Abstract
Organic fertilisers and biostimulants play a very important role in the germination, seedling stage, yield and quality of plants. Seaweed and humic acid are organic substances known for their positive effects. Therefore, the aim of this study was to investigate the effects of humic acid, seaweed extract and their combined applications against salt stress in oilseed rape at the seedling development stage. The effects of foliar application of seaweed extract (SW1, SW2, SW3) (0, 10, 20 mL), humic acid (HA1, HA2, HA3) (0, 10, 20 mL) and SW + HA (SW1 + HA1, SW2 + HA2, SW3 + HA3) (0, 10(5 + 5 mL L− 1 SW + HA), 20 mL L− 1 (10 + 10 mL L− 1 SW + HA)) on resistance to salt concentrations (S1, S2, S3) (0, 50, 100 mM) in oilseed rape cultivar (Elvis) under greenhouse conditions. In this study, seedling length (SL), root length (RL), total chlorophyll (Total Chl), carotenoids (Crt), electrolyte leakage (EL), malondialdehyde (MDA), superoxide dismutase (SOD), total phenolic compounds (TPC), relative water content (RWC) and proline (Pro) parameters were examined. Both applications played a significant role in reducing the negative effects of salt stress on all parameters studied. The combined application of SW + HA resulted in a remarkable increase in SL, RL TPC, SOD and RWC. In particular, the SW3 + HA3 dose played a more effective role in the antioxidant protective mechanism against stress. According to the PCA analysis, the studied parameters could be divided into 3 different groups. It was concluded that SW and HA can be evaluated as an effective natural, eco-friendly material that can be used to increase the resistance of plants to salt stress. In conclusion, in this study all applications are compared with each other, it has been determined that SW + HA is more effective application in reducing the effects of salt stress than others.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Today, with a growing population, it is necessary to increase the yield and quality of crop production to ensure that this population has access to nutrition and safe food (Sogoni et al. 2021). However, worldwide, abiotic stresses are among the most important factors negatively affecting yield and quality (Chanthini et al. 2022; Mndi et al. 2023). The stress factors that have the greatest impact on the development of plants are cold, heat, drought, salt stress, etc. (Gürsoy 2023).
Salt, which is one of the most effective elements, causes the formation of osmotic stress in plants, which leads to the accumulation of salt ions and a decrease in water in plants (Munns and Tester 2008; Baz et al. 2020). In addition, the formation of reactive oxygen species (ROS) in plant cells is triggered by the increase in sodium ion (Na+) concentration resulting from salt stress in plants (Gürsoy 2023). Excessive ROS production leads to lipid peroxidation and enzyme inhibition (Boussadia et al. 2023).
Excessive ROS production leads to salt ion accumulation and water loss in plants (Munns and Tester 2008; Baz et al. 2020). In addition, reactive oxygen species (ROS) production in plant cells is triggered by the increase in sodium ion (Na+) concentration caused by salt stress in plants (Gürsoy 2023). Excessive ROS production leads to lipid peroxidation and enzyme inhibition (Boussadia et al. 2023). As a result, plant yield is reduced.
Plant salt sensitivity varies with different developmental stages. Salinity can affect the percentage and rate of germination, leading to unstable plant growth, resulting in lower yield and quality (Luo et al. 2021). Plants are affected by salt stress throughout their lives, but the first seedling stage is much more sensitive (Ali et al. 2021). In addition to its effects on early plant developmental stages, salinity reduces chlorophyll synthesis and limits the process of photosynthesis, which directly affects plant production (Hussain et al. 2021; Sobrinho et al. 2023). In addition, this salinity also reduces some traits such as root length and shoot length at the seedling stage (Haq et al. 2014).
Sustainable agricultural practices are gaining importance due to the increasing negative effects of stress factors (Rasouli et al. 2022). Some of these practices include the use of organic fertilisers, biostimulants, etc. (Gürsoy 2022a). Researchers have reported that natural biostimulants have positive effects on yield and quality, and have protective effects on photosynthetic pigments along with phytochemical activity (Michalak et al. 2021). Similarly, the use of natural biostimulants as a promising, environmentally friendly, new approach increases flowering, plant growth, plant production and nutrient use efficiency in plants, while increasing resistance to many abiotic stresses (Rouphael and Colla 2020).
In order to reduce these negative effects, environmentally friendly biostimulant applications have an important place today. Many researchers have reported that the use of biostimulants in agriculture increases the resistance of plants to various stress factors (Gürsoy 2020, 2022a). According to the classification made by Du Jardin (2015), biostimulants are divided into 7 categories. These are (1) humic and fulvic acids, (2) protein hydrolysates and other N-containing compounds, (3) seaweed and plant extracts, (4) chitosan and other biopolymers, (5) inorganic compounds, (6) beneficial fungi and (7) beneficial bacteria.
Seaweed extract is a biostimulant that has been used as a soil amendment to develop plant growth mechanisms (Hurtado et al. 2009). Seaweed (SW) application is one of the new applications that are being intensively studied today. SW application is a natural regulator and is effective in increasing plant growth and yield against various environmental factors (Chanthini et al. 2022). SW, which is a sustainable and environmentally friendly practice, plays an important role in reducing environmental problems and increasing product yield (Chanthini et al. 2022). It has been observed that seaweed has a remarkable effect on plant growth, including stem, root and shoot length (Craigie 2011).
Humic acid (HA) is one of the most important components of biostimulants and biofertilisers (El-Tanahy et al. 2019; Gürsoy 2022b). Although humic substances contribute significantly to soil fertility by influencing the physical, physico-chemical, chemical and biological properties of the soil, they have a positive effect on the nutrition of plant roots due to the biostimulant effect of humic substances (Du Jardin 2015). HA is believed to play an important role in plant growth regulation as a biostimulant (Saidimoradi et al. 2019). Humic acid is a component of humic substances, which are derived from the residue of biological matter that is soil (Shukry et al. 2023).
Rapeseed (Brassica napus L.) is an important oil crop as well as an important protein crop (Nima et al. 2018). Rapeseed is an important oil crop with winter and summer varieties, with 38–50% oil and 16–24% protein in its seeds, as well as an important oil crop with its very high content of unsaturated fatty acids (Gürsoy 2016, 2019).
The aim of the present study is to determine the effects of SW, HA and their combinations (SW + HA) applications on seedling growth, total chlorophyll, carotenoids, proline, relative water content, total phenolic compounds, electrolyte leakage, malondialdehyde (MDA) and superoxide dismutase (SOD) parameters of rapeseed seedlings under salt stress in greenhouse conditions.
2 Materials and Methods
In this study, rapeseed seeds were obtained from Kırşehir Ahi Evran University, Kırşehir, Türkiye. The research was carried out at Aksaray University Güzelyurt Vocational School, Güzelyurt, Aksaray, Türkiye. Before starting the experiment, all seeds were pre-treated with 5% sodium hypochlorite solution for 5 min and then rinsed with distilled water for sterilisation. The seeds were then dried to their initial weight. The research was carried out in a randomised factorial design with 3 replications. The experimental pots were filled with surrounding soil that was considered fertile. According to the soil analysis made on the sample taken from the same soil, the saturation is 50.05%, pH 7.71, lime 1.41%, organic matter 1.07%, phosphorus 5.75 kg da− 1, potassium 146.46 kg da− 1, nitrogen 0.90% and it has a loamy structure. Ten rape seeds were planted in each pot. The plants were watered with tap water to saturation. Germination was observed in the pots 5 days after planting. After 15 days, the plants were thinned out to 5 plants per pot. After thinning, the plants were watered every two days with saline irrigation water (0, 50, 10 mM) and different concentrations of SW (commercial) (0, 10, 20 mL L− 1), HA (0, 10, 20 mL L− 1) and SW + HA (0, 10 (5 + 5 mL L− 1 SW + HA) 20 mL L− 1 (10 + 10 mL L− 1 SW + HA) were sprayed once a week for 4 weeks with a hand sprayer. The pots in the control group were sprayed with tap water only and the study was terminated at the end of the 8th week after sowing.
2.1 Measurements
2.1.1 Seedling Lenght (cm)
The seedlings and roots (fresh weight) of the plants in each pot were separated and the length of the seedling part was measured with a ruler.
2.1.2 Root Lenght (cm)
This was determined by measuring the roots of the plants whose seedling and root parts had been separated with a ruler.
2.1.3 Total Chlorophll Content (mg g− 1 FW)
To determine the chlorophyll content, 0.25 g of leaves were weighed on a precision balance and homogenised in acetone (80%). The extract was made up to 25 ml with acetone. The absorbance of the extract was measured at 663 and 645 nm using a spectrophotometer. The total chlorophyll content was determined according to the formula based on the values obtained (Lichtenthaler and Welburn 1983).
V = volume of leaf extract in 80% acetone
W = fresh weight of leaf material
2.1.4 Carotenoid Content (mg g− 1 FW)
A sample of 0.25 g was taken from the leaves of rape seedlings, homogenised with 80% acetone and filtered. The homogenate was then made up to 25 mL with acetone and the samples were measured in a spectrophotometer at 450 nm. The amount of carotenoids was calculated using the following formula (Lichtenthaler and Welburn 1983).
2.1.5 Lipid Peroxidation (MDA) (nmol mL− 1)
A leaf sample (0.5 g) taken from the plants was homogenised with 5 mL of 0.1% trichloroacetic acid (TCA) and then centrifuged at 15,000 rpm for 10 min. TBA (thiobarbituric acid) 0.5 mL was dissolved in 4 mL of 20% TCA taken from the upper phase or supernatant of the centrifuged samples. The samples were then centrifuged at 10,000 rpm for 10 min and the absorbance was determined at 532 and 600 nm using the clear fraction. The malondialdehyde (MDA) content was calculated from the values obtained using the formula (Heath and Packer 1968).
2.1.6 Superoxide Dismutase (SOD) (U g− 1 FW)
Fresh leaf samples (0.5 g) were homogenised with 10 mL sodium phosphate buffer and centrifuged at 15,000 rpm for 15 min to obtain the plant extract. Na-phosphate buffer (50 mM) (Na2HPO4×H2O2), Na-EDTA (0.1 mM), NBT (33 µM), riboflavin (75 µM), methionine (13 mM) was used as reaction solution (pH: 7.0). The reaction solution (2.5 mL) and the plant extract solution (0.1 mL) were then mixed. The control and reaction solutions were measured at 560 nm (Rahnama and Ebrahimzadeh 2005).
2.1.7 Electrolyte Leakeage (EL) (%)
EL was determined according to Bajji et al. (2002). Leaves (0.1 g) of the seedlings were placed in test tubes and, after the addition of 10 mL of deionised water, they were kept at room temperature for 24 h. At the end of this period, the samples were measured with an EC meter (EC1). The test tubes were then heated to 100 °C for 15 min. They were then cooled to 25 °C and measured again with an EC meter (EC2). The EL was calculated using the following formula:
2.1.8 Proline (Pro) (µmol g− 1 FW)
To calculate the proline content (Bates 1973), a leaf sample (0.5 g) was homogenised with 10 mL of sulphosalicylic acid. After filtering the extract obtained with filter paper, 2 mL of acetic acid and 2 mL of ninhydrin were added. The mixture was heated to 100 °C for 1 h. The reaction was terminated by immersion in an ice bath and 4 mL of toluene was added and mixed. The absorbance was read in a spectrophotometer at 520 nm using toluene as a blank.
2.1.9 Total Phenolic Content (TPC) (mg GAE g− 1 FW)
To determine the total phenolic content, the fresh leaf sample is homogenised in 80% acetone and centrifuged at 10,000 rpm for 10 min. 1 mL of Folin-Ciocalteu, 2 mL of distilled water and 5 mL of Na2CO3 are added to the extract obtained. It is then made up to a total of 10 ml with distilled water. It is read in a spectrophotometer at 750 nm. (Julkunen-Titto 1985).
2.1.10 Relative Water Content (RWC) (%)
Leaf samples taken from the plants in the pots were weighed, their fresh weights determined and placed in glass tubes containing 5 mL of deionised water and kept in the light for 24 h. At the end of this period, the leaf samples were weighed again and their turgor weights were determined. The leaf samples were dried in an oven at 80oC for 48 h and their dry weights were determined again. Finally, the relative water content was calculated according to the following formula (Ritchie et al. 1990).
FW: wet weight, TW: turgor weight, DW: dry weight.
2.1.11 Data Analysis
The data obtained at the end of the research were subjected to analysis of variance using MSTAT-C computer software. The Duncan test was used to determine the significance levels of the differences between the means of the applications. SPSS was used for the PCA analysis.
3 Results
In this study, the triple interaction of applications (SW, HA, SW + HA), application doses and salt doses were found to be significant at p < 0.01 level (except for the carotenoid parameter). There was a significant difference at the p < 0.01 level between salt doses in carotenoid.
3.1 Seedling Lenght (cm)
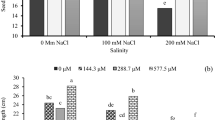
From Fig. 1, it was observed that seedling height decreased with increasing salt doses. However, in the SW2 + HA2 and SW3 + HA3 applications, where SW and HA were applied together, it was found that the seedling height was less affected by salt stress and the seedling height did not shorten compared to the control even at S2 and S3 doses. However, it was found that the SW3 + HA3 dose was the most effective application in reducing the negative effects of stress.
Interaction effects of salt (S1, S2, S3), seaweed (SW1, SW2, SW3), humic acid (HA1, HA2, HA3) and seaweed + humic acid (SW1 + HA1, SW2 + HA2, SW3 + HA3) applications on seedling length (SL). Different letters indicated significant differences among priming applications and salt doses to Duncan’s multiple range tests at p < 0.01
3.2 Root Lenght (cm)
When examining Fig. 2 in terms of root length, the longest root length was recorded in the SW3 + HA3 application. When the applications were compared, it was found that the HA was more effective in protecting against the negative effects of stress than the SW. In all applications, the shortest root length was recorded in the control. Root length was found to be longer in SW, HA, SW + HA and salt applications compared to the control. This shows that the positive effects of the treatments are important in increasing root length despite salt stress.
Interaction effects of salt (S1, S2, S3), seaweed (SW1, SW2, SW3), humic acid (HA1, HA2, HA3) and seaweed + humic acid (SW1 + HA1, SW2 + HA2, SW3 + HA3) applications on root length (RL). Different letters indicated significant differences among priming applications and salt doses to Duncan’s multiple range tests at p < 0.01
3.3 Total Chlorophyll (mg g− 1 FW)
Total chlorophyll was significantly (p < 0.01) affected by all treatments and their combinations. When looking at the interaction (Fig. 3), it can be seen that the amount of chlorophyll in all applications is highest in the S1 and SW3 + HA3 combination. It was observed that the chlorophyll content decreased with increasing salinity for all. The lowest chlorophyll content was found in the SW1 application, i.e. the control. In the HA3 and SW2 + HA2 combination, the chlorophyll content was less affected by the salt application at the S2 dose compared to the control.
Interaction effects of salt (S1, S2, S3), seaweed (SW1, SW2, SW3), humic acid (HA1, HA2, HA3) and seaweed + humic acid (SW1 + HA1, SW2 + HA2, SW3 + HA3) applications on total chlorophyll (Total Chl). Different letters indicated significant differences among priming applications and salt doses to Duncan’s multiple range tests at p < 0.01
3.4 Carotenoid (mg g− 1 FW)
Looking at Fig. 4 for the carotenoid content, there is only a significant difference between the salt doses at the 0.01 level. However, the highest carotenoid was found in S3, which is the highest salt dose and in the SW + HA application where seaweed and humic acid were used together.
3.5 Electrolyte Leakage (%)
The EL content was significantly influenced by the interaction between treatments and salt doses (p < 0.01) (Fig. 5). EL decreased with increasing salt dose in all treatments. However, in the SW3 + HA3 application, it can be seen that the amount of EL increased in S3 compared to S2. It can be seen that the EL increases with the increase of the applied dose, even if the salt doses increase.
Interaction effects of salt (S1, S2, S3), seaweed (SW1, SW2, SW3), humic acid (HA1, HA2, HA3) and seaweed + humic acid (SW1 + HA1, SW2 + HA2, SW3 + HA3) applications on electrolyte leakeage (EL). Different letters indicated significant differences among priming applications and salt doses to Duncan’s multiple range tests at p < 0.01
3.6 Lipid Peroxidation (nmol g− 1)
The MDA content of all plants grown under salt stress was significantly affected by all treatments (p < 0.01) (Fig. 6). The highest MDA content was obtained from the S3 salt dose and the control application of SW and HA applications. Although MDA increased due to stress in all salt applications, MDA in SW3 application was lower than S2 at S3 dose. The effect of application caused a decrease in MDA. Similarly, the increase in MDA in the SW3 + HA3 application was not much compared to the control. It was found that the applications are effective in reducing the negative effects of MDA.
Interaction effects of salt (S1, S2, S3), seaweed (SW1, SW2, SW3), humic acid (HA1, HA2, HA3) and seaweed + humic acid (SW1 + HA1, SW2 + HA2, SW3 + HA3) applications on malondialdehyde (MDA). Different letters indicated significant differences among priming applications and salt doses to Duncan’s multiple range tests at p < 0.01
3.7 Superoxide Dismutase (U g− 1 FW)
When the treatments were examined in terms of SOD activity (Fig. 7), the SOD content of all salt-stressed plants was significantly affected by all treatments (p < 0.01). When the mean values were examined, the lowest SOD content was found in application SW1S1. The highest was found in SW3HA3 and S3. It was observed that the SOD content increased with increasing salinity in all treatments. In the treatments made together with the effect of stress, there was an increase in the SOD enzyme and significant increases were determined compared to the control.
Interaction effects of salt (S1, S2, S3), seaweed (SW1, SW2, SW3), humic acid (HA1, HA2, HA3) and seaweed + humic acid (SW1 + HA1, SW2 + HA2, SW3 + HA3) applications on superoxide dismutase (SOD). Different letters indicated significant differences among priming applications and salt doses to Duncan’s multiple range tests at p < 0.01
3.8 Total Phenolic Content (mg GAE g− 1 FW)
TPC in the leaves of salt-stressed plants was also significantly affected by the treatments (p < 0.01). TPC increased in all treatments, but the highest TPC was observed in SW2 + HA2 application (Fig. 8). The lowest TPC was found in SW1 and HA1. When the treatments were compared, it was found that the HA treatment was more effective in increasing TPC against stress than the SW.
Interaction effects of salt (S1, S2, S3), seaweed (SW1, SW2, SW3), humic acid (HA1, HA2, HA3) and seaweed + humic acid (SW1 + HA1, SW2 + HA2, SW3 + HA3) applications on total phenolic content (TPC). Different letters indicated significant differences among priming applications and salt doses to Duncan’s multiple range tests at p < 0.01
3.9 Relative Water Content (%)
Looking at the research results in terms of RWC (Fig. 9), the highest RWC was obtained from the SW3 + HA3 application. The lowest RWC was obtained from the control application of SW. In addition, all treatments were also effective in terms of RWC content. When all the treatments were compared, it was found that the SW + HA was more effective in protecting the water content of the plants against stress.
Interaction effects of salt (S1, S2, S3), seaweed (SW1, SW2, SW3), humic acid (HA1, HA2, HA3) and seaweed + humic acid (SW1 + HA1, SW2 + HA2, SW3 + HA3) applications on relative water content (RWC). Different letters indicated significant differences among priming applications and salt doses to Duncan’s multiple range tests at p < 0.01
3.10 Proline (µmol g− 1 FW)
In this study, proline production in leaves under salt stress increased in all treatments compared to the control. However, in HA3 and SW3 + HA3 applications, the amount of proline was found to be lower in S2 than in the control (S1) (Fig. 10). The highest levels were found in Pro SW1 + HA1 and S3, the lowest in SW2 and HA2 in S1 and HA3 in S2.
Interaction effects of salt (S1, S2, S3), seaweed (SW1, SW2, SW3), humic acid (HA1, HA2, HA3) and seaweed + humic acid (SW1 + HA1, SW2 + HA2, SW3 + HA3) applications on proline (Pro). Different letters indicated significant differences among priming applications and salt doses to Duncan’s multiple range tests at p < 0.01
3.11 PCA Analysis
The PCA analysis was performed separately for all applications (Fig. 11). In all cases, the components were divided into 3 groups. For SW application (Fig. 11A) Crt, SOD, TPC were in the first group, EL, MDA, Pro were in the second group, RL, SL, Total Chl, RWC were in the third group. For HA application (Fig. 11B) RL, SL, Total Chl, RWC are in the first group, SOD, Crt, TPC are in the second group and EL, MDA, Pro are in the third group. For SW + HA (Fig. 11C), RL, SL, Total Chl, RWC were in the first group, TPC, SOD, Crt were in the second group and EL, MDA, Pro were in the third group. The characteristics within each group have positive and strong correlations. Since the angle between the parameters in each group is more than 90°, it was determined that the relationship between them is weak (Kızılgeçi et al. 2019).
Principal component analysis (PCA) (A) seaweed, (B) humic acid, (C) seaweed + humic acid for different parameters of rapeseed under salinity stress conditions. SL: Seedling Length, RL: Root Length, Total Chl: Total Chlorophyll, Crt: Carotenoid, EL: Electrolyte Leakeage, MDA: Molandialdehyde, SOD: Superoxide Dismutase, TPC: Total Phenolic Content, RWC: Relative Water Content, Pro: Proline
4 Discussion
Previous studies have reported that salt stress causes shortening of plant and root length, as in this study. It was reported by researchers that SW and HA increased vegetative growth in pepper plants in all applications under salt stress (Yildiztekin et al. 2018). Chanthini et al. (2022) reported that they found an increase in radicle and plumule length with the effect of SW in their study where SW was applied to rice plants under salt stress. Similarly, various researchers reported that root and seedling growth increased with humic acid application (Jindo et al. 2011). According to Barekati et al. (2019), in their study, HA significantly increased the height of growing plants in the form of foliar application of HA on rapeseed plants. Gürsoy (2022b) played a significant role in reducing the negative effects of salt stress in the study where she applied HA priming to the sunflower plant. In the study, she reported that root and seedling length increased with the effect of HA. Similar results have been reported by other researchers (Gürsoy and Kolsarıcı 2017; Çöl and Akınerdem 2017).
Salt stress affects the photosynthetic system due to the increase in reactive oxygen species (ROS). This situation causes oxidative stress, which leads to the degradation of chlorophyll and ultimately to a decrease in the amount of chlorophyll (Keshavarz Mirzamohammadi et al. 2021). Photosynthetic rate has a positive relationship with relative chlorophyll content (Arora et al. 2012). They reported that the effect of SW was more significant, especially at high doses. Researchers reported that the reason for the increase in chlorophyll with SW applications is that the SW extract contains glycine betaine, and glycine betaine also reduces the breakdown of chlorophyll (Genard et al. 1991). Ramarajan et al. (2015) applied SW in the form of foliar spray to soybean plants under salt stress. As a result of the study, they found an increase in leaf pigment (especially chl a).
Foliar applications of SW extract, HA and SW + HA were found to have a positive effect on carotenoids as well as SL, RL and chlorophyll. Carotenoids are antioxidants that play a very important biological role in photosynthetic activity (Çoka and Akınoğlu 2023). Chlorophyll also increased with the increase in photosynthetic activity, and carotenoids also play a role in this increase. In addition, carotenoids are a group of pigments that protect against oxidative stress and pigment loss in plants (Doğru and Torlak 2020). Thirumaran et al. (2009) reported that the application of seaweed to the plant Abelmoschus esculentus caused an increase in carotenoids. Sofi et al. (2021) reported that they found the highest carotenoid in HA + 1.5 dS m− 1 S application in their study where HA was applied to Trifolium alexandrium plant under salt stress.
Membrane permeability and membrane lipid peroxidation in cells also increase due to stress-induced increased ROS levels. Electrolyte leakage (EL) induces cell membrane damage and membrane stability when plants are grown under salt stress (Hegazi et al. 2015; Naeem et al. 2020). However, applications of EL played an important role in reducing cell membrane damage and increasing plant resistance to stress. The application of humic acid to bean plants in combination with salt stress led to a decrease in ROS production, an increase in the activity of antioxidant enzymes (SOD, CAT, etc.) and ultimately a decrease in EL (Aydin et al. 2012). Abu-Ria et al. (2023) reported that they detected a reduction in the EL ratio with salt stress in rice plants. Shukry et al. (2023) reported that the growth retardation of the plant due to salt stress was related to Na+ accumulation, which triggered electrolyte leakage as a result of their study in which they applied humic acid to rice plant under salt stress.
In terms of lipid peroxidation (MDA), humic acid can improve the stability of cell membranes and therefore reduce Na+ accumulation and improve K+ accumulation, thus reducing salinity-induced lipid peroxidation (Saidimoradi et al. 2019). Ali et al. (2019) reported that in their study where they applied humic acid to forage sorghum plants under salt stress, MDA increased with the effect of salt stress and humic acid showed a positive effect on MDA. Farooq et al. (2022) found that reduced MDA levels (65–75%) and regulated ROS production resulted in improved membrane stability.
Due to stress, the activities of various antioxidant enzymes such as superoxide dismutase (SOD) are inhibited; thus, increasing levels of ROS lead to membrane deterioration and ultimately cell death (Hasanuzzaman et al. 2012). Humic acid increases antioxidant capacity under various abiotic stresses and regulates the metabolism of secondary metabolites (Manonmani et al. 2022). There was a significant development in antioxidant enzyme activity such as superoxide dismutase (SOD), catalase (CAT) etc. activities in the plant under salt stress conditions and it was treated with SW and HA. It suggests that SW and HA can increase salt stress resistance and cause protection of pepper plant against oxidative stress. (Yildiztekin et al. 2018). Humic acid improves plant tolerance to salinity due to various biochemical and physiological activities, such as antioxidant enzymes like SOD (Abbas et al. 2022). The SOD enzyme plays an important role in the conversion of superoxide radicals to H2O2 and it has been reported that the application of humic acid to wheat genotypes under salt stress conditions causes an increase in the SOD enzyme (Abbas et al. 2022). The increase in SOD activity in this study shows that it is effective in defending against stress.
In general, seaweeds are rich in phenolic compounds. Phenolic compounds are secondary metabolites that are synthesised under stress to protect cells and cell components (Wang et al. 2009). Important roles of phenolic compounds include antioxidant activity, scavenging radicals such as singlet oxygen, superoxide and hydroxyl etc. (Andjelkovi’c et al. 2006). In this study, TPC has increased with the effect of applications and it is extremely important in terms of plant defence mechanisms against stress. Chernane et al. (2015) reported that the application of seaweed extract to wheat plants under salt stress had an increasing effect on total phenolic compounds. According to Frioni et al. (2021), the effects of a seaweed extract were also investigated and similar positive results on phenolic concentration were observed in grape plants.
Leaf water content was affected by salt stress. In addition, SW and HA practices were also effective on RWC content. Dekhordi et al. (2021) reported that the application of SW to fenugreek ecotypes resulted in an increase in RWC. Gürsoy (2022b) reduced RWC in sunflower cultivars under salt stress.
Proline is considered to be one of the major accumulated osmolytes in plant tissues and the increase in proline content can be considered as one of the tolerance capacity criteria under different stress conditions and plays different roles in stress resistance in plants (Manonmani et al. 2022; Nezamdoost et al. 2022). However, Saidimoradi et al. (2019) observed that plants treated with humic acid had lower proline content, which could be related to a mechanism to reduce the unfavourable effects of salinity stress. The significant increase in proline content observed in the present study is consistent with that previously reported for safflower (Farooq et al. 2020). In this study, the amount of proline increased with increasing salinity stress, but decreased in some SW, HA and SW + HA applications.
5 Conclusion
In this study, seaweed, humic acid and seaweed + humic acid treatments were found to play an important role in reducing the negative effects of salt stress. All treatments had a positive effect on seedling growth and biochemical properties of oilseed rape seeds. In this study, when both treatments were compared, it was found that the seaweed + humic acid application was more effective in reducing the effects of stress. Therefore, the combined application of seaweed and humic acid to rapeseed can improve salt tolerance and increase growth, as well as antioxidant enzymes that mitigate damage caused by salinity stress. Furthermore, the third dose of this seaweed + humic acid application was found to be the most effective dose in reducing the effects of salinity stress. The use of these treatments in salinity stress for salt-sensitive plants may be useful, but more extensive research in this area is recommended.
References
Abbas G, Rehman S, Siddiqui MH, Ali HM, Farooq MA, Chen Y (2022) Potassium and humic acid synergistically increase salt tolerance and nutrient uptake in contrasting wheat genotypes through ionic homeostasis and activation of antioxidant enzymes. Plants 11:263. https://doi.org/10.3390/plants11030263
Abu-Ria M, Shukry W, Abo-Hamed S, Albaqami M, Almuqadam L, Ibraheem F (2023) Humic acid modulates ionic homeostasis, osmolytes content, and antioxidant defense to improve salt tolerance in rice. Plants 12:1834. https://doi.org/10.3390/plants12091834
Ali AYA, Ibrahim MEH, Zhou G, Nimir N, Jiao X, Zhu G, Elsiddig A, Zhi W, Chen X, Lu H (2019) Ameliorative effects of jasmonic acid and humic acid on antioxidant enzymes and salt tolerance of forage sorghum under salinity conditions. Agron J 111:1–10. https://doi.org/10.2134/agronj2019.05.0347
Ali AYA, Ibrahim MEH, Zhou G, Nimir NEA, Elsiddig AMI, Jiao X et al (2021) Gibberellic acid and nitrogen efficiently protect early seedlings growth stage from salt stress damage in sorghum. Sci Rep 11:1–11. https://doi.org/10.1038/s41598-021-84713-9
Andjelkovi´c M, Van Camp J, De Meulenaer B, Depaemelaere G, Socaciu C, Verloo M, Verhe R (2006) Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem 98:23–31. https://doi.org/10.1016/j.foodchem.2005.05.044
Arora S, Sharma P, Kumar S et al (2012) Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul 66:303–310. https://doi.org/10.1007/s10725-011-9649-z
Aydin A, Kant C, Turan M (2012) Humic acid application alleviate salinity stress of bean (Phaseolus vulgaris L.) plants decreasing membrane leakage. Afr J Agric Res 7:1073–1086. https://doi.org/10.5897/AJAR10.274
Bajji M, Lutts S, Kinet JM (2002) The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul 36:61–70. https://doi.org/10.1023/A:1014732714549
Barekati F, Hervan EM, Rad ASS, Mohamedi GN (2019) Effect of sowing date and humic acid foliar application on yield and yield components of canola cultivars. J Agricultural Sci 25:70–78. https://doi.org/10.15832/an-kutbd.539003
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Baz H, Creech M, Chen J, Gong H, Bradford K, Huo H (2020) Water-soluble carbon nanoparticles improve seed germination and post-germination growth of lettuce under salinity stress. Agronomy 10:1192. https://doi.org/10.3390/agronomy10081192
Boussadia O, Zgallai H, Mzid N, Zaabar R, Braham M, Doupis G, Koubouris G (2023) Physiological responses of two olive cultivars to salt stress. Plants 12:1926. https://doi.org/10.3390/plants12101926
Chanthini KMP, Senthil-Nathan S, Pavithra GS, Malarvizhi P, Murugan P, Deva-Andrews A, Janaki M, Sivanesh H, Ramasubramanian R, Stanley-Raja V et al (2022) Aqueous seaweed extract alleviates salinity-induced toxicities in rice plants (Oryza sativa L.) by modulating their physiology and biochemistry. Agriculture 12:2049. https://doi.org/10.3390/agriculture12122049
Chernane H, Latique S, Mansori M, Kaoua ME (2015) Salt stress tolerance and antioxidative mechanisms in wheat plants (Triticum durum L.) by seaweed extracts application. IOSR J Agric Veterinary Sci 8:36–44. https://doi.org/10.9790/2380-08313644
Çoka İ, Akınoğlu G (2023) Bitkilerde karotenoidlerin işlevleri. 5. International Ankara Multidisciplinary studies Congress January 27–29, 2023 full text book. Ankara, Türkiye, pp 819–824. (in Turkish)
Çöl N, Akınerdem F (2017) The Effect of humic acid applications in different quantities on the yield and some yield components on potato plant (Solanum tuberosum L). Selcuk J Agr Food Sci 31:24–32. https://doi.org/10.15316/SJAFS.2017.31
Craigie JS (2011) Seaweed extract stimuli in plant science and agriculture. J Appl Phycol 23:371–393. https://doi.org/10.1007/s10811-010-9560-4
Dekhordi AR, Roghani SR, Mafakheri S, Asghari B (2021) Effect of biostimulants on morpho-physiological traits of various ecotypes of fenugreek (Trigonella foenum-graecum L.) under water deficit stress. Sci Hort 283:11007. https://doi.org/10.1016/j.scienta.2021.110077
Doğru A, Torlak E (2020) Effects of exogenous ascorbic acid application in maize plants under salt stress. YYU J Agricultural Sci 30:919–927
Du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic 196:3–14. https://doi.org/10.1016/j.scienta.2015.09.021
EL-Tanahy AMM, Marzouk NM, Mahmoud AR, Ali AH (2019) Influence of humic acid application and yeast extract on growth and productivity of Okra plants. Middle East J Agric Res 8:418–424
Farooq A, Bukhari SA, Akram NA, Ashraf M, Wijaya L et al (2020) Exogenously applied ascorbic acid-mediated changes in osmoprotection and oxidative defense system enhanced water stress tolerance in different cultivars of safflower (Carthamus tinctorious L). Plants 9:104. https://doi.org/10.3390/plants9010104
Farooq T, Akram MN, Hameed A, Ahmed T, Hameed A (2022) Nanopriming-mediated memory imprints reduce salt toxicity in wheat seedlings by modulating physiobiochemical attributes. BMC Plant Biol 22:540. https://doi.org/10.1186/s12870-022-03912-2
Frioni T, VanderWeide J, Palliotti A, Tombesi S, Poni S, Sabbatini P (2021) Foliar vs. soil application of Ascophyllum nodosum extracts to improve grapevine water stress tolerance. Sci Hortic 277:109807. https://doi.org/10.1016/j.scienta.2020.109807
Genard H, Le-Saos J, Billard JP, Tremolieres A, Boucaud J (1991) Effect of salinity on lipid composition, glycine betaine content and photosynthetic activity in chloroplasts of Suaeda maritime. Plant Physiol Biochem 29:421–427
Gürsoy M (2016) The role of differential humic acid levels on quantative and qualitive characteristics of summer rapeseed plant (Brassica napus ssp. oleifera L.):An Investigation of Leonardite under Ankara Conditions. PhD Thesis, 80 p
Gürsoy M (2019) Importance of some oil crops in Hu-man nutrition. Turkish J Agriculture-Food Sci Technol 7:2154–2158. https://doi.org/10.24925/turjaf.v7i12.2154-2158.2916
Gürsoy M (2020) Effect of Chitosan pretreatment on seedling growth and antioxidant enzyme activity of safflower (Carthamus tinctorius L.) cultivars under saline conditions. Appl Ecol Environ Res 18:6589–6603. https://doi.org/10.15666/aeer/1805_65896603
Gürsoy M (2022a) Biostimulant applications in agriculture. 7th International Zeugma Conference on Scientific Research, January 21–23, Gaziantep/Türkiye pp:41–47
Gürsoy M (2022b) Role of biostimulant priming applications on germination, growth and chlorophyll content of sunflower (Helianthus annuus L.) cultivars under salinity stress. Selcuk J Agr Food Sci 36:75–81. https://doi.org/10.15316/SJAFS.2022.011
Gürsoy M (2023) An overview of the effects of salt stress on plant development. 9th Internatıonal Zeugma Conference on Scientıfic Research. February 19–21, 2023/ Gaziantep, Türkiye 508–513
Gürsoy M, Kolsarıcı Ö (2017) Effects of different humic acid doses in leonardite environment on the oil percentage and fatty acids of summer rapeseed (Brassica napus ssp. Oleifera L.) under Central Anatolian conditions. Fresenius Environ Bull 26:6447–6456
Haq TU, Akhtar J, Ali A, Maqbool MM, Ibrahim M (2014) Evaluating the response of some canola (Brassica napus L.) cultivars to salinity stress at seedling stage. Pakistan J Agricultural Sci 51:571–579
Hasanuzzaman M, Hossain MA, daSilva JAT, Fujita M (2012) Plant responses and tolerance to abiotic oxidative stress: antioxidant defenses is a key factor. In: Bandi V, Shanker AK, Shanker C, Mandapaka M (eds) Crop stress and its management: perspectives and strategies. Springer, Berlin, pp 261–316
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hegazi A, El-Shraiy A, Ghoname A (2015) Alleviation of salt stress adverse effect and enhancing phenolic antioxidant content of eggplant by seaweed extract. Gesunde Pflanzen 67:21–31. https://doi.org/10.1007/s10343-014-0333-x
Hurtado AQ, Yunque DA, Tibubos K, Critchley AT (2009) Use of Acadian Marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J Appl Phycol 21:633–639. https://doi.org/10.1007/s10811-008-9395-4
Hussain S, Ali B, Ren X, Chen X, Li Q, Ahmad N (2021) Recent progress in understanding salinity tolerance in plants: story of Na+/K+ balance and beyond. Plant Physiol Biochem 160:239–256. https://doi.org/10.1016/j.plaphy.2021.01.029
Jindo K, Martim SA, Navarro EC, Pérez-Alfocea F, Hernandez T, Garcia C, Aguiar NO, Canellas LP (2011) Root growth promotion by humic acids from composted and non-composted urban organic wastes. Plant Soil. https://doi.org/10.1007/s11104-011-1024-3
Julkunen-Titto R (1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33:213–217
Keshavarz Mirzamohammadi H, Tohidi-Moghadam HR, Hosseini SJ (2021) Is there any relationship between agronomic traits, soil properties and essential oil profile of peppermint (Mentha Piperita L.) treated by fertilizer treatments and irrigation regimes? Ann Appl Biol 179:331–344. https://doi.org/10.1111/aab.12707
Kızılgeçi F, Yıldırım M, Akıncı C, Albayrak Ö (2019) Genotype and environment effects on the grain yield and quality traits of some in barley genotypes. KSU J Agric Nat 22:346–353. https://doi.org/10.18016/ksutarimdoga.vi.499013
Lichtenthaler HK, Wellburn AR (1983) Determinations of total careteonids and chlorophylls a and b of leaf extracts indifferent solvents. Biomchem Soc Transac 11:591–592
Luo X, Dai Y, Zheng C, Yang Y, Chen W, Wang Q, Chandrasekaran U, Du J, Liu W, Shu K (2021) The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol 229:950–962. https://doi.org/10.1111/nph.16921
Manonmani S, Senthilkumar S, Manivannan S (2022) Multilevel functionality of biostimulants in sustainable horticulture for modern era. Pharma Innov J 11:1283–1288
Michalak I, Norrie J, Critchley AT (2021) Biostimulants for Crops from Seed Germination to Plant Development. Chapter 7- Enhancing Plant Pigments using Natural Biostimulants. PP:165–196
Mndi O, Sogoni A, Jimoh MO, Wilmot CM, Rautenbach F, Laubscher CP (2023) Interactive effects of salinity stress and irrigation intervals on plant growth, nutritional value, and phytochemical content in Mesembryanthemum crystallinum L. Agriculture 13:1026. https://doi.org/10.3390/agriculture13051026
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Naeem M, Basit A, Ahmad I, Mohamed H, Wasila H (2020) Effect of salicylic acid and salinity stress on the performance of tomato plants. Gesunde Pflanzen 72:393–402. https://doi.org/10.1007/s10343-020-00521-7
Nezamdoost D, Ghahremani Z, Akbari MB, Barzegar T, Ranjbar ME (2022) Can seed priming with seaweed extract neutralize the effects of salinity on new red fire leafy lettuce characteristics? https://doi.org/10.1007/s10343-022-00738-8. Gesunde Pflanzen
Nima D, Mahmoud T, Mostafa V, Ali B (2018) The proteome response of salt sensitive rapeseed (Brassica napus L.) genotype to salt stress. Not Bot Horti Agrobo 47:17–23. https://doi.org/10.15835/nbha47111133
Rahnama H, Ebrahimzadeh H (2005) The effect of NaCl on antioxidant enzyme activities in potato seedlings. Biol Plant 49:93–97. https://doi.org/10.1007/s10535-005-3097-4
Ramarajan S, Beschi Antony Rayan S, Henry Joseph L, Saravana Ganthi A (2015) Effect of seaweed extracts mediated biochemical changes in soybean under NaCl salt stress condition. RAC J Res 1:7–9
Rasouli F, Nasiri Y, Asadi M et al (2022) Fertilizer type and humic acid improve the growth responses, nutrient uptake, and essential oil content on Coriandrum sativum L. Sci Rep 12:7437. https://doi.org/10.1038/s41598-022-11555-4
Ritchie SW, Nguyen HT, Haloday AS (1990) Leaf water content and gas exchange parameters of two wheat genotypes differing in drought resistance. Crop Sci 30:105–111. https://doi.org/10.2135/cropsci1990.0011183X003000010025x
Rouphael Y, Colla G (2020) Editorial: biostimulants in agriculture. Front. Plant Sci 11:40. https://doi.org/10.3389/fpls.2020.00040
Saidimoradi D, Ghaderi N, Javadi T (2019) Salinity stress mitigation by humic acid application in strawberry (Fragaria x ananassa Duch). Sci Hort 15:256108594. https://doi.org/10.1016/j.scienta.2019.108594
Shukry WM, Abu-Ria ME, Abo-Hamed SA et al (2023) The efficiency of humic acid for improving salinity tolerance in salt sensitive rice (Oryza sativa): growth responses and physiological mechanisms. Gesunde Pflanzen. https://doi.org/10.1007/s10343-023-00885-6
Sobrinho TG, da Silva AAR, de Lima GS, de Lima VLA, Borges VE, Nunes KG, Soares LA, Saboya LMF, Gheyi HR, Gomes JP et al (2023) Foliar applications of salicylic acid on boosting salt stress tolerance in sour passion fruit in two cropping cycles. Plants 12. https://doi.org/10.3390/plants12102023
Sofi A, Ebrahimi M, Shirmohammadi E (2021) Influence of humic acid on germination, morphological characteristics and photosynthesis pigments of Trifolium Alexandrium L. under salinity stress. Ecopersia 9:287–297
Sogoni A, Jimoh MO, Kambizi L, Laubscher CP (2021) The impact of salt stress on plant growth, mineral composition, and antioxidant activity in Tetragonia decumbens Mill.: an underutilized edible halophyte in South Africa. Horticulturae 7:140. https://doi.org/10.3390/horticulturae7060140
Thirumaran G, Arumugam M, Arumugam R, Anantharaman P (2009) Effect of seaweed liquid fertilizer on growth and pigment concentration of Abelmoschus esculentus (I) Medikus. Am -Eurasian J Agron 2:57–66
Wang T, Jonsdottir R, Ólafsdóttir G (2009) Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem 116:240–248. https://doi.org/10.1016/j.foodchem.2009.02.041
Yildiztekin M, Tuna AL, Kaya C (2018) Physiological effects of the brown seaweed (Ascophyllum nodosum) and humic substances on plant growth, enzyme activities of certain pepper plants grown under salt stress. Acta Biol Hung 69:325–335. https://doi.org/10.1556/018.68.2018.3.8
Funding
No funds, grants, or other support was received.
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research Involving Human Participants and/or Animals
No applicable.
Informed Consent
No applicable.
Financial Interests
The author declare she has no financial or non-financial interests to disclose.
Conflict of Interest
The author declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gürsoy, M. Alone or Combined Effect of Seaweed and Humic Acid Applications on Rapeseed (Brassica napus L.) under Salinity Stress. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-01759-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-01759-0