Abstract
Salinity stress has become a major threat to worldwide crop production. Exogenous melatonin (MT) has appeared as a promising treatment against salt stress in several plant species. However, MT effect on the tolerance of sorghum plants under different saline conditions (moderate and severe) remains ambiguous. This study was carried out to explore the impact of MT (0, 50, 100 and 200 µM) as a foliar application on sorghum seedlings grown under moderate and severe saline conditions using sodium chloride, NaCl (75 and 150 µM NaCl). Salinity treatments were applied as solution in sand medium in pots. The results demonstrated that rising salinity level negatively affected plant growth, photosynthetic pigments (chlorophylls and carotenoids), leaf water status and ionic homeostasis (sodium, potassium, and calcium ions). Applied-MT specifically at 100 or 200 µM enhanced the osmotic balance, cell membrane stabilizing and leaf relative water content. These effects were associated with an obvious restriction to the level of hydrogen peroxide, lipid peroxidation (malondialdehyde content) and methylglyoxal. Moreover, antioxidant activities of peroxidase, catalase, superoxide dismutase, and ascorbate peroxidase enzymes were modulated by MT treatments. Molecular docking modeling assessment illustrated top-ranked confirmations between MT and the target antioxidant enzymes. MT forms multiple hydrogen bonds with key amino acid residues for glycine (A: 162), tryptophan (A: 41), leucine (A: 165), tyrosine (A: 235) in the active site of ascorbate peroxidase. The alkyl interactions with leucine (A: 37), arginine (A: 38) and cysteine (A: 168) also contribute to its high affinity. Despite sorghum plant is commonly moderately tolerant to salinity stress, the results of this study confirmed its high sensitivity to a wide range of saline conditions at early growth stages. Melatonin spraying led to improvements in various morphological, physiological and biochemical mechanisms that harmonized together to confer stress resistance to salt-stressed sorghum seedlings.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Climate changes have generated critical issues of unfavorable conditions or stresses that negatively influence crop production and food security (El-Bially et al. 2018; Saudy and El-Metwally 2019; Abd–Elrahman et al. 2022; Sattar et al. 2023). Crop growth and yield are dramatically influenced by ecological pressures which involve salinity, inappropriate temperatures, water scarcity, heavy metal toxicity, and nutrient deficiency (El-Yazeid and Abou-Aly 2011; Saudy 2014; El-Bially et al. 2022a; El-Metwally et al. 2022a; Saudy et al. 2023a; Shaaban et al. 2023a; Shahin et al. 2023). Further, biological pressures have also pernicious influences on crop productivity and quality (Saudy 2015Saudy and Mubarak 2015; Saudy et al. 2020a, 2021b; Abou El-Enin et al. 2023). The ecological pressures which known as abiotic stresses cause disturbance in plant metabolism, suppressing crop potential (Abd El-Mageed et al. 2022; Hadid et al. 2023; Ramadan et al. 2023a; Saudy et al. 2023b). A major stress in this respect is soil salinization which poses a significant risk to crop productivity and world food security particularly with climate change intensifies (Mukhopadhyay et al. 2021; Shalaby et al. 2023). It may be naturally occurred through global warming and water evaporation, leading to accumulate huge amounts of salts in the soil surface (Corwin 2021). Moreover, there are many anthropogenic reasons including unsustainable agricultural practices and industrial wastes can increase the issues of salt affected soils worldwide (Litalien and Zeeb 2020).
Exposing plants to salinity stress can seriously affect water acquisition, osmotic equilibrium and ionic homeostasis (El Nahhas et al. 2021; Abdelaal et al. 2022; Darwish et al. 2023; Mansha et al. 2023). These effects occur due to reducing cell membrane stability and the toxic effect of the considerable amounts of the accumulated Na+ ions on the functioning of membrane transporter proteins (Alsamadany et al. 2022). Dysfunction of photosynthetic machinery and cellular oxidative burst are two other major biochemical markers of high salinity stress (Farag et al. 2022; Shehata and Ibrahim 2023; Vineeth et al. 2023). These responses have been found to be due to the excessive release of reactive oxygen species (ROS), such as superoxide radicals, hydroxyl radicals, and hydrogen peroxide (H2O2) through hindering electron transport systems in chloroplast (Sun et al. 2016; Din et al. 2020; Gashash et al. 2022) and mitochondria (Analin et al. 2020). Methylglyoxal (MG) is also cytotoxic molecule in its high concentrations under saline conditions (Yadav et al. 2005). It is derived from glycolysis and can be involved in parallel with ROS in lipid peroxidation, photosynthesis disruption, and increasing the rates of DNA damage and cell death under adverse conditions (Wang et al. 2019; El-Metwally and Saudy 2021a; El-Mogy et al. 2022; Doklega et al. 2023). Moreover, salinity plays a primary role in controlling the photosynthetically fixed carbon by reducing the stomatal conductance and altering photosynthetic pigments (Youssef et al. 2021; Dourado et al. 2022). Hence, tolerance of plants to high saline conditions has been found to be due to maintain the performance of growth, photosynthetic apparatus, upregulation antioxidant defense systems, ion homeostasis and enhancing plant water relations (Mansour et al. 2021; Youssef et al. 2021).
Sorghum (Sorghum bicolor (L.) Moench; family; poaceae) is the most important cereal crop in the world after wheat, rice, maize, and barley (Jardim et al. 2020). It has been characterized as a C4 plant and tolerant to moderate saline environments (Huang 2018; Mansour et al. 2021). However, sorghum may encounter with the risk of high soil salinization in the arid and semi-arid regions, where temperatures and evaporation rates are high leading to accumulate toxic amounts of Na+ in the rhizosphere (Youssef et al. 2021). It has been found that sorghum can produce high biomass under salinity level up to 6.8 dS m− 1, but this production was declined to 50% when salinity level reached 10 dS m− 1 (Hossain et al. 2018). As well, both germination and seedling growth are the most susceptible stages to saline conditions which may determine the final plant performance of sorghum crop (Rajabi Dehnavi et al. 2020; Youssef et al. 2021). Therefore, it is robustly important to find a successful approach to improve seedling tolerance and survival under high salinity conditions. Plants have evolved various strategies to cope a variety of stressed environments while maintaining their survival and potential to grow and reproduce (Noureldin et al. 2013; Saudy et al. 2018, 2022; El-Metwally and Saudy 2021b). Plant growth responses to stresses are intricately modulated via multiple phytohormones which have vital works to orchestrate the various plant life processes and adaptability to stresses (Saudy et al. 2020b; El-Metwally et al. 2022b; Saudy et al. 2021d; Rizk et al. 2023).
Melatonin (N-acetyl-5-methoxytryptamine; MT) has been found to have an essential role in plant survival under biotic and abiotic stresses (Zhao et al. 2021a; El-Beltagi et al. 2023). As a powerful antioxidant, it can serve with high efficiency to scavenge ROS under various harsh environmental conditions i.e. drought (Huang et al. 2019; El-Metwally et al. 2021; Ramadan et al. 2023b; Shaaban et al. 2023b), heat stress (Buttar et al. 2020), cold (Qari et al. 2022), heavy metals (Hoque et al. 2021) and salinity (Li et al. 2019; Ali et al. 2021). Additionally, MT has been known as a phytohormone with a wide spectrum of functions and relationships with other phytohormones (Khan et al. 2022; Ali et al. 2024). Melatonin can stimulate the lateral root formation and regulate the root architecture by modulating auxin responses (Liang et al. 2017). Furthermore,, MT demonstrated an obvious ability to stimulate seed germination (Xiao et al. 2019), delay leaf senescence (Zhao et al. 2021b), alter flowering, fruit set and fruit ripening (Arnao and Hernández-Ruiz 2020) and enhance photosynthesis (Jahan et al. 2021). Under salinity stress, melatonin has been found to have the ability to regulate the antioxidant system, proline and carbohydrates metabolism (Siddiqui et al. 2019).
Very little research has been focused on the role of melatonin in mitigating the harmful effects of different salinity levels on sorghum plants at early sensitive stage of vegetative growth. As well, the optimal concentration of melatonin in this regard still needs to be determined based on stress level. The present study hypothesised that the appropriate MT concentration for alleviating salinity injuries could change as the salt stress level changed Therefore, the potential effects of different MT concentrations (0, 50, 100 and 200 µM) on alleviating the impacts of salinity stress at moderate (75 µM NaCl) and severe (150 µM NaCl) level were examined. Our results included different mechanisms were orchestrated together to confer salinity tolerance to sorghum plants i.e., regulating the vegetative growth, photosynthetic pigments and osmolytes, achieving cell membrane stability and ion homeostasis, suppressing the oxidative and carbonyl stress biomarkers and enhancing the antioxidant systems. These response mechanisms will help researchers to better understand the potential complex roles of melatonin in sorghum tolerance to saline conditions.
2 Materials and methods
2.1 Plant Materials and Treatments
Sorghum grains (Sorghum bicolor L. Moench; Cv. Dorado; purchased from Agricultural Research Center, Giza, Egypt) sterilized with 0.1% sodium hypochlorite for 5 min and washed 5 times with distilled water then sown in black plastic pots filled with 8 kg pre-washed sand. Each pot (20 cm diameter × 25 cm height) contained ten seedlings and was watered every two days with 250 ml of ½ strength Hoagland’s solution. After three weeks (full seedlings emergence), to explore the effects of moderate and severe salinity stress, the nutrient solution was modified by adding 75 and 150 µM NaCl, representing 6.85 and 13.70 dS m− 1. Over a period of 2 weeks after complete emergence, the pots received the salt solution at 2-day intervals. Three concentraions of melatonin, MT (50, 100 and 200 µM), in addition to a control treatment (0, MT, distilled water) were applied.The melatonin stock solution was prepared by dissolving melatonin in ethanol (100 mg of melatonin in 2 mL of ethanol absolute). After that different concentrations were prepared by dilution. Seedlings were foliar sprayed four times at 24, 27, 30 and 33 days after sowing with different MT concentrations. Everytime, each pot received 200 ml of MT solution. Tween-20, 0.05% (Sigma-Aldrich) were used as a surfactant with all MT (MT; Bio Basic, Markham, Canada) treatments. The experiment was terminated by collecting plant samples at 35 days after sowing for different physiological and biochemical investigations.
2.2 Assessements
2.2.1 Determination of Roots and Shoots Biomass and Leaf Pigments
At the end of the experiment, seedlings were collected to assess the fresh weights of roots and shoots per plant. Further, the concentration of chlorophyll a, chlorophyll b and carotenoids were determined spectrophotometry (Alan 1994). Pigment extraction in 80% acetone was performed using a fresh weight (0.5 g) of completely grown young leaves. The pigment extract was measured at 663, 644, and 452.5 nm for chlorophyll a, chlorophyll b, and carotenoids respectively, versus a blank of pure 80% acetone.
2.2.2 Leaf Relative Water Content (RWC) and Osmotic Molecules Determination
Ten fresh leaf discs were accurately weighed (FW) and maintained in distilled water for 1 h to gain turgidity (TW). The leaf discs were oven-dried for 24 h at 80 ℃ to determine their dry weight (DW). The RWC was calculated according to Hayat et al. (2007) using formula 1:
where FW, DW and TW represent fresh, dry and turgid weights of leaf discs respectively. The percentage of relative water content (RWC) was measured according to Smart and Bingham (1974).
The ninhydrin reagent was used to estimate proline as described by Bates et al. (1973). For estimating total soluble sugars (TSS), the phenol-sulfuric acid technique was used (Chow and Landhäusser 2004). Free amino acids (FAA) were measured as glycine using the ninhydrin reagent (Hamilton et al. 1943).
2.2.3 Quantification of Hydrogen Peroxide (H2O2), Malondialdehyde (MDA), Methylglyoxylate (MG) and Cell Membrane Stability Index
For H2O2 determination, 0.3 g of leaf sample was homogenized in 3 mL of Trichloroacetic acid (TCA) 0.1% (w/v), followed by centrifugation at 10,000 x g for 10 min at 4 °C (Velikova et al. 2000). The supernatant (500 µL) was then combined with equivalent volume of 0.1 M potassium phosphate buffer (pH 7.8) and 1 mL of 1 M potassium iodine (KI). The combination was utilised to calculate the H2O2 by reading its absorbance at 390 nm. The level of lipid peroxidation was determined by measuring the amount of MDA at 535 nm and corrected for non-specific turbidity at 600 nm using thiobarbituric acid (TBA) reaction as performed by Heath and Packer (1968).
MG was measured in accordance with Hossain et al. (2009), with some adjustments. Fresh leaves (0.5 g) were homogenized in 3 mL of 0.5 M perchloric acid and incubated on ice for 15 min. The mixture was centrifuged at 4 °C for 10 min at 10,000 rpm. The supernatant was discolored by adding charcoal, then centrifuged at 10,000 rpm for 10 min. This supernatant was neutralized for 15 min at room temperature with a saturated potassium carbonate solution before being centrifuged at 10,000 rpm for 10 min. The reaction mixture contained 750 µL of 7.2 µM 1, 2-diaminobenzene, 250 µL of 5 M perchloric acid, and 2 mL of the supernatant was incubated at room temperature for 25 min then the absorbance was recorded at 335 nm. The stability of membrane was assessed as carried out by Premachandra et al. (1990) using the readings of EC meter for leaf discs before (EC1) and after (EC2) placing in autoclave at 120 °C for 20 min. The membrane stability index (MSI) of cell was estimated using the formula 2:
2.2.4 Nutrients Estimation
Inductively coupled plasma mass spectrometry (ICP-MS) was used to measure the amounts of sodium (Na+), potasium (K+) and calcium (Ca+ 2) ions according to Jones Jr (2001). Further, K+/Na+ ratio was computed.
2.2.5 Antioxidant Enzymes Assay
For extraction of antioxidant enzymes, 0.2 g of fresh leaves were homogenized in 4 mL of 0.1 M ice-cold sodium phosphate buffer (pH 7.0) containing 1% (w/v) PVP and 0.1 µM EDTA, then centrifuged at 10,000 xg for 20 min at 4 C. The supernatant was used to measure peroxidase (POX, EC 1.11.1.7), catalase (CAT, EC EC 1.11.1.6), superoxide dismutase (SOD, EC 1.15.1.1) and ascorbate peroxidase (APX, EC 1.11.1.11) activities. Total soluble protein in the supernatant was also determined as described by Bradford (1976) to calculate the specific activity of different enzymes. POX activity was determined according to Reddy et al. (1985) at 470 nm. CAT activity was assayed following Cakmak et al. (1993) by reading the absorbance at 240 nm. The capability of SOD to inhibit the photochemical reduction of nitro blue tetrazolium (NBT) at 560 nm was determined according to Beyer and Fridovich (1987). APX was determined at 290 nm (Nakano and Asada 1981),
2.2.6 Molecular Docking
The three-dimensional model structure of the receptors used in this molecular docking study were retrieved from UniProt and the Protein Data Bank (PDB) (PDB: https://doi.org/10.2210/pdb1EEA/ pdb). The following receptors were selected: (1) Ascorbate peroxidase (APX) (pdb ID: 8djs) with a binding pocket centered at coordinates (center_x = − 29.586, center_y = 28.561, center_z = 63.093); (2) Catalase (UniProt ID: C5Z2J6) with a binding pocket centered at coordinates (center_x = -1.311, center_y = − 3.311, center_z= -1.688); (3) Glutathione peroxidase (UniProt ID: Q6JAG4) with a binding pocket centered at coordinates (center_x = - -1.946, center_y = -13.748, center_z = -2.224); (4) superoxide dismutase (Uniprot ID: A0A194YHS4) with a binding pocket centered at coordinates (center_x = -8.466, center_y = -14.551, center_z = 13.116).Each with sized: 20 × 20 × 20The active sites on these receptors were identified using the Deepsite/PlayMolecule software (https://www.playmolecule.com/deepsite). The Deepsite results were retrieved from the PlayMolecule platform. All ligands’ SMILES and SDF (structure data files) were retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The selected ligands were prepared for docking by performing energy minimization using Avogadro 1.2.0 with the MMFF94 force field. This step ensured that the ligands were in an energetically favourable conformation for docking. Melatonin was retrieved from pubchem with ID 896. The receptor proteins were prepared for docking using AutoDock Tools 1.5.6. This involved the addition of hydrogen atoms, removal of water molecules, and assignment of charges to the protein structure. The receptor’s grid box was centered on the active site coordinates mentioned earlier. Molecular docking was performed using AutoDock Vina. The prepared ligands were docked into the active sites of the receptors, and the software calculated the binding affinities and predicted binding poses. Multiple docking runs were conducted to explore different binding conformations. The docking results were analyzed to identify potential binding modes and interactions between ligands and receptors. The binding affinities and RMSD (Root Mean Square Deviation) values were examined to assess the quality of the docking poses.
2.3 Statistical Analysis and Data Visualization
The correlation analysis was performed according to hierarchical clustering and two-dimensional heatmap plotting was constructed using R-studio and Orange3 module. Statistical analysis was conducted using the one-way analysis of variance (ANOVA) test followed by SAS software. Tukey’s multiple range test at 5% was carried out to determine the significance of means using triple replication.
3 Results
3.1 Effect of Melatonin on Plant Biomass under Salt Stress
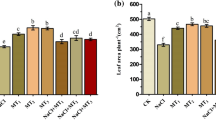
Plants subjected to moderate (75 µM) and high concentrations (150 µM) of NaCl stress showed significant (p > 0.05) reductions in the root and shoot fresh weight (FW), which was partially improved by the application of exogenous melatonin (MT) compared to the control (Fig. 1). Root and shoot FW was observed to be improved under each slinity level with supplying MT at the concentrations of 50, 100 and 200 µM without significant differences (p < 0.05) between them. However, under severe salinity (150 µM), MT did not differe than the control treatment for root FW.
Influence of melatonin (MT) concentration on roots and shoots fresh weight of sorghum seedling under salinity levels. MT0, MT50, MT100 and MT200: application of MT concentration at 0, 50, 100 and 200 µM, respectively; S1, S2 and S3: salinity level at 0, 75 nad 150 NaCl µM, respectively. The means in each column followed by the same lower-case-letter refer to that there is no significant difference according Tukey’s Honestly Significant Difference (HSD) test at P < 0.05. Data were expressed as the mean ± standard deviation of 3 replications
3.2 Effect of Melatonin on Pigments Content under Salt Stress
NaCl stress (both moderate and high levels) brought about significant (p > 0.05) reductions in chlorophyll a, chlorophyll b, total chlorophyll and carotenoids (Fig. 2). Generaly, melatonin supply improved chlorophylls and carotenoids pigment compared to the control plants either in the presence or absence of salinity stress. Different MT levels showed differential effects on chlorophylls and carotenoids content at moderate and higher levels of salinity stress. Herein, MT100 was more effective in increasing chlorophyll a, and carotenoids at moderate concentration of NaCl stress, surpassing MT0, MT50 and MT200 treatments by 40.3 and 40.7%, 18.4 and 19.7% and 10.1 and 19.1%, respectively. Also, MT100 along MT200 exhibited greater values of chlorophyll b and total chlorophyll under modertae slinity and carotenoids under high salinity than MT0 and MT50.
Influence of melatonin (MT) concentration on chlorophyll a, chlorophyll b, total chlorophyll and carotenoids of sorghum seedling under salinity levels. MT0, MT50, MT100 and MT200: application of MT concentration at 0, 50, 100 and 200 µM, respectively; S1, S2 and S3: salinity level at 0, 75 nad 150 NaCl µM, respectively. The means in each column followed by the same lower-case-letter refer to that there is no significant difference according Tukey’s Honestly Significant Difference (HSD) test at P < 0.05. Data were expressed as the mean ± standard deviation of 3 replications
3.3 Effect of Melatonin on RWC and Osmolyte Accumulation under Salt Stress
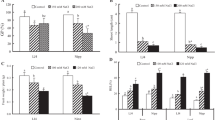
The effects of NaCl stress and melatonin supplementation on the modulation of relative water content, proline, sugar and free amino acids were significant (p > 0.05) as dipected in Fig. 3. Salinity stress markedly reduced RWC at 75 µM and 150 µM NaCl stress, where the later resulted in a serious deterioration in RWC content. Conversely, exogenous MT treatments resulted in an obvious improvement in RWC either in the presence of 75 µM or 150 µM NaCl stress. With exception on unstressed conditions (no salinity), MT100 and MT200 under 75 µM NaCl and MT100 under 150 µM NaCl wer the efficient practices for maintining RWC showig increases of 3.3, 2.4 and 3.4%, compared to the counterpart control treatment (MT0), respectiveky. Concenrning the osmolytes, the accumulation of proline, total soluble sugars and free amino acids showed significant (p > 0.05) increases in the presence of NaCl stress (Fig. 3). Overall, the highet increase in proline was observed with MT100 or MT200 under 75 µM NaCl. While MT100 and MT50 showed the maximal total soluble and free amino acids, respectively, under150 µM NaCl.
Influence of melatonin (MT) concentration on relative water content (RWC), total soulable sugars (TSS), proline and free amino acids (FAA) of sorghum seedling under salinity levels. FW: fresh weight; MT0, MT50, MT100 and MT200: application of MT concentration at 0, 50, 100 and 200 µM, respectively; S1, S2 and S3: salinity level at 0, 75 nad 150 NaCl µM, respectively. The means in each column followed by the same lower-case-letter refer to that there is no significant difference according Tukey’s Honestly Significant Difference (HSD) test at P < 0.05. Data were expressed as the mean ± standard deviation of 3 replications
3.4 Effect of Melatonin on H2O2, MDA, MG Accumulation and MSI under Salt Stress
Plants grown under NaCl stress exhibited a sharp increase in H2O2 content accompanied by elevation in MDA and MG content (Fig. 4). Thus, under each of moderate or severe salinity, no MT supply (MT0) treatment gave the highest H2O2, MDA and MG accumulation. Application of exogenous MT resulted in substaincial (p > 0.05) reductions in H2O2, MDA and MG accumulation. Both the intermediate and high concentrations of MT (100 and 200 µM) were effective in reducing the effect of NaCl stress of H2O2, MDA and MG. Accordingly, compared to the counterpart control treatment (MT0), reductions in H2O2, MDA and MG were 17.8 and 20.7%; 47.6 and 43.4% and 27.5 and 28.3% due to application of MT100 and MT200, respectively, under 75 µM NaCl stress. As well, the corresponding reductions under 150 µM NaCl stress were 22.0 and 23.9%; 29.1 and 23.8%; and 26.0 and 40.4%, respectively.
Membrane stability index (MSI %) was seen to be reduced in the presence of NaCl stress. However, an obvious and significant (p > 0.05) improvement was observed implementing MT treatments (Fig. 4). The impact of MT levels was more evident under severe salt stress, since MT100 along MT50 possessed the best improvement in MSI %. While, the difference between MT50, MT100 and MT200 in MSI % under moderate salt stress was not significant (p < 0.05).
Influence of melatonin (MT) concentration on hydrogen peroxide (H2O2), malondialdehyde (MDA), methyglycoxal (MG) and membrane stablity index (MSI) of sorghum seedling under salinity levels. FW: fresh weight; MT0, MT50, MT100 and MT200: application of MT concentration at 0, 50, 100 and 200 µM, respectively; S1, S2 and S3: salinity level at 0, 75 nad 150 NaCl µM, respectively. The means in each column followed by the same lower-case-letter refer to that there is no significant difference according Tukey’s Honestly Significant Difference (HSD) test at P < 0.05. Data were expressed as the mean ± standard deviation of 3 replications
3.5 Melatonin Regulates Ion Homeostasis under Salt Stress
The effect of NaCl stress on ion levels was analyzed by measuring Na+, K+, Ca2+ content and calculating Na+/K+ ratio in sorghum roots and shoots (Fig. 5). Na+ showed 1.8 and 3.4-fold increase in roots and 3.5 and 6.2-fold increase in shoots under moderate and severe salinity level compared to the unstressed plants. While, K+ levels showed decreases of 28.0 and 41.3% in roots and 19.5 and 34.3% in shoots of plants subjected to 75 and 150 µM NaCl stress, respectively. Accordingly, Na+/K+ ratio was observed to exhibit sharp increase up 2.2 and 5.9% in roots as well as 4.4 and 9.5% in shoots of plants subjected to 75 and 150 µM NaCl stress, respectively. Further, NaCl stress at 75 and 150 µM NaCl resulted in reduction in Ca2+ content by 27.4 and 40.8% in roots and 33.9 and 50.9% in shoots, respectively. Exogenous MT significantly reduced the accumulation of Na in both root and shoot systems under moderate and higher salinity levels. In this context, these responses were in parallel with enhancing the content of K+ in both organs (root and shoot) and consequently reducing the ratio of Na+/K+. The treatments of MT at 100 and 200 µM were more potent in this respect. Moreover, MT treatments specifically at 100 and 200 µM exhibited a significant (p > 0.05) increase in Ca+ 2 content in the root and shoot systems of the saline stressed plants whether with 75 or 150 µM NaCl.
Influence of melatonin (MT) concentration on sodium (Na+), potassium (K+), Na+/K+ ratio and calcium (Ca+ 2) of sorghum seedling under salinity levels. DW: dry weight; MT0, MT50, MT100 and MT200: application of MT concentration at 0, 50, 100 and 200 µM, respectively; S1, S2 and S3: salinity level at 0, 75 nad 150 NaCl µM, respectively. The means in each column followed by the same lower-case-letter refer to that there is no significant difference according Tukey’s Honestly Significant Difference (HSD) test at P < 0.05. Data were expressed as the mean ± standard deviation of 3 replications
3.6 Melatonin Regulates the Activities of Antioxidant Enzymes under Salt Stress
Plants subjected to salinity stress (75 µM and 150 µM) displayed dramatic increase in the activity of antioxidant enzymes (POX, CAT, SOD and APX) compared to the unstressed plants (Fig. 6). The treatment of MT at 100 µM led to the highest significant (p > 0.05) increase in the activity of POX compared to the untreated plants under both moderate and higher salinity levels. Under moderate salinity stress (75 µM NaCl), application of MT100 and MT200 showed the highest significant (p > 0.05) increase in the activity APX and CAT, respectively. Meanwhile, maximum activity of SOD was observed under the higher salinity level (150 µM NaCl) with supplying MT100.
Influence of melatonin (MT) concentration on the activity of peroxidase (POX, µmol mg− 1 protein min− 1), catalse (CAT, µmol mg− 1 protein min− 1), superoxide dismutase (SOD, Unit mg− 1 protein) and ascorbate peroxidase (APX, µmol mg− 1 protein min− 1) of sorghum seedling under salinity levels. DW: dry weight; MT0, MT50, MT100 and MT200: application of MT concentration at 0, 50, 100 and 200 µM, respectively; S1, S2 and S3: salinity level at 0, 75 nad 150 NaCl µM, respectively. The means in each column followed by the same lower-case-letter refer to that there is no significant difference according Tukey’s Honestly Significant Difference (HSD) test at P < 0.05. Data were expressed as the mean ± standard deviation of 3 replications
3.7 Molecular Docking
As illustrated in Fig. 7a, b, the test samples had very good interactions with the target enzymes, as shown by binding energies and binding mode. Melatonin has top-ranked confirmations with target antioxidant enzymes, according to obtained docking results. The molecular docking results reveal that there were several favourable binding affinities between MT and antioxidant enzymes APX and CAT (Fig. 7a) and POX and SOD (Fig. 7b). MT with APX and CAT shows the strongest binding affinities with ΔG values of − 8.7 and − 8.3 kcal/mol respectively. MT forms multiple hydrogen bonds with key amino acid residues for glycine (A: 162), tryptophane (A: 41), leucine (A: 165), tyrosine (A: 235) in the active site of ascorbate peroxidase. The alkyl interactions with leucine (A: 37), arginine (A: 38) and cysteine (A: 168) also contribute to its high affinity.
Docking view of melatonin on the binding sites of antioxidant enzymes; (A) Ascorbate peroxidase; (B) Catalase. Left are the 2D interaction diagrams, and right are the complex structures in 3D. Docking view of melatonin on the binding sites of antioxidant enzymes; (C) Peroxidase; (D) Superoxide dismutase. Left are the 2D interaction diagrams, and right are the complex structures in 3D
4 Discussion
Salinity stress has been considered a major threat for crop production due to its destructive impacts on plant growth and development in many regions worldwide. In this study, roots and shoots fresh weights were significantly suppressed by exposing to moderate or sevre saline levels (75 and 150 µM NaCl). According to its level, salinity stress can affect plant cell cycle regulation, leading to reduce cell division rates and cell numbers in leaves, roots, or the shoot meristem (Qi and Zhang 2020). Melatonin can act as a plant growth regulator with a similar action of indolyl-3-acetic acid (Arnao and Hernández-Ruiz 2014). It has different signalling pathways and crosstalk reactions with other phytohormones under normal and stressful conditions (Khan et al. 2022). Under saline conditions, many previous studies have revealed that exogenous MT can stimulate plant growth and development in different plant species i.e., maize (Chen et al. 2018), cucumber (Zhang et al. 2020), tomato (Ali et al. 2021), snap bean (El-Beltagi et al. 2023) and sorghum (Nie et al. 2023). In the present study, applied-MT at different concentrations (50, 100 and 200 µM) significantly improved shoot fresh weight under unstressed and various saline conditions (75 and 150 µM NaCl). Meanwhile, root fresh weight was enhanced by MT-treatments under unstressed and moderate saline conditions (75 µM NaCl), but this effect was less clear under higher salinity level (150 µM NaCl). This effect might be attribute to that high salinity level can affect the rate and direction of carbon flow between different plant tissues (Dong and Beckles 2019).
Subjecting sorghum plants to salinity stress sharply reduced the leaf content of chlorophyll a, chlorophyll b, total chlorophyll and carotenoids compared to the unstressed conditions. Salinity stress can provoke the photosynthetic apparatus to release excessive amounts of ROS, accelerating degradation rate of leaf pigments, inducing leaf senescence, and eventually causing leaf cell death (Liang et al. 2015; Youssef et al. 2021; El-Bially et al. 2023). However, applied-MT led to an obvious and significant increase in chlorophylls (a & b) and carotenoids of sorghum leaf. Several earlier reports have revealed that under saline conditions, applied-MT plays an important role in improving the photosynthetic efficiency and maintaining the leaf pigments (Wang et al. 2016; Chen et al. 2018; Altaf et al. 2020; Jiang et al. 2021). These influences may be due to the ability of MT to regulate the transcription of leaf-pigments related genes, protecting of photosynthetic proteins, promoting antioxidant systems and activating the xanthophyll cycle (Yang et al. 2022).
Relative water content (RWC) appears to be an important parameter for measuring the ability of plants to be survived under saline conditions. In this study, MT-treated plants exhibited a significant improvement in RWC compared to those of unstressed conditions. The accumulation of osmoprotectants i.e., proline, soluble sugars and free amino acids are common adaptive responses in several plants species under saline conditions (Ali et al. 2021; Youssef et al. 2021; Farag et al. 2022). As well, these organic molecules can be orchestrated together to regulate plant water uptake and preventing the dehydration of tissues through readjustment the cellular osmotic potential under adverse conditions (Batista-Silva et al. 2019; Dong and Beckles 2019; Ramadan et al. 2022). Earlier works have ascertained the role of MT in improving different osmolytes concentration under salinity stress (Siddiqui et al. 2019; Ali et al. 2021; Nie et al. 2023). In the present work, increase of proline, soluble sugars and the oscillation in free amino acids in MT-treated plants implies that MT may be involved in the biosynthesis and metabolism of osmolytes under saline conditions.
NaCl stress (moderate and high level) led to a marked elevation in H2O2 content, lipid peroxidation (MDA), MG content, while reduced MSI %. Application of exogenous MT was effective in reducing the levels of H2O2, MDA and MG content. According to Zhang et al. (2021b) MT is effective in instigating the gene expression of antioxidative enzymes including ascorbate-peroxidase, hence exogenous melatonin reduced H2O2 levels and restored cell membrane integrity in NaCl-stressed sugar beets. Findings of the present work revealed that exogenous MT possibly appears beneficial in normalizing the redox imbalance by reducing H2O2 content and maintain cell membrane stability (low MDA content and increased MSI %). Various reports have provided instances of the effect of MT in reducing H2O2 and MDA content under salt stress (Li et al. 2012; Wang et al. 2016; Jiang et al. 2021; El-Beltagi et al. 2023).
Alharbi et al. (2021) have reported the modulatory role of MT in regulation of glyoxalase system. In the present study, NaCl stress significantly increased MG content which was however, partially reduced by MT treatment. Thus, modulation of glyoxalase system in the presence of MT is evident to exert regulatory effects on MG content under NaCl stress. Glyoxalase system is known to be an important regulator of ROS and MG content during salinity and other abiotic stress in plants (Hasanuzzaman et al. 2014; Nahar et al. 2016; El-Yazied et al. 2022). Previous investigations also reported the effect of MT in improvement the membrane stability index (MSI%) under abiotic stress (Ali et al. 2021; Chen et al. 2021). In the present work, MT treatments reduced lipid peroxidation under salinity stress which was accompanied by a concomitant increase in MSI%. Therrfore, reduced H2O2 and MG content in the presence of exogenous MT was observed to be correlated with improving membrane stability and oxidative tolerance in plants subjected to NaCl stress.
As for ion homeostasis, it has been reported that the unfavorable circumistances cause dengerous impacts of nutrients uptake and utilization affecting the related plant metabolism (Saudy et al. 2020c, 2021c; Salem et al. 2022; Ali et al. 2023). NaCl stress significantly increased Na+ content in both roots and shoots which was accompanied by a reduction in K+ and Ca2+ content. Contrariwise, exogenous MT (at all concentrations) reduced the accumulation of Na+ in both roots and shoots which was accompanied by subsequent increase in K+ and Ca2+ content. Thus, observations recorded in the present work imply that exogenous MT effectively reduced Na+/K+ ratio during NaCl stress. Apart from its role as a potent antioxidant, MT has been ascertained to be an important biostimulant which alleviates NaCl stress-induced ionic imbalance in plants. Herein, maize seedlings treated with NaCl stress have shown a reduction in Na+/K+ ratio in the presence of MT treatment (Yan et al. 2020). Furthermore, seedlings of Malus sp. have also exhibited an obvious increase in K+ content and higher K+/Na+ ratio in the presence of MT treatment (Li et al. 2012). Interestingly, MT-induced ion homeostasis during NaCl stress indicates increased activity of SOS2, AKT and NHX (Zhan et al. 2019). Although MT-induced regulation of ion homeostasis during NaCl stress requires further investigations, it is well evident that MT functions as a positive regulator of Na+/H+ antiporter and K+ influx system in cell and tonoplast. Zhang et al. (2021a) reported that melatonin treatment improved Na+ efflux and K+ influx systems in NaCl-stressed sugar beet plants. Thus, Na+ compartmentalization, and its efflux is accompanied by increase in K+ content during MT supplementation under NaCl stress. We also recorded a marked increase in Ca2+ which indicates that MT was effective in restoring ion and electropotential gradient across the plasma membrane of plants subjected to NaCl stress. An interesting report by Liu et al. (2020) has provided evidence of melatonin-induced instigation of Ca2+ signalling during NaCl stress. The authors reported MT-induced increase in calcineurin B-like/calcineurin B-like-interacting protein kinase and calcium-dependent protein kinase expression in salinity stressed rice plants. Moreover, endogenous and exogenous melatonin has been advocated to be important for improving Ca+ content and Ca2+-associated signalling pathways during salinity tolerance in cotton plants (Zhang et al. 2021b) Thus, briefly, our present work substantiates the fact that exogenous MT in variable concentrations are effective in reducing Na+/K+ ratio and improving Ca2+ content under moderate and high levels of NaCl stress.
Salinity stress can disturb the physiological/biochemical processes in plants by stimulating the excessive generation of free radicals as superoxide radical and hydrogen peroxide which can directly attack membrane lipids and led to the loss in MSI (Miller et al. 2010; Youssef et al. 2021). In order to prevent the damage triggered by oxidative stress, plants can evolve enzymatic antioxidant scavenging mechanism for eliminating the adverse effect of ROS (Mansour et al. 2021; Makhlouf et al. 2022; Saudy and El-Metwally 2023). In the current study, the activities of some antioxidant enzymes as SOD, CAT, POX and APX were significantly increased under salinity stress. The higher activities of these enzymes afford the tolerance mechanism in plants by scavenging ROS induced by salinity stress (Chen et al. 2018; El-Beltagi et al. 2023; Nie et al. 2023). As well, the results in this investigation showed that the exogenous application of MT induced further increase in the activities of antioxidant enzymes (SOD, CAT, POX and APX) under salinity stress, leading to attenuate the accumulation of ROS levels (El-Bially et al. 2022b; (El-Metwally et al. 2022c). These effects may confirm the protecting effect of MT as powerful antioxidant under saline conditions (Zhang et al. 2020; Ali et al. 2021).
Furthermore, in this study, it was observed that MT had high affinity with different studied antioxidant enzymes (SOD, CAT, POX and APX) with docking score of -8.3. The treatments of MT showed three polar interactions with the active residues of the target CAT enzyme. It was observed that Arg A:62, Arg A: 344 and Gly A:173 established hydrogen bond, also additional pi-sigma, alkyl, and pi-alkyl interactions with residues Ala A: 123, Typ A: 348, Val A: 130 and Arg A: 102. Melatonin form van der waals force, alkyl and pi-alkyl contacts with POX but have lower affinity (-5.1 kcal/mol) than APX and CAT enzymes. In summary, MT with target antioxidant enzymes confer better binding potential which can be explained by their ability to form multiple hydrogen bonds and pi-pi stacked conformations with amino acids in the active site. Structural modifications of antioxidant enzymes to integrate more hydrogen bonding functional groups could further improve their affinity and activator potential. The ligand melatonin shows the strongest predicted binding affinity with SOD, having a ΔG of -6.8 kcal/mol. It forms a hydrogen bond interaction with the key active site residue Arg A: 339 and Ser A:292. It forms alkyl and pi-alkyl interactions with residues Val A:72, Lue A: 258 and leu A: 287. The combination of hydrogen bonds and hydrophobic interactions with multiple active site residues leads to its positive and expected binding. To link the results of the molecular docking study with the estimation of target antioxidant enzymes activity, based on previous studies, MT has a stimulant effect on these enzymes. This feature has been related to the influence of MT, which reduced lipid peroxidation and improved ROS metabolism (Sati et al. 2023). In another study, Jiao et al. (2022) found the enhanced antioxidant properties in MT -treated kiwifruit by increasing SOD activity. As well, Onik et al. (2021) observed a rising trend in CAT, SOD, and POX activity in apples treated with MT followed by a corresponding decrease in MDA level.
5 Conclusions
Despite sorghum plant is a relatively moderate tolerant to salinity stress, the results of this study confirmed its high sensitivity to a wide range of saline conditions at early growth stages. Applied-melatonin enhanced different morphological, physiological and biochemical mechanisms which orchestrated together to confer the stressed seedlings the required tolerance against salt stress. These mechanisms included strengthening photosynthesis by elevating the biosynthesis of photosynthetic pigments, stabilizing cell membranes, enhancing osmotic balance, and nutrients homeostasis. Moreover, melatonin had the ability to enhance the antioxidant capacity by altering the activities of antioxidant enzymes. The molecular docking modeling studies showed top-ranked confirmations between melatonin and the target antioxidant enzymes. The highest powerful affinity has been showed between melatonin treatments and ascorbate peroxidase compared to the other enzymes.
Abbreviations
- Ala:
-
Alanine
- APX:
-
Arginine
- CAT:
-
catalase
- Cys:
-
Cysteine
- Gly:
-
Glycine
- Leu:
-
Leucine
- MDA:
-
Malondialdehyde
- MSI:
-
Membrane stability index
- POD:
-
Peroxidase
- ROS:
-
Reactive Oxygen Species
- RWC:
-
Relative water content
- Ser:
-
Serine
- SOD:
-
Superoxide dismutase
- Trp:
-
Tryptophan
- Val:
-
Valine
References
Abdelaal K, Alsubeie MS, Hafez Y, Emeran A, Moghanm F, Okasha S, Omara R, Basahi MA, Darwish DBE, Ibrahim MFM, El-Yazied AA, Rashwan EA, Elkelish A, Mady MA, Ibraheem F (2022) Physiological and biochemical changes in vegetable and field crops under drought, salinity and weeds stresses: Control strategies and management. Agric 12:2084. https://doi.org/10.3390/agriculture12122084
Abd El-Mageed TA, Mekdad AAA, Rady MOA, Abdelbaky AS, Saudy HS, Shaaban A (2022) Physio-biochemical and agronomic changes of two sugar beet cultivars grown in saline soil as influenced by potassium fertilizer. J Soil Sci Plant Nutr 22:3636–3654. https://doi.org/10.1007/s42729-022-00916-7
Abd–Elrahman SH, Saudy HS, Abd El–Fattah DA, Hashem FA (2022) Effect of irrigation water and organic fertilizer on reducing nitrate accumulation and boosting lettuce productivity. J Soil Sci Plant Nutr 22:2144–2155. https://doi.org/10.1007/s42729-022-00799-8
Abou El-Enin MM, Sheha AM, El-Serafy Rasha S, Ali OAM, Saudy HS, Shaaban A (2023) Foliage-sprayed nano-chitosan-loaded nitrogen boosts yield potentials, competitive ability, and profitability of intercropped maize-soybean. Int J Plant Prod 17:517–542. https://doi.org/10.1007/s42106-023-00253-4
Alan R (1994) The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Alharbi BM, Elhakem AH, Alnusairi GS, Soliman MH, Hakeem KR, Hasan MM, Abdelhamid MT (2021) Exogenous application of melatonin alleviates salt stress-induced decline in growth and photosynthesis in Glycine max (L.) seedlings by improving mineral uptake, antioxidant and glyoxalase system. Plant Soil Environ 67:208–220. https://doi.org/10.17221/659/2020-PSE
Ali IAA, Hassan SE, Abdelhafez AA, Hewidy M, Nasser MA, Saudy HS, Hassan KM, Abou-Hadid AF (2023) Modifying the growing media and bio stimulants supply for healthy gerbera (Gerbera jamesonii) flowers Gesun Pflanz. https://doi.org/10.1007/s10343-023-00943-z
Ali MAA, Nasser MA, Abdelhamid AN, Ali IAA, Saudy HS, Hassan KM (2024) Melatonin as a key factor for regulating and relieving abiotic stresses in harmony with phytohormones in horticultural plants — a review. J Soil Sci Plant Nutr 24:54–73. https://doi.org/10.1007/s42729-023-01586-9
Ali M, Kamran MG, Abbasi HM, Saleem H, Ahmad S, Parveen A, Malik Z, Afzal S, Ahmar S, Dawar KM (2021) Melatonin-induced salinity tolerance by ameliorating osmotic and oxidative stress in the seedlings of two tomato (Solanum lycopersicum L.) cultivars. J Plant Growth Reg 40:2236–2248. https://link.springer.com/article/https://doi.org/10.1007/s00344-020-10273-3
Alsamadany H, Mansour H, Elkelish A, Ibrahim MF (2022) Folic acid confers tolerance against salt stress-induced oxidative damages in snap beans through regulation growth, metabolites, antioxidant machinery and gene expression. Plants 11:1459. https://doi.org/10.3390/plants11111459
Altaf M, Shahid R, Ren M, Naz S, Altaf M, Qadir A, Anwar M, Shakoor A, Hayat F (2020) Exogenous melatonin enhances salt stress tolerance in tomato seedlings. Biol Plantar 64:604–615. https://doi.org/10.32615/bp.2020.090
Analin B, Mohanan A, Bakka K, Challabathula D (2020) Cytochrome oxidase and alternative oxidase pathways of mitochondrial electron transport chain are important for the photosynthetic performance of pea plants under salinity stress conditions. Plant Physiol Biochem 154:248–259. https://doi.org/10.1016/j.plaphy.2020.05.022
Arnao M, Hernández-Ruiz J (2020) Melatonin in flowering, fruit set and fruit ripening. Plant Reprod 33:77–87. https://doi.org/10.1007/s00497-020-00388-8
Arrnao MB, Hernández-Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci 19:789–797. https://doi.org/10.1016/j.tplants.2014.07.006
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant soil 39:205–207. https://doi.org/10.1007/BF00018060
Batista-Silva W, Heinemann B, Rugen N, Nunes-Nesi A, Araújo WL, Braun H-P, Hildebrandt TM (2019) The role of amino acid metabolism during abiotic stress release. Plant Cell Environ 42:1630–1644. https://doi.org/10.1111/pce.13518
Beyer WF Jr, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566. https://doi.org/10.1016/0003-2697(87)90489-1
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Buttar ZA, Wu SN, Arnao MB, Wang C, Ullah I, Wang C (2020) Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 9:809. https://www.mdpi.com/2223-7747/9/7/809#
Cakmak I, Strbac D, Marschner H (1993) Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J Exp Bot 44:127–132. https://doi.org/10.1093/jxb/44.1.127
Chen YE, Mao JJ, Sun LQ, Huang B, Ding CB, Gu Y, Liao JQ, Hu C, Zhang ZW, Yuan S (2018) Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol Planta 164:349–363. https://doi.org/10.1111/ppl.12737
Chen Y, Li R, Ge J, Liu J, Wang W, Xu M, Zhang R, Hussain S, Wei H, Dai Q (2021) Exogenous melatonin confers enhanced salinity tolerance in rice by blocking the ROS burst and improving Na+/K+ homeostasis. Environ Exp Bot 189:104530. https://doi.org/10.1016/j.envexpbot.2021.104530
Chow PS, Landhäusser SM (2004) A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol 24:1129–1136. https://doi.org/10.1093/treephys/24.10.1129
Corwin DL (2021) Climate change impacts on soil salinity in agricultural areas. Euro J Soil Sci 72:842–862. https://doi.org/10.1111/ejss.13010
Darwish H, Al-Osaimi GS, Al Kashgry NAT, Sonbol H, Alayafi AAM, Alabdallah NM, Al-Humaid A, Al-Harbi NA, Al-Qahtani SM, Abbas ZK, Darwish DBE, Ibrahim MFM, Noureldeen A (2023) Evaluating the genotoxicity of salinity stress and secondary products gene manipulation in lime, Citrus aurantifolia, plants. Front Plant Sci 14:1211595. https://doi.org/10.3389/fpls.2023.1211595
Din AFMZE, Ibrahim, MFM, Farag R, El-Gawad HGA, El-Banhawy A, Alaraidh IA, Rashad YM, Lashin I, El-Yazied AA, Elkelish A, Abd Elbar OH (2020) Influence of polyethylene glycol on leaf anatomy, stomatal behavior, water loss, and some physiological traits of date palm plantlets grown in vitro and ex Vitro. Plants 9:1440 https://doi.org/10.3390/plants9111440
Doklega SMA, Saudy HS, El-Sherpiny MA, Abou El-Yazied A, Abd El-Gawad HG, Ibrahim MFM, Abd El-Hady MAM, Omar MMA, Metwally AA (2023) Rhizospheric addition of hydrogel polymer and zeolite plus glutathione mitigate the hazard effects of water deficiency on common bean plants through enhancing the defensive antioxidants. Gesun Pflanz. https://doi.org/10.1007/s10343-023-00947-9
Dong S, Beckles DM (2019) Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J Plant Physiol 234:80–93. https://doi.org/10.1016/j.jplph.2019.01.007
Dourado PRM, de Souza ER, Santos MAD, Lins CMT, Monteiro DR, Paulino MKSS, Schaffer B (2022) Stomatal regulation and osmotic adjustment in sorghum in response to salinity. Agric 12:658. https://www.mdpi.com/2077-0472/12/5/658#
El-Beltagi HS, El-Yazied AA, El-Gawad HGA, Kandeel M, Shalaby TA, Mansour AT, Al-Harbi NA, Al-Qahtani SM, Alkhateeb AA, Ibrahim MF (2023) Synergistic impact of melatonin and putrescine interaction in mitigating salinity stress in snap bean seedlings: reduction of oxidative damage and inhibition of polyamine catabolism. Hortic 9:285. https://www.mdpi.com/2311-7524/9/2/285#
El-Bially MA, El-Metwally IM, Saudy HS, Aisa KH, Abd El-Samad GA (2023) Mycorrhiza-inoculated biochar as an eco-friendly tool improves the broomrape control efficacy in two faba bean cultivars. Rhizosphere 26:100706. https://doi.org/10.1016/j.rhisph.2023.100706
El-Bially MA, Saudy HS, El-Metwally IM, Shahin MG (2018) Efficacy of ascorbic acid as a cofactor for alleviating water deficit impacts and enhancing sunflower yield and irrigation water–use efficiency. Agric Wat Manage 208:132–139. https://doi.org/10.1016/j.agwat.2018.06.016
El-Bially MA, Saudy HS, El-Metwally IM, Shahin MG (2022a) Sunflower response to application of L–ascorbate under thermal stress associated with different sowing dates. Gesun Pflanz 74:87–96. https://doi.org/10.1007/s10343-021-00590-2
El-Bially MA, Saudy HS, Hashem FA, El–Gabry YA, Shahin MG (2022b) Salicylic acid as a tolerance inducer of drought stress on sunflower grown in sandy soil. Gesun Pflanz 74:603–613. https://doi.org/10.1007/s10343-022-00635-0
El-Metwally IM, Geries L, Saudy HS (2022a) Interactive effect of soil mulching and irrigation regime on yield, irrigation water use efficiency and weeds of trickle–irrigated onion. Archiv Agron Soil Sci 68:1103–1116. https://doi.org/10.1080/03650340.2020.1869723
El-Metwally IM, Sadak MSh, Saudy HS (2022b) Stimulation effects of glutamic and 5-Aminolevulinic acids on photosynthetic pigments, physio-biochemical constituents, antioxidant activity, and yield of peanut. Gesun Pflanz 74:915–924. https://doi.org/10.1007/s10343-022-00663-w
El-Metwally IM, Saudy HS (2021a) Interactional impacts of drought and weed stresses on nutritional status of seeds and water use efficiency of peanut plants grown in arid conditions. Gesun Pflanz 73:407–416. https://doi.org/10.1007/s10343-021-00557-3
El-Metwally IM, Saudy HS (2021b) Interactive application of zinc and herbicides affects broad–leaved weeds, nutrient uptake, and yield in rice. J Soil Sci Plant Nutr 21:238–248. https://doi.org/10.1007/s42729-020-00356-1
El-Metwally IM, Saudy HS, Abdelhamid MT (2021) Efficacy of benzyladenine for compensating the reduction in soybean productivity under low water supply. Italian J Agromet 2:81–90. https://doi.org/10.36253/ijam-872
El-Metwally IM, Saudy HS, Elewa TA (2022c) Natural plant by-products and mulching materials to suppress weeds and improve sugar beet (Beta vulgaris L.) yield and quality. J Soil Sci Plant Nutr 22:5217–5230. https://doi.org/10.1007/s42729-022-00997-4
El-Mogy MM, Atia MAM, Dhawi F, Fouad AS, Bendary ESA, Khojah E, Samra BN, Abdelgawad KF, Ibrahim MFM, Abdeldaym EA (2022) Towards better grafting: SCoT and CDDP analyses for prediction of the tomato rootstocks performance under drought stress. Agron 12:153. https://doi.org/10.3390/agronomy12010153
El Nahhas N, AlKahtani MD, Abdelaal KA, Al Husnain L, AlGwaiz HI, Hafez YM, Attia KA, El-Esawi MA, Ibrahim MF, Elkelish A (2021) Biochar and jasmonic acid application attenuates antioxidative systems and improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water. Plant Physiol Biochem 166:807–817. https://doi.org/10.1016/j.plaphy.2021.06.033
El-Yazeid AA, Abou-Aly HE (2011) Enhancing growth, productivity and quality of tomato plants using phosphate solubilizing microorganisms. Aust J Basic Appl Sci 5:371–379
El-Yazied AA, Ibrahim MF, Ibrahim MA, Nasef IN, Al-Qahtani SM, Al-Harbi NA, Alzuaibr FM, Alaklabi A, Dessoky ES, Alabdallah NM (2022) Melatonin mitigates drought induced oxidative stress in potato plants through modulation of osmolytes, sugar metabolism, ABA homeostasis and antioxidant enzymes. Plants 11:1151. https://www.mdpi.com/2223-7747/11/9/1151#
Farag HA, Ibrahim MF, El-Yazied A, El-Beltagi HS, El-Gawad HGA, Alqurashi M, Shalaby TA, Mansour AT, Alkhateeb AA, Farag R (2022) Applied selenium as a powerful antioxidant to mitigate the harmful effects of salinity stress in snap bean seedlings. Agron 12:3215. https://www.mdpi.com/2073-4395/12/12/3215#
Gashash EA, Osman NA, Alsahli AA, Hewait HM, Ashmawi AE, Alshallash KhS, El-Taher AM, Azab ES, Abd El-Raouf HS, Ibrahim MFM (2022) Effects of plant-growth-promoting rhizobacteria (PGPR) and cyanobacteria on botanical characteristics of tomato (Solanum lycopersicon L.) plants. Plants 11:2732 https://doi.org/10.3390/plants11202732
Hadid ML, Ramadan KhMA, El-Beltagi HS, Ramadan AA, El-Metwally IM, Shalaby TA, Bendary ESA, Saudy HS (2023) Modulating the antioxidant defense systems and nutrients content by proline for higher yielding of wheat under water deficit. Not Bot Horti Agrobo 51:13291. https://doi.org/10.15835/nbha51313291
Hamilton PB, Van Slyke DD, Lemish S (1943) The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J Biol Chem 150:231–250. https://doi.org/10.1016/S0021-9258(18)51268-0
Hasanuzzaman M, Alam MM, Nahar K, Ahamed KU, Fujita M (2014) Exogenous salicylic acid alleviates salt stress-induced oxidative damage in Brassica napus by enhancing the antioxidant defense and glyoxalase systems. Aust J Crop Sci 8:631–639
Hayat S, Ali B, Hasan SA, Ahmad A (2007) Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41. https://doi.org/10.1016/j.envexpbot.2006.06.002
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hoque MN, Tahjib-Ul-Arif M, Hannan A, Sultana N, Akhter S, Hasanuzzaman M, Akter F, Hossain MS, Sayed MA, Hasan MT (2021) Melatonin modulates plant tolerance to heavy metal stress: Morphological responses to molecular mechanisms. Int J Mol Sci 22:11445. https://www.mdpi.com/1422-0067/22/21/11445#
Hossain A, Razzak MA, Tajkia JE, Sagar A, Roy R (2018) Effect of salt stress on growth of sorghum germplasms at vegetative stage. J Bangladesh Agric Univ 16:67–72. https://doi.org/10.3329/jbau.v16i1.36483
Hossain MA, Hossain MZ, Fujita M (2009) Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci 3:53
Huang B, Chen Y-E, Zhao Y-Q, Ding C-B, Liao J-Q, Hu C, Zhou L-J, Zhang Z-W, Yuan S, Yuan M (2019) Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front Plant Sci 10:677. https://doi.org/10.3389/fpls.2019.00677
Huang R-d (2018) Research progress on plant tolerance to soil salinity and alkalinity in sorghum. J Integr Agric 17:739–746. https://doi.org/10.1016/S2095-3119(17)61728-3
Jahan MS, Guo S, Sun J, Shu S, Wang Y, Abou El-Yazied A, Alabdallah NM, Hikal M, Mohamed MH, Ibrahim MF (2021) Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol Biochem 167:309–320. https://doi.org/10.1016/j.plaphy.2021.08.002
Jardim AMRF, da Silva GÍN, Biesdorf EM, Pinheiro AG, da Silva MV, Júnior GNA, dos Santos A, Alves HKMN, de Sá Souza M, de Morais JEF, Alves CP, da Silva TGF (2020) Production potential of Sorghum bicolor (L.) Moench crop in the Brazilian semiarid. Rev Pubvet 14. https://doi.org/10.31533/pubvet.v14n4a550.1-13
Jiang D, Lu B, Liu L, Duan W, Meng Y, Li J, Zhang K, Sun H, Zhang Y, Dong H (2021) Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol 21:1–19. https://doi.org/10.1186/s12870-021-03082-7
Jiao J, Jin M, Liu H, Suo J, Yin X, Zhu Q, Rao J (2022) Application of melatonin in kiwifruit (Actinidia chinensis) alleviated chilling injury during cold storage. Sci Hortic 296:110876. https://doi.org/10.1016/j.scienta.2022.110876
Jones JB Jr (2001) Laboratory guide for conducting soil tests and plant analysis. CRC
Khan M, Ali S, Manghwar H, Saqib S, Ullah F, Ayaz A, Zaman W (2022) Melatonin function and crosstalk with other phytohormones under normal and stressful conditions. Genes 13:1699. https://doi.org/10.3390/genes13101699
Lasheen FF, Hewidy M, Abdelhamid AN, Thabet RS, Abass MMM, Fahmy Asmaa A, Saudy HS, Hassan KM (2023) Exogenous application of humic acid mitigates salinity stress on pittosporum (Pittosporum tobira) plant by adjusting the osmolytes and nutrient homeostasis. Gesun Pflanz. https://doi.org/10.1007/s10343-023-00939-9
Liang C, Li A, Yu H, Li W, Liang C, Guo S, Zhang R, Chu C (2017) Melatonin regulates root architecture by modulating auxin response in rice. Front Plant Sci 8:134. https://doi.org/10.3389/fpls.2017.00134
Liang C, Zheng G, Li W, Wang Y, Hu B, Wang H, Wu H, Qian Y, Zhu XG, Tan DX (2015) Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J Pineal Res 59(1):91–101. https://doi.org/10.1111/jpi.12243
Li C, Wang P, Wei Z, Liang D, Liu C, Yin L, Jia D, Fu M, Ma F (2012) The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J Pineal Res 53:298–306. https://doi.org/10.1111/j.1600-079x.2012.00999.x
Li J, Liu J, Zhu T, Zhao C, Li L, Chen M (2019) The role of melatonin in salt stress responses. Int J Mol Sci 20:1735. https://doi.org/10.3390/ijms20071735
Litalien A, Zeeb B (2020) Curing the earth: a review of anthropogenic soil salinization and plant-based strategies for sustainable mitigation. Sci Total Environ 698:134235. https://doi.org/10.1016/j.scitotenv.2019.134235
Liu J, Shabala S, Zhang J, Ma G, Chen D, Shabala L, Zeng F, Chen ZH, Zhou M, Venkataraman G (2020) Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ 43:2591–2605. https://doi.org/10.1111/pce.13759
Makhlouf BSI, Khalil SRA, Saudy HS (2022) Efficacy of humic acids and chitosan for enhancing yield and sugar quality of sugar beet under moderate and severe drought. J Soil Sci Plant Nutr 22:1676–1691. https://doi.org/10.1007/s42729-022-00762-7
Mansha MZ, Aatif HM, Ikram K, Hanif CMS, Sattar A, Iqbal R, Zaman Qu, Al-Qahtani SM, Al-Harbi NA, Omar WA, Ibrahim, MFM (2023) Impact of various salinity levels and fusarium oxysporum as stress factors on the morpho-physiological and yield attributes of onion. Hortic 9:786. https://doi.org/10.3390/horticulturae9070786
Mansour MMF, Emam MM, Salama KHA, Morsy AA (2021) Sorghum under saline conditions: responses, tolerance mechanisms, and management strategies. Planta 254:24. https://doi.org/10.1007/s00425-021-03671-8
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33(4):453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mubarak M, Salem EMM, Kenawey MKM, Saudy HS (2021) Changes in calcareous soil activity, nutrient availability, and corn productivity due to the integrated effect of straw mulch and irrigation regimes. J Soil Sci Plant Nutr 21:2020–2031. https://doi.org/10.1007/s42729-021-00498-w
Mukhopadhyay R, Sarkar B, Jat HS, Sharma PC, Bolan NS (2021) Soil salinity under climate change: challenges for sustainable agriculture and food security. J Environ Manage 280:111736. https://doi.org/10.1016/j.jenvman.2020.111736
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf 126:245–255. https://doi.org/10.1016/j.ecoenv.2015.12.026
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nie M, Ning N, Chen J, Zhang Y, Li S, Zheng L, Zhang H (2023) Melatonin enhances salt tolerance in sorghum by modulating photosynthetic performance, osmoregulation, antioxidant defense, and ion homeostasis. Open Life Sci 18:20220734. https://doi.org/10.1515/biol-2022-0734
Noureldin NA, Saudy HS, Ashmawy F, Saed HM (2013) Grain yield response index of bread wheat cultivars as influenced by nitrogen levels. Ann Agric Sci Ain Shams Univ 58:147–152. https://doi.org/10.1016/j.aoas.2013.07.012
Onik JC, Wai SC, Li A, Lin Q, Sun Q, Wang Z, Duan Y (2021) Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chem 337:127753. https://doi.org/10.1016/j.foodchem.2020.127753
Premachandra GS, Saneoka H, Ogata S (1990) Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soybean. J Agric Sci 115:63–66. https://doi.org/10.1017/S0021859600073925
Qari SH, Hassan MU, Chattha MU, Mahmood A, Naqve M, Nawaz M, Barbanti L, Alahdal MA, Aljabri M (2022) Melatonin induced cold tolerance in plants: physiological and molecular responses. Front Plant Sci 13:843071. https://doi.org/10.3389/fpls.2022.843071
Qi F, Zhang F (2020) Cell cycle regulation in the plant response to stress. Front Plant Sci 10:1765. https://doi.org/10.3389/fpls.2019.01765
Rajabi Dehnavi A, Zahedi M, Ludwiczak A, Cardenas Perez S, Piernik A (2020) Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) genotypes. Agron 10:859. https://www.mdpi.com/2073-4395/10/6/859#
Ramadan KMA, Alharbi MM, Alenzi AM, El-Beltagi HS, Darwish DB, Aldaej MI, Shalaby TA, Mansour AT, El-Gabry YA, Ibrahim MFM (2022) Alpha lipoic acid as a protective mediator for regulating the defensive responses of wheat plants against sodic alkaline stress: physiological, biochemical and molecular aspects. https://doi.org/10.3390/plants11060787. Plants 11.787
Ramadan KMA, El-Beltag HS, Abd El Mageed TA, Mazrou KE, Mohamed GF, El-Saadony MT, El-Saadony FMA, Roby MHH, Saudy HS, Abou-Sreea AIB (2023a) Significance of selenium in ameliorating the effects of irrigation deficit via improving photosynthesis efficiency, cell integrity, osmo-protectants, and oil profile of anise crop. Not Bot Horti Agrobo 51(4):13437. https://doi.org/10.15835/nbha51413437
Ramadan KMA, El-Beltagi HS, Abd El-Mageed TAA, Saudy HS, Al-Otaibi HH, Mahmoud MAA (2023b) The changes in various physio-biochemical parameters and yield traits of faba bean due to humic acid plus 6-benzylaminopurine application under deficit irrigation. Agron 13:1227. https://doi.org/10.3390/agronomy13051227
Reddy K, Subhani S, Khan P, Kumar K (1985) Effect of light and benzyladenine on dark-treated growing rice (Oryza sativa) leaves II. Changes in peroxidase activity. Plant Cell Physiol 26:987–994
Rizk TY, kholousy ASO, Saudy HS, Sultan ShS, Abd Alwahed SHA (2023) Breaking dormancy and enhancing germination of Avena sterilis L. and Amaranthus retroflexus L. weeds by gibberellic acid and potassium nitrate to keep soil and crops healthy. Gesun Pflanz 75:757–763. https://doi.org/10.1007/s10343-022-00780-6
Salem EMM, Kenawey MKM, Saudy HS, Mubarak M (2021) Soil mulching and deficit irrigation effect on sustainability of nutrients availability and uptake, and productivity of maize grown in calcareous soils. Comm Soil Sci Plant Anal 52:1745–1761. https://doi.org/10.1080/00103624.2021.1892733
Salem EMM, Kenawey MKM, Saudy HS, Mubarak M (2022) Influence of silicon forms on nutrient accumulation and grain yield of wheat under water deficit conditions. Gesun Pflanz 74:539–548. https://doi.org/10.1007/s10343-022-00629-y
Sati H, Khandelwal A, Pareek S (2023) Effect of exogenous melatonin in fruit postharvest, crosstalk with hormones, and defense mechanism for oxidative stress management. Food Front 4:233–261. https://doi.org/10.1002/fft2.180
Sattar A, Sher A, Ijaz M, Ul-Allah S, Hussain S, Rasheed U, Hussain J, Al-Qahtani SM, Al-Harbi NA, Mahmoud SF, Ibrahim, MFM (2023) Modulation of antioxidant defense mechanisms and morpho-physiological attributes of wheat through exogenous application of silicon and melatonin under water deficit conditions. Sustain 15:7426. https://doi.org/10.3390/su15097426
Saudy HS (2014) Chlorophyll meter as a tool for forecasting wheat nitrogen requirements after application of herbicides. Archiv Agron Soil Sci 60:1077–1090. https://doi.org/10.1080/03650340.2013.866226
Saudy HS (2015) Maize–cowpea intercropping as an ecological approach for nitrogen-use rationalization and weed suppression. Archiv Agron Soil Sci 61:1–14. https://doi.org/10.1080/03650340.2014.920499
Saudy HS, Abd El–Momen WR, El–khouly NS (2018) Diversified nitrogen rates influence nitrogen agronomic efficiency and seed yield response index of sesame (Sesamum indicum, L.) cultivars. Comm Soil Sci Plant Anal 49:2387–2395. https://doi.org/10.1080/00103624.2018.1510949
Saudy HS, El-Bially MA, El-Metwally IM, Shahin MG (2021a) Physio–biochemical and agronomic response of ascorbic acid–treated sunflower (Helianthus annuus) grown at different sowing dates and under various irrigation regimes. Gesun Pflanz 73:169–179. https://doi.org/10.1007/s10343-020-00535-1
Saudy HS, El-Bially MA, Hashem FA, Shahin MG, El–Gabry YA (2023a) The changes in yield response factor, water use efficiency, and physiology of sunflower owing to ascorbic and citric acids application under mild deficit irrigation. Gesun Pflanz 75:899–909. https://doi.org/10.1007/s10343-022-00736-w
Saudy HS, El-Bially MA, Ramadan KA, Abo El–Nasr EKh, El-Samad A (2021b) GA Potentiality of soil mulch and sorghum extract to reduce the biotic stress of weeds with enhancing yield and nutrient uptake of maize crop. Gesun Pflanz 73:555–564. https://doi.org/10.1007/s10343-021-00577-z
Saudy HS, El-Metwally IM (2019) Nutrient utilization indices of NPK and drought management in groundnut under sandy soil conditions. Comm Soil Sci Plant Anal 50:1821–1828. https://doi.org/10.1080/00103624.2019.1635147
Saudy HS, El-Metwally IM (2023) Effect of irrigation, nitrogen sources and metribuzin on performance of maize and its weeds. Comm Soil Sci Plant Anal 54:22–31. https://doi.org/10.1080/00103624.2022.2109659
Saudy HS, El-Metwally IM, Abd El-Samad GA (2020a) Physio–biochemical and nutrient constituents of peanut plants under bentazone herbicide for broad–leaved weed control and water regimes in dry land areas. J Arid Land 12:630–639. https://doi.org/10.1007/s40333-020-0020-y
Saudy HS, El-Metwally IM, Shahin MG (2021c) Co–application effect of herbicides and micronutrients on weeds and nutrient uptake in flooded irrigated rice: does it have a synergistic or an antagonistic effect? Crop Prot 149:105755. https://doi.org/10.1016/j.cropro.2021.105755
Saudy HS, El-Metwally IM, Sobieh ST, Abd-Alwahed SHA (2022) Mycorrhiza, charcoal, and rocket salad powder as eco-friendly methods for controlling broomrape weed in inter-planted faba bean with flax. J Soil Sci Plant Nutr 22:5195–5206. https://doi.org/10.1007/s42729-022-00995-6
Saudy HS, Hamed MF, Abd El–Momen WR, Hussein H (2020b) Nitrogen use rationalization and boosting wheat productivity by applying packages of humic, amino acids and microorganisms. Comm Soil Sci Plant Anal 51:1036–1047. https://doi.org/10.1080/00103624.2020.1744631
Saudy HS, Hamed MF, El–Metwally IM, Ramadan KA, Aisa KH (2021d) Assessing the effect of biochar or compost application as a spot placement on broomrape control in two cultivars of faba bean. J Soil Ci Plant Nutr 21:1856–1866. https://doi.org/10.1007/s42729-021-00485-1
Saudy HS, Mubarak M (2015) Mitigating the detrimental impacts of nitrogen deficit and fenoxaprop-p-ethyl herbicide on wheat using silicon. Comm Soil Sci Plant Anal 46:913–923. https://doi.org/10.1080/00103624.2015.1011753
Saudy HS, Noureldin NA, Mubarak M, Fares W, Elsayed M (2020c) Cultivar selection as a tool for managing soil phosphorus and faba bean yield sustainability. Archiv Agron Soil Sci 66:414–425. https://doi.org/10.1080/03650340.2019.1619078
Saudy HS, Salem EMM, Abd El–Momen WR (2023b) Effect of potassium silicate and irrigation on grain nutrient uptake and water use efficiency of wheat under calcareous soils. Gesun Pflanz 75:647–654. https://doi.org/10.1007/s10343-022-00729-9
Shaaban A, Abd El-Mageed TA, Abd El-Momen WR, Saudy HS, Al-Elwany OAAI (2023a) The integrated application of phosphorous and zinc affects the physiological status, yield and quality of canola grown in phosphorus-suffered deficiency saline soil. Gesun Pflanz 75:1813–1821. https://doi.org/10.1007/s10343-023-00843-2
Shaaban A, Mahfouz H, Megawer EA, Saudy HS (2023b) Physiological changes and nutritional value of forage clitoria grown in arid agro-ecosystem as influenced by plant density and water deficit. J Soil Sci Plant Nutr 23:3735–3750. https://doi.org/10.1007/s42729-023-01294-4
Shahin MG, Saudy HS, El-Bially ME, Abd El-Momen WR, El-Gabry YA, Abd El-Samad GA, Sayed AN (2023) Physiological and agronomic responses and nutrient uptake of soybean genotypes cultivated under various sowing dates. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-023-01389-y
Shalaby OA, Farag RE, Ibrahim MFM (2023) Effect of hydrogen sulfide and hydrogen peroxide on growth, yield and nutrient content of broccoli plants grown under saline conditions. Sci Hortic 316:112035. https://doi.org/10.1016/j.scienta.2023.112035
Shehata SA, Ibrahim MFM (2023) Grafting, seed soaking/priming, soil amendment, and foliar application as tools to increase abiotic stress tolerance of crops. Crop Sustainability and Intellectual Property Rights, 1st ed. Apple Academic Press, p 151–190 http://dx.doi.org/10.1201/9781003383024-9
Siddiqui MH, Alamri S, Al-Khaishany MY, Khan MN, Al-Amri A, Ali HM, Alaraidh IA, Alsahli AA (2019) Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int J Mol Sci 20:353. https://doi.org/10.3390/ijms20020353
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiol 53:258–260. https://doi.org/10.1104/pp.53.2.258
Sun Z, Ren L, Fan J, Li Q, Wang K, Guo M, Wang L, Li J, Zhang G, Yang Z, Chen F, Li X (2016) Salt response of photosynthetic electron transport system in wheat cultivars with contrasting tolerance. Plant Soil Environ 62:515–521. https://doi.org/10.17221/529/2016-PSE
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vineeth T, Krishna G, Pandesha P, Sathee L, Thomas S, James D, Ravikiran K, Taria S, John C, Vinaykumar N, Lokeshkumar B, Jat H, Bose J, Camus D, Rathor S, Krishnamurthy S, Sharama P (2023) Photosynthetic machinery under salinity stress: Trepidations and adaptive mechanisms. Photosynthetica 61:73–93. https://doi.org/10.32615/ps.2023.002
Wang L, Liu J, Wang W, Sun Y (2016) Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 54:19–27. https://doi.org/10.1007/s11099-015-0140-3
Wang Y, Ye X-Y, Qiu X-M, Li Z-G (2019) Methylglyoxal triggers the heat tolerance in maize seedlings by driving AsA-GSH cycle and reactive oxygen species-/methylglyoxal-scavenging system. Plant Physiol Biochem 138:91–99. https://doi.org/10.1016/j.plaphy.2019.02.027
Xiao S, Liu L, Wang H, Li D, Bai Z, Zhang Y, Sun H, Zhang K, Li C (2019) Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L). PLoS ONE 14:e0216575. https://doi.org/10.1371/journal.pone.0216575
Yadav SK, Singla-Pareek SL, Ray M, Reddy MK, Sopory SK (2005) Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem Biophys Res Commun 337:61–67. https://doi.org/10.1016/j.bbrc.2005.08.263
Yan F, Wei H, Li W, Liu Z, Tang S, Chen L, Ding C, Jiang Y, Ding Y, Li G (2020) Melatonin improves K + and na + homeostasis in rice under salt stress by mediated nitric oxide. Ecotoxicol Environ saf 206:111358. https://doi.org/10.1016/j.ecoenv.2020.111358
Yang S, Zhao Y, Qin X, Ding C, Chen Y, Tang Z, Huang Y, Reiter RJ, Yuan S, Yuan M (2022) New insights into the role of melatonin in photosynthesis. J Exp Bot 73:5918–5927. https://doi.org/10.1093/jxb/erac230
Youssef MHM, Raafat A, El-Yazied AA, Selim S, Azab E, Khojah E, El Nahhas N, Ibrahim MFM (2021) Exogenous application of alpha-lipoic acid mitigates salt-induced oxidative damage in sorghum plants through regulation growth, leaf pigments, ionic homeostasis, antioxidant enzymes, and expression of salt stress responsive genes. Plants 10:2519. https://doi.org/10.3390/plants10112519
Zhang P, Liu L, Wang X, Wang Z, Zhang H, Chen J, Liu X, Wang Y, Li C (2021a) Beneficial effects of exogenous melatonin on overcoming salt stress in sugar beets (Beta vulgaris L). Plants 10:886. https://doi.org/10.3390/plants10050886
Zhang T, Shi Z, Zhang X, Zheng S, Wang J, Mo J (2020) Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci Hortic 262:109070. https://doi.org/10.1016/j.scienta.2019.109070
Zhang Y, Fan Y, Rui C, Zhang H, Xu N, Dai M, Chen X, Lu X, Wang D, Wang J, Wang Q, Wang S, Chen C, Guo L, Zhao L, Ye W (2021b) Melatonin improves cotton salt tolerance by regulating ROS scavenging system and Ca2+ signal transduction. Front Plant Sci 12:693690. https://doi.org/10.3389/fpls.2021.693690
Zhan H, Nie X, Zhang T, Li S, Wang X, Du X, Tong W, Song W (2019) Melatonin: a small molecule but important for salt stress tolerance in plants. Int J Mol Sci 20:709. https://doi.org/10.3390/ijms20030709
Zhao D, Wang H, Chen S, Yu D, Reiter RJ (2021a) Phytomelatonin: an emerging regulator of plant biotic stress resistance. Trends Plant Sci 26:70–82. https://doi.org/10.1016/j.tplants.2020.08.009
Zhao Y-Q, Zhang Z-W, Chen Y-E, Ding C-B, Yuan S, Reiter RJ, Yuan M (2021b) Melatonin: a potential agent in delaying leaf senescence. Crit Rev Plant Sci 40:1–22. https://doi.org/10.1080/07352689.2020.1865637
Acknowledgements
The authors would like to thank Ain Shams University, Cairo, Egypt for technically supporting this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Helal, N.M., Saudy, H.S., Hamada, M.M.A. et al. Potentiality of Melatonin for Reinforcing Salinity Tolerance in Sorghum Seedlings via Boosting Photosynthetic Pigments, Ionic and Osmotic Homeostasis and Reducing the Carbonyl/Oxidative Stress Markers. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-01830-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-01830-w