Abstract
Purpose
Careless burning, discarding, or overfertilization of spent mushroom substrate (SMS) is environmentally harmful. The reuse of SMS resources is a matter of concern.
Methods
Spent seafood mushroom substrate solid-state fermentation combined with Rhizobium pusense solution was added to soil to evaluate its environmental and Cenchrus fungigraminus seedling growth effects in pot trial experiments.
Results
The 40% fermented seafood mushroom SMS (fresh weight) with R. pusense treatment had significantly higher total nitrogen (TN), ammonium nitrogen (AMN), organic matter, etc., and decreased soil electrical conductivity (EC) and increased pH compared with other treatment. Soil catalase (CAT), dehydrogenase (DHA), and urease (UE) activities were significantly improved; there were no differences in CAT and UE activities between 40% (T4) and 50% (T5) SMS additions. C. fungigraminus seedling height and leaf length increased gradually and were significantly different from those of the positive control. The 30–50% seafood mushroom SMS treatment increased plant TN by 5.76–10.00% and total phosphorus by 15.44–16.55%. Plant root length and root activity peaked in T4; however, plant split root number and root activity in T5 decreased by 43.96% and 26.97% compared with those in T4. According to Pearson correlation and one-dimensional regression analyses, the seafood mushroom SMS content affected fertility > enzyme activity > plant growth. Principal component analysis and redundancy analysis showed that the effect of the seafood mushroom SMS concentration on the soil environment was mainly reflected in plant aboveground and root activity.
Conclusions
Under an SMS addition amount of 40%, plant growth-promoting rhizobacteria promoted the growth of C. fungigraminus seedlings to a greater extent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hypsizygus marmoreus (Peck) H. E. Bigelow (seafood mushroom or crab mushroom) has excellent potential for commercialization due to its remarkable flavor, delicious taste, chewy texture, nutritional and medicinal value and being famous in Asia and Europe (Gong et al. 2022; Oliveira et al. 2019; Sun et al. 2020a, b). Shunchang County is the “Hometown of Seafood Mushrooms” in Southeast China, and the annual total output accounts for nearly 70% of the global output. Spent mushroom substrate (SMS) is an abundant and organic agricultural byproduct after mushroom cultivation and is mainly composed of sawdust, grass, corncob, and rice bran. SMS increases with the rise in the consumption of seafood mushrooms, and the more than 80 million bags of SMS made of cottonseed shells and corncobs produced annually have been shown to cause substantial environmental pollution and resource waste by burning or discarding (Li et al. 2022; Martín et al. 2023). Fungal hyphae have been reported to intertwine with soil, wrap around plant roots and increase water and mineral availability for plants, thus improving yields. A 20% yield increase was reported when Stropharia rugoso-annulata was grown together with maize and with sprouts (Brassica oleracea var. gemmifera), while a 25% yield increase was observed when Hypsizygus ulmarius and Brussels sprouts were grown together (Prabu et al. 2014; Jasińska 2018). Two strains of beneficial bacterial (Pseudonocardia mangrovi)-Auricularia cornea interactions fostering growth and yield enhancement were identified from the spent mushroom substrate of Auricularia cornea (Phithakrotchanakoon et al. 2022). SMS gathered after Pleurotus ostreatus cultivation was investigated for its economic potential as a new raw material for xylooligosaccharides, a novel functional food and feed application (Seekram et al. 2021). Pleurotus eryngii SMS was successfully generated for the remediation of soil historically contaminated with chlorimuron-ethyl and remediated the microbial community structure and P (Zang et al. 2020). Solid-state fermentation (composting) is a reliable way for manure treatment and nutrient recycle, which might reduce the ability of nitrogen assimilation, but increases the degradation of lignin, cellulose, and hemicellulose in continuous composting system (Chen et al. 2022). After compost fermentation, SMS can also be used in mushroom production by increasing the protein content and yield of mushroom fruiting bodies (Pardo-Giménez et al. 2016; Ye et al. 2023). SMS is a multifunctional material used in fertilizers, feeds, soil conditioners, antibacterial agents, new materials, etc., that could feasibly be recycled as a new commercially sustainable agriculture cycle when amended with supplements (Du et al. 2022; Liu et al. 2015; Shi et al. 2020; Tso et al. 2021). Due to growing environmental concerns, the proper disposal and handling of excess SMS accumulation is essential. Therefore, it is necessary to develop new cultivation methods to improve the economic efficiency of seafood mushroom SMS.
Cenchrus fungigraminus Z. X. Lin & D. M. Lin & S. R. Lan sp. Nov. (Pennisetum giganteum Z. X. Lin, Jujuncao and giant Juncao have been used before) is a new species of grass with ecological and energetic value that is widely used and rapidly developed in Asia as well as in Africa. The high yield and photosynthesis of C. fungigraminus make it have significant application prospects in the fields of mushroom cultivation, ecological management, animal feed, bioenergy, and panel production (Lin et al. 2022; Zheng et al. 2023). According to Lin et al. (2019), the total nitrogen content of C. fungigraminus may exceed its total nitrogen uptake from soil and fertilizer, presumably due to the presence of the endophytic nitrogen-fixing bacteria (Klebsiella and Bradyrhizobium) that were shown to be the main nitrogen-fixing bacteria present in the roots and stems of C. fungigraminus. Meanwhile, Rhizobacteria in the C. fungigraminus rhizosphere play an important role as plant growth-promoting rhizobacteria (PGPR) (Li et al. 2023). C. fungigraminus has been vigorously introduced into Shunchang County because it has constituted an agricultural and industrial production industry since 2019. Current research has clearly indicated that agricultural application has been shown to be the most efficient method for recycling SMS due to its high fertilization value (Kwiatkowski and Harasim 2021; Owaid et al. 2017; Velusami et al. 2021). This material has great development prospects in alleviating food security and producing functional bacterial fertilizer. However, excessive inorganic N fertilization or overfertilization was shown to reduce tomato yield, soil pH and total carbon in a 12-year continuous cropping system, and bacterial diversity and richness decreased by 6% and 31%, respectively (Song et al. 2022). Approximately 30% of the N used as fertilizer in the world is consumed in China (Ali et al. 2020). Poudel et al. (2023) reported that SMS had high electrical conductivity and salt concentration, which resulted in poor seed germination and significantly influenced the production and nutritional quality with a small yield decrease in radish and peas. As a result of these studies, rational fertilization and improved nitrogen use efficiency are now promoted as effective strategies to mitigate greenhouse gas emissions and sustain global food security (Zhang et al. 2020).
Currently, there is limited research on the effects of agronomic measures on soil environmental properties and the soil enzyme activities associated with C. fungigraminus growth promotion by SMS and PGPR treatment. To this end, it is helpful to have a better understanding of the suitability of seafood mushroom SMS combined with PGPR as organic fertilizer for C. fungigraminus seedlings, including plant growth, soil enzyme activity, and fertility analysis. The present study aimed to investigate the influence of SMS application on (i) dynamic changes in soil nutrients and enzyme activity; (ii) C. fungigraminus seedling growth and root activity; and (iii) correlations between SMS concentration and soil nutrients, enzyme activity, and plant growth. To solve the problem of environmental pollution caused by SMS, SMS has been developed as a soil fertilizer to regulate soil fertility and plant growth.
2 Material and Methods
2.1 Experimental Material
Seafood mushroom SMS was collected from Fujian Nanping Shunchang Xinjundu Mushroom Industry Development Co., Ltd. All SMS (mainly containing 50% cottonseed shell, 30% corncob and 10% bran, moisture of approximately 59%) materials were fresh, without diseases or insect pests, and were obtained 1–2 days after mushroom fruiting body harvest. PGPR liquid microbial fertilizer (containing 2 strains of Rhizobium pusense (v:v = 1:1), GenBank Accession Nos. OQ119905 and OQ119906), experimental soil and C. fungigraminus seedlings were supplied by the National Engineering Center of Juncao Technology at Fujian Agricultural and Forestry University (FAFU). According to previous research, Rhizobium pusense was found in rhizosphere soil of C. fungigraminus and effectively improved plant growth as a PGPR (Chaudhary et al. 2020; Li et al. 2023). The following pot trial experiments were carried out on seedlings with basic growth recovery for 7 days in a greenhouse at temperatures from 26 to 28 °C. All reagents or kits in this experiment were obtained from Solarbio Technology Co., Ltd. (Peking, China), and all experimental work was completed at FAFU (26°5′12″N, 119°14′9″E) from April to November 2022.
2.2 Seafood Mushroom SMS Solid-State Fermentation
Seafood mushroom SMSs were torn into 1–3 cm pieces after bagging, the moisture was adjusted to 62%, and the SMSs were composted to 2 m (long) × 1 m (wide) × 1.5 m (high) stacks for solid-state fermentation. During fermentation, the stack was turned when the SMS temperature reached 55–60 °C, and fermentation lasted for 5–7 days. After 35 days, fermentation ended when the fermented SMS became brown and soft, without a peculiar smell, and the temperature was below 35 °C for nearly 1 week. SMS pH was determined with distilled water (1:2.5 water to SMS suspension) and analyzed by a pH meter (Sartorius, Germany) (Chen et al. 2017). SMS electrical conductivity (EC) was measured following Motsara and Roy (2008). SMS moisture was determined after drying in an oven (Jiangnan, China). The chemical characteristics of SMS, including organic matter (OM), total nitrogen (TN), total phosphorus (TP), total potassium (TK), available nitrogen (AN), available phosphorus (AP), available potassium (AK), nitrate nitrogen (NIN), and ammonium nitrogen (AMN), were analyzed as described below. OM and TN were determined using the potassium dichromate volumetric method and micro-Kjeldahl method (Conklin 2014) followed by colourimetric measurement using a microplate reader (Molecular, USA). NIN was determined using the dual wavelength colourimetric method, and AMN was determined using the indophenol blue colourimetric method according to the China national guideline LY/T 1228–2015. The TP and TK contents were processed by the wet oxidation method following the Chinese national guidelines HJ 632–2011 and GB 9836–88, respectively. TP was measured by using the molybdenum blue method (Corey 1973). AN, AP, and AK were determined by alkaline diffusion, sodium bicarbonate extraction with molybdenum antimony anti-colourimetric and ammonium acetate-atomic absorption methods, respectively (Bao 2000).
2.3 Experimental Treatment and C. fungigraminus Seedling Promotion
Ten percent (T1), 20% (T2), 30% (T3), 40% (T4), and 50% (T5) fermented seafood mushroom SMS (fresh weight) was mixed with C. fungigraminus seedling soil (700 g per pot), and 15 mL of 2 × 109 cfu mL−1 PGPR microbial fertilizer with Luria–Bertani (LB) culture medium was watered near the roots of C. fungigraminus seedlings every week. PGPR microbial fertilizer only was used as the positive control (CK), and LB culture medium only was used as the negative control (NK). Each group had 5 reduplicates in the greenhouse, and each pot was watered with 300 mL distilled water every 2 days. After 6 weeks of treatment, the plant height, leaf number (NO.), split root NO., root length, and root activity in each group were recorded. Split root NO. and root length were measured by a root scanning instrument (Epson, Japan). The root activity was determined by the triphenyl tetrazolium chloride (TTC) method (Liu et al. 2021). The aboveground parts of plants were smashed, and samples were collected randomly. The TN of C. fungigraminus seedlings was determined by the micro-Kjeldahl method, while the TP content was determined by colourimetry (Su et al. 2019).
2.4 Determination of Soil Nutrient Dynamics and Enzyme Activity
Five soil samples (100 g pot−1) from each group were randomly collected from the whole pot using a clean soil auger and placed in clean, labeled polyethylene bags. Each sample was divided into two parts, and onepart was stored at − 20 °C until physical and chemical analyses were performed; the other part was stored at − 80 °C for further analysis. Soil samples were ground to a smaller particle size and then sieved through a 2 mm mesh (Parajuli and Duffy 2013). The soil physical and chemical characteristics (pH, EC, OM, TN, TP, TK, AN, AP, AK, NIN, and AMN) of the seafood mushroom SMS were analyzed.
Soil enzymes are the products of plant roots and soil microorganisms, which regulate the conversion and circulation of soil nutrients, and can characterize the process and strength of soil nutrient conversion. In this study, we determined the activities of soil urease (S-UE), soil dehydrogenase (S-DHA), soil sucrase (S-SC), soil neutral phosphatase (S-NP), and soil catalase (S-CAT) using the following methods. S-UE can promote the transformation of organic nitrogen into available nitrogen that can be utilized by plants (Chapin et al. 2012), which can reflect the nitrogen supply capacity of soil and soil fertility (Vincent et al. 2019), and was assayed according to Li et al. (2015). The released ammonium in the solution was quantified colourimetrically at 578 nm on a spectrophotometer. S-SC activity was determined using the dinitro salicylic method, the sodium phenol colourimetric method and spectrophotometric measurements performed at 508 nm (Mendes et al. 2019). S-DHA activity detection followed the method of Stevenson (1959). Soil dehydrogenase is an enzyme that reflects the abundance of active microorganisms in the soil system and their degradation of organic matter, with a maximum absorption peak at 485 nm. S-NP activity was measured according to the p-nitrophenyl phosphate method at 660 nm, as described by Tabatabai and Bremner (1969). Determination of S-CAT activity followed the method of Alef and Nannipieri (1995). H2O2 (1 mmol) was dripped into 1 g soil, which was defined as the enzyme activity at 240 nm.
2.5 Correlation Between SMS, Soil Environment, and Plant Growth
Pearson correlation analysis was used to study the correlation between the seafood mushroom SMS concentration, soil environment, and C. fungigraminus seedling growth. One-dimensional linear regression analysis was performed to quantify the relationship between the seafood mushroom SMS content and each indicator. Then, principal component analysis (PCA) and redundancy analysis (RDA) were used to identify the main influential indicators of SMS in soil.
2.5.1 Pearson Correlation Analysis
Pearson correlation analysis (to measure the linear relationship between two or more variables with a continuous normal distribution) was carried out by correlating the seafood SMS concentration, soil environment, and C. fungigraminus seedling growth data, with coefficient values between − 1 and 1 (Eq. 1).
2.5.2 One-Dimensional Regression Analysis
In this experiment, the least squares method was used to establish a one-dimensional linear regression equation between the SMS concentration and soil environment and plant growth, in which the causal relationship between the SMS concentration, soil environment, and plant growth was revealed to predict the dependent variable from the independent variable. This was done by finding the regression coefficient of the regression equation \(\widehat{y}=a+bx\) when the residual sum of squares \(\sum {\left(y-\widehat{y}\right)}^{2}\) (\(y\): measured value; \(\widehat{y}\): predicted value) was at its minimum. \({R}^{2}\) (Eq. 2) was used as the coefficient of determination for the goodness of fit of the equation; the closer \({R}^{2}\) is to 1, the stronger the relationship between the independent and dependent variables, and the opposite is true when the number is lower.
2.5.3 Principal Component Analysis
Principal component analysis (PCA) uses the idea of dimensional reduction to convert multiple indicators into a smaller number of complementary and related composite indicators.
-
According to the results of the correlation analysis, the new variables \({p}_{1}\) and \({p}_{2}\) are linear functions of the original variables \(h\) and \(w\), and the 2 new variables are uncorrelated.
-
The actual variable \({x}_{1}-{x}_{m}\), with a total of \(n\) results collected, was standardized for the sample, and the correlation between the new variables and the derived variables can be expressed as Eq. 3:
$$\begin{array}{c}{p}_{1}={l}_{11}{x}_{1}+{l}_{12}x2+{l}_{13}{x}_{3}+\cdots +{l}_{1m}{x}_{m}\\ {p}_{3}={l}_{12}{x}_{1}+{l}_{22}x2+{l}_{23}{x}_{3}+\cdots +{l}_{2m}{x}_{m}\\ {p}_{3}={l}_{31}{x}_{1}+{l}_{32}x2+{l}_{33}{x}_{3}+\cdots +{l}_{3m}{x}_{m}\\ \cdots \cdots \cdots \cdots \cdots \cdots \cdots \cdots \cdots \cdots \cdots \cdots \cdots \cdots \cdots \cdots \cdots \\ {p}_{m}={l}_{m1}{x}_{1}+{l}_{m2}x2+{l}_{m3}{x}_{3}+\cdots +{l}_{mm}{x}_{m}\end{array}$$(3) -
The variance of principal component \(i\) is the result of the total variance being assigned to component \(i\) after redistribution across the principal scores and is numerically equal to eigenvalue \(i\) (Eq. 4).
$${S}_{{p}_{1}}=\frac{\sum_{i=1}^{m}{\left({p}_{i}-\overline{{p }_{i}}\right)}^{2}}{n-1}={\lambda }_{i}$$(4)\(\sum_{i=1}^{m}{\lambda }_{i}=m\) The number of original variables \(m\) is equal to the number of eigenvalues \(m\), and the sum of the variances of \(m\) eigenvalues is equal to the sum of \(m\) eigenvalues, which is equal to \(m\), the sum of the variances of the original variables normalized.
-
The contribution of each component is a measure of the amount of information included in the variable in terms of variance, and the variance provided by each component as a percentage of the total variance \(\left(m\right)\) is the contribution of the component (Eq. 5).
$$\frac{{\lambda }_{i}}{\sum_{i=1}^{m}{\lambda }_{i}}=\frac{{S}_{{p}_{1}}}{\sum_{i=1}^{m}{S}_{{p}_{1}}}=\frac{{\lambda }_{i}}{m}$$(5)The cumulative contribution of the top k components is
$$\sum_{i=1}^{k}\frac{{\lambda }_{i}}{\sum_{i=1}^{m}{\lambda }_{i}}=\sum_{i=1}^{k}\frac{{\lambda }_{i}}{m}$$(6) -
The components were considered principal components based on their cumulative contribution ≥ 70%.
2.5.4 Redundancy Analysis
The effects of seafood mushroom SMS in soil are ultimately directly expressed through plant growth. Redundancy analysis was used to investigate the correlation between the explanatory variables (soil physical and chemical properties, enzyme activity) and the response variables (plant growth). Then, we decomposed and filtered the indicator variables by ranking them to obtain the dominant factors and to test the significance, which complements and validates the regression analysis and PCA.
2.6 Statistical Analysis
Analysis of variance (ANOVA) was performed with the means of quintuplicate values of enzyme productivity, and Duncan’s multiple range test was employed to determine significant differences between five means with 95% or 99% least significant difference (P < 0.05 or P < 0.01) by SPSS Statistics 26 (IBM, USA). Pearson correlation analysis, principal component analysis, and redundancy analysis were performed using SPSS Statistics 26 and Canoco 5.0 (Microcomputer Power, USA). All figures were generated using GraphPad Prism 9 (GraphPad Software, USA), Adobe Photoshop 2020 (Adobe, USA) and Microsoft Office Excel 2010 (Microsoft, USA).
3 Results
3.1 Physical and Chemical Properties of Seafood Mushroom SMS Solid-State Fermentation
Solid-state fermentation, with a long history in China and largely developed for the treatment of agricultural byproducts, was explored here as a fertilizer processing technology suitable for SMS. With the duration of the solid-state fermentation of SMS, the temperature of SMS increased to 55 °C after 1 week, and the peak temperature reached 61.18 °C after the compost were turned. The fermentation ended when the compost stack temperature stabilized above 50 °C for 15 days and dropped to 35 °C for nearly one week (Fig. 1). Solid-state fermentation at 55 °C for 7–15 days is able to help kill pathogenic microorganisms and pest eggs in SMS and ensure that the compost is thoroughly decomposed. The moisture content of the seafood mushroom SMS after fermentation was 66.925 ± 0.711%. The SMS pH increased from 5.47 to 8.89 because microorganisms produced NH3 residues (Fig. 1). After fermentation, the OM, AMN, and NIN of the seafood mushroom SMS (alkalinity) increased to 33.121 ± 0.359 mg kg−1, 32.333 ± 0.412 mg kg−1, and 14.460 ± 0.230 mg kg−1, respectively, while the contents of OM, AMN, and NIN in the experimental soil before treatment were only 3.814 ± 0.518 mg kg−1, 25.632 ± 0.220 mg kg−1, and 7.282 ± 0.094 mg kg−1, respectively (Table 1).
3.2 C. fungigraminus Seedling Soil Environmental Properties and Enzyme Activity
Soil environmental properties are closely associated with soil-forming and environmentally dependent factors, such as topography, climate, and parent material (Sandor et al. 2005). After 6 weeks of treatment, the TN, TP, TK, AN, AP, AK, AMN, NIN, and OM of C. fungigraminus seedling soil were significantly different compared to those of CK soil (P < 0.05, Table 1). For instance, OM and TN in soil increased 3.7 and 3.9 times, respectively; NIN increased 55.70% and TP increased 33.28%; AN, AP, and AK increased 44.59%, 67.41%, and 45.05%, respectively (P < 0.05) in T4. However, between T4 and T5, there were no differences in the TN, TP, TK, AN, AP, AK, OM, and EC of C. fungigraminus seedling soil (P > 0.05). The soil pH of C. fungigraminus seedlings was effectively increased from 5.67 ± 0.018 to 7.89 ± 0.010 by SMS additions, with a change in the soil to be neutral or weakly alkaline, which influenced the microbes in this ecosystem by modifying enzyme activity as well as controlling the accessibility of nutrient and moisture supplements by altering the ionization balance in soil (Fierer and Jackson 2006; Ren et al. 2022). Seafood mushroom SMS solid-state fermentation is feasible as a soil pH conditioner to adjust the soil pH. Based on Table 1, with the increase in seafood mushroom SMS in soil, the EC value was gradually reduced from 0.700 ± 0.005 to 0.540 ± 0.001 mS cm−1 in each group, which prevented soil acidification, while there was no significant difference between T4 and T5 (P > 0.05) (Table 1).
Enzyme activity also affects soil fertility and the microecological environment and further affects plant growth and development (Galhardi et al. 2020; Alkorta et al. 2003). Table 2 shows that the activities of the 5 enzymes differed significantly as the seafood mushroom SMS concentration increased compared to the PGPR-only treatment (P < 0.05). Under this treatment, S-SC and S-DHA were positively correlated with soil OM. These values were highest (54.968 ± 3.818 U g−1 and 218.686 ± 14.305 U g−1) under 40% seafood mushroom SMS and PGPR microbial fertilizer. S-DHA is a typical soil enzyme that represents the activity of soil microorganisms and effectively promotes the purification of organic pollutants in soil (Boleas et al. 2005) and reached its highest value (218.686 ± 14.305 U g−1) in T4. This result also indicated that 20% seafood mushroom SMS was able to stimulate S-UE to 585.829 ± 21.691 U g−1, without showing significant differences from T3, T4, and T5 (P > 0.05). S-UE has been shown to be one of the most active hydrolases in soil and catalyzes the hydrolysis of urea in soil to produce NH3 and CO2 (Huang et al. 2012) and improve the nitrogen fertilizer utilization rate. S-CAT, which is an important enzyme of soil microbial metabolism and plays an important role in the H2O2 removal system, was not different between the 40% and 50% SMS treatments (P > 0.05). The TC, AP, and pH of soil are significantly influenced by phosphatase (Tan et al. 2014). S-NP gradually increased in each group without any difference between T3 and T4 (P > 0.05).
3.3 Growth Promotion of C. fungigraminus Seedlings
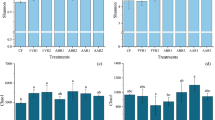
The plant height, leaf NO., leaf length, and root length of C. fungigraminus were significantly promoted by the interaction of different concentrations of SMS solid-state fermentation and PGPR microbial fertilizer (Fig. 2). Compared to CK, 20–40% seafood SMS with PGPR significantly increased plant height by 8.93–19.89% (P < 0.05) and increased leaf length by 34.72–62.86% (P < 0.05), while there was no significant change in the C. fungigraminus seedling leaf NO. (P > 0.05, Table 3). The results also showed that the root length, split root NO., and root activity reached their highest values, i.e., 1816.710 ± 179.439 cm, 18,051.000 ± 2495.566, and 33.185 ± 2.087 μg g−1 h−1, respectively, in T4. The level of root activity is directly able to affect the nutritional status and yield of the aboveground parts of the plant. However, the split root NO. and root activity of C. fungigraminus seedlings in T5 decreased by 43.96% and 26.97%, respectively, compared with those in T4 (P < 0.05). Roots are important plant organs that not only absorb water and mineral nutrients but also carry out anabolism. OM can significantly promote root growth, increase root distribution, delay the decline in root growth in the late stage, and improve root activity. In this work, the addition of 40% SMS solid-state fermentation increased the soil OM content, loosened the soil layer, and adjusted the soil structure and improved soil nutrition and aeration (Table 1, 3). Moreover, 30–50% SMS addition increased the plant total nitrogen (P-TN) by 5.76–10.00% and total phosphorus (P-TP) by 15.44–16.55% compared to CK (P < 0.05, Table 2).
Plant height, leaf length, and root length of C. fungigraminus seedlings treated with seafood mushroom spent substrate solid-state fermentation combined with plant growth-promoting rhizobacteria microbial fertilizer after 6 weeks. Note: Plant growth-promoting rhizobacteria microbial fertilizer only was used as the positive control (CK), 10% (T1), 20% (T2), 30% (T3), 40% (T4), and 50% (T5) fermented seafood mushroom spent substrate (fresh weight). Luria–Bertani culture medium only was used as the negative control (NK)
3.4 Correlation Between Seafood Mushroom SMS and the Soil Environment
The correlation between soil properties and soil-forming factors is an important issue that is being studied worldwide (Li et al. 2018; Rodríguez-Lado and Lado 2016). In this study, a strong correlation between SMS concentrations and soil environmental factors was observed, and the correlation coefficients were in the order of TK = AMN > TN > OM > AN > AK > NIN > TP > AP > pH > EC, with correlation coefficients of 0.969, 0.969, 0.968, 0.964, 0.951, 0.950, 0.935, 0.934, 0.928, 0.811, and − 0.705, respectively. The least negative correlation was found between EC and pH (correlation coefficient was − 0.356), and the strongest negative correlation was found between soil EC and SMS concentration (correlation coefficient was − 0.705) (Fig. 3 (a)). Figure 3 (b) shows that there was a highly significant correlation between the seafood mushroom SMS concentration and S-DHA, S-UE, and S-SC, with correlation coefficients of 0.852, 0.667, and 0.598, respectively; there was a significant correlation between S-DHA and S-NP, with a correlation coefficient of 0.494 (P < 0.05). In addition, there was a highly significant correlation between S-UE and S-CAT and between S-UE and S-DHA with correlation coefficients of 0.805 and 0.596 (P < 0.01), respectively; there was a significant correlation between S-UE and S-SC with a correlation coefficient of 0.499 (P < 0.05) and a significant correlation between S-SC and S-DHA with a correlation coefficient of 0.550 (P < 0.05). Figure 3 (c) shows that there was a highly significant correlation between SMS concentration and root activity and between seafood mushroom SMS concentration and leaf length, with correlation coefficients of 0.751 and 0.660 (P < 0.01), respectively.
Correlation analysis between seafood mushroom spent substrate concentration and soil physicochemical factors (a), soil enzyme activity (b), and plant growth (c) (* is P < 0.05; ** is P < 0.01). Note: Spent mushroom substrate (SMS), total nitrogen (TN), total phosphorus (TP), total potassium (TK), available nitrogen (AN), available phosphorus (AP), available potassium (AK), nitrate nitrogen (NIN), ammonium nitrogen (AMN), soil organic matter (OM), electrical conductivity (EC), soil neutral phosphatase (S-NP), soil catalase (S-CAT), soil dehydrogenase (S-DHA), soil sucrose (S-SC), soil urase (S-UE), leaf number (leaf NO.), and split root number (split root NO.)
To quantify the direction and degree of significant correlations between SMS concentrations and soil environmental status, enzyme activity, and plant growth indicators, the dependent variables (\(x\): soil environmental indicators, enzyme activity and plant growth indicators) and independent variables (\(y\): SMS concentration) were subjected to a one-dimensional linear regression, and the equations and fits (R2) were calculated (Figs. 4, 5, and 6). Figure 4 (a, b, c, d, and e) shows that the SMS concentration showed a highly positive correlation with TK, TN, AN, AMN, and OM; the SMS concentration showed a negative correlation with EC (Fig. 4 (f)). The graph of the one-dimensional linear regression between the seafood mushroom SMS concentration and soil enzyme activity is shown in Fig. 5, indicating that the seafood mushroom SMS concentration was highly positively correlated with S-DHA (R2 = 0.7255) (Fig. 5 (a)) and generally positively correlated with other enzyme activities (Fig. 5 (b, c, d, and e)). Figure 6 (a) displays a generally positive correlation between the seafood mushroom SMS concentration and root activity (R2 = 0.5644) with a consistent direction of influence.
One-dimensional linear regression analysis between the seafood mushroom spent substrate concentration and TK (a), TN (b), AN (c), AMN (d), OM (e), and EC (f). Note: Spent mushroom substrate (SMS), the soil of total nitrogen (TN), total potassium (TK), available nitrogen (AN), ammonium nitrogen (AMN), organic matter (OM), and electrical conductivity (EC)
3.5 Principal Component Analysis
Principal component analysis (PCA) was carried out on soil physical and chemical properties, soil enzyme activity, and plant growth indicators. When the Kaiser–Meyer–Olkin (KMO) value > 0.5 and Bartlett’s spherical test significance < 0.001, the covariance between the factors is demonstrated, and it follows that they could be validly presented and suitable for PCA. When the extraction of factors exceeds 0.8, the common factors have good explanatory power. Moreover, the common factors with eigenvalues greater than 1 and cumulative variance higher than 70% were selected as principal components. The number of principal components that explained the original data was obtained.
3.5.1 PCA of Soil Physical and Chemical Characteristics
The PCA of the soil physical and chemical characteristics, with a KMO value of 0.711, i.e., > 0.5, and Bartlett’s spherical test significance level less than 0.001, indicated that these factors could be effectively presented as a common factor for PCA. Table S1 shows the variance for each factor, and the extraction degrees of TN, TP, TK, AN, AP, AK, OM, AMN, and NIN exceeded 0.8, which indicated that these factors were able to explain these nine indicators better than others; the extraction degree of EC’s common factor was only 0.476, indicating a poor explanation. The total variance explained for the soil physical and chemical factors is shown in Table 4, with an overall explanation of 90.735% from 11 factors, indicating a good explanation. The component matrix analysis showed that positive factors (except for the EC value) were associated with principal component 1 (Table S4). The principal component eigenvectors (Y1) were calculated from the composition matrix and were derived as follows (Table S5):
3.5.2 PCA of Soil Enzyme Activity
The PCA of soil enzyme activity had a KMO value of 0.510, i.e., > 0.5, and Bartlett’s spherical test significance < 0.001, which indicated that soil enzyme activity was suitable for PCA. The extraction values of S-CAT and S-UE exceeded 0.8 (Table S2); the extraction value of S-NP was only 0.485, indicating a poor explanation. Two principal components were extracted from the five enzymes, with variances of 53.421% and 24.541% (Table 5). The two principal components explained 77.961% of the original data variance. The composition matrices and eigenvectors for enzyme activity are shown in Tables S6 and S7. S-UE, S-DHA, and S-SC were the main factors in principal component 1, while S-CAT and S-NP were the main factors in principal component 2. The loadings of S-UE and S-CAT in principal component 2 were both negatively correlated with seafood SMS. Based on the eigenvectors, the expressions for principal components Y2 and Y3 were as follows:
3.5.3 PCA of Growth Indicators for C. fungigraminus Seedlings
The PCA of the growth indicators for C. fungigraminus showed a KMO value of 0.410, i.e., < 0.5, and Bartlett’s test of sphericity significance was 0.001, indicating that there was no collinearity between the plant growth indicators in the principal component analysis. The total variance of each plant growth indicator is presented in Table 6, and the common factor variance is shown in Table S3. The composition matrix and the eigenvectors are shown in Tables S8 and S9. Based on the eigenvectors, the expressions for principal components Y4 and Y5 were as follows:
3.6 Redundancy Analysis
Soil composition and soil enzyme activity can significantly affect plant growth. The growth of C. fungigraminus seedlings and the soil environment were used as response variables and explanatory variables for redundancy analysis (RAD) in Canoco 5. In Fig. 7 (a), RDA1 mainly reflected the changes in pH, AN, AMN, OM, and TN; RDA2 mainly reflected the changes in EC. pH primarily affected the aboveground plant parts. In T4 and T5, there was a closer correlation with AN, AMN, OM, and TN and a stronger effect on plant height and root activity. According to Fig. 7 (b), RDA1 mainly reflected the changes in S-UE, S-CAT, S-DHA, and S-SC, while RDA2 mainly reflected the change in S-NP. All soil enzyme activities were positively correlated with C. fungigraminus seedling growth indicators except for root length and split root NO. The results suggested that increasing SMS concentrations in soil were able to alter the growth of aboveground plant parts by affecting soil enzyme activity and root activity.
a RDA of plant growth and soil environmental factors; b RDA of plant growth and soil enzyme activities. Note: Blue arrows represent plant growth, while red arrows represent soil physical and chemical properties and enzyme activities. The arrow length indicates the contribution of each indicator; the longer the length of the arrow, the greater the contribution to the model and vice versa. The angle between the red and blue arrows indicates the correlation; the smaller the angle, the stronger the correlation. The larger the vertical projection of the red arrow on the blue arrow, the greater the influence on the plant growth indicators. The soil of total nitrogen (TN), available nitrogen (AN), ammonium nitrogen (AMN), soil organic matter (OM), electrical conductivity (EC). The soil neutral phosphatase (S-NP), soil catalase (S-CAT), soil dehydrogenase (S-DHA), soil sucrose (S-SC), soil urase (S-UE). The plant leaf number (leaf NO.) and split root number (split root NO.)
4 Discussion
It was estimated that approximately 64 million tons of SMS were produced worldwide in 2018, which is forecast to rise to more than 100 million tons by 2026. (Martín et al. 2023). Careless discarding or burning SMS may cause environmental pollution, so farmland consumption is one of the important ways to utilize agricultural wastes (such as SMS) as resources. SMS contains a high amount of organic matter and a microbial community, with high nitrogen nutrient levels, high cation exchange capacity and slow mineralization, making SMS efficient for plant growth as an organic fertilizer, soil conditioner, feed, plant-disease controller, source of enzymes, and bioactive compounds and replacing metallurgical coke as a reducing agent for electric-arc furnace dust (Chang et al. 2022; Ishihara et al. 2018; Martín et al. 2023; Zhou et al. 2019). Our work examined whether soil supplemented with fermented seafood mushroom SMS combined with PGPR microbial fertilizer was able to enhance C. fungigraminus seedling root metabolism and respiration by improving soil AMN, TN, OM, pH, and enzyme activity while decreasing soil EC, thereby improving the absorption of plant nutrients, root activity, and growth. The results showed that the seafood mushroom SMS solid fertilizer was weakly alkaline. The 10–50% addition increased the soil pH from 7.32 ± 0.020 to 7.89 ± 0.010 and reduced the EC value from 0.770 ± 0.023 to 0.540 ± 0.010 mS cm−1 (Table 1). According to Pearson correlation analysis, SMS can be used to significantly affect soil pH (correlation coefficient was 0.811) and optimize the soil structure (EC correlation coefficient was − 0.755). Abnormal soil pH can accelerate the activation of harmful heavy metals, reduce the number of beneficial microorganisms and enzyme activity, aggravate soil hardening and soil degradation, and pose a potential threat to agricultural production, the ecological environment and human health (Chen et al. 2021a, b; Naz et al. 2022; Niu et al. 2023; Souza and Billings 2022). Adding SMS can regulate soil acidity and improve soil environment. EC is the ability to transmit electric current in soil, which is closely correlated with the soluble salt concentration. Soluble salt in soil is a strong electrolyte that displays strong conductivity after dissolution. The EC level usually reflects the salt content in the soil, which can be determined by the soluble salt concentration (Mirzakhaninafchi et al. 2022). Higher soil EC will reduce plant yield and quality degradation, cause permanent damage to the leaves, and even cause plant death. The addition of seafood mushroom SMS (≤ 50%) effectively reduced the EC value and made it stable and suitable for C. fungigraminus seedlings. Our study demonstrated that adding a certain proportion of fermented seafood mushroom SMS was able to significantly improve soil pH and permeability.
Nitrogen is the most abundant element in the Earth’s atmosphere and is important for plant growth, being a key factor affecting soil fertility and participating in the ecosystem cycle. Soil inorganic nitrogen mainly exists in the form of ammonium and nitrate, which can be directly used by plants. Based on our work, the seafood mushroom SMS concentration had the most significant impact on AMN in soil. It significantly affected the contents of AMN, TN, and OM in T4 (Table 1, Fig. 3 (a)). Moreover, after 6 weeks, TN, TP, TK, AN, AP, AK, AMN, AIN, and OM in soil nearly reached levels as high as those of seafood SMS after soil fertilization (Table 1). Soil OM can also make the soil loose, form a granular structure, improve the physical properties, provide nutrients, water, and fertilizer and act as a buffer for crops. Many studies have shown that there is a significant positive correlation between the soil OM content and crop yield, which is an important indicator of soil fertility (Oldfield et al. 2018; Sáez-Sandino et al. 2023). Compared to inorganic fertilizer application, organic fertilizer application was able to significantly improve the content and effectiveness of soil OM (Ladha et al. 2011; Ghisman et al. 2023). According to Dong et al. (2022), different application rates of biochar and N fertilizer application increased SOC by 20–58% and total N content by 9.3–15% and had a varied effect on the activity of enzymes associated with soil carbon, N, and phosphorus cycling. On the other hand, excessive N toxicity in plants engenders several harmful mechanisms such as the production of high contents of nitrate (NO3−) and reactive oxygen species (ROS), including hydrogen peroxide (H2O2) and superoxide (O2−), which may cause nitrogen leaching and runoff loss, negatively influencing the soil environmental quality and threatening human health in agricultural production systems (Belal et al. 2023; Juan et al. 2021; Zhang et al. 2023). Our work indicated that SMS is rich of OM and TN, and excessive SMS can reduce plant tillering and root vitality. Therefore, SMS discarded or applied arbitrarily should be noted in actual production, otherwise it may cause adverse consequences.
Soil enzymes are referred to as “biocatalysts” and participate in biochemical reactions in the soil; they are the most sensitive soil fertility indicators responding to agricultural management and tillage methods (Bandick and Dick 1999). Fertilization is an important factor that causes complex and interrelated changes in soil-microbial enzyme activity and crops and establishes a new ecological balance (Chen et al. 2021a, b). Based on our work, as the SMS content increased to 40%, the S-NP, S-CAT, S-SC, and S-UE activities increased by 40.47%, 31.95%, 153.67%, and 194.40%, respectively. Moreover, Pearson correlation analysis showed that fundamental alterations in soil enzyme activity (S-DHA, S-UE, and S-SC) depended on SMS concentrations, with correlation cofficients of 0.598 to 0.852 (Fig. 3 (b)). S-DHA activity correlated most positively with the seafood mushroom content (R2 = 0.7255) (Fig. 5 (a)). Soil hydrolytic enzymes (S-UE, S-CAT, and S-SC) have been shown to mediate soil OM decomposition, transformation, and mineralization and to play a pivotal role in decomposition and nutrient cycling (Chen et al. 2017). The soil pH, soil AP content, and activities of S-UE, S-CAT, and alkaline phosphatase were the main factors affecting the soil bacterial community richness, soil nutrient contents, and enzyme activities (Sun et al. 2020a, b). Soil enzyme activity is a suitable bioindicator or parameter for monitoring soil pollution due to its sensitivity in quickly responding to any soil disturbance and has been predicted to regulate the soil rhizosphere environment as well as promote root activity (Maphuhla et al. 2020; Tian et al. 2023).
According to the obtained results, the seafood mushroom SMS and PGPR were able to significantly promote C. fungigraminus seedling height, leaf length, and root activity in T5 (P < 0.05). Between T4 and T5, the aboveground part of the plant (plant height and leaf length) did not display any difference (P > 0.05), while the belowground part (root number and root activity) of the C. fungigraminus seedlings was inhibited by 50% SMS addition (P < 0.05). The inoculation of rhizobia without fertilizer treatment has been shown to promote the average yield of soybean nitrogen fixation by 127 kg hm−2. When 200 kg hm−2 of nitrogen fertilizer was applied, the biological nitrogen fixation of soybean was inhibited, resulting in yield reduction and resource waste (Hungria et al. 2006). Integrated fertilization generally results in higher soil nutrient contents, soil fertility, and crop yields. Beneficial rhizosphere plant-microbial interactions under N fertilization suggest that rational fertilization is critical to promote beneficial rhizosphere interactions for sustainable agricultural production (Kang et al. 2022). Our results shown that the appropriate addition of seafood mushroom SMS resulted in a positive correlation between root activity, soil enzyme activities, and soil fertility (Table 1, 2, 3, Figs. 7, 8).
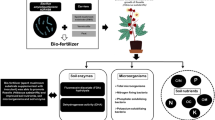
Flowchart of this study. Forty percent seafood mushroom spent substrate plus plant growth-promoting rhizobacteria fertilizer significantly promoted soil fertility, plant growth, and root activity. An excessive abundance of spent mushroom substrate negatively affected plant root length, split root NO., and root activity. Seafood mushroom spent substrate can absolutely be used as a solid microbial fertilizer to promote sustainable agriculture of “fungus-fertilizer-plant.” Note: Spent mushroom substrate (SMS), plant growth-promoting rhizobacteria (PGPR), plant total nitrogen (P-TN), plant total phosphorus (P-TP), Leaf number (leaf NO.), split root number (split root NO.), total nitrogen (TN), total potassium (TK), available nitrogen (AN), ammonium nitrogen (AMN), electrical conductivity (EC), soil neutral phosphatase (S-NP), soil catalase (S-CAT), soil dehydrogenase (S-DHA), soil sucrose (S-SC), and soil urase (S-UE)
5 Conclusion
Our research showed that the optional conditions of seafood mushroom spent substrate and plant growth-promoting rhizobacteria promoted the growth of C. fungigraminus seedlings to a better extent than rhizobacteria only as microbial fertilizer, which provides necessary theoretical support for maintaining the soil environment and nutrition for plant cultivation. The development of spent mushroom substrate as a microbial fertilizer has realized the sustainable cycle of “fungus-fertilizer-plant,” which has significant scientific guidance for the recyclic utilization of agricultural wastes.
Data availability
Data will be made available upon request.
References
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic Press, New York
Ali A, Ghani MI, Ding HY, Iqbal M, Cheng ZH, Cai ZC (2020) Garlic substrate induces cucumber growth development and decreases fusarium wilt through regulation of soil microbial community structure and diversity in replanted disturbed soil. Int J Mol Sci 21:6008–6030. https://doi.org/10.3390/ijms21176008
Alkorta I, Aizpurua A, Riga P, Albizu I, Amézaga I, Garbisu C (2003) Soil enzyme activities as biological indicators of soil health. Rev Environ Health 18:65–73. https://doi.org/10.1515/reveh.2003.18.1.65
Bandick AK, Dick RP (1999) Field management effects on soil enzyme activities. Soil Biol Biochem 31:1471–1479. https://doi.org/10.1016/S0038-0717(99)00051-6
Bao SD (2000) Soil agrochemical analysis. China Agriculture Press, Peking
Belal HEE, Abdelpary MAM, Desoky EM, Ali EF, Al Kashgry NAT, Rady MM, Semida WM, Mahmoud AEM, Sayed AAS (2023) Effect of eco-friendly application of bee honey solution on yield, physio-chemical, antioxidants, and enzyme gene expressions in excessive nitrogen-stressed common bean (Phaseolus vulgaris L.) plants. Plants (Basel, Switzerland) 12: 3435. https://doi.org/10.3390/plants12193435
Boleas S, Alonso C, Pro J, Fernández C, Carbonell G, Tarazona JV (2005) Toxicity of the antimicrobial oxytetracycline to soil organisms in a multi-species-soil system (MS.3) and influence of manure co-addition. J Hazard Mater 122:233–241. https://doi.org/10.1016/j.jhazmat.2005.03.003
Chang HH, Chen IG, Yu HY, Tsai MY, Wu KT, Liu SH (2022) Spent mushroom substrate and electric arc furnace dust recycling by carbothermic reduction method. Materials (basel) 15:2639. https://doi.org/10.3390/ma15072639
Chapin FS, Matson PA, Vitousek PM (2012) Principles of terrestrial ecosystem ecology. Springer, New York
Chaudhary T, Gera R, Shukla P (2020) Deciphering the potential of Rhizobium pusense MB-17a, a plant growth-promoting root endophyte, and functional annotation of the genes involved in the metabolic pathway. Front Bioeng Biotech 8:617034. https://doi.org/10.3389/fbioe.2020.617034
Chen BB, Jiao S, Luo SW, Ma BB, Qi W, Cao CD, Zhao ZG, Du GZ, Ma XJ (2021a) High soil pH enhances the network interactions among bacterial and archaeal microbiota in alpine grasslands of the Tibetan Plateau. Environ Microbiol 23:464–477. https://doi.org/10.1111/1462-2920.15333
Chen J, Luo YQ, Li JW, Zhou XH, Cao JJ, Wang RW, Wang YQ, Shelton S, Jin Z, Walker LM, Feng ZZ, Niu SL, Feng WT, Jian SY, Zhou LY (2017) Costimulation of soil glycosidase activity and soil respiration by nitrogen addition. Glob Chang Biol 23:1328–1337. https://doi.org/10.1111/gcb.13402
Chen QQ, Liu B, Che JM, Liu GH, Shi H, Wang JP, Zhong LL (2022) Succession of bacterial community function in a continuous composting system with spent mushroom substrate and sawdust as bulking agents. J Soil Sci Plant Nut 22:4587–4597. https://doi.org/10.1007/s42729-022-00928-3
Chen WJ, Guo XD, Guo QE, Tan XL, Wang ZG (2021b) Long-term Chili monoculture alters environmental variables affecting the dominant microbial community in Rhizosphere soil. Front Microbiol 12:681953. https://doi.org/10.3389/fmicb.2021.681953
Conklin AR (2014) Introduction to soil chemistry: analysis and instrumentation. Soil Sci Soc Am J 78:1828. https://doi.org/10.2136/sssaj2014.0002br
Corey RB (1973) A textbook of soil chemical analysis. Soil Sci Soc Am J 37:iv–iv. https://doi.org/10.2136/sssai1973.03615995003700020003x
Dong ZJ, Li HB, Xiao JN, Sun JL, Liu RL, Zhang AP (2022) Soil multifunctionality of paddy field is explained by soil pH rather than microbial diversity after 8-years of repeated applications of biochar and nitrogen fertilizer. Sci Total Environ 853: 158620. https://doi.org/10.1016/j.scitotenv.2022.158620
Du BH, Xuan HN, Geng LL, Li WH, Zhang J, Xiang WS, Liu RM, Shu CL (2022) Microflora for improving the Auricularia auricula spent mushroom substrate for Protaetia brevitarsis production. iScience 25: 105307. https://doi.org/10.1016/j.isci.2022.105307
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. PNAS 103:626–631. https://doi.org/10.1073/pnas.0507535103
Galhardi JA, Fraceto LF, Wilkinson KJ, Ghoshal S (2020) Soil enzyme activities as an integral part of the environmental risk assessment of nanopesticides. J Agric Food Chem 68:8514–8516. https://doi.org/10.1021/acs.jafc.0c04344
Ghisman V, Georgescu PL, Ghisman G, Buruiana DL (2023) A new composite material with environmental implications for sustainable agriculture. Materials 16:6440. https://doi.org/10.3390/ma16196440
Gong M, Huang TY, Li Y, Li JX, Tang LH, Su EZ, Zou G, Bao DP (2022) Multi-Omics analysis of low-temperature fruiting highlights the promising cultivation application of the nutrient accumulation in Hypsizygus marmoreus. J Fungi (Basel) 8:695. https://doi.org/10.3390/jof8070695
Huang L, Gao X, Liu M, Du G, Guo JS, Ntakirutimana T (2012) Correlation among soil microorganisms, soil enzyme activities, and removal rates of pollutants in three constructed wetlands purifying micro-polluted river water. Ecol Eng 46:98–106. https://doi.org/10.1016/J.ECOLENG.2012.06.004
Hungria M, Franchini JC, Campo RJ, Crispino CC, Moraes JZ, Sibaldelli RNR, Mendes IC, Arihara J (2006) Nitrogen nutrition of soybean in Brazil: contributions of biological N2 fixation and N fertilizer to grain yield. Can J Plant Sci 86:927–939. https://doi.org/10.4141/P05-098
Ishihara A, Goto N, Kikkawa M, Ube N, Ushijima S, Ueno M, Ueno K, Osaki-Oka K (2018) Identification of antifungal compounds in the spent mushroom substrate of Lentinula edodes. J Pestic Sci 43:108–113. https://doi.org/10.1584/jpestics.D17-094
Jasińska A (2018) Spent mushroom compost (SMC) - retrieved added value product closing loop in agricultural production. Acta Agraria Debreceniensis 185–202. https://doi.org/10.34101/actaagrar/150/1715
Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E (2021) The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, Lipids and Proteins) and induced pathologies. Int J Mol Sci 22:4642. https://doi.org/10.3390/ijms22094642
Kang A, Zhang N, Xun WB, Dong XY, Xiao M, Liu ZH, Xu ZH, Feng HC, Zou JW, Shen QR, Zhang RF (2022) Nitrogen fertilization modulates beneficial rhizosphere interactions through signaling effect of nitric oxide. Plant Physiol 188:1129–1140. https://doi.org/10.1093/plphys/kiab555
Kwiatkowski CA, Harasim E (2021) The effect of fertilization with spent mushroom substrate and traditional methods of fertilization of common thyme (Thymus vulgaris L.) on yield quality and antioxidant properties of herbal material. Agronomy 11:329. https://doi.org/10.3390/agronomy11020329
Ladha JK, Reddyb CK, Padrea AT, Kessel C (2011) Role of nitrogen fertilization in sustaining organic matter in cultivated soils. J Environ Qual 40:1756–1766. https://doi.org/10.2134/jeq2011.0064
Li HJ, Yoshida S, Mitani N, Egusa M, Takagi M, Izawa H, Matsumoto T, Kaminaka H, Ifuku S (2022) Disease resistance and growth promotion activities of chitin/cellulose nanofiber from spent mushroom substrate to plant. Carbohydr Polym 284:119233. https://doi.org/10.1016/j.carbpol.2022.119233
Li J, Zhou BX, Li TT, Lin H, Lin ZX, Lu GD, Liu YL, Lin BS, Lin DM (2023) Isolation of rhizobacteria from the Cenchrus fungigraminus rhizosphere and characterization of their nitrogen-fixing performance and potential role in plant growth promotion. Plant Soil 486:1–17. https://doi.org/10.1007/s11104-023-06028-0
Li X, Mccarty GW, Karlen DL, Cambardella CA (2018) Topographic metric predictions of soil redistribution and organic carbon in iowa cropland fields. CATENA 160:222–232. https://doi.org/10.1016/j.catena.2017.09.026
Li ZP, Wu FZ, Yang WQ, Xu ZF, Gou XL, Xiong L, Yin R, Huang L (2015) Soil invertase and urease activities at different periods in subalpine forest gap in western Sichuan. Acta Ecol Sin 35:3919–3925. https://doi.org/10.5846/STXB201308302177
Lin BS, Chen Y, Zheng SZ, Jia YL, Wang LF, Lu GD, Lin ZX (2019) Construction of nitrogen-fixing Klebsiella variicola GN02 expression vector pET28a-Lac-EGFP and its colonization of Pennisetum sinense Roxb roots. Biotechnol Biotec 33:1074–1084. https://doi.org/10.1080/13102818.2019.1638301
Lin ZX, Lin DM, Liu ZJ, Lan SR (2022) Cenchrus fungigraminus Z. X. Lin & D. M. Lin & S. R. Lan sp. nov., a new species of Panicoideae (Poaceae): evidence from morphological, nuclear and plastid genome data. Journal of Forest and Environment 42: 514–520. https://doi.org/10.13324/j.cnki.jfcf.2022.05.009
Liu CK, Wang X, Tu BJ, Li YS, Chen H, Zhang QY, Liu XB (2021) Root k affinity drivers and photosynthetic characteristics in response to low potassium stress in k high-efficiency vegetable soybean. Front Plant Sci 12:732164. https://doi.org/10.3389/fpls.2021.732164
Liu YL, Zhao C, Lin DM, Lin H, Lin ZX (2015) Effect of water extract from spent mushroom substrate after Ganoderma balabacense cultivation by using JUNCAO technique on production performance and hematology parameters of dairy cows. Anim Sci J 86:855–862. https://doi.org/10.1111/asj.12371
Maphuhla NG, Lewu FB, Oyedeji OO (2020) The effects of physicochemical parameters on analysed soil enzyme activity from alice landfill site. Int J Env Res Pub He 18:221. https://doi.org/10.3390/ijerph18010221
Martín C, Zervakis GI, Xiong SJ, Koutrotsios G, Strætkvern KO (2023) Spent substrate from mushroom cultivation: exploitation potential toward various applications and value-added products. Bioengineered 14:2252138. https://doi.org/10.1080/21655979.2023.2252138
Mendes MS, Latawiec AE, Sansevero JBB, Crouzeilles R, Moraes LFD, Castro A, Alves-Pinto HN, Brancalion PHS, Rodrigues RR, Chazdon RL, Barros FSM, Santos J, Iribarrem A, Mata S, Lemgruber L, Rodrigues A, Korys K, Strassburg BBN (2019) Look down-there is a gap-the need to include soil data in Atlantic Forest restoration. Restor Ecol 27:361–370. https://doi.org/10.1111/rec.12875
Mirzakhaninafchi H, Mani I, Hasan M, Nafchi AM, Parray RA, Kumar D (2022) Development of prediction models for soil nitrogen management based on electrical conductivity and moisture content. Sensors (basel) 22:6728. https://doi.org/10.3390/s22186728
Motsara MR, Roy RN (2008) Guide to laboratory establishment for plant nutrient analysis. Food and Agriculture Organization of the United Nations, Rome
Naz M, Dai Z, Hussain S, Tariq M, Danish S, Khan IU, Qi S, Du D (2022) The soil pH and heavy metals revealed their impact on soil microbial community. J Environ Manage 321:115770. https://doi.org/10.1016/j.jenvman.2022.115770
Niu B, Lei T, Chen Q, Shao M, Yang X, Jiao H, Yang Y, Guggenberger G, Zhang G (2023) pH: a core node of interaction networks among soil organo-mineral fractions. Environ Int 178:108058. https://doi.org/10.1016/j.envint.2023.108058
Oldfield EE, Wood SA, Bradford MA (2018) Direct effects of soil organic matter on productivity mirror those observed with organic amendments. Plant Soil 423:363–373. https://doi.org/10.1007/s11104-017-3513-5
Oliveira RS, Biscaia SMP, Bellan DL, Viana SRF, Di-Medeiros Leal MC, Vasconcelos AFD, Lião LM, Trindade ES, Carbonero ER (2019) Structure elucidation of a bioactive fucomannogalactan from the edible mushroom Hypsizygus marmoreus. Carbohydr Polym 225:115203. https://doi.org/10.1016/j.carbpol.2019.115203
Owaid MN, Abed IA, Al-Saeedi S (2017) Applicable properties of the bio-fertilizer spent mushroom substrate in organic systems as a byproduct from the cultivation of Pleurotus spp. Inform Process Agr 4:78–82. https://doi.org/10.1016/j.inpa.2017.01.001
Parajuli P, Duffy S (2013) Evaluation of soil organic carbon and soil moisture content from agricultural fields in Mississippi. Open J Soil Sci 3:81–90. https://doi.org/10.4236/ojss.2013.32009
Pardo-Giménez A, Catalán L, Carrasco J, Álvarez-Ortí M, Zied D, Pardo J (2016) Effect of supplementing crop substrate with defatted pistachio meal on Agaricus bisporus and Pleurotus ostreatus production. J Sci Food Agr 96:3838–3845. https://doi.org/10.1002/jsfa.7579
Phithakrotchanakoon C, Mayteeworakoon S, Siriarchawatana P, Kitikhun S, Harnpicharnchai P, Wansom S, Eurwilaichitr L, Ingsriswang S (2022) Beneficial bacterial-Auricularia cornea interactions fostering growth enhancement identified from microbiota present in spent mushroom substrate. Front Microbiol 13:1006446. https://doi.org/10.3389/fmicb.2022.1006446
Poudel P, Duenas AEK, Di Gioia F (2023) Organic waste compost and spent mushroom compost as potential growing media components for the sustainable production of microgreens. Front Plant Sci 14:1229157. https://doi.org/10.3389/fpls.2023.1229157
Prabu M, Jeyanthi C, Kumuthakalavalli R (2014) Spent mushroom substrate: an enriched organic manure for improving the yield of Vigna unguiculata (L.) Walp. (Cowpea) leguminous crop. SIRJ-APBBP 1: 7–14. https://www.researchgate.net/publication/225088933
Ren YL, Dong CB, Shao QY, Zhang ZY, Liang ZQ, Han YF (2022) Application of redundancy analysis in microbial ecology. J Mount Agric Biol 41: 41–48. https://doi.org/10.15958/j.cnki.sdnyswxb.2022.01.006
Rodríguez-Lado L, Lado M (2016) Relation between soil forming factors and scaling properties of particle size distributions derived from multifractal analysis in topsoils from Galicia (NW spain). Geoderma 287:147–156. https://doi.org/10.1016/j.geoderma.2016.08.005
Sáez-Sandino T, Delgado-Baquerizo M, Egidi E, Singh BK (2023) New microbial tools to boost restoration and soil organic matter. Microb Biotechnol 16:2019–2025. https://doi.org/10.1111/1751-7915.14325
Sandor J, Burras CL, Thompson M (2005) Factors of soil formation: human impacts. Encyclopedia Soils in the Environ 520–532. https://doi.org/10.1016/B0-12-348530-4/00032-1
Seekram P, Thammasittirong A, Thammasittirong SN (2021) Evaluation of spent mushroom substrate after cultivation of Pleurotus ostreatus as a new raw material for xylooligosaccharides production using crude xylanases from Aspergillus flavus KUB2. 3 Biotech 11: 176. https://doi.org/10.1007/s13205-021-02725-8
Shi YJ, Wang ZY, Wang YR (2020) Optimizing the amount of pig manure in the vermicomposting of spent mushroom (Lentinula) substrate. PeerJ 8:e10584. https://doi.org/10.7717/peerj.10584
Song QB, Fu HD, Shi QW, Shan X, Wang Z, Sun ZP, Li TH (2022) Overfertilization reduces tomato yield under long-term continuous cropping system via regulation of soil microbial community composition. Front Microbiol 13:952021. https://doi.org/10.3389/fmicb.2022.952021
Souza LFT, Billings SA (2022) Temperature and pH mediate stoichiometric constraints of organically derived soil nutrients. Glob Chang Biol 28:1630–1642. https://doi.org/10.1111/gcb.15985
Stevenson IL (1959) Dehydrogenase activity in soils. Can J Microbiol 5:229–235. https://doi.org/10.1139/m59-026
Su F, Li ZA, Li YW, Xu L, Li YX, Li SY, Chen HF, Zhuang P, Wang FG (2019) Removal of total nitrogen and phosphorus using single or combinations of aquatic plants. Int J Env Res Pub He 16:4663. https://doi.org/10.3390/ijerph16234663
Sun JH, Yang L, Wei J, Quan JN, Yang XT (2020a) The responses of soil bacterial communities and enzyme activities to the edaphic properties of coal mining areas in Central China. PLoS ONE 15:e0231198. https://doi.org/10.1371/journal.pone.0231198
Sun SJ, Li F, Xu X, Liu YC, Kong XQ, Chen JQ, Liu T, Chen LD (2020b) Study on the community structure and function of symbiotic bacteria from different growth and developmental stages of Hypsizygus marmoreus. BMC Microbiol 20:311. https://doi.org/10.1186/s12866-020-01998-y
Tabatabai MA, Bremner JG (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Boil Biochem 1:301–307. https://doi.org/10.1016/0038-0717(69)90012-1
Tan XP, Xie BN, Wang JX, He WX, Wang XD, Wei GH (2014) County-scale spatial distribution of soil enzyme activities and enzyme activity indices in agricultural land: implications for soil quality assessment. Scientific World J 535768. https://doi.org/10.1155/2014/535768
Tso KH, Lumsangkul C, Ju JC, Fan YK, Chiang HI (2021) The potential of peroxidases extracted from the spent mushroom (Flammulina velutipes) substrate significantly degrade mycotoxin deoxynivalenol. Toxins 13:72. https://doi.org/10.3390/toxins13010072
Tian L, An M, Wu M, Liu F, Zhang Y (2023) Habitat ecological characteristics and soil fungal community structure of Paphiopedilum subgenus Brachypetalum Hallier (Orchidaceae) plants in Southwest China. Plant Signal Behav 18:2227365. https://doi.org/10.1080/15592324.2023.2227365
Velusami B, Jordan SN, Curran T, Grogan H (2021) Fertiliser characteristics of stored spent mushroom substrate as a sustainable source of nutrients and organic matter for tillage, grassland and agricultural soils. Irish J Agr Food Res 60: 1–11. https://doi.org/10.15212/ijafr-2020-0121
Vincent Q, Chartin C, Krüger I, Van Wesemael B, Carnol M (2019) CARBIOSOL: Biological indicators of soil quality and organic carbon in grasslands and croplands in Wallonia. Belgium Ecology 100:e02843. https://doi.org/10.1002/ecy.2843
Ye D, Hu Q, Bai X, Zhang W, Guo H (2023) Increasing the value of Phragmites australis straw in a sustainable integrated agriculture model (SIAM) comprising edible mushroom cultivation and spent mushroom substrate compost. Sci Total Environ 869:161807. https://doi.org/10.1016/j.scitotenv.2023.161807
Zang HL, Liu WJ, Cheng Y, Wang HL, An XJ, Sun SS, Wang Y, Hou N, Cui CY, Li CY (2020) Bioremediation of historically chlorimuron-ethyl-contaminated soil by co-culture chlorimuron-ethyl-degrading bacteria combined with the spent mushroom substrate. Microorganisms 8:369. https://doi.org/10.3390/microorganisms8030369
Zhang JT, Tian HQ, Shi H, Zhang JF, Wang XK, Pan SF, Yang J (2020) Increased greenhouse gas emissions intensity of major croplands in China: Implications for food security and climate change mitigation. Glob Chang Biol 26:6116–6133. https://doi.org/10.1111/gcb.15290
Zhang W, Ni K, Long L, Ruan J (2023) Nitrogen transport and assimilation in tea plant (Camellia sinensis): a review. Front Plant Sci 14:1249202. https://doi.org/10.3389/fpls.2023.1249202
Zheng HK, Wang BY, Hua XT, Gao RT, Wang YH, Zhang ZX, Zhang YX, Mei J, Huang YJ, Huang YM, Lin H, Zhang XT, Lin DM, Lan SR, Liu ZJ, Lu GD, Wang ZH, Ming R, Zhang JS, Lin ZX (2023) A near-complete genome assembly of the allotetrapolyploid Cenchrus fungigraminus (JUJUNCAO) provides insights into its evolution and C4 photosynthesis. Plant Commun 3:100633. https://doi.org/10.1016/j.xplc.2023.100633
Zhou H, Fang H, Zhang Q, Wang Q, Chen C, Mooney SJ, Peng X, Du Z (2019) Biochar enhances soil hydraulic function but not soil aggregation in a sandy loam. Eur J Soil Sci 70:291–300. https://doi.org/10.1111/ejss.12732
Acknowledgements
We are grateful to Shunchang Juncao Science and Technology Backyard and Fujian Nanping Shunchang Xinjundu Mushroom Industry Development Co., Ltd., for their help during the seafood mushroom SMS collection. We thank Bingxin Zhou, Ling Man, Shimei Peng, and Hui Lin from the National Engineering Research Center of Juncao Technology for their help during the pretreatment of the experimental samples.
Funding
This study was supported by the Special Project for the Protection and Utilization of Agricultural Resources of the Department of Agriculture and Rural Affairs of Fujian Province, “Research and Application of Key Technologies for Innovation and Industrialized Utilization of Juncao and Juncao Maushroom” (22001XA), and Fujian Agricultural and Forestry University’s Top Priority Ecological Discipline Construction Funds (722022003).
Author information
Authors and Affiliations
Contributions
Jing Li, Dongmei Lin, and Zhanxi Lin conceived and designed the experiments; Jing Li, Yufang Lei, Zheng Qin, and Junyuan Liu performed the experiments, analyzed the data, prepared the figures, and wrote and revised the draft. Christopher Rensing revised and edited the manuscript. All authors edited, reviewed, and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, J., Lei, Y., Qin, Z. et al. Effects of Seafood Mushroom Spent Substrate Solid-State Fermentation Combined with PGPR as a Microbial Fertilizer on the Soil Environment and Growth Promotion of Cenchrus fungigraminus. J Soil Sci Plant Nutr 24, 1261–1277 (2024). https://doi.org/10.1007/s42729-024-01627-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-024-01627-x