Abstract
Purpose
Medicinal plants show a relevant importance in human life and health. The use of natural substances in crop management is not only significant for the plant healthy growth and yield, but also for obtaining safe products. The study aimed to examine the effect of seaweed extract (SW) as a natural source of nutrition and salicylic acid (SA) on plant health, physiology and essential oil profile of French marigold (Tagetes patula L.).
Methods
Three levels of salicylic acid, SA50, SA100 and SA200, (50, 100, and 200 mg L− 1) and three levels of seaweed extract, SW2, SW4 and SW6 (2.0, 4.0, and 6.0 ml L− 1), in comparison with control treatment (tap water) were applied as foliar sprayings. The seven treatments were arranged in randomized complete block design and were replicated thrice in two growing seasons (2020 and 2021).
Results
Findings pointed out that SA200 increased plant height, branches number plant− 1, stem diameter, leaf area, leaves number plant− 1, plant fresh weight, and plant dry weight by 32.9, 112.2, 59.2, 34.4, 44.3, 33.0 and 56.9% (averages of the two seasons), respectively, compared to the control treatment (tap water). SW6 increased carotenoids content by 34.8 and 46.4% in 2020 and 2021 seasons, respectively. Salicylic acid at the rate of 200 mg L− 1 along seaweed extract at 6 ml L− 1 gave the highest values of total sugars and free amino acids. SA200 alone recorded the maximal value of peroxidase activity surpassing the other treatments. SW6 possessed the greatest essential oil content in both seasons, statistically equaling SA200 in the first season. Huge variations in essential oil profile were recorded under the tested trial treatments.
Conclusion
It could be concluded that salicylic acid and seaweed extract foliar application unveiled their involvement in diverse physiological and developmental responses; pigment formation, enzyme activities, flower induction, nutrient uptake, essential oil metabolism, and overall plant growth and development. It is recommended to use the highest concentrations of the tested substances (SA at 200 mg L− 1 or SW at 6.0 ml L− 1) for achieving distinctive improvements in physiological responses of French marigold plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, the ornamental and medicinal plants industry is one of the most agricultural production sectors (Vita et al. 2015; Barna et al. 2021). Therefore, cultivation of these plant species while following appropriate and effective practices has received great attention recently (Ali et al. 2024a; Lasheen et al. 2024). One of these plants is Tagetes patula L. that belongs to the family Asteraceae. Tagetes patula L. is called French marigold and it is an important annual flower crop used for ornamental and commercial purposes (Ilbi et al. 2020). The main components of French marigold are phenols, essential oil, vitamins (carotenoids), and flavonoids (Kurkina et al. 2021. French marigold was used to treat various diseases, for example, cough, colic, constipation, diarrhea, rheumatism, and eye problems. Nowadays, marigolds are used as an antimicrobial, antiseptic, hepatoprotective, blood purifying, and diuretic agent (Liu et al. 2020).
Plant growth regulators play a crucial role within the life cycle of plants and these are often produced naturally by leaves factories of the plant or synthetically by chemical laboratories (Saudy et al. 2021a; El-Bially et al. 2022a; El-Metwally et al. 2022a). The exogenous supply of growth regulators showed good potential to improve crop physiological status (Hadid et al. 2023; Ramadan et al. 2023; Saudy et al. 2023; Doklega et al. 2024) while enhancing growth and productivity (El-Bially et al. 2018; El-Metwally et al. 2021; Rizk et al. 2023). One of the most important external applications of the industrial plant growth regulators is ortho‒hydroxybenzoic acid named salicylic acid (SA), which is inexpensive and functions as a plant natural hormone, derivatized from phenols (Kulak et al. 2021).
SA had a mainly important role in multiple functions in physiological and biochemical processes of plant metabolism including modulating growth by improving morphological characteristics, photosynthesis, cellular metabolism, protein synthesis, enzymatic activities, stomatal closure, ion uptake, avoiding leaf senescence, transpiration, cell elongation, cellular division, cell differentiation, and gas exchange (Shadmehri and Khatiby 2020). Moreover, it can effectively alleviate the harmful effects caused by abiotic stresses by enhancing the defense system of the plant, which leads to an increase in enzymatic and non-enzymatic antioxidants (Zulfiqar et al. 2021). SA foliar application enhanced growth, root, and flowering parameters, photosynthesis, transformation, and storage carbohydrates, additionally improved mineral nutrients percentages of leaves, thus it had a positive significant effect on gazania (Gazania linearis) plant (Saeed 2020). SA functions as an endogenous regulator on flowering and together with plant regulators, like gibberellin, induced flowering (Sewedan et al. 2018). SA application led to a rise production of additional petals and enhanced the biosynthesis of volatile oil (Elsadek 2018).
Under traditional agriculture conditions, as in most developing countries, synthetic chemicals are widely used in crop production despite their harmful impacts on environment and human health (Saudy et al. 2020, 2021b; Abou El-Enin et al. 2023; Shaaban et al. 2023). Therefore, many organic alternatives have shown immense potential for use as safe agricultural inputs in plant production to increase plant quality while preserving and sustaining environmental resources (Abd–Elrahman et al. 2022; Dookie et al. 2021; El-Metwally et al. 2022b; Elgala et al. 2022; Makhlouf et al. 2022). Since seaweed extract has elevated levels of macro and micronutrients and many vitamins and plant hormones that promote plant growth and flowering, enhancing the tolerance to various stresses, significant increases in growth and reproductive parameters in several crop types were reported (Shukla et al. 2019). Also, noticed significant increases in plant height and leaves number of Chinese carnation and gazania due to spraying of seaweed extract at different concentrations (Al-Hamzawi 2019). However, little knowledge related to the response of French marigold plants toward seaweed application is available. In this connection, the current research hypothesized that diversified concentrations of salicylic acid and seaweed extract could have varied influences on French marigold performance. Therefore, this work aimed to assess the alternations in physiology, growth, nutritional status and essential oil constituents owing to exogenous supply of salicylic acid and seaweed extract, at different concentrations, as efficient eco-friendly alternatives for Tagetes patula production.
2 Materials and Methods
2.1 Experimental Location
Along two successive seasons of 2020 and 2021, greenhouse experiments were conducted at Ornamental Horticulture Department, Faculty of Agriculture, Cairo University, Giza, Egypt (30º 01’ 02.7ꞌꞌ N, 31º 12’ 32.0ꞌꞌ E). The study area has typical Mediterranean climatic conditions with no rainfall and hot–dry in summer. The averages of air temperatures, humidity and solar radiation during the plant growth period (March-June) were approximately 25.0 ºC, 52.4% and 28.7 MJ m–2 day–1, respectively. The used soil media was formed by mixing clay loam and loamy sand (1:1 v/v) having the physicochemical properties shown in Table 1. Soil analysis was performed according to Piper (1950).
2.2 Trial Design and Treatments Application
On the first of March in 2020 and 2021, 3–5 seeds of French marigold were sown in plastic pots (30-cm in diameter), filled with the previously prepared mixture of clay and sand (1:1 v/v). At 4–6 leaf old, 35 days after sowing (DAS), seedlings were thinned to secure one plant per pot. Seven treatments were arranged in a randomized complete block design with three replicates. In addition to the control treatment (tap water), three levels of salicylic acid (50, 100, and 200 mg L− 1) and three levels of seaweed extract (2.0, 4.0, and 6.0 ml L− 1) were applied as foliar sprayings. The spray solutions of both salicylic acid and seaweed extract were sprayed separately three times, 50, 65 and 80 DAS, using manual back‒pack knapsack sprayer fitted with a flat-fan nozzle. Seaweeds extract was obtained from Algeser product from Shoura Chemicals Company, Cairo, Egypt. The constituents of seaweed extract were determined (Bonner 1994) and illustrated in Table 2.
2.3 Assessments
2.3.1 Vegetative and Flowering Traits
At the end of June in 2020 and 2021, the experiment was completed and data were recorded on the different vegetative growth characteristics (plant height, branches number plant− 1, stem diameter, leaf area, leaves number plant− 1, fresh and dry weights of shoots (stems + leaves). Also, data were recorded on the flowering including the number, fresh weigh and dry weight of flowers plant− 1.
2.3.2 Plant Pigments
Based on the Eqs. 1–4, described by Moran (1982) and Wellburn (1994) chlorophylls and carotenoids were estimated. The leaf samples of 100 mg fresh weight were ground using a mortar and extracted with 85% methanol. Then, the extracts were centrifuged for 10 min at 8000 rpm. The pigment quantification was performed by spectrophotometry, with wavelengths at 665, 646 and 470 nm for chlorophyll a, chlorophyll b and carotenoids, respectively. After extraction, the absorbance readings were performed in a digital spectrophotometer (Edutec EEQ 9023).
2.3.3 Biochemical Constituents
Total sugars were determined by using the method of phenol sulphuric acid reagent as described by Dubois et al. (1956). Total free amino acids analysis was determined by spectrophotometer at 650 nm, using ninhydrine reagent (Singleton et al. 1999).
2.3.4 Antioxidant Activity
The activity of antioxidant enzymes was determined in leaves samples. Peroxidase (POX; EC 1.11.1.7), according to Hammerschmidt et al. (1982) using a spectrophotometer at 470 nm and Glutathione-s-transferase (EC 2.5.1.13) determined by the method of Habig and Jakoby (1981) at 340 nm. Furthermore, Vitamin C was extracted and estimated according to the method mentioned by Obouayeba et al. (2014).
2.3.5 Leaf Macronutrient Content
Nitrogen content (N) was determined using the micro-Kjeldahl method, according to Pregl (1945). Phosphorus (P) content was estimated using the method described by King (1951). Potassium (K) content was assessed according to the methods described by Cottenie et al. (1982).
2.3.6 Essential Oil Content and Composition
In the fresh herb, essential oil percentage was extracted for 2 h by water distillation of 20 g of aerial parts of plant (Guenther 1961). The essential oil extracted was dehydrated with anhydrous sodium sulphate. Furthermore, the GC-MS analysis of the essential oil of the different treatments was carried out using gas chromatography/mass spectrometry instrument stands in the Research Institute of Medicinal & Aromatic plants (RIMAP) with the following specifications. Instrument: a traces GC Ultra Gas Chromatographs (THERMO Scientific Corp., USA), coupled with a thermo mass spectrometer detector (ISQ Single Quadruple Mass Spectrometer). The GC-MS system was equipped with a Tr-5 MS column (30 m x 0.32 mm i.d., 0.25 μm film thickness). Analyses were carried out using helium as carrier gas at a flow rate of 1.3 ml/min and a split ratio of 1:10 using the following temperature program: 60 °C for 1 min; rising at 4 oC /min to 160 °C and held for 6 min; rising at 6 C/min to 210 °C and held for 1 min. The injector and detector were held at 210 °C. Diluted samples (1:10 hexane, v/v) of 0.1µL of the mixtures were injected. Mass spectra were obtained by electron ionization (EI) at 70 eV, using a spectral range of m/z 40–450.
2.4 Statistical Analysis
The measured data were statistically analyzed based on the analysis of variance technique (ANOVA) for randomized complete blocks design in triplicates by Genstat computer software package (VSN International Ltd., Oxford, UK). For post-ANOVA-mean separation, Duncan’s multiple range test (p ≤ 0.05) was used. Treatments of SA and SW were considered fixed variables, while replications and seasons were regarded as random factors. Pearson’s correlation analysis expressed in heat map was performed to quantify relationships among observed parameters.
3 Results
3.1 Vegetative and Flowering Traits
The treatments of salicylic acid and seaweed extract showed significant (p ≤ 0.05) variation in vegetative and flowering parameters (Table 3). SA200 was the efficient treatment for enhancing all growth traits in both seasons of 2020 and 2021. As averages of the two seasons, SA200 increased plant height, branches number plant− 1, stem diameter, leaf area, leaves number plant− 1, plant fresh weight, and plant dry weight by 32.9, 112.2, 59.2, 34.4, 44.3, 33.0 and 56.9%, respectively, compared to the control treatment (tap water). However, the improvements in plant height (with SW4, SW6 and SA100), branches number plant− 1 (with SW6 and SA100), plant fresh weight (with SW6), and plant dry weight (with SW6 and SA100) were as similar as that of SA200 in the first season. In the second season, the values of plant height (with SW4, SW6 and SA100), branches number plant− 1 (with SW6), and stem diameter (with SA100) as well as leaf area, leaves number plant− 1 and plant dry weight (with SW6 and SA100) significantly equaled (p ≥ 0.05) the corresponding values of SA200 treatment.
For flowering traits, spraying of SA200 was the most effective practice for enhancing flowers number plant− 1, flower fresh weight plant− 1 and flower dry weight plant− 1 in both growing seasons, surpassing the other treatments, except SW6 for flower dry weight plant− 1 in the first season and SW6 and SA100 for flower fresh weight plant− 1 in the second season (Table 4). In this context, SA200 increased flowers number plant− 1 by 2.26 and 1.96 times, flower fresh weight plant− 1 by 1.54 and 1.53 times and flower dry weight plant− 1 by1.64 and 1.75 times, in the first and second seasons, respectively, compared to the control treatment.
3.2 Plant Pigments
The pigment values of leaves increased significantly (p ≤ 0.05) after spraying salicylic acid or seaweed extract when compared to control in both seasons (Table 5). Chlorophylls content in plants treated with SA at 200 mg L− 1 were significantly higher than that treated with seaweed and control, but there was higher significant (p ≤ 0.05) increase in carotenoids content when plants were treated with seaweed extract at 6 ml L− 1. Result revealed also that the concentration of chlorophyll a, chlorophyll b, and total chlorophyll in the leaves increased with increasing the concentration of salicylic acid and seaweed. Increases were 56.5 and 86.1% in chlorophyll a, 81.2 and 186.7% in chlorophyll b and 63.8 and 109.1% total chlorophyll, owing to application of SA200 compared to tap water, in the first and second seasons, respectively. While, SW4 and SW6 increased carotenoids content by 21.7 and 34.8% in 2020 season and 17.8 and 46.4% in 2021 season, respectively, compared to the control. Moreover, in the first season SA200 treatment possessed 17.4% increase in carotenoids content.
3.3 Biochemical Constituents
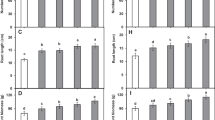
Total sugar and free amino acids increased gradually (p ≤ 0.05) with increase the concentrations of salicylic acid and seaweed extract (Fig. 1). Salicylic (200 mg L− 1) and seaweed extract (6 ml L− 1) gave the highest values of total sugars and free amino acids. In this situation, SA200 and SW6 gave rise to about 2.09- and 1.86-fold increments in total sugars and 1.66- and 1.84-fold increments in free amino acids, respectively, compared to the control (tap water).
Effect of salicylic acid (mg L− 1) and seaweed extract (ml L− 1) foliar application on total sugar and free amino acid contents (mg g− 1 FW) in Tagetes patula L. leaves. SW2, SW4 and SW6: seaweed extract concentrations of 2, 4 and 6 ml L− 1, respectively; SA50, SA100 and SA 200: salicylic acid at 50, 100 and 200 mg L− 1, respectively. Means values in each bar followed by different letters are significantly different based on the Duncan test (p ≤ 0.05)
3.4 Antioxidant Activity
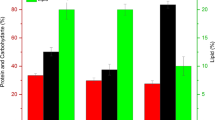
As depicted in Fig. 2, peroxidase activity and vitamin C remarkably (p ≤ 0.05) influenced by salicylic acid and seaweed extract treatments, while glutathione-s-transferase was not modified. SA200 alone recorded the maximal value of peroxidase surpassing the other treatments, recording increase of 28.8% compared to the control. Except SA50 treatment, all other salicylic acid and seaweed extract treatments showed statistically similar values of vitamin C greater than the control treatment.
Effect of salicylic acid (mg L− 1) and seaweed extract (ml L− 1) foliar application on glutathione-s-transferase (GST) and peroxidase (POX) in Tagetes patula L. leaves. SW2, SW4 and SW6: seaweed extract concentrations of 2, 4 and 6 ml L− 1, respectively; SA50, SA100 and SA 200: salicylic acid at 50, 100 and 200 mg L− 1, respectively. Means values in each bar followed by different letters are significantly different based on the Duncan test (p ≤ 0.05)
3.5 Leaf Macronutrient Content
As shown in Table 6, macronutrients content of Tagetes patula L. leaves significantly (p ≤ 0.05) responded to foliar applications of SA or SW. SA200 showed the maximum increases in nitrogen, phosphorus and potassium content in both growing seasons, surpassing the other treatments, except SW6 for potassium content in the first season and SW6 or SA100 for phosphorus content in the second season. Leaves of Tagetes patula L. had increased values of N, P and K by about 2.15 and1.94 folds, 1.79 and 1.74 folds as well as 1.52 and 2.51 folds in 2020 and 2021 seasons, respectively, due to SA200.
3.6 Essential Oil Content and Composition
Figure 3 illustrates the significant (p ≤ 0.05) impact of salicylic acid and seaweed extract treatments on leaf essential oil content of Tagetes patula L. in 2020 and 2021 growing seasons. It is interesting to observe that SW6 possessed the greatest essential oil content in both seasons, statistically equaling (p ≥ 0.05) SA200 in the first season. Comparing with the control treatment (tap water), increases in essential oil content reached 1.26 and 1.27 times due to SW6 in first and second seasons, respectively, and 1.25 times due to SA200 in the first season.
Effect of salicylic acid and seaweed extracts foliar application on essential oil content of Tagetes patula L. leaves in 2020 and 2021 seasons. SW2, SW4 and SW6: seaweed extract concentrations of 2, 4 and 6 ml L− 1, respectively; SA50, SA100 and SA 200: salicylic acid at 50, 100 and 200 mg L− 1, respectively. Means values in each bar followed by different letters are significantly different based on the Duncan test (p ≤ 0.05)
As for essential oil composition defined using GC-MS, there were variations in essential oil profile due to application of salicylic acid and seaweed extract (Supplementary Table 1). Both of SA200 and SW6 showed increments in majority of essential oil components detected with notable presence of identified components when compared to other treatments and approximately 1.22-fold increment when compared to control. SA200 increased D-Limonene, cis-Ocimene, α-terpinolene, Linalool, (-)- Carvyl Acetate, 1,3,8-p-Menthatriene, cis-p-Mentha-2,8-dien-1-ol and Piperitone. Nevertheless, SA200 showed a notable reduction in one of the major components of essential oil which is Caryophyllene. SW6 showed notable increase in some component such as α-terpinolene, p-Cymen-8-ol, dihydroedulan II and Neophytadiene.
3.7 Correlation Coefficient
The associations relationship expressed in correlation coefficients between the studied traits of Tagetes patula L. are presented in heat map (Fig. 4). Significant and positive associations were obtained between chlorophyll b and each of branches number plant− 1, leaf area, plant fresh weight, flower number plant− 1 and chlorophyll a as well as carotenoids and each of potassium, vitamin C and essential oil. While, glutathione-s-transferase showed significant and negative correlations with plant height, branches number plant− 1, and stem diameter. Furthermore, highly significant and negative correlations were observed with glutathione-s-transferase and each of leaf area, leaves number plant− 1, plant fresh weight, plant dry weight, flower number plant− 1, flower fresh weight plant− 1, flower dry weight plant− 1, and chlorophyll a. On the contrary, there were no noticeable correlation between chlorophyll b and each of plant height and leaves number plant− 1 as well as carotenoids and each of plant height, branches number plant− 1, stem diameter, leaf area, leaves number plant− 1, plant fresh weight, plant dry weight, flower number plant− 1, flower fresh weight plant− 1, flower dry weight plant− 1, and chlorophyll a. On the other hand, the other remained possible correlations between Tagetes patula L. studied traits were positive and highly significant.
4 Discussion
Obviously, French marigold (Tagetes erecta L.) physiology, growth and essential oil were distinctively affected by exogenous supply of salicylic acid and seaweed extract. Generally, findings of the current work exhibited that application of salicylic acid at 200 mg L− 1 and seaweed extract at 6 ml L− 1 achieved promising enhancements in plant growth, antioxidant activity, sugar and amino acid contents as well as the nutritional status, and final economical yield. Herein, SA had the potency to enhance the activity of photosynthesis and enzymatic antioxidant (Abdelaal 2015), while stimulates the metabolisms of proline (Khan et al. 2014). Also, plant growth was improved by exogenous supply of SA (Abbaszadeh et al. 2020), since it serves as protectant against lipid peroxidation (Kang et al. 2013). Therefore, the improvements in French marigold growth with application of SA could be ascribed to the important action of SA in maintaining the photosynthetic apparatus and activity. SA, as a plant hormone (Davies 2010) can stimulate, plant pigments, growth, development and flowering, in addition to photosynthesis (Arif et al. 2020; El–Bially et al. 2022b). Furthermore, growth and flowering and antioxidant system were improved as well as photosynthesis and enzymatic scavenging activity of free radicals was up-regulated owing to SA application (Arif et al. 2020; Zulfiqar et al. 2021). The enhancement in photosynthetic pigments with SA supply could be attributed to its potential to quench the reactive oxygen species (ROS) that naturally produced during photosynthesis process and caused several injuries to chlorophyll molecules and oxidative stress to plant tissues (El-Bially et al. 2022b; Ali et al. 2024b). Wen et al. (2005) proved that SA stimulated the stress tolerance via induction and activation of phenylalanine ammonia lyase (PAL) gene expression. The action of PAL to reduce oxidative damage through the antioxidant pathway involves high accumulation of phenolics and flavonoids, resulting in better photosynthesis and low membrane damage (Li et al. 2020). It has been documented that phenolics and flavonoids and antioxidant enzymes are effective scavengers of ROS in plants (Dias et al. 2021). Thus, PAL and other enzymatic activities are often used as enzymes of the antioxidant system to evaluate the roles of genes in stresses (Zhang et al. 2019). Exogenous SA treatment induced PAL gene expression in Salvia officinalis and Salvia virgata (Ejtahed et al. 2015) and Salvia miltiorrhiza (Li et al. 2016). Salicylic acid-induced cytosolic acidification increases the accumulation of phenolic acids in Salvia miltiorrhiza cells and secondary metabolic components (Li et al. 2016). Moreover, SA treatment increased the activities of the enzymes of phenylpropanoid pathway in Citrus sinensis leading to the accumulation of phenolic acids in the fruits while enhanced the level of lignin (Zhou et al. 2018). The increase in the secondary metabolites was mediated via the elevated activity of the enzyme PAL (Cappellari et al. 2020). Thus, SA regulates the expression of the genes corresponding to the enzymes associated with secondary metabolite biosynthesis, including the key enzymes; PAL and isochorismate synthase (Ali 2021). Cicer arietinum plants responded very quickly to SA at 1.5 mM and showed higher induction of peroxidase and polyphenol oxidase activities, besides the higher accumulation of phenols, H2O2 and proteins (War et al. 2011). Accordingly, foliar spray of SA significantly increased vegetative growth because of its positive influences on cell membrane functions by raising nutrient uptake, ion absorption, and nucleic acid (Es-sbihi et al. 2020). CO2 fixation was augmented via enhancing ribulose diphosphate carboxylase activity as a result of affording SA (Tan et al. 2020), hence photosynthesis, respiration, vegetative growth, thermogenesis, flower formation, seed production, and senescence were regulated (An and Mou 2011). Additionally, SA enhanced flowering and flower longevity, while suppressed ethylene biosynthesis and reversed abscisic acid action (Rocher et al. 2009). SA treatment resulted in significant increases of intracellular protein and the amounts of DNA and RNA while decrease of proteolytic activity (Kirillova et al. 2011). Thus, using SA gave the better growth and flower production of marigold plant (Choudhary et al. 2016; Basit et al. 2018), while increased biomass production (Pacheco et al. 2013).
As for essential oil content, Mirzajani et al. (2015) recorded an increase in total essential oil production in the leaves and the stems of sweet basil (Ocimum basilicum), in response to 1 mM dose of SA. SA application not only affects essential oil content but also oil composition. Herein, foliar application of SA significantly enhanced the content and yield of essential oil in Thymus daenensis plants while carvacrol, α-thujene, α-pinene and p-cymene increased and thymol and, β-caryophyllene declined (Pirbalouti et al. 2014). Limonene, linalool and linalyl acetate of essential oil of Citrus aurantium leaves were elevated due to SA treatment (Sarrou et al. 2015). SA at 1.00 mM increased the yield of artemisinin up to 50.0% in Artemisia annua plants (Aftab et al. 2010). Monoterpene oxygenated and sesquiterpenes in lemon balm (Melissa officinalis) plants were considerably enhanced by SA spray, while essential oil yield was not affected (Pirbalouti et al. 2019).
Regarding the use of natural materials in crop growing, it is worthily to notice that seaweed extract (SW) had several significant compounds involving nutrients and growth regulators (Table 2) that are useful for plant growth. Seaweed extract contains amino acids, laminaran, fucoidan, alginate, and betaine, which stimulate metabolic activity and thus, increase plant growth and yield (Khan et al. 2009). It has been reported that alginic acid significantly enhanced radish growth with adjusting the osmotic potential (Wang et al. 2020). Alginate oligosaccharide improved photosynthetic performance and accumulation of sugars in citrus (Li et al. 2023). Thus, the exogenous supply of seaweed was expected to promote French marigold plants. However, the appropriate concentration was not well known. This research elucidated that SW at the concentration of 6 ml L− 1 was the promising treatment for enhancing physiological status, hence growth and yield. In this regard, spraying the vegetative part of Amaranthus tricolor plant with two concentrations of seaweed caused significant increases in plant height and number of branches in addition to the fresh and dry weights of shoot and root (Abdel-Aziz et al. 2011). Similar results were obtained in Tagetes erecta plants treated with seaweed extract at 2.0, 4.0, 6.0 and 8.0 ml L− 1 after 30 days of germination (Sridhar and Rengasamy 2010). Similarly, significant increases in chlorophyll content, stomatal conductance, photosynthetic rate, and transpiration rate were recorded in asparagus plants treated with seaweed extract (Al-Ghamdi and Elansary 2018). Treatment of willow (Salix sp.) plants with an extract of Ecklonia maxima enhanced the electron transfer rates of both photosystems (Digruber et al. 2018). Applications of seaweed extract on cottonwood (Fei et al. 2017) and mustard (Stasio et al. 2017) significantly increased leaves potassium uptake. The physiological status was adjusted in favor of plant development by Ecklonia maxima seaweed extract via enhancing stomata opening and conductance, chlorophyll content assimilation of CO2, while raising transpiration and nutrient uptake (Rouphael et al. 2017; Lefi et al. 2023). Therefore, application of seaweed extracts in maize showed that leaves were able to significantly absorb more Zn, Fe, B, Cu, Mo, S, Mg, Ca, and Mn than the control treatment (Ertani et al. 2018). Accordingly, seaweed extract is represented a promising natural source in nourishing medicinal crops such as marigold plants.
5 Conclusions
In potted-cultivated French marigold plants, foliar salicylic acid or seaweed extract applications exhibited promising findings on vegetative and flowering growth, physiological response, nutrients and essential oil. Leaves biomass, which is the most significant product for obtaining oil of marigold plants, remarkably increased with increasing salicylic acid and seaweed extract concentrations. Salicylic acid or seaweed extract concentrations not only affected yield biomass and essential oil content but also, oil profile. Accordingly, using salicylic acid (200 mg L− 1) and seaweed extract (6.0 ml L− 1) is advisable to gain better profits from marigold plants cultivation. Since salicylic acid and seaweed extract achieved individually distinctive improvements in French marigold physiology and end marketable product, further studies should be implemented to assess their combined application effect.
References
Abbaszadeh B, Layeghhaghighi M, Azimi R, Hadi N (2020) Improving water use efficiency through drought stress and using salicylic acid for proper production of Rosmarinus officinalis L. Indus Crops Prod 144:111893. https://doi.org/10.1016/j.indcrop.2019.111893
Abd–Elrahman SH, Saudy HS, Abd El–Fattah DA, Hashem FA (2022) Effect of irrigation water and organic fertilizer on reducing nitrate accumulation and boosting lettuce productivity. J Soil Sci Plant Nutr 22:2144–2155. https://doi.org/10.1007/s42729-022-00799-8
Abdel-Aziz NG, Mahgoub MH, Siam HS (2011) Growth, flowering and chemical constituent performance of Amaranthus tricolor plants as influenced by seaweed (Ascophyllum nodosum) extract application under salt stress conditions. J App Sci Res 7:1472–1484
Abdelaal KAA (2015) Effect of salicylic acid and abscisic acid on morpho-physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. J Plant Prod 6:1771–1788. https://doi.org/10.21608/jpp.2015.52096
Abou El-Enin MM, Sheha AM, El-Serafy Rasha S, Ali OAM, Saudy HS, Shaaban A (2023) Foliage-sprayed nano-chitosan-loaded nitrogen boosts yield potentials, competitive ability, and profitability of intercropped maize-soybean. Inter J Plant Prod 17:517–542. https://doi.org/10.1007/s42106-023-00253-4
Aftab T, Masroor M, Khan A, Idrees M, Moinuddin NM (2010) Salicylic acid acts as potent enhancer of growth, photosynthesis and artemisinin production in Artemisia annua L. J Crop Sci Biotech 13:183–188. https://doi.org/10.1007/s12892-010-0040-3
Al-Ghamdi AA, Elansary HO (2018) Synergetic effects of 5-aminolevulinic acid and Ascophyllum nodosum seaweed extracts on Asparagus phenolics and stress related genes under saline irrigation. Plant Physiol Bioch 129:273–284. https://doi.org/10.1016/j.plaphy.2018.06.008
Al-Hamzawi MK (2019) Effect of Seaweed Extract and micronutrients mixture on some growth characters and flowering of Dianthus chinensis L. and Gazania Splender L. plants. J Phys Conf Ser 1294:092001. https://doi.org/10.1088/1742-6596/1294/9/092001
Ali B (2021) Salicylic acid: an efficient elicitor of secondary metabolite production in plants. Biocat Agri Biotech 31:101884. https://doi.org/10.1016/j.bcab.2020.101884
Ali IAA, Hassan Soheir E, Abdelhafez AA, Hewidy M, Nasser MA, Saudy HS, Hassan KM, Abou-Hadid AF (2024a) Modifying the growing media and bio stimulants supply for healthy gerbera (Gerbera jamesonii). Flowers Gesun Pflanz 76:337–345. https://doi.org/10.1007/s10343-023-00943-z
Ali MAA, Nasser MA, Abdelhamid AN, Ali IAA, Saudy HS, Hassan KM (2024b) Melatonin as a key factor for regulating and relieving abiotic stresses in harmony with phytohormones in horticultural plants– a review. J Soil Sci Plant Nutr 24:54–73. https://doi.org/10.1007/s42729-023-01586-9
An C, Mou Z (2011) Salicylic acid and its function in plant immunity. J Integr Plant Biol 53:412–428. https://doi.org/10.1111/j.1744-7909.2011.01043.x
Arif Y, Sami F, Siddiqui H, Bajguz A, Hayat S (2020) Salicylic acid in relation to other phytohormones in plant: a study towards physiology and signal transduction under challenging environment. Environ Exp Bot 175:104040. https://doi.org/10.1016/j.envexpbot.2020.104040
Barna D, Kisvarga S, Kovacs S, Csatari G, Toth IO, Fari MG, Alshaal T, Bakonyi N (2021) Raw and fermented alfalfa brown juice induces changes in the germination and development of French marigold (Tagetes patula L.) plants. Plants (Basel) 10:1076. https://doi.org/10.3390/plants10061076
Basit A, Shah K, Rahman MU, Xing L, Zuo X, Han M, Alam N, khalid MA (2018) Salicylic acid an emerging growth and flower inducing hormone in marigold (Tagetes sp. L). Pure Appl Biol 7:1301–1308. https://doi.org/10.19045/bspab.2018.700151
Bonner J (1994) Chapters from my life. Annu Rev Plant Physiol Plant Mol Biol 4:1–23. https://doi.org/10.1146/annurev.pp.45.060194.000245
Cappellari LDR, Santoro MV, Schmidt A, Gershenzon J, Banchio E (2020) Improving phenolic total content and monoterpene in Mentha x piperita by using salicylic acid or methyl jasmonate combined with Rhizobacteria inoculation. Int J Mol Sci 21:50. https://doi.org/10.3390/ijms21010050
Choudhary A, Mishra A, Bola PK, Moond SK, Dhayal M (2016) Effect of foliar application of zinc and salicylic acid on growth, flowering and chemical constitute of African marigold cv. pusa narangi gainda (targets erecta L). J Appl Nat Sci 8:1467–1470. https://doi.org/10.31018/jans.v8i3.984
Cottenie A, Verloo M, Kiekens L, Velghe G, Camerlynck R (1982) Chemical analysis of Plant and Soil. Laboratory of Analytical and Agrochemistry, State Univ. Ghent. Belgium. PP. 100–129
Davies PJ (2010) The plant hormones: their nature, occurrence, and functions. In: Davies PJ (ed) Plant hormones: Biosynthesis, Signal Transduction, Action! 3rd edn. Springer, Dordrecht, The Netherlands, pp 1–12. https://doi.org/10.1007/978-1-4020-2686-7_1.
Dias MC, Pinto D, Silva A (2021) Plant flavonoids: Chemical characteristics and biological activity. Molecul 26:5377. https://doi.org/10.3390/molecules26175377
Digruber T, Sass L, Cseri A, Paul K, Nagy AV, Remenyik J, Molnár I, Vass I, Toldi O, Gyuricza C, Dudits D (2018) Stimulation of energy willow biomass with triacontanol and seaweed extract. Ind Crop Prod 120:104–112. https://doi.org/10.1016/j.indcrop.2018.04.047
Doklega SMA, Saudy HS, El-Sherpiny MA, Abou El-Yazied A, Abd El-Gawad HG, Ibrahim MFM, Abd El-Hady MAM, Omar MMA, Metwally AA (2024) Rhizospheric addition of hydrogel polymer and zeolite plus glutathione mitigate the hazard effects of water deficiency on common bean plants through enhancing the defensive antioxidants. Gesun Pflanz 76:235–249. https://doi.org/10.1007/s10343-023-00947-9
Dookie M, Ali O, Ramsubhag A, Jayaraman J (2021) Flowering gene regulation in tomato plants treated with brown seaweed extracts. Sci Hortic (Amsterdam) 276:109715. https://doi.org/10.1016/j.scienta.2020.109715
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith FF (1956) Colorimetric method for the determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Ejtahed RS, Radjabian T, Hoseini Tafreshi SA (2015) Expression analysis of phenylalanine ammonia lyase gene and rosmarinic acid production in Salvia officinalis and Salvia virgata shoots under salicylic acid elicitation. Appl Biochem Biotech 176:1846–1858. https://doi.org/10.1007/s12010-015-1682-3
El-Bially MA, Saudy HS, El-Metwally IM, Shahin MG (2018) Efficacy of ascorbic acid as a cofactor for alleviating water deficit impacts and enhancing sunflower yield and irrigation water–use efficiency. Agric Wat Manage 208:132–139. https://doi.org/10.1016/j.agwat.2018.06.016
El-Bially MA, Saudy HS, El-Metwally IM, Shahin MG (2022a) Sunflower response to application of L–ascorbate under thermal stress associated with different sowing dates. Gesun Pflanz 74:87–96. https://doi.org/10.1007/s10343-021-00590-2
El-Bially MA, Saudy HS, Hashem FA, El-Gabry YA, Shahin MG (2022b) Salicylic acid as a tolerance inducer of drought stress on sunflower grown in sandy soil. Gesunde Pflanz 74:603–613. https://doi.org/10.1007/s10343-022-00635-0
El-Metwally IM, Sadak MSh, Saudy HS (2022a) Stimulation effects of glutamic and 5-Aminolevulinic acids on photosynthetic pigments, physio-biochemical constituents, antioxidant activity, and yield of peanut. Gesun Pflanz 74:915–924. https://doi.org/10.1007/s10343-022-00663-w
El-Metwally IM, Saudy HS, Abdelhamid MT (2021) Efficacy of benzyladenine for compensating the reduction in soybean productivity under low water supply. Ital J Agromet 2:81–90. https://doi.org/10.36253/ijam-872
El-Metwally IM, Saudy HS, Elewa TA (2022b) Natural plant by-products and mulching materials to suppress weeds and improve sugar beet (Beta vulgaris L.) yield and quality. J Soil Sci Plant Nutr 22:5217–5230. https://doi.org/10.1007/s42729-022-00997-4
Elgala AM, Abd-Elrahman SH, Saudy HS, Nossier MI (2022) Exploiting Eichhornia crassipes shoots extract as a natural source of nutrients for producing healthy tomato plants. Gesun Pflanz 74:457–465. https://doi.org/10.1007/s10343-022-00622-5
Elsadek MA (2018) Improvement yield and quality of dahlia flowers by exogenous application of gibberellic acid and salicylic acid under sandy soil conditions. J Plant Prod 9:289–297. https://doi.org/10.21608/jpp.2018.35498
Ertani A, Francioso O, Tinti A, Schiavon M, Pizzeghello D, Nardi S (2018) Evaluation of seaweed extracts from Laminaria and Ascophyllum Nodosum spp. as biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front Plant Sci 9:428. https://doi.org/10.3389/fpls.2018.00428
Es-sbihi FZ, Hazzoumi Z, Benhima R, Amrani Joutei K (2020) Effects of salicylic acid on growth, mineral nutrition, glandular hairs distribution and essential oil composition in Salvia officinalis L. grown under copper stress. Envir Sustain 3:199–208. https://doi.org/10.1007/s42398-020-00109-x
Fei H, Crouse M, Papadopoulos Y, Vessey JK (2017) Enhancing the productivity of hybrid poplar (Populus× hybrid) and switchgrass (Panicum virgatum L.) by the application of beneficial soil microbes and a seaweed extract. Biomass Bioenerg 107:122–134. https://doi.org/10.1016/j.biombioe.2017.09.022
Guenther E (1961) The essential oils, 4th Vol. III. edn. D. Van Nostrand Comp., New York, London
Habig WH, Jakoby WB (1981) [51] Assays for differentiation of glutathione S-Transferases. In Methods in enzymology. Academic press 77, pp. 398–405
Hadid ML, Ramadan KhMA, El-Beltagi HS, Ramadan AA, El-Metwally IM, Shalaby TA, Bendary ESA, Saudy HS (2023) Modulating the antioxidant defense systems and nutrients content by proline for higher yielding of wheat under water deficit. Not Bot Horti Agrobo 51:13291. https://doi.org/10.15835/nbha51313291
Hammerschmidt R, Nuckles EM, Kuć J (1982) Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol 20:73–82. https://doi.org/10.1016/0048-4059(82)90025-X
Ilbi H, Powell AA, Alan O (2020) Single radicle emergence count for predicting vigour of marigold (Tagetes spp.) seed lots. Seed Sci Technol 48:381–389. https://doi.org/10.15258/sst.2020.48.3.06
Kang G, Li G, Liu G, Xu W, Peng X, Wang C, Zhu Y, Guo T (2013) Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate-glutathione cycle. Biol Plant 57:718–724. https://doi.org/10.1007/s10535-013-0335-z
Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, Critchley AT, Craigie JS, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399. https://doi.org/10.1007/s00344-009-9103-x
Khan MIR, Asgher M, Khan NA (2014) Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L). Plant Physiol Biochem 80:67–74. https://doi.org/10.1016/j.plaphy.2014.03.026
King EJ (1951) Micro-analysis in Medical Biochemistry, 2nd edn. Churchill Publishing Co., London, p 260
Kirillova NV, Belykh YV, Spasenkov AI (2011) Influence of salicylic acid on biosynthesis of nucleic acids in Polyscias filicifolia cell culture under the action of unfavorable temperatures. App Biochem Microbiol 47:431–434
Kulak M, Jorrín-Novo JV, Romero-Rodriguez MC, Yildirim ED, Gul F, Karaman S (2021) Seed priming with salicylic acid on plant growth and essential oil composition in basil (Ocimum basilicum L.) plants grown under water stress conditions. Ind Crop Prod 161:113235. https://doi.org/10.1016/j.indcrop.2020.113235
Kurkina AV, Savel’eva AE, Kurkin VA (2021) Quantitative determination of total flavonoids in Tagetes patula marigold flowers. Pharm Chem J 55:165–169. https://doi.org/10.1007/s11094-021-02387-z
Lasheen FF, Hewidy M, Abdelhamid AN, Thabet RS, Abass MMM, Fahmy AA, Saudy HS, Hassan KM (2024) Exogenous application of humic acid mitigates salinity stress on pittosporum (Pittosporum tobira) plant by adjusting the osmolytes and nutrient homeostasis. Gesun Pflanz 76:317–325. https://doi.org/10.1007/s10343-023-00939-9
Lefi E, Badri M, Hamed SB, Talbi S, Mnafgui W, Ludidi N, Chaieb M (2023) Influence of brown seaweed (Ecklonia maxima) extract on the morpho-physiological parameters of melon, cucumber, and tomato plants. Agron 13:2745. https://doi.org/10.3390/agronomy13112745
Li X, Guo H, Qi Y, Liu H, Zhang X, Ma P, Liang Z, Dong J (2016) Salicylic acid-induced cytosolic acidification increases the accumulation of phenolic acids in Salvia miltiorrhiza cells. Plant Cell Tiss Org Cult 126:333–341. https://link.springer.com/article/10.1007/s11240-016-1001-x
Li X, Zhang X, Liu G, Tang Y, Zhou C, Zhang L, Lv J (2020) The spike plays important roles in the drought tolerance as compared to the flag leaf through the phenylpropanoid pathway in wheat. Plant Physiol Bioch 152:100–111. https://doi.org/10.1016/j.plaphy.2020.05.002
Li Z, Duan S, Lu B, Yang C, Ding H, Shen H (2023) Spraying alginate oligosaccharide improves photosynthetic performance and sugar accumulation in citrus by regulating antioxidant system and related gene expression. Front Plant Sci 13:1108848. https://doi.org/10.3389/fpls.2022.1108848
Liu LL, Luo SM, Yu M, Metwaly AM, Ran XK, Ma CY, Dou DQ, Cai DC (2020) Chemical constituents of Tagetes patula and their neuroprotecting action. Nat Prod Commun 5:1–8. https://doi.org/10.1177/1934578x20974507
Makhlouf BSI, Khalil SRA, Saudy HS (2022) Efficacy of humic acids and chitosan for enhancing yield and sugar quality of sugar beet under moderate and severe drought. J Soil Sci Plant Nutr 22:1676–1691. https://doi.org/10.1007/s42729-022-00762-7. efficinc
Mirzajani Z, Hadavi E, Kashi A (2015) Changes in the essential oil content and selected traits of sweet basil (Ocimum basilicum L.) as induced by foliar sprays of citric acid and salicylic acid. Ind Crop Prod 76:269–274. https://doi.org/10.1016/j.indcrop.2015.06.052
Moran R (1982) Formulae for determination of chlorophyllous pigments extracted with N, N-dimethylformamide. Plant Physiol 69:1376–1381. https://doi.org/10.1104/pp.69.6.1376
Obouayeba AP, Djyh NB, Diabate S, Djaman AJ, N’guessan JD, Kone M, Kouakou TH (2014) Phytochemical and antioxidant activity of Roselle (Hibiscus Sabdariffa L.) petal extracts. Res J Pharm Biol Chem Sci 4:P1454
Pacheco AC, da Silva Cabral C, da Silva Fermino ES, Aleman CC (2013) Salicylic acid-induced changes to growth, flowering and flavonoids production in marigold plants. J Med Plant Res 7:3158–3163. https://doi.org/10.5897/JMPR2013.5208
Piper CS (1950) Soil and plant analysis. the University of Adelaide, Adelaide, Australia, p 368
Pirbalouti AG, Samani RM, Hashemi M, Zeinali H (2014) Salicylic acid affects growth, essential oil and chemical compositions of thyme (Thymus daenensis celak.) Under reduced irrigation. Plant Growth Regul 72:289–301. https://doi.org/10.1007/s10725-013-9860-1
Pirbalouti AG, Nekoei M, Rahimmalek M, Malekpoor F (2019) Chemical composition and yield of essential oil from lemon balm (Melissa officinalis L.) under foliar applications of jasmonic and salicylic acids. Biocat Agric Biotech 19:101144. https://doi.org/10.1016/j.bcab.2019.101144
Pregl P (1945) Quantitative organic microanalysis, 4th edn. Churchill Publ. Co., London, p 200
Ramadan KMA, El-Beltagi HS, Abd El-Mageed TAA, Saudy HS, Al-Otaibi HH, Mahmoud MAA (2023) The changes in various physio-biochemical parameters and yield traits of faba bean due to humic acid plus 6-benzylaminopurine application under deficit irrigation. Agron 13:1227. https://doi.org/10.3390/agronomy13051227
Rizk TY, kholousy ASO, Saudy HS, Sultan ShS, Abd Alwahed SHA (2023) Breaking dormancy and enhancing germination of Avena sterilis L. and Amaranthus retroflexus L. weeds by gibberellic acid and potassium nitrate to keep soil and crops healthy. Gesun Pflanz 75:757–763. https://doi.org/10.1007/s10343-022-00780-6
Rocher F, Chollet JF, Legros S, Jousse C, Lemoine R, Faucher M, Bush DR, Bonnemain JL (2009) Salicylic acid transport in Ricinus communis involves a pH-dependent carrier system in addition to diffusion. Plant Physiol 150:2081–2091. https://doi.org/10.1104/pp.109.140095
Rouphael Y, De Micco V, Arena C, Raimondi G, Colla G, De Pascale S (2017) Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J Appl Phycol 29:459–470. https://link.springer.com/article/10.1007/s10811-016-0937-x
Saeed AAJM (2020) Effect of ascorbic and salicylic acids on growth and flowering of Gazania Cv. Frosty Kiss Mixed Ornam Hortic 26:537–544. https://doi.org/10.1590/2447-536X.v26i4.2118
Sarrou E, Chatzopoulou P, Dimassi-Theriou K, Therios I, Koularmani A (2015) Effect of melatonin, salicylic acid and gibberellic acid on leaf essential oil and other secondary metabolites of bitter orange young seedlings. J Essent Oil Res 27:487–496. https://doi.org/10.1080/10412905.2015.1064485
Saudy HS, Hamed MF, Abd El–Momen WR, Hussein H (2020) Nitrogen use rationalization and boosting wheat productivity by applying packages of humic, amino acids and microorganisms. Comm Soil Sci Plant Anal 51:1036–1047. https://doi.org/10.1080/00103624.2020.1744631
Saudy HS, El-Bially MA, El-Metwally IM, Shahin MG (2021a) Physio–biochemical and agronomic response of ascorbic acid–treated sunflower (Helianthus annuus) grown at different sowing dates and under various irrigation regimes. Gesun Pflanz 73:169–179. https://doi.org/10.1007/s10343-020-00535-1
Saudy HS, El-Bially MA, Ramadan KA, Abo El–Nasr EKh, El-Samad A (2021b) GA Potentiality of soil mulch and sorghum extract to reduce the biotic stress of weeds with enhancing yield and nutrient uptake of maize crop. Gesun Pflanz 73:555–564. https://doi.org/10.1007/s10343-021-00577-z
Saudy HS, El-Bially MA, Hashem FA, Shahin MG, El–Gabry YA (2023) The changes in yield response factor, water use efficiency, and physiology of sunflower owing to ascorbic and citric acids application under mild deficit irrigation. Gesun Pflanz 75:899–909. https://doi.org/10.1007/s10343-022-00736-w
Sewedan E, Osman AR, Moubarak M (2018) Effect of methyl jasmonate and salicylic acid on the production of Gladiolus Grandiflorus. Nat Sci 16:40–47. https://doi.org/10.7537/marsnsj160618.07
Shaaban A, Abd El-Mageed TA, Abd El-Momen WR, Saudy HS, Al-Elwany OAAI (2023) The integrated application of phosphorous and zinc affects the physiological status, yield and quality of canola grown in phosphorus-suffered deficiency saline soil. Gesun Pflanz 75:1813–1821. https://doi.org/10.1007/s10343-023-00843-2
Shadmehri AA, Khatiby A (2020) The effect of salicylic acid on different plant processes– a review. Cerc Agron Mold 53:233–242. https://doi.org/10.46909/cerce-2020-020
Shukla P, Mantin E, Adil M, Bajpai S, Critchley A, Prithiviraj B (2019) Ascophyllum nodosum-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front Plant Sci 10:655. https://doi.org/10.3389/fpls.2019.00655
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Sridhar SG, Rengasamy RN (2010) Significance of seaweed liquid fertilizers for minimizing chemical fertilizers and improving yield of Arachis hypogaea under field trial. Rec Res Sci Techn 2:73–80
Stasio ED, Rouphael Y, Colla G, Raimondi G, Giordano M, Pannico A, El-Nakhel C (2017) The influence of Ecklonia maxima seaweed extract on growth, photosynthetic activity and mineral composition of Brassica rapa L. subsp. sylvestris under nutrient stress conditions. Eur J Hortic Sci 82:286–293. https://doi.org/10.17660/eJHS.2017/82.6.3
Tan S, Abas M, Verstraeten I, Glanc M, Molnár G, Hajný J, Lasák P, Petřík I, Russinova E, Petrášek J, Novák O, Pospíšil J, Friml J (2020) Salicylic acid targets protein phosphatase 2A to attenuate growth in plants. Curr Biol 30:381–395e8. https://doi.org/10.1016/j.cub.2019.11.058
Vita GD, Allegra V, Zarbà AS (2015) Building scenarios: a qualitative Approach to forecasting Market Developments for Ornamental plants. Int J Bus Glob 15:130–151. https://doi.org/10.1504/IJBG.2015.071152
Wang H, Wang L, Yong M, Wang S, Cheng F, Li Q, Hu J (2020) Effects of alginic acid on radish growth and osmotic adjustment substance content under cadmium stress. IOP Conf Series: Earth Environ Sci 480:012006. https://doi.org/10.1088/1755-1315/480/1/012006
War AR, Paulraj MG, War MY, Ignacimuthu S (2011) Role of salicylic acid in induction of plant defense system in chickpea (Cicer arietinum L). Plant Signal Behav 6:1787–1792. https://doi.org/10.4161/psb.6.11.17685
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Wen P-F, Chen J-Y, Kong W-F, Pan Q-H, Huang WS-B W-D (2005) Salicylic acid induced the expression of phenylalanine ammonia-lyase gene in grape berry. Plant Sci 169:928–934. https://doi.org/10.1016/j.plantsci.2005.06.011
Zhang F, Wang Y, Liu C, Chen F, Ge H, Tian F, Yang T, Ma K, Zhang Y (2019) Trichoderma Harzianum mitigates salt stress in cucumber via multiple responses. Ecotoxicol Environ Saf 170:436–445. https://doi.org/10.1016/j.ecoenv.2018.11.084
Zhou Y, Ma J, Xie J, Deng L, Yao S, Zeng K (2018) Transcriptomic and biochemical analysis of highlighted induction of phenylpropanoid pathway metabolism of citrus fruit in response to salicylic acid, Pichia membranaefaciens and oligochitosan. Postharvest Biol Tec 142:81–92. https://doi.org/10.1016/j.postharvbio.2018.01.021
Zulfiqar F, Chen J, Finnegan PM, Younis A, Nafees M, Zorrig W, Hamed KB (2021) Application of trehalose and salicylic acid mitigates drought stress in sweet basil and improves plant growth. Plants 10:1078. https://doi.org/10.3390/plants10061078
Author information
Authors and Affiliations
Contributions
This study was designed and implemented by its authors. All authors contributed to writing the manuscript, interpreting the information presented, and have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Ziat, R.A.M., Saudy, H.S. & Hewidy, M. The Alteration in Physiological Status, Growth and Essential Oil Profile of French Marigold (Tagetes patula L.) Owing to Seaweed Extract and Salicylic Acid Application. J Soil Sci Plant Nutr 24, 3909–3922 (2024). https://doi.org/10.1007/s42729-024-01811-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-024-01811-z