biomaterials 2021;273:120806.
Qian YN, Li LH, Song Y, Dong LL, Chen PX, Li XM, Cai KY, Germershaus O, Yang L, Fan YB. Surface modification of nanofibrous matrices via layer-by-layer functionalized silk assembly for mitigating the foreign body reaction. Biomaterials 2018;164:22.
Nakkala JR, Yao Y, Zhai Z, Duan Y, Zhang D, Mao Z, Lu L, Gao C. Dimethyl itaconate-loaded nanofibers rewrite macrophage polarization, reduce inflammation, and enhance repair of myocardic infarction. Small 2021;17:e2006992.
Tang SW, Tong WY, Pang SW, Voelcker NH, Lam YW. Deconstructing, replicating, and engineering tissue microenvironment for stem cell differentiation. Tissue Eng Part B Rev 2020;26:540.
Shores LS, Kelly SH, Hainline KM, Suwanpradid J, Macleod AS, Collier JH. Multifactorial design of a supramolecular peptide anti-IL-17 vaccine toward the treatment of psoriasis. Front Immunol 1855;11:1855.
Syed O, Kim JH, Keskin-Erdogan Z, Day RM, El-Fiqi A, Kim HW, Knowles JC. SIS/aligned fibre scaffold designed to meet layered oesophageal tissue complexity and properties. Acta Biomater 2019;99:181.
Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 2012;33:1771.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (32071350, 31771048), Fundamental Research Funds for the Central Universities (2232018A3-07, 2232019A3-06), International Cooperation Fund of the Science and Technology Commission of Shanghai Municipality (19440741600).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, L., Feng, Q., Chen, Y. et al. Nanofibers for the Immunoregulation in Biomedical Applications. Adv. Fiber Mater. 4, 1334–1356 (2022). https://doi.org/10.1007/s42765-022-00191-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-022-00191-2