Abstract
Naturally, under field conditions, plants are regularly experienced by a mixture of two or more stress factors. Drought is a major abiotic stress, and fungal pathogens characterize a main biotic stress challenge faced by plants and impact negatively on plant development and productivity. We propose that foliar application of nitric oxide (NO) donors can have positive effects on the induction of tolerance to biotic and abiotic stress on groundnut plants. This investigation was carried out to study the changes in growth, some biochemical aspects, and yield and quality of groundnut plants as well as induction of resistance to Cercospora leaf spot disease in response to nitric oxide (NO) donors, sodium nitroprusside (SNP), and arginine (Arg) (2.5, 5.0, and 7.5 mM) under two water irrigation levels 100% and 75% of water irrigation requirements (WIR), in two field experiments through two successive growing seasons of 2021 and 2022. Decreasing irrigation water significantly reduced shoot length, branches numberplant-1, shoot fresh and dry weight, photosynthetic pigments components, endogenous indole acetic acid (IAA) contents, and yield components. Furthermore, root fresh and dry weight, phenols, total soluble sugars (TSS), proline contents, and the accumulation of hydrogen peroxide (H2O2) and lipid peroxidation of groundnut leaves increased significantly. Contrarily, foliar application with Arg and SNP alleviated the negative influences of drought on growth and productivity of groundnut plants via enhancing photosynthetic pigments, IAA, phenolic compounds, TSS, and proline contents. Additionally, SNP and Arg significantly decreased oxidative damage through decreasing H2O2 and lipid peroxidation by the induction of antioxidant enzymes. Remarkably, the increase of drought level led to a reduction in Cercospora leaf spot (CLS) disease with the use of high concentrations of both Arg and SNP. Interestingly, in both stressed and unstressed plants, SNP treatment at 7.5 mM was the most effective in reducing the incidence and severity of disease, while Arg at 2.5 mM recorded the lowest reduction compared to other treatments. In conclusion, foliar treatment of either SNP or Arg is a profound effect on modulating the drought stress and induction of resistance to Cercospora leaf spot disease of groundnut plants throughout regulating physiological and biochemical processes associated with photosynthesis and oxidative responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Groundnut or Arachis hypogaea L. is known as the world’s fourth most valuable oilseed crop (Zanjare et al. 2023) and staple plant grown mostly in semiarid areas of the world (Zheng et al. 2018; El-Metwally et al. 2022). In Egypt, groundnut is one of the most important crops for both export and local consumption (Hilal et al. 1994). The higher percentage of unsaturated fatty acids (48–50%), easily absorbed protein (26–28%), half of the required vitamins, and one-third of the critical minerals makes groundnut seed more valuable (Bakry et al. 2020). A recent assessment found that several abiotic and biotic factors such as unfavorable climate, nutritional imbalance, and plant diseases caused by fungi and bacteria impede groundnut production. Among abiotic stresses, drought solely led to a yearly decrease in worldwide groundnut yield of about 6 million tone (Bhalani et al. 2019; Ghorbani et al. 2023b).
Abiotic stresses such as drought, salinity, temperature, metal, and flood adversely affect plant productivity during their life cycle. About 20% of the agricultural areas all over the world are under drought stress, making it among the most effective environmental constraints to plant production (Nabi et al. 2022). According to predictions, drought severity could rise by 1% by 2100 as a result of global warming (Price et al. 2022). The increased global population and intense human activities have led to an increase in the amount of water used for agriculture. Water deficiency is now a worldwide problem that is among the most effective environmental stresses that are limiting crop yields globally (Lobell et al. 2011; Fahad et al. 2017). Water deficiency impacts via causing physiological, biochemical, morphological, and molecular changes in plants. Drought stress causes water scarcity in plants, which causes a decrease in tissue water content as its main effect (Seleiman et al. 2021). Furthermore, the drop in water content has a significant impact on growth, water intake, nutrient uptake and mobilization, photosynthesis, and transpiration and assimilates translocation (Hasanuzzaman et al. 2018). Plant growth is significantly hampered by stress that causes stunting, wilted leaves, chlorosis, and yellowing. Furthermore, water deficiency damage photosynthetic pigments, alter cell structure and function, impair metabolism, slow down nutrient uptake and transportation rate, increased plant energy consumption, reduced plant growth, decrease plant quality, and even death (Zhu 2016; Chakraborty et al. 2016; Nan et al. 2018; Bakhoum et al. 2019).
Biotic stress such as early and late Cercospora leaf spot disease is the most important foliar disease of groundnut worldwide (Maninderpal 2011). Early leaf spots are caused by the fungi Passalora arachidicola (Hori) U Braun [Cercospora arachidicola S. Hori] (telemorph = Mycosphaerella arachidis Deighton) and late leaf spots are caused by Nothopassalora personata (Berk. and M.A. Curtis) U. Braun, C. Nakash, Videira and Crous [Cercosporidium personatum [Berk and Curtis] Deighton] (telemorph = Mycosphaerella berkeleyi Jenk.) (Denwar et al. 2021). These fungi’s symptoms are easily visible on the lower leaf surface. Early CLS is distinguished by light-brown spots surrounded by a yellow halo, whereas late CLS spots are black and usually lack the yellow halo (Shokes and Culbreath 1997). Leaf spots can attack all the aerial parts of groundnut resulting in yield losses of up to 50% (Mohammed et al. 2018; Kankam et al. 2022). Responses to biotic and abiotic variables might interplay positively or negatively. Drought stress may affect the intensity along with the distribution of plant diseases (Dikilitas et al. 2016). Mostly, drought declines or prevents the expansion of plant diseases caused by pathogens that grow under moist conditions (Sunarti et al. 2022). However, some diseases are preferred by drought. This is because when plants are stressed due to deficiency of moisture, they become more susceptible to these diseases (Wegulo et al. 2013). It is also necessary to recognize the actual effects of numerous combined stresses on yield-related parameters in field-grown crops because all these complex stress interaction potentials and their product have not yet been explored (Pandey et al. 2017).
Environmental and biotic stresses induce certain changes by which plant cells can sense stress stimuli or signals and respond through ways which help plants to cope with those challenging circumstances (Rasheed et al. 2022). The sensing of biotic or abiotic stress triggers signaling cascades that stimulate the formation of signaling molecules among reactive oxygen species (ROS), calcium (Ca+2), salicylic acid, and nitric oxide (NO) (Jaspers and Kangasjärvi 2010; Khan et al. 2023). The improvement of endogenous NO level, in addition to its exogenous treatment, could reduce the negative effects of drought and biotic stresses via variety of mechanisms. Sodium nitroprusside (SNP) is a bioactive diffusible signaling molecule that is commonly employed as a NO donor which is essential for plant growth, metabolic processes, and yield in both normal and stressful environment (Sadak 2016). Such signaling molecules may be applied exogenously in order to elicit a defense mechanism against the encountered stresses. Moreover, NO can mediate plant growth regulators and ROS metabolism, as well as motivate in signal transduction and responses to biotic and abiotic stresses (Chakraborty and Acharya 2017; Rai et al. 2020). NO is also, may be act as a phytohormone, serving as a signal in hormonal and defense responses like stomatal closure, root development, apoptosis, and stress responses (Oz et al. 2015). It regulates both plant life processes and environmental stress tolerance. In several plant species, NO regulates programmed cell death (PCD), salinity, drought, high and low-temperature stresses, and metal toxicity-induced responses (Wendehenne et al. 2005). During stress conditions, NO and ROS play a critical role in the restoration of various pathophysiological processes in plants (Fancy et al. 2017; Sarkar et al. 2021).
Arginine is one of the most functionally diverse amino acids and is commonly applied for improving plant stress resistance, because it is a primary precursor for the biosynthesis of polyamines (PAs), proline, and the cell signaling molecule (NO) (Liu et al. 2015; Ghorbani et al. 2023a) as well as increasing antioxidant system activity (Babalar et al. 2018). Polyamines are required for development and responding to abiotic and biotic stresses. They regulate several biological activities in plants, including cellular defense against oxidative damage via inhibition of lipid peroxidation and free radical scavenging (Winter et al. 2015). Arginine is a major nitrogen storage and transport form in tree and other plant underground storage organs and roots (Rennenberg et al. 2010). As a result, arginine metabolism plays a key role in nitrogen distribution and recycling in plants (Polacco et al. 2013). Plants can absorb amino acids from exogenous applications in addition to endogenous arginine synthesis, which can contribute to a variety of physiological and biochemical processes (Bai et al. 2018).
As far as we are aware, not much study has examined how arginine and sodium nitroprusside treatment affects the morphological, physiological, and yield attributes of groundnut plants under the combinations of stresses. Therefore, the aim of this investigation was to study the effect of arginine (a substrate for NO synthesis) and sodium nitroprusside (NO - donor) as a promising plant regulatory materials to improve groundnut tolerance to drought stress and biotic stress via growth, yield, induction of resistance to Cercospora leaf spot disease, and other physiological parameters.
2 Material and Methods
2.1 Experimental Procedure
Two field experiments were conducted during two successive growing seasons 2021 and 2022 at the experimental farm of National Research Center (latitude 30° 30′ 1.4″ N, longitude 30° 19′ 10.9″ E, and 21 m+ MSL (mean sea level)), Al Nubaryia district, El-Behaira Governorate, Egypt. Prior to initiation of each experiment, soil samples to 30 cm depth were collected to assess physical and chemical properties of the soil of the experimentation site. Soil samples were analyzed according to the standard published procedures of Carter and Gregorich (2006). Soil texture was sandy and had the following characteristics: sand 94.7%, pH 8.6, organic matter 0.8%, CaCO3 2.4%, EC 0.13 mmhos/cm2, available N 18.0 ppm, available P 18.0 ppm, available K 104 ppm, and available Zn 0.05 ppm.
The experimental design was split plot design with three replications, the irrigation water requirements IW 100% and 75% in the main plots, while arginine (Arg) or sodium nitroprusside (SNP) 0.0, 2.5, 5.0, and 7.5 mM were randomly applied in sub-plots and applied twice, the first foliar application after 30 days from sowing and the second after 45 days from sowing. The plot area was 10.5 m2 consisting of five rows (3.5 m length and 60 cm between rows).
The seeds of groundnut (Arachis hypogaea L.) variety Gize-6 was procured from Oil Crops Research Section, Field Crops Research Institute, Agricultural Research Center–Giza–Egypt. The seeds were inoculated just before sowing with the specific rhizobium bacteria inoculants. Seeds of groundnut were sown in the first week of May in both seasons. Phosphorus fertilizer as calcium superphosphate (15.5% P2O5) was applied during the seed bed preparation at rate of 60 kg P2O5/ha−1. Potassium sulfate (48% K2O) at the rate of 50 Kg ha−1 was applied at sowing. Nitrogen fertilizer was added at the rate of 30 kg N/ha−1 as ammonium sulfate (20.6% N) in two equal portions, the first half at sowing and the second after 30 days later.
2.2 Irrigation Water Requirements
Irrigation water requirements were calculated using Penman Monteith equation and crop coefficient according to Allen et al. (1989). The average amount of irrigation water applied with sprinkler irrigation system were 5950, and 4460 m3 ha.−1 season−1 as 100% and 75% respectively in both the seasons (Keller and Karmeli 1975).
2.3 Agronomical Traits
Growth parameters: plant samples were taken after 60 days of sowing to measure morphological parameters such as plant height (cm), number of branches plant−1, shoot fresh and dry weight (g), as well as root length (cm) and root fresh weight (g).
2.4 Isolation, Purification, and Identification of the Pathogen
The work was carried out at the laboratory of Plant Pathology Department, Faculty of Agriculture, Ain Shams University, Qalyubia Governorate, Egypt. The fungal pathogens were isolated from naturally infected leaves of groundnut showing characteristic symptoms of Cercospora leaf spot disease according to Rangaswami and Mahadevan (2006) and Kumari et al. (2013). The culture was purified by using the hyphal tip technique according to Barnett and Hunter (1999) and maintained on potato dextrose agar medium and grown for 14–15 days. The identification was based on their cultural properties and morphological and microscopical characteristics according to Kolte (1984) and McDonald et al. (1985).
2.5 Assessment of Disease Incidence and Severity
Disease incidence and severity of early and late Cercospora leaf spots combined were assessed after harvesting. Number of infected leaves was obtained from 20 random plants per treatment. Percentage of disease incidence was calculated for each individual treatment using the formula adopted from Subrahmanyam et al. (1995) as follows:
Disease severity was assessed using a rating scale of 1–9 as described by Subrahmanyam et al. (1995) where percent infected leaf area was 1 = 0%, 2 = 1–5%, 3 = 6–10%, 4 = 11–20%, 5 = 21–30%, 6 = 31–40%, 7 = 41–60%, 8 = 61–80%, and 9 = 81–100%.
The percentage of disease severity index (DSI) was estimated using the following formula.
where ∑n = sum of individual ratings
N = total number of plant samples−1 examined in each replicate
9 = the maximum disease rating
2.6 Measurement of Yield Characters and Its Components
Samples of five plants were taken randomly from each plot at harvest time (120 days from sowing date), and data was recorded on seed yield characters like plant height (cm), biological yield plant−1 (g), number of branches plant−1, number of pods plants−1, shoot fresh weight plant−1 (g), pod yield plant−1 (g), and seed yield plant−1 (g). Whole plot was harvested, and the pods were air dried to calculate pod yield (Kg ha−1), seed yield (Kg ha−1), oil yield (Kg ha−1), and protein yield (Kg ha−1).
2.7 Biochemical Analysis
Photosynthetic Pigments
Total chlorophyll a and b and carotenoid contents in fresh leaves of groundnut plants were estimated using the method of Lichtenthaler and Buschmann (2001). The fresh tissue was ground in a mortar and pestles using 80% acetone. The optical density (OD) of the solution was recorded at 662 and 645 nm (for chlorophyll a and b, respectively) and 470 nm (for carotenoids) using a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan). The values of photosynthetic pigments were expressed in mgg−1 FW.
Indole Acetic Acid Content
A known weight of the fresh samples was taken and extracted with 85% cold methanol (v/v) for three times at 0 °C. The combined extracts were collected and made up to a known volume with cold methanol. Then, 1 mL of the methanolic extract was taken, and 4 mL of PDAB reagent (1 g of para-dimethylaminobenzoic acid dissolved in 50 mL HCl and 50 mL of 95% ethanol) was added and left for 60 minutes (min) at 30–40 °C. The developing color was spectrophotometrically measured at 530 nm (Gusmiaty et al. 2019).
Total Phenol Content
The leaf tissue was extracted as IAA extraction, and then, 0.5 mL of the extraction was added to 0.5 mL Folin-Ciocalteu reagent, shaken, and allowed to stand for 3 min. Then, 1 mL of saturated sodium carbonate was added followed by distilled water, shaken, and allowed to stand for 60 min. The optical density was determined at 725 nm using spectrophotometer as described by Gonzalez et al. (2003).
Total Soluble Sugars (TSS)
Total soluble carbohydrates (TSS) were extracted by overnight submersion of dry tissue in 10 mL of 80% (v/v) ethanol at 25 °C with periodic shaking and centrifuged at 600 × g. The supernatant was evaporated till completely dried and then dissolved in a known volume of distilled water to be ready for determination of soluble carbohydrates (Homme et al. 1992). TSS were analyzed by the reaction of 0.1 mL of ethanolic extract with 3.0 mL freshly prepared anthrone (150 mg anthrone + 100 mL of 72% H2SO4) in boiling water bath for 10 min, and the cooled samples were read at 625 nm using Spekol Spectrophotometer VEB Carl Zeiss (Chow and Landhausser 2004).
Proline
Proline was assayed according to the method described by Bates et al. (1973). Two milliliters of proline extract, 2 mL of acid ninhydrin, and 2 mL of glacial acetic acid were added and incubated for 1 h in a boiling water bath followed by an ice bath. The absorbance was measured at 520 nm using Spekol Spectrophotometer VEB Carl Zeiss. A standard curve was obtained using a known concentration of authentic proline.
Lipid Peroxidation
The levels of lipid peroxidation were measured by determining the levels of malondialdehyde (MDA). Malondialdehyde is a product of lipid peroxidation and was assayed by thiobarbituric acid reactive substrates (TBARS) using the method of Hodges et al. (1999) with some modifications. Fresh leaf samples (0.2 g) were ground in 5 mL of 0.1% (w/v) trichloroacetic acid (TCA), at 4 °C. Following the centrifugation at 12,000 × g for 5 min, an aliquot of 1 mL from the supernatant was added to 4 mL of 0.5% (w/v) thiobarbituric acid (TBA) in 20% (w/v) TCA. Samples were heated at 90 °C for 30 min. Thereafter, the reaction was stopped in ice bath. Centrifugation was performed at 10,000 × g for 5 min, and absorbance of the supernatant was recorded at 532 nm on a spectrophotometer (Model CamspecM330 UV/Vis) and corrected for non-specific turbidity by subtracting the absorbance at 600 nm. The following formula was applied to calculate malondialdehyde content using its absorption coefficient (ɛ) and expressed as nmol malondialdehyde g−1 fresh mass (FM):
where ɛ is the specific extinction coefficient (= 155 mMcm−1), V is the volume of crushing medium, W is the fresh weight of leaf, A600 is the absorbance at 600 nm, and A532 is the absorbance at 532 nm.
Hydrogen Peroxide Concentration
Hydrogen peroxide (H2O2) concentration was determined according to Velikova et al. (2000). Leaf samples of 0.5 g were homogenized in 3 mL of 1% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 10,000 rpm and 4 °C for 10 min. Subsequently, 0.75 mL of the supernatant was added to 0.75 mL of 10 mM K-phosphate buffer (pH 7.0), and 1.5 mL of 1M KI. H2O2 concentration of the supernatant was evaluated by comparing its absorbance at 390 nm to a standard calibration curve. The concentration of H2O2 was calculated from a standard curve plotted in the range from 100 to 1000 μmol mL−1. H2O2 concentration was expressed as μmol g−1 DW.
Assay of Enzyme Activities
Enzyme extractions were collected according to the method described by Chen and Wang (2006). Leaf tissues were homogenized in ice-cold phosphate buffer (50 mM, pH 7.8), followed by centrifugation at 8000 rpm and 4 °C for 15 min. The supernatant was used immediately to determine the activities of enzymes.
Catalase Activity
Catalase enzyme (CAT, EC 1.11.1.6) was measured using spectrophotometer by following the reduction in absorbance at 240 nm (Chen and Wang 2006). The deduction in absorbance at 240 nm per minute was determined as one unit of CAT activity (Kong et al. 1999).
Superoxide Dismutase Activity
Assay of superoxide dismutase (SOD) was carried according to the method of Chen and Wang (2006). One milliliter of 125 mM sodium carbonate, 0.4 mL of 25 μM NBT, and 0.2 mL of 0.1 mM EDTA were added to 0.5 mL of plant extract. The reaction was initiated by adding 0.4 mL of 1 mM hydroxylamine hydrochloride, and the absorbance was read at 560 nm using spectrophotometer at 1 min intervals. Units of SOD were expressed as amount of enzyme required for inhibiting the reduction of NBT by 50%.
Peroxidase Activity
Peroxidase (POX, EC 1.11.1.7) activity was assayed by the method of Bergmeyer (1974). The reaction mixture used for estimating the peroxidase enzyme (POX) contained 2 mL of 0.1 M phosphate buffer (pH 6.8), 1 mL of 0.01 M pyrogallol, 1 mL of 0.005 M H2O2, and 0.5 mL of the enzyme extract. The solution was incubated for 5 min at 25 °C after which the reaction was terminated by adding 1 mL of 2.5 N H2SO4. The amount of purpurogallin formed was determined by measuring the absorbance at 420 nm against a reagent blank prepared by adding the extract after the addition of 2.5 N H2SO4 at the zero time.
Total Carbohydrate
Determination of total carbohydrates was carried out according to method described by Albalasmeh et al. (2013). A known weight (0.2–0.5 g) of dried tissue was placed in a test tube, and then, 10 mL of sulphuric acid (1 N) was added. The tube was sealed and placed overnight in an oven at 100 °C. The solution was then filtered into a measuring flask (100 mL) and filled to the mark with distilled water. The total sugars were determined colorimetrically as follows: an aliquot of 1 mL of sugar solution was transferred into test tube and treated with 1 mL of 5% aqueous phenol solution followed by 5.0 mL of concentrated sulphuric acid. The tubes were thoroughly shaken for 10 min, then placed in a water bath at 23–30 °C for 20 min. The optical density of the developed color was measured at 490 nm using Shimadzu spectrophotometer model UV 1201.
Oil Contents
The oil of groundnut seeds was extracted according to Das et al. (2002); the powdered seed is shaken overnight with isopropanol:chloroform (1:1). The solvent was evaporated under reduced pressure of CO2 atmosphere. The lipid residue is taken up in a chloroform:methanol (2:1 v/v) and given a folch’s wash. The dissolved total oils were purified by washing with 1% aqueous saline solution. The aqueous phases were washed with chloroform, and that was combined with the pure oil solution. Chloroform was evaporated, and the total pure oil was weighed.
Protein
Total protein concentration of the supernatant was determined according to the method described by Pedrol and Tamayo (2001) with bovine serum albumin as a standard. An amount of 2 g of sample was ground in mortar with 5 mL of phosphate buffer (pH 7.6) and was then transferred to the centrifuge tubes. The homogenate was centrifuged at 8000 rpm for 20 min. The supernatant of different samples was put in separate tubes. The volume of all the samples in tubes was then made equal by adding phosphate buffer solution, and the extraction was stored in the refrigerator at 4 °C for further analysis. After extraction, 30 μL of different samples were taken out in separate tubes and were mixed with 70 μL of distilled water separately. In all these separate sample tubes, 2.9 mL of Coomassie Brilliant Blue solution was added and mixed thoroughly. All these tubes were incubated for 5 min at room temperature, and absorbance at 600 nm was recorded against the reagent blank. A standard curve of absorbance (600 nm) versus concentration (μg) of protein was calculated.
Flavonoid Content
Flavonoid content of crude extract was determined by the aluminium chloride colorimetric method (Chang et al. 2002). In brief, 50 μl of crude extract (1 mg mL−1 ethanol) was made up to 1 mL with methanol, mixed with 4 mL of distilled water and then 0.3 mL of 5% NaNO2 solution; 0.3 mL of 10% AlCl3 solution was added after 5 min of incubation, and the mixture was allowed to stand for 6 min. Then, 2 mL of 1 M NaOH solution were added, and the final volume of the mixture was brought to 10 mL with double-distilled water. The mixture was allowed to stand for 15 min, and absorbance was measured at 510 nm. The total flavonoid content was calculated from a calibration curve, and the result was expressed as mg rutin equivalent per g dry weight.
Free Radical Scavenging Activity (DPPH%)
The free radical scavenging activity by different plant extracts was done according to the method reported by Gyamfi et al. (2002). Fifty microliters of the plant extract in methanol, yielding 100 μg mL−1 in each reaction was mixed with 1 mL of 0.1 mM DPPH in methanol solution and 450 μL of 50 mM Tris-HCl buffer (pH 7.4). Methanol (50 μL) only is used as control. After 30 min of incubation at room temperature, the reduction of the DPPH free radical was measured reading the absorbance at 517 nm. L-Ascorbic acid and BHT were used as controls. The percent inhibition was calculated from the following equation:
2.8 Statistical Analysis
Analyses of variance of split plot design (ANOVA) for all data presented in this investigation were calculated using SPSS v20.0 (SPSS Inc., Chicago, USA) analyzing software. Since the trend was similar in both seasons, the Duncan’s test at p ≤ 0.05 levels was applied, and the combined analysis of the two seasons was done according to the method, using SPSS software (SPPS Institute Inc. 2008; Steel et al. 1997)
3 Results
3.1 Impact of Arginine (Arg) and Sodium Nitroprusside (SNP) on Growth Indices of Groundnut Plants Grown Under Drought Stress
The data presented in Table 1 shows that decreasing water irrigation levels from I100% to I75% significantly decreased different morphological characters such as number of branches plant−1 and shoot fresh and dry weight (g), while in case of plant height (cm) and root length (cm), the decrease was non-significant. Furthermore, I75% increased significantly fresh weight of roots (g) as compared with plants irrigated normally (I100%). In this respect, plant height decreased from 18.33 to 15.00 cm, recording a reduction of 18.17% compared to the control. Additionally, the shoot-related traits, namely, number of branches plant−1 and plant fresh and dry weight in addition to root length, were attenuated from 6.00, 25.95, 21.59, and 8.67 (control plants) to 4.00, 7.73, 2.16, and 8.33 (stressed plants), with reductions of 33.33%, 85.39%, 89.98%, and 3.85%, respectively. Furthermore, fresh roots of ground nut plants were positively affected by drought stress, where it increased from 1.40 (control) to 1.54 (stressed plants), with a percent increment of 10.50%. On the contrary, different treatments of either arginine or SNP (2.5, 5.0, and 7.5 mM) increased various growth parameters studied under both normal and drought stressed conditions. Additionally, the data clearly show that SNP was more effective than arginine on increasing shoot different growth criteria (Table 1). Furthermore, the highest values of growth parameters were obtained at 5.0 mM SNP followed by 7.5 mM arginine under normal irrigation conditions and drought stress conditions (Table 1).
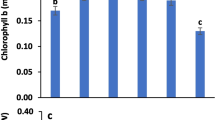
3.2 Impact of Arginine (Arg) and Sodium Nitroprusside (SNP) on the Photosynthetic Pigments of Groundnut Plants Grown Under Drought Stress
Irrigation of groundnut plants with low water irrigation requirement 75% WIR (drought stress) induced a significant decrease in chlorophyll a, chlorophyll b, carotenoids, and total pigments (Fig. 1) relative to the non-stressed plants. Meanwhile, foliar treatments with either arginine or SNP with different levels not only improved photosynthetic pigment constituents but also restrained the resulted reduction in chlorophyll a, chlorophyll b, carotenoids, and total pigments as compared with the untreated controls under normal irrigation as well as drought stress conditions. Furthermore, data show that under normal irrigation condition, 5.0 mM of SNP caused the highest increases in chlorophyll a, chlorophyll b, carotenoids, and total pigments followed by 7.5 mM arginine, while under drought stress conditions (75% WIR), arginine 7.5 mM caused the highest increases in various studied photosynthetic pigments followed by 5.0 SNP as compared with the other treatments.
Impact of arginine (Arg) 2.5, 5.0, and 7.5 mM and sodium nitroprusside (SNP) 2.5, 5.0, and 7.5 mM on photosynthetic pigments (μg/100 g fresh weight) of groundnut plants under drought stress (D0 = 100% IW, D1 = 75% IW). IW = irrigation water; all the statistical differences were presented relative to the untreated control. Data are means of three replicates ± SE; means with different letters within the same column are significantly different (p ≤ 0.05)
3.3 Impact of Arginine (Arg) and Sodium Nitroprusside (SNP) on Endogenous Indole Acetic Acid and Phenol Contents of Groundnut Plants Grown Under Drought Stress
The endogenous indole acetic acid (IAA) content of groundnut plants exposed to drought stress was significantly lower than that of unstressed control plant. Compared to un-drought stressed plants, phenolic content showed significant increases in response to drought stress. Furthermore, treatment with arginine or SNP with different concentrations not only alleviated the decrease in IAA but significantly enhanced its concentrations when compared to control (untreated) plants. Moreover, the applied protectants boosted phenolic compounds, whereas plants treated with 5 mM arginine or SNP and irrigated with 75% WIR showed the greatest stimulation (Fig. 2).
Impact of arginine (Arg) 2.5, 5.0, and 7.5 mM and sodium nitroprusside (SNP) 2.5, 5.0, and 7.5 mM on IAA (μg/g fresh wt) and phenols (mg/g dry weight) of groundnut plants under drought stress (D0 = 100% IW, D1 = 75% IW). IW = irrigation water; all the statistical differences were presented relative to the untreated control. Data are means of three replicates ± SE; means with different letters within the same column are significantly different (p ≤ 0.05)
3.4 Impact of Arginine (Arg) and Sodium Nitroprusside (SNP) on Osmoprotectants of Groundnut Plants Grown Under Drought Stress
Drought stress caused significant increases in the TSS and proline contents of the leaves of groundnut plants as compared to the control unstressed plants. Furthermore, external application of arginine or SNP significantly increased the contents of the above mentioned osmoprotectants regardless of the water irrigation conditions. Comparatively, the 5.0 mM of either arginine or SNP was more effective than the other two concentrations (2.5 and 7.5 mM) either under normal irrigation or under drought-stressed conditions (Fig 3).
Impact of arginine (Arg) 2.5, 5.0, and 7.5 mM and sodium nitroprusside (SNP) 2.5, 5.0, and 7.5 mM on total soluble sugars (TSS) and proline (mg/g dry weight) of groundnut plants under drought stress (D0 = 100% IW, D1 = 75% IW). IW = irrigation water; all the statistical differences were presented relative to the untreated control. Data are means of three replicates ± SE; means with different letters within the same column are significantly different (p ≤ 0.05)
3.5 Impact of Arginine (Arg) and Sodium Nitroprusside (SNP) on Hydrogen Peroxide (H2O2) and Lipid Peroxidation of Groundnut Plants Grown Under Drought Stress
The presented data in Fig. 4 showed that irrigation of groundnut plants with low irrigation water (75%) significantly increased H2O2 and lipid peroxidation expressed in the MDA content as compared with those plants irrigated with 100% IWR. On the other hand, the exogenous application using different concentrations of arginine or SNP caused significant decreases in the contents of H2O2 and MDA as compared to their corresponding untreated controls. It was noted that 5.0 mM was the most effective concentration of either arginine or SNP as it has higher capacity to decrease H2O2 and lipid peroxidation than the other two used concentrations.
Impact of arginine (Arg) 2.5, 5.0, and 7.5 mM and sodium nitroprusside (SNP) 2.5, 5.0, and 7.5 mM on MDA and H2O2 (μg g−1 fresh weight) of groundnut plants under drought stress (D0 = 100% IW, D1 = 75% IW). IW= irrigation water; MDA = malondialdehyde, H2O2 = hydrogen peroxide; all the statistical differences were presented relative to the untreated control. Data are means of three replicates ± SE; means with different letters within the same column are significantly different (p ≤ 0.05)
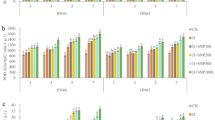
3.6 Impact of Arginine (Arg) and Sodium Nitroprusside (SNP) on Antioxidant Enzymes of Groundnut Plants Grown Under Drought Stress
Regarding the antioxidant enzymes of drought stressed plants, CAT, SOD, and POX were improved in comparison with control plants (Fig. 5). Moreover, treating groundnut plants with arginine or SNP under normal or drought stressed conditions caused further increases in CAT, SOD, and POX as compared with their corresponding untreated controls.
Impact of arginine (Arg) 2.5, 5.0, and 7.5 mM and sodium nitroprusside (SNP) 2.5, 5.0, and 7.5 mM on antioxidant enzymes (U min−1 g−1 fresh weight/h) of groundnut plants under drought stress (D0 = 100% IW, D1 = 75% IW). IW = irrigation water; CAT = catalase; SOD = superoxide dismutase; POX = peroxidase; all the statistical differences were presented relative to the untreated control. Data are means of three replicates ± SE; means with different letters within the same column are significantly different (p ≤ 0.05)
3.7 Impact of Arginine (Arg) and Sodium Nitroprusside (SNP) on Yield and Its Components of Groundnut Plants Grown Under Drought Stress
The adverse effects of drought stress and the alleviation effects of NO donors (arginine and SNP) on yield and morphological traits of harvested plants were assessed (Table 2). The yield attributes expressed as plant height, biological yield plant−1, number of branches plant−1, pods number and weight plant−1, seeds weightplant−1, as well as biological yield (Kg ha−1), pods and seeds yield (Kg ha−1), and 100 seeds weight of groundnut plants under drought stress were reduced drastically and significantly as compared with unstressed control plants (Table 2). Drought stress reduced biological yield (Kg ha−1) by 57.7%, pods yield (Kg ha−1) by 53.4%, seeds yield (Kg ha−1) by 52.1%, and 100 seeds weight (g) by 8.8%, while different foliar treatments of Arg or SNP increased significantly for the studied yield and its components under 100% WIR. Additionally, those treatments could alleviate the negative effect of drought stress by causing significant increase in yield parameters of groundnut as compared with untreated control.
3.8 Impact of Arginine (Arg) and Sodium Nitroprusside (SNP) on the Nutritional Traits of Groundnut Plants Grown Under Drought Stress
As shown in Table 3, drought stress at 75% WIR induced significant decreases in carbohydrates %, protein %, and oil % as well as flavonoids and DPPH of the yielded groundnut seeds compared to full irrigated plants (100% WIR). However, exogenous foliar treatments with arginine and SNP caused significant increases in different studied attributes of groundnut plants as compared with untreated plant. However, exogenous arginine or SNP not only increased nutritional components but also alleviated the negative impacts of drought stress on groundnut seeds by causing significant increase in the above mentioned parameters.
3.9 Morphological Differentiation of Early and Late Cercospora Leaf Spots in Groundnut Plants Under Field Conditions
Early and late leaf spots in groundnut can be separated on basis of color of spot and yellow halo. Symptoms of ELS is dark brown spots surrounded by a yellow halo, while in case of LLS, it is dark brown or black surrounded by a faint yellow halo or without a halo (Fig. 6).
3.10 Isolation and Identification of the Pathogen
The fungal pathogens Passalora arachidicola and Nothopassalora personata were isolated from infected leaves and confirmed as the causative agents of Cercospora leaf spot diseases of groundnut. Stroma, conidiophores, and conidia are seen on slide made from host tissue kept under extremely favorable environmental conditions using a light microscope (Leica DM 2500), equipped with a digital camera (Leica, Wetzlar). Conidium of Passalora arachidicola is sub-hyaline or pale yellow, obclavate or cylindrical and septate with rounded base and sub-acute tip (Fig. 7). However, in the case of Nothopassalora personata, conidium was obclavate or cylindrical and light colored. The base is shortly tapered with a conspicuous hilum (Fig. 8).
3.11 Effect of Arginine and SNP on Incidence and Severity of Cercospora Leaf Spot Disease of Groundnuts at Different Levels of Drought Stress Under Field Conditions
The influence of arginine and sodium nitroprusside (SNP) as foliar treatments for induction of resistance in groundnut plants to Cercospora leaf spot (CLS) disease was evaluated under two levels of water condition (100% as normal irrigated and 75% as drought stress). Data in Fig. 9 revealed that all treatments and their concentrations significantly decreased incidence and severity of groundnut CLS diseaseas compared to control either in unstressed plants (100%) or those under drought stress conditions (75%). Additionally, the reduction of disease incidence and severity was higher in stressed than unstressed plants. The obtained results proved that treatment with SNP was higher than arginine in reducing disease incidence and severity of CLS disease in both stressed and unstressed plants. Data in Fig. 9 showed that treatment with SNP at concentration of 7.5 mM was the most effective for reducing incidence (20.47–27.23%) and severity (12.33–19.98%) of the disease, while arginine at 2.5 mM recorded the lowest reduction of both incidence (49.8–57.92%) and severity (41.83–49%) of the disease compared to other treatments and corresponding control in both stressed and unstressed plants, respectively. Further, there is a positive correlation between treatment concentrations and their effect on the disease incidence and severity. It is interesting to note that increasing the drought level led to a reduction in disease incidence and severity with the use of high concentrations of both arginine and SNP.
Effects of arginine (Arg) and sodium nitroprusside (SNP) on incidence (A) and severity (B) of Cercospora leaf spot disease of groundnut under field conditions at different levels of drought stress (D0 = 100% IW, D1= 75% IW). IW = irrigation water. Arg1 = 2.5 mM, Arg2 = 0.5 mM, Arg3 = 7.5 mM, SNP1 = 2.5 mM, SNP2 = 0.5 mM, and SNP 3= 7.5 mM. All the statistical differences were presented relative to the untreated control. Data are means of three replicates ± SE; means with different letters within the same column are significantly different (p ≤ 0.05)
4 Discussion
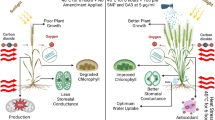
Abiotic stress, especially drought stress, significantly influenced growth, different metabolic processes, productivity, and nutrient composition of groundnut seeds. Exogenous application with NO donors to decrease the negative impact of drought stress on groundnut plants was studied. The decreased effect of drought on different growth attributes of groundnut plants are in accordance with the results obtained for groundnut, wheat, Moringa oleifera, and white lupine (Sadak et al. 2020; Abd Elhamid et al. 2021; Sadak 2022; Bakhoum et al. 2022a). The reduction in growth parameters may be resulted via decreased cell division, cell expansion, and suppression of apical growth (Acosta-Motos et al. 2017; Bakhoum et al. 2022b). Additionally, drought stress reduces the amount of CO2 that can be fixed by closing stomata, which interferes with Calvin cycle’s regular electron flow for reducing carbon. The decrease in fresh and dry matter production is a result of this disruption which is associated with low fresh and dry matter production (Mbarki et al. 2018).
According to the current research, exogenous application of arginine and SNP reduced the detrimental effects of drought stress on growth attributes of groundnut. Those results are consistent with the earlier data obtained in sunflower treated with arginine and SNP, which improved the growth parameters of the plants under salinity stress (Ramadan et al. 2019) and wheat plants (Ragaey et al. 2022) via preserving ion homeostasis and restoring the redox balance (Groß et al. 2013). Furthermore, NO is important in regulating plant metabolism, photosynthetic pigments, alleviating oxidative stress, and plant hormones (Groß et al. 2013). On the contrary, the role of arginine in enhancing growth parameters may be resulted via the increase in polyamine biosynthesis that used in a wide range of biological processes including growth (Nasibi et al. 2011). The results of the current investigation also show that the increase in leaf water content and photosynthetic pigments as well as over production of osmoprotectant compounds and an antioxidative defense system may be responsible for the promotion impact of SNP or arginine on groundnut drought tolerance. The NO molecule penetrates membranes and functions as a crucial signal molecule in plants. Based on previous findings, the breakdown of photosynthetic pigments, that results in decreased photosynthesis and growth retardation, is a known sign of drought stress (Sadak and Ramadan 2021; Abdalla et al. 2022). These findings are supported by other research on wheat (Bakry et al. 2019), Moringa oleifera (Abd Elhamid et al. 2021), and quinoa (Sadak and Bakhoum 2022). These reductions might be due to overaccumulation of reactive oxygen species (ROS) under drought stress which hampered the biosynthesis of different photosynthetic pigment constituents and downregulated the photosynthetic electron transport chain in apple (Wang et al. 2018). Furthermore, this reduced effect is caused by diffusion limitation due to closure of the stomata and the reduced rubisco content, suggesting co-dominance and biochemical restriction of the stomata under water stress conditions which can affect CO2 assimilation rates (Flexas et al. 2016; Li et al. 2022). On the contrary, exogenous treatments with arginine or SNP reversed those diminished impacts of water stress on photosynthetic pigments constituents. This might be resulted due to the impact of NO in scavenging ROS, decreasing oxidative damage of the photosynthetic apparatus, raising chlorophyll contents, and preserving chlorophyll-containing cell organelles from drought-induced damage (Kausar et al. 2013). According to Ragaey et al. (2022), applying arginine or SNP increased the chlorophyll content in wheat plants under stress.
Endogenous indole-3-acetic acid (IAA), the most prevalent naturally found auxin, is a plant growth regulator formed by plants, fungi, and bacteria. IAA plays a key role in regulating plant growth and development. Drought stress decreased endogenous indole acetic acid (IAA) content of groundnut plants. The decrease was linked with reduced vegetative growth. Low level of IAA during drought stress might be attributed to suppression the biosynthesis of IAA or conversion to inactive form. Kazan (2013) considered this reduction in IAA as a stress-responsive transcription factor involved in regulation of auxin and root developmental. In the current study, external application of arginine or SNP at various doses reduced the negative effect of IAA level caused by drought stress on groundnut. Those results are in agreement with previous studies of Elewa et al. (2017), Sadak et al. (2019), and Ragaey et al. (2022). The increased contents of phenolic have the ability to alleviate the negative effect of drought stress. Phenols are antioxidant substances which support the defense system and scavenge ROS over accumulated by various stresses (Dawood et al. 2021; Wang et al. 2023). This process leads to stabilize and delocalize the unpaired electron (chain-breaking function) and from their ability to chelate transition metal ions (Jasim et al. 2016). Applying arginine or SNP can increase IAA and phenol contents of groundnut plants whether they were under drought stress or under normal conditions (Ramadan et al. 2019). The activation or increasing of phenols was further up regulated by exogenous treatment using NO sources.
Osmotic adjustment in plant cells occurs via increases in amounts of osmoprotectant substances as TSS and proline. Therefore, to alleviate osmotic stress, plant cells under drought stress increase soluble proteins and carbohydrates as a cellular defense against oxidative damage through lipid peroxidation inhibition and free radical scavenging (Ahmed et al. 2016). TSS and proline contents were shown to increase in the current investigation in groundnut plants grown under drought stress. The improving impact of drought stress on osmoprotectant was confirmed earlier in sunflower and wheat plants (Ramadan et al. 2019; Sadak and Bakhoum 2022; Ragaey et al. 2022). Proline is a non-toxic osmotic solute which maintains the macromolecule and organelle structure (Polash et al. 2018). Moreover, proline serves a variety of functions in plant cells including regulating the pressure potential and membrane integrity, scavenging ROS, and enhancing growth (Acosta-Motos et al. 2019). The present findings are in qualitative agreement with the earlier on chickpea (Latef et al. 2017), sunflower (Ramadan et al. 2019), flax (Sadak and Bakry 2020), and wheat (Ragaey et al. 2022).
The present investigation of drought stress induced high oxidative damage in groundnut leaves, as denoted by the increase in H2O2 that was reflected in the induction of the MDA content. However, it is possible to attribute the inhibition of oxidative burst caused by NO to increase the flexibility and stability of cells through fortifying a phospholipid bilayer, for promoting membrane fluidity and controlling ROS (Singh et al. 2016). NO as a free gas can function as a chain-disintegrating agent during lipid peroxidation reaction through collaboration with lipid alkoxyl and peroxyl radicals (Gan et al. 2015). Recent researches also show a connection between the accumulation of H2O2 in the leaves and the decreased chlorophyll content in groundnut plant under drought stress.
The state of the cellular ROS balance is mediated by the implication of enzymatic antioxidants that combat oxidative damage. Groundnut drought stressed plants showed increased CAT, SOD, and POX activities which might be involved in quenching over accumulated ROS from stressed cells, decreasing cellular damage, and improving plants’ oxidative ability to tolerate drought stress (Sofy et al. 2021). Additionally, elevated CAT, SOD, and POX activities improved the removal of excessive ROS molecules, giving groundnut plants greater stress tolerance. The protecting impact of the release of NO may be the cause of either arginine’s or SNP’s promotion of antioxidant enzymes of groundnut plants (Ragaey et al. 2022). However, NO functions as a signal molecule that increases drought tolerance by raising the frequency at which different antioxidant enzymes are produced in the mitochondria. Within that approach, NO protects stressed plants from oxidative damage by controlling redox homeostasis and increasing the activity of H2O2-scavenging enzymes (Stefanov et al. 2023). Foliar treatment of NO sources could therefore stimulate endogenous NO production, which has signaling or scavenger abilities for considerable period of time even after the NO donor is depleted.
As a result of drought stress and metabolic abnormalities, significant reductions in all yield parameter attributes along with several growth features were observed. Additionally, drought reduced the amount of carbohydrates, oil, and protein of groundnut. The decreases in groundnut yield were coincided with significant declines in growth indices and photosynthetic pigments. Changing the level of carbohydrate buildup is extremely important since they have a direct impact on other physiological functions like respiration and required for seeds filling (Sadak et al. 2023). Delay in flowering, decreased flower number, and reduced pod set are all factors that contribute to decreased yield (Khan et al. 2016). Used NO sources (either arginine or SNP) significantly improved yield and its components as well as carbohydrate, oil, and protein contents of groundnut either under normal conditions or drought stress conditions. These findings are consistent with those obtained by Ali et al. (2017), Ramadan et al. (2019), and Hanafy and Sh (2023).
Cercospora leaf spot (CLS) disease is the most common and serious groundnut disease in Egypt and around the world. Passalora arachidicola and Nothopassalora personata, the causative agents of Cercospora leaf spot diseases, were isolated and identified from infected groundnut leaves. The conidium of Passalora arachidicola was sub-hyaline and septate with a rounded base and sub-acute tip. While the conidium of Nothopassalora personata was obclavate, and the base was shortly tapered with a conspicuous hilum. This morphological description is identical to that reported by Ijaz (2011). Exogenous application of nitric oxide sources (arginine and SNP) can induce disease resistance in plants (Wu et al. 2016; Zhu et al. 2019). Furthermore, the NO can mediate plant growth regulators and ROS metabolism which involves in signal transduction and responses to biotic and abiotic stresses (Domingos et al. 2015; Kolbert et al. 2019). In terms of the effect of inducer treatments on the incidence and severity of groundnut Cercospora leaf spot (CLS) disease, the results revealed that all treatments had a significant effect in reducing disease parameters. SNP treatment at 75 mM was the most effective for reducing incidence and severity of the disease compared to other treatments in both stressed and unstressed plants. Also, increasing the concentration of the tested inducers caused an increasing in their reducing efficiency of disease parameters. The obtained results were in agreement with the findings of Pietrowska et al. (2015), Chakraborty et al. (2019), Chakraborty (2021), Sarkar et al. (2021), and Chang et al. (2002) which showed about protective function of nitric oxide in different host and pathogen combinations like Botrytis cinerea in tomato, Colletotrichum gloeosporioides in common bean, Alternaria alternata in chilli, and Fusarium oxysporum in tomato. The present data also showed that increasing the drought level led to reducing the disease incidence and severity. These findings are supported by previous studies Ramegowda and Senthil-Kumar (2015), Abu-Ellail and El-Mansoub (2020), and Hoheneder et al. (2021). Recent studies have revealed that combined stresses (biotic and a biotic) induce different physiological and molecular responses in plants, including rewiring of the hormonal pathways, accumulation of various metabolites, and induction or suppression of immunity genes (Pandey and Kumar 2019; Saijo and Loo 2020; Zarattini et al. 2021). In the case of foliar pathogens, stomatal closure is the first physiological barrier in the defense response. Stomatal closure is also a drought prevention strategy, so drought-induced stomatal closure reduces pathogen entry into the plant tissues. Similarly, pathogen-induced stomatal closure improves the plant in efficient use of water (Sjokvist et al. 2019). Early exposure of plants to drought stress results in the initiation of drought stress responses such as increased abscisic acid (ABA), the primary regulator of drought stress response, which is also known to alter pathogen response of plants depending on the severity of each stress and ROS levels which play antagonistic role in defeating or minimizing the effect of pathogen infection (Telles et al. 2017). Several metabolites have been linked to CLS disease resistance in groundnut. Phenols generally play an important role in groundnut resistance to fungal diseases. Phenols are required for the biosynthesis of lignin, which helps to reinforce the cell wall structure and thus prevent disease development (Zhang et al. 2013). When plants are stressed by biotic and abiotic factors, cell signaling pathways are activated, which can induce the production of secondary metabolites associated with resistance, such as phenolics, flavonoids, and lignin (Dukare et al. 2019). Furthermore, our investigation revealed a link between induced resistance to disease and some biochemical changes in groundnut plants. These biochemical changes were an increase in oxidative enzyme activity, accumulation of phenols and flavonoid compounds, as well as total proline and percentage of crude protein in both stressed and non-stressed plants. As compared to control, stressed plants are achieving higher results than non-stressed plants. These biochemical changes became a marker for resistance induction. Increase of flavonoid and phenols as a part of active defense responses was detected by application of different biotic and abiotic elicitor treatment (Chandra et al. 2015).
5 Conclusion
Results proved that exogenous foliar treatments with sodium nitroprusside (SNP) and arginine could improve growth and yield of groundnut plants via counteracting some of the reduced influence of drought stress and induction of resistance to Cercospora leaf spot (CLS) disease. Treatment with SNP and arginine improved photosynthetic pigments, increasing indole acetic acid (IAA) levels, phenolic biosynthesis, and antioxidant enzyme via decreasing ROS accumulated in cells. Moreover, SNP and arginine alleviated oxidative stress disturbances, accumulating osmoprotectants and inducing hydrogen-peroxide-scavenging enzymes. All the above modifications had a good effect on growth, yield quantity, and oil productivity in the stressed groundnut plants. Furthermore, NO sources improved different nutritional components of the yielded groundnut seeds such as carbohydrates, oil and protein contents as well as flavonoids and DPPH activities. Also, our finding revealed that treatment with nitric oxide sources (arginine and SNP) can induce disease resistance to Cercospora leaf spot disease in groundnut plants. All treatments had a significant effect in reducing disease incidence and severity in both stressed and unstressed plants. Interestingly, it was observed that stressed plants are achieving higher resistance than unstressed plants as compared to control. Additionally, we exposed a link between induction of resistance to disease and some biochemical changes in plants. These biochemical changes were an increase in oxidative enzyme activity, accumulation of phenols and flavonoid compounds, as well as total proline and percentage of crude protein in both stressed and unstressed plants. These changes became a marker for resistance induction. Eventually, it may be suggested to use sodium nitroprusside (SNP) and arginine as a foliar application to induce tolerance to biotic and abiotic stress by improving the growth and productivity of groundnut plants under normal and drought-stress conditions.
Abbreviations
- SNP:
-
sodium nitroprusside
- NO:
-
nitrogen oxide
- Arg:
-
arginine
- TSS:
-
total soluble sugars
- IAA:
-
indole acetic acid
- DPPH:
-
antioxidant activity percentage
- POX:
-
peroxidase enzyme
- CAT:
-
catalase
- SOD:
-
super oxide dismutase
References
Abd Elhamid EMA, Sadak MSh, Ezzo M, Abdalla AM et al (2021) Impact of glycine betaine on drought tolerance of Moringa oleifera plant grown under sandy soil. Asian J Plant Sci 20:578–589. https://doi.org/10.3923/ajps.2021.578.589
Abdalla AM, Sadak MSh, Abd Elhamid EM, Ezzo M (2022) Amelioration of drought stress reduced effects by exogenous application of L-Phenylalanine on Moringa oleifera . Egypt J Chem 65:523–532. https://doi.org/10.21608/EJCHEM.2022.109253.4978
Abu-Ellail FB, El-Mansoub MA (2020) Impact of water stress on growth, productivity and powdery Mildew disease. Alexandria Sci Exchange J 41:2. https://doi.org/10.21608/ASEJAIQJSAE.2020.91325
Acosta-Motos JR, Diaz-Vivancos P, Acosta M, Hernandez JA (2019) Effect of bio stimulants on plant responses to salt stress. In: Hasanuzzaman M, Fujita M, Oku H, Tofazzal Islam M (eds) Plant tolerance to environmental stress. CRC Press, Boca Raton, F L, USA, pp 363–380
Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18. https://doi.org/10.3390/agronomy7010018
Ahmed MA, Sh BG, EL- Housini EA, Badr EA (2016) Alleviation of water stress on wheat by Benzyl adenine. Int J Pharm Tech Res 9:109–119
Albalasmeh AA, Berhe AA, Ghezzehei TA (2013) A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydrate Polymers 97:253–261. https://doi.org/10.1016/j.carbpol.04.072
Ali Q, Daud MK, Haider MZ, Ali S, Rizwan M, Aslam N, Noman A, Iqbal N, Shahzad F, Deeba F et al (2017) Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol Biochem 119:50–59. https://doi.org/10.1016/j.plaphy.2017.08.010
Allen RG, Jensen ME, Wright JL, Burman RD (1989) Operational estimates of reference evapotranspiration. Agron J 81:650–662. https://doi.org/10.2134/agronj1989.00021962008100040019x
Babalar M, Pirzad F, SarcheshmehMa A, Talaei A, Lessani H (2018) Arginine treatment attenuates chilling injury of pomegranate fruit during cold storage by enhancing antioxidant system activity. Postharvest Biol Technol 137:31–37. https://doi.org/10.1016/j.postharvbio.2017.11.012
Bai Y, Kissoudis C, Yan Z, Visser RGFF, van der Linden G (2018) Plant behavior under combined stress: tomato responses to combined salinity and pathogen stress. Plant J 93:781–793. https://doi.org/10.1111/tpj.13800
Bakhoum GS, Badr EAELM, Sadak MS, Kabesh MO, Amin GA (2019) Improving growth, some biochemical aspects and yield of three cultivars of soybean plant by methionine treatment under sandy soil condition. Int J Environ Res 13:35–43. https://doi.org/10.1007/s41742-018-0148-1
Bakhoum GSh, Amin G, Sadak MSh (2022a) Biochemical study of faba bean ( Vicia faba L.) cultivars under different water regimes in sandy soil. Egypt J Chem 65:87–101. https://doi.org/10.21608/EJCHEM.2022.117184.5288
Bakhoum GS, Sadak MS, Tawfik MM (2022b) Chitosan and chitosan nanoparticle effect on growth, productivity and some biochemical aspects of Lupinustermis L plant under drought conditions. Egypt J Chem 65:537–549. https://doi.org/10.21608/EJCHEM.2021.97832.4563
Bakry AB, Sadak MSh, Abd El-Monem AA (2020) Physiological aspects of tyrosine and salicylic acid on morphological, yield and biochemical constituents of peanut plants. Pak J Biol Sci 23:375–384. https://doi.org/10.3923/pjbs.2020.375.384
Bakry AB, Sh SM, El-Karamany MF, Tawfik MM (2019) Sustainable production of two wheat cultivars under water stress conditions. Plant Archives 19:2307–2315
Barnett HL, Hunter BB (1999) Illustrated genera of imperfect fungi, 4th edn. Academic Press, San Diego California, United States of America, p 218
Bates LS, Waldan RP, Teare LD (1973) Rapid determination of free proline under water stress studies. Plant Soil 39:205–207
Bergmeyer HU (1974) Methods of enzymatic analysis I, 2nd edn. Academic Press, New York. https://doi.org/10.1016/B978-0-12-091302-2.X5001-4
Bhalani H, Thankappan R, Mishra GP, Sarkar T, Bosamia TC, Dobaria JR (2019) Regulation of antioxidant mechanisms by AtDREB1A improves soil-moisture deficit stress tolerance in transgenic peanut (Arachis hypogaea L.). PloS One 14:e0216706. https://doi.org/10.1371/journal.pone.0216706
Carter MR, Gregorich EG (2006) Soil sampling and methods of analysis. Univ. Canadian Society of Soil Science. by Taylor & Francis Group L L C https://www.aweimagazine.com
Chakraborty K, Mahatma M, Thawait L, Bishi S, Kalariya K, Singh A (2016) Water deficit stress affects photosynthesis and the sugar profile in source and sink tissues of groundnut (Arachis hypogaea L.) and impacts kernel quality. J Appl Botany Food Quality 89:98–104. https://doi.org/10.5073/JABFQ.2016.089.012
Chakraborty N (2021) Salicylic acid and nitric oxide cross-talks to improve innate immunity and plant vigor in tomato against Fusarium oxysporum stress. Plant Cell Rep 40:1415–1427. https://doi.org/10.1007/s00299-021-02729-x
Chakraborty N, Acharya K (2017) “NO way”! Says the plant to abiotic way’s. Plant Gene 11:99–105. https://doi.org/10.1016/j.plgene.2017.05.001
Chakraborty N, Mukherjee K, Sarkar A, Acharya (2019) Interaction between Bean and Colletotrichum gloeosporioides: understanding through a biochemical approach. Plants 8: 345. https://doi.org/10.3390/plants8090345
Chandra S, Chakraborty N, Dasgupta A et al (2015) Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Sci Rep 5:15195. https://doi.org/10.1038/srep15195
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182. https://doi.org/10.38212/2224-6614.2748
Chen JX, Wang XF (2006) Plant physiology experimental guide. Higher Education Press, Beijing
Chow PS, Landhausser SM (2004) A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiology 24:1129–1136
Das M, Das SK, Suthar SH (2002) Composition of seed and characteristics of oil from Karingda. Int J Food Sci Technol 37:893–896. https://doi.org/10.1046/j.1365-2621.2002.00638.x
Dawood MFA, Tahjib-Ul-Arif M, Sohag AAM, Abdel Latef AAH, Ragaey MM (2021) Mechanistic insight of allantoin in protecting tomato plants against ultraviolet C stress. Plants 10:11. https://doi.org/10.3390/plants10010011
Denwar NN, Simpson CE, Starr JL, Wheeler TA, Burow MD (2021) Evaluation and selection of interspecific lines of groundnut (Arachis hypogaea L.) for resistance to leaf spot disease and for yield improvement. Plants 10:873. https://doi.org/10.3390/plants10050873
Dikilitas M, Karakas S, Hashem A, Allah EA, Ahmad P (2016) Oxidative stress and plant responses to pathogens under drought conditions. In: Water Stress and Crop Plants. Wiley, Hoboken, NJ, USA, pp 102–123. https://doi.org/10.1002/9781119054450.ch8
Domingos P, Prado AM, Wong A, Gehring C, Feijo JA (2015) Nitric oxide: a multitasked signaling gas in plants. Mol Plant 8:506–520. https://doi.org/10.1016/j.molp.2014.12.010
Dukare AS, Paul S, Nambi VE et al (2019) Exploitation of microbial antagonists for the control of postharvest diseases of fruits: a review. Critic Rev Food Sci Nutri 59:1498–1513. https://doi.org/10.1080/10408398.2017.1417235
Elewa TA, Sadak MSh, Dawood MG (2017) Improving drought tolerance of quinoa plant by foliar treatment of trehalose. Agric Eng Int Special 245
El-Metwally IM, Sadak MSh, Saudy HS (2022) Stimulation effects of glutamic and 5-aminolevulinic acids on photosynthetic pigments, physio-biochemical constituents, antioxidant activity, and yield of peanut. Gesunde Pflanzen 74:915–924. https://doi.org/10.1007/s10343-022-00663-w
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147. https://doi.org/10.3389/fpls.2017.01147
Fancy NN, Bahlmann AK, Loake GJ (2017) Nitric oxide function in plant abiotic stress. Plant Cell Environ 40:462–472. https://doi.org/10.1111/pce.12707
Flexas J, Díaz-Espejo A, Conesa MA, Coopman RE, Douthe C, Gago J, Gallé A, Medrano H, Ribas-Carbo M, Tomás M et al (2016) Mesophyll conductance to CO2 and Rubisco as targets for improving intrinsic water use efficiency in C3 plants. Plant Cell Environ 39:965–982. https://doi.org/10.1111/pce.12622
Gan L, Wu X, Zhong Y (2015) Exogenously applied nitric oxide enhances the drought tolerance in hulless barley. Plant Prod Sci 91:52–56. https://doi.org/10.1626/pps.18.52
Ghorbani A, Pishkar L, Saravi KV, Chen MX (2023b) Melatonin-mediated endogenous nitric oxide coordinately boosts stability through proline and nitrogen metabolism, antioxidant capacity, and Na+/K+ transporters in tomato under NaCl stress. Front Plant Sci 14:1135943. https://doi.org/10.1134/S1021443718060079
Ghorbani A, Ghasemi-Omran VO, Chen M (2023a) The effect of glycine betaine on nitrogen and polyamine metabolisms, expression of glycoside-related biosynthetic enzymes, and K/Na balance of stevia under salt stress. Plants 12:1628. https://doi.org/10.3390/plants12081628
Gonzalez M, Guzman B, Rudkyk R, Romano E, Molina MA (2003) Spectrophotometric determination of phenolic compounds in propolis Lat. Am J Pharm 22:243–248
Groß F, Durner J, Gaupels F (2013) Nitric oxide; antioxidants and prooxidants in plant defence responses. Front Plant Sci 4:419. https://doi.org/10.3389/fpls.2013.00419
Gusmiaty M, Restu A, Payangan RY (2019) Production of IAA (Indole Acetic Acid) of the rhizosphere fungus in the Suren community forest stand. IOP Conf Series: Earth Environ Sci 343:012058. https://doi.org/10.1088/1755-1315/343/1/012058
Gyamfi MA, Yonamine M, Aniya Y (2002) Free radical scavenging action of medicinal herbs from Ghana: Thonningia sanguine on experimentally induced liver injuries. Gen Pharmacol 32:661–667. https://doi.org/10.1016/S0306-3623(98)00238-9
Hanafy RS, Sadak MSh (2023) Foliar spray of stigmasterol regulates physiological processes and antioxidant mechanisms to improve yield and quality of sunflower under drought stress. J Soil Sci Plant Nutri 23:2433–2450. https://doi.org/10.1007/s42729-023-01197-4
Hasanuzzaman M, Nahar K, Rahman A, Inafuku M, Oku H, Fujita M (2018) Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiol Mol Biol Plants 24:993–1004. https://doi.org/10.1007/s12298-018-0531-6
Hilal AA, Metwally AH, Khaled SA, El-Deeb AA (1994) Evaluation of peanut cultivars, date of sowing and NPK as integrated control measurement against soil borne diseases. Zagazig J Agric Res 21:1151–1162
Hodges DM, De Long JM, Forney C, Prange PK (1999) Improving the thiobarbaturic acid reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611. https://doi.org/10.1007/s004250050524
Hoheneder F, Hofer K, Groth J, Herz M, Heß M, Hückelhoven R (2021) Ramularia leaf spot disease of barley is highly host genotype-dependent and suppressed by continuous drought stress in the field. J Plant Dis Prot 128:749–767. https://doi.org/10.1007/s41348-020-00420-z
Homme PM, Gonzalez B, Billard J (1992) Carbohydrate content, frutane and sucrose enzyme activities in roots, stubble and leaves of rye grass (Lolium perenneL.) as affected by sources/link modification after cutting. J Plant Physiol 140:282–291. https://doi.org/10.1016/S0176-1617(11)81080-1
Ijaz M (2011) Epidemiology and management of Cercospora leaf spot of groundnut (Arachis hypogaeaL.) in Punjab. Doctor of Philosophy Thesis. Mehr Ali Shah Arid Agriculture University Rawalpindi, Pakistan Pir, pp 339–346
Jasim AH, Abo Al Timmen WM, Abid AS (2016) Effect of salt stress on plant growth and free endogenous hormones of primed radish (Raphanus Sativus, L.) seeds with salicylic acid. Int J Chem Tech Res 9(6):339–346
Jaspers P, Kangasjärvi J (2010) Reactive oxygen species in abiotic stress signaling. Physiol Plant 138:405–413. https://doi.org/10.1111/j.1399-3054.2009.01321.x
Kankam F, Akpatsu IB, Tengey TK (2022) Leaf spot disease of groundnut: a review of existing research on management strategies. Cogent Food Agric 8:2118650. https://doi.org/10.1080/23311932.2022.2118650
Kausar F, Shahbaz M, Ashraf M (2013) Protective role of foliar-applied nitric oxide in Triticum aestivum under saline stress. Turk J Bot 37:1155–1165. https://doi.org/10.3906/bot-1301-17
Kazan K (2013) Auxin and the integration of environmental signals into plant root development. Annal Botany 112:1655–1665. https://doi.org/10.1093/aob/mct229
Keller J, Karmeli D (1975) Trickle irrigation design parameters. Rain Bird Sprinkler Manufacturing Corporation, Glendora, California, p 24
Khan HA, Siddique KHM, Colmer TD (2016) Vegetative and reproductive growth of salt-stressed chickpea are carbon-limited, sucrose infusion at the reproductive stage improves salt tolerance. J Exp Bot 68:2001–2011. https://doi.org/10.1093/jxb/erw177
Khan M, Al Azzawi TNI, Ali S, Yun BW, Mun BG (2023) Nitric oxide, a key modulator in the alleviation of environmental stress-mediated damage in crop plants: a meta-analysis. Plants 12:2121. https://doi.org/10.3390/plants12112121
Kolbert ZS et al (2019) A forty-year journey: the generation and roles of NO in plants. Nitric Oxide 93:53–70. https://doi.org/10.1016/j.niox.2019.09.006
Kolte SJ (1984) Disease of annual edible oilseed crops Vol. I. Peanut Disease, CRC Press, Inc., Boka, Raton, Florida, p 143
Kong FX, Hu W, Chao WL, Sang WL, Wang LS (1999) Physiological responses of Mexicana to oxidative stress of SO2. Environ Exp Bot 42:201–209. https://doi.org/10.1016/S0098-8472(99)00034-9
Kumari A, Kumar R, Maurya S, Choudhary JS, Kumar S (2013) Antifungal efficacy of aqueous extracts of Neem cake, Karanj cake and vermicompost against some phytopathogenic fungi. The Bioscan 8:671–674
Latef AAHA, Srivastava AK, Saber H, Alwaleed EA, Tran LSP (2017) Sargassum muticum and Janiarubens regulate amino acid metabolism to improve growth and alleviate salinity in chickpea. Sci Rep 7:10537. https://doi.org/10.1038/s41598-017-07692-w
Li M, Xia Q, Lv S, Tong J, Wang Z, Nie Q, Yang J (2022) Enhanced CO2 capture for photosynthetic lycopene production in engineered Rhodopseudomonas palustris, a purple nonsulfur bacterium. Green chemistry 24:7500–7518. https://doi.org/10.1039/D2GC02467E
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–VIS spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P (eds) Current protocols in food analytical chemistry (CPFA). Wiley, New York, pp F4.3.1–F4.3.8
Liu JH, Wang W, Wu H, Gong X, Moriguchi T (2015) Polyamines function in stress tolerance: from synthesis to regulation. Front Plant Sci 6:827. https://doi.org/10.3389/fpls.2015.00827
Lobell DB, Schlenker W, Costa-Roberts J (2011) Climate trends and global crop production since 1980. Science 333:616–620. https://doi.org/10.1126/science.1204531
Maninderpal S (2011) Physiological consequence of late leaf spot on peanut (Arachis hypogaeaL.) cultivars of differing resistance. Ph.D. Thesis, Florida Univ., p 138
Mbarki S, Sytar O, Cerda A, Zivcak M, Rastogi A, He X, Zoghlami A, Abdelly C, Brestic M (2018) Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In: Kumar V (ed) Salinity responses and tolerance in plants, vol 1. Springer, Cham, Switzerland, pp 85–136
McDonald D, Subrahmanyam P, Gibbons RW, Smith DH (1985) Early and late leaf spot of groundnut. In: Information Bulletin no. 21. Patancheru, A P. 502324, India. International Crops Research Institute for the Semi-Arid Tropics, p 24
Mohammed KE, Afutu E, Odong TL, Okello DK, Nuwamanya E, Grigon O, Rubaihayo PR, Okori P (2018) Assessment of groundnut (Arachis hypogaea L.) genotypes for yield and resistance to late leaf spot and rosette diseases. J Exp Agric Int 21:1–13. https://doi.org/10.9734/JEAI/2018/39912
Nabi RBS, Lee MH, Kim S, Kim J, Kim MY, Cho KS, Oh E (2022) Physiological and biochemical responses of diverse peanut genotypes under drought stress and recovery at the seedling stage. Plant Breed Biotech 10:15–30. https://doi.org/10.9787/PBB.2022.10.1.15
Nan M, Zhao GQ, Li J, Cai JK (2018) Correlation analysis and synthesize evaluation of yield and quality introduced oat varieties in the semiarid of Northwest. Acta Agrestia Sinica 26:125–133. https://doi.org/10.11733/j.issn.1007-0435.2018.01.015
Nasibi F, Yaghoobi M, Kalantari K (2011) Effect of exogenous arginine on alleviation of oxidative damage in tomato plant under water stress. J Plant Interact 6:291–296. https://doi.org/10.1080/17429145.2010.539708
Oz MT, Eyidogan F, Yucel MO, Ktem HA (2015) Functional role of nitric oxide under abiotic stress conditions. In: Khan MN, Mobin M, Mohammad F, Corpasn FJ (eds) Nitric oxide action in abiotic stress responses in plants. Springer, New York, pp 21–41
Pandey P, Kumar M (2019) S (2019) Plant-pathogen interaction in the presence of abiotic stress: what do we know about plant responses? Plant Physiol Rep 24:541–549. https://doi.org/10.1007/s40502-019-00483-7
Pandey P, Irulappan V, Bagavathiannan MV, Senthil-Kumar M (2017) Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio - morphological traits. Front Plant Sci 8:537. https://doi.org/10.3389/fpls.2017.00537
Pedrol N, Tamayo PR (2001) Protein content quantification by Bradford method. In: Handbook of plant ecophysiology techniques chapter: 19. Kluwer Academic Publishers, Dordrecht, The Netherlands Editors: Reigosa MJ
Pietrowska E, Różalska S, Kaźmierczak A, Nawrocka J, Małolepsza U (2015) Reactive oxygen and nitrogen (ROS and RNS) species generation and cell death in tomato suspension cultures - Botrytis cinerea interaction. Protoplasma 252:307–319. https://doi.org/10.1007/s00709-014-0680-6
Polacco JC, Mazzafera P, Opinion TT (2013) Nickel and urease in plants: still many knowledge gaps. Plant Sci 199–200:79–90. https://doi.org/10.1016/j.plantsci.2012.10.010
Polash MAS, Sakil MA, Tahjib-Ul-Arif M, Hossain MA (2018) Effect of salinity on osmolytes and relative water content of selected rice genotypes. Trop Plant Res 5:227–232. https://doi.org/10.22271/tpr.2018.v5.i2.029
Price J, Warren R, Forstenhäusler N et al (2022) Quantification of meteorological drought risks between 1.5 °C and 4 °C of global warming in six countries. Climatic Change 174:12. https://doi.org/10.1007/s10584-022-03359-2
Ragaey MM, Sadak MSh, Dawood MFA, Mousa NHS, Hanafy RS, Latef AAHA (2022) Role of signaling molecules sodium nitroprusside and arginine in alleviating salt-induced oxidative stress in wheat. Plants 11:1786. https://doi.org/10.3390/plants11141786
Rai KK, Pandey N, Rai SP (2020) Salicylic acid and nitric oxide signaling in plant heat stress. Physiol Plant 168:241–255. https://doi.org/10.1111/ppl.12958
Ramadan AA, Abd Ebtihal EM, Sadak MS (2019) Comparative study for the effect of arginine and sodium nitroprusside on sunflower plants grown under salinity stress conditions. Bull Natl Res Cent 43:118. https://doi.org/10.1186/s42269-019-0156-0
Ramegowda V, Senthil-Kumar M (2015) The interactive effects of simultaneous biotic and abiotic stresses on plants: mechanistic understanding from drought and pathogen combination. J Plant Physiol 176:47–54. https://doi.org/10.1016/j.jplph.2014.11.008
Rangaswami G, Mahadevan A (2006) Diseases of crops plants in India, 4th edn. Prentice Hall of India Pvt. Ltd., New Delhi, pp 335–336
Rasheed R, Ashraf MA, Ali S, Iqbal M, Zafar S (2022) Plant metabolism adjustment in exogenously applied NO under stress. In: Nitric Oxide in Plant Biology. Academic Press, Cambridge, MA, USA, pp 261–296
Rennenberg H, Wildhagen H, Ehlting B (2010) Nitrogen nutrition of poplar trees. Plant Biol (Stuttg.) 12:275–291. https://doi.org/10.1111/j.1438-8677.2009.00309.x
Sadak MS, Bakry BA (2020) Alleviation of drought stress by melatonin foliar treatment on two flax varieties under sandy soil. Physiol Mol Biol Plants 26:907–919. https://doi.org/10.1007/s12298-020-00789-z
Sadak MS, Bakry AB, Taha MH (2019) Physiological role of trehalose on growth, some biochemical aspects and yield of two flax varieties grown under drought stress. Plant Arch 19:215–225
Sadak MS, El-Enany MAM, Bakry BA, Abdallah MMS, El-Bassiouny HMS (2020) Signal molecules improving growth, yield and biochemical aspects of wheat cultivars under water stress. Asian J Plant Sci 19:35–53. https://doi.org/10.3923/ajps.2020.35.53
Sadak MS, Hanafy RS, Elkady FMAM, Mogazy AM, Abdelhamid MT (2023) Exogenous calcium reinforces photosynthetic pigment content and osmolyte, enzymatic, and non-enzymatic antioxidants abundance and alleviates salt stress in bread wheat. Plants 12:1532. https://doi.org/10.3390/plants12071532
Sadak SM (2016) Physiological role of signal molecules in improving plant tolerance under abiotic stress. Inter J of Chem Tech Res 9:46–60
Sadak SM (2022) Nitric oxide and hydrogen peroxide as signaling molecules for better growth and yield of wheat plant exposed to water deficiency. Egyptian J Chem 65:209–223. https://doi.org/10.21608/ejchem.2022.117465.5297
Sadak SM, Ramadan AA (2021) Impact of melatonin and tryptophan on water stress tolerance in white lupine (Lupinustermis L.). Physiol Mol Biol Plants 27:469–481. https://doi.org/10.1007/s12298-021-00958-8
Sadak MS, Sh BG (2022) Selenium-induced modulations in growth, productivity and physiochemical responses to water deficiency in Quinoa (Chenopodium quinoa) grown in sandy soil. Biocatal Agric Biotechnol 44:102449. https://doi.org/10.1016/j.bcab.2022.102449
Saijo Y, Loo EP (2020) Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol 225:87–104. https://doi.org/10.1111/nph.15989
Sarkar A, Chakraborty N, Acharya K (2021) Unraveling the role of nitric oxide in regulation of defense responses in chilli against Alternaria leaf spot disease. Physiologic Mol Plant Pathol 114:101621. https://doi.org/10.1016/j.pmpp.2021.101621
Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, Dindaroglu T, Abdul-Wajid HH, Battaglia ML (2021) Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10:259. https://doi.org/10.3390/plants10020259
Shokes FM, Culbreath AK (1997) Early and late leaf spots. In: Kokalis-Burelle N, Porter DM, Rodriguez-Kabana R, Smith DH, Subrahmanyam P (eds) Compendium of peanut diseases, 2nd edn. APS Press, Saint Paul, MN, USA, pp 17–20
Singh NB, Yadav K, Amist N (2016) Positive effects of nitric oxide on Solanum lycopersicum. J Plant Interact 9:10–18. https://doi.org/10.1080/17429145.2012.748937
Sjokvist E, Lemcke R, Kamble M, Turner F, Blaxter M, Havis NH, Lyngkjær MF, Radutoiu S (2019) Dissection of Ramularia leaf spot disease by integrated analysis of barley and Ramularia collo- cygni transcriptome responses. Mol Plant Microbe Interact 32:176–193. https://doi.org/10.1094/MPMI-05-18-0113-R
Sofy M, Mohamed H, Dawood M, Abu-Elsaoud A, Soliman M (2021) Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Arch Agron Soil Sci 21:1–22
SPSS Statistics 17.0.0 (2008) SPSS for Windows. SPSS Inc.
Steel RGD, Torrie GH, Dickey DA (1997) Principles and procedures of statistics: a biometrical approach, 3rd edn. McGraw-Hill, New York, USA, p 450
Stefanov MA, Rashkov GD, Yotsova EK, Borisova PB, Dobrikova AG, Apostolova EL (2023) Protective effects of sodium nitroprusside on photosynthetic performance of Sorghum bicolor L. under salt stress. Plants 12:832. https://doi.org/10.3390/plants12040832
Subrahmanyam PD, Donald M, Waliyar FR, Nigam LJ, Gibbons SN, Rao RW, Singh VR, Pande AKS, Reddy PM, Rao SPV (1995) Screening methods and sources of resistance to rust and late leaf spot of groundnut. Information Bulletin no. 47. International Crops Research Institute for the Semi-Arid Tropics
Sunarti S, Kissoudis C, Van DerHoek Y, Van Der Schoot H, Visser RGF, Van Der Linden CG, VanDe WC, Bai Y (2022) Drought stress interacts with powdery Mildew infection in tomato. Front Plant Sci 13:845379. https://doi.org/10.3389/fpls.2022.845379
Telles AC, Kupski L, Furlong EB (2017) Phenolic compound in beans as protection against mycotoxins. Food Chem 214:293–299. https://doi.org/10.1016/j.foodchem.2016.07.079
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci 5:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Wang L, Li X, Gao F, Liu Y, Lang S, Wang C, Zhang D (2023) Effect of ultrasound combined with exogenous GABA treatment on polyphenolic metabolites and antioxidant activity of mung bean during germination. Ultrasonics Sonochem 94:106311. https://doi.org/10.1016/j.ultsonch.2023.106311
Wang Z, Li G, Sun H, Ma L (2018) Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biology Open 7:035279. https://doi.org/10.1242/bio.035279
Wegulo S, Loren G, Robert H, Jackson-Ziems Tamra A, Bo L, Kevin K (2013) Impacts of drought on disease development and management. Plant Pathology 537 http://digitalcommons.unl.edu/plantpathpapers/537
Wendehenne D, Gould K, Lamotte O, Durner J, Vandelle E, Lecourieux D, Courtois C, Barnavon L, Bentejac M, Pugin A (2005) NO signaling functions in the biotic and abiotic stress responses. BMC Plant Biol 5:S3
Winter G, Todd CD, Trovato M, Forlani G, Funck D (2015) Physiological implications of arginine metabolism in plants Front. Plant Sci 6:1–14. https://doi.org/10.3389/fpls.2015.00534
Wu ZL, Yin XB, Bañuelos GS et al (2016) Effect of selenium on control of postharvest gray mold of tomato fruit and the possible mechanisms involved. Front Microbiol 6:1441. https://doi.org/10.3389/fmicb.2015.01441
Zanjare SR, Suryawanshi AV, Zanjare SS, Shelar VR, Balgude YS (2023) Screening of Groundnut (Arachishy pogaea L.) Genotypes for identification of sources of resistance against leaf spot disease. Legume Research 46:288–294. https://doi.org/10.18805/LR-4370
Zarattini M, Farjad M, Launay A, Cannella D, Soulié MC, Bernacchia G et al (2021) Every cloud has a silver lining: how abiotic stresses affect gene expression in plant-pathogen interactions. J Exp Bot 72:1020–1033. https://doi.org/10.1093/jxb/eraa531
Zhang XH, Shen L, Li FJ et al (2013) Arginase induction by heat treatment contributes to amelioration of chilling injury and activation of antioxidant enzymes in tomato fruit. Postharvest Biol Technol 79:1–8. https://doi.org/10.1016/J.POSTHARVBIO.2012.12.019
Zheng Z, Sun Z, Fang Y, Qi F, Liu H, Miao L et al (2018) Genetic diversity, population structure, and botanical variety of 320 global peanut accessions revealed through tunable genotyping-by-sequencing. Sci Rep 8:14500. https://doi.org/10.1038/s41598-018-32800-9
Zhu J (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324. https://doi.org/10.1016/j.cell.2016.08.029
Zhu LQ, Du HY, Wang W et al (2019) Synergistic effect of nitric oxide with hydrogen sulfide on inhibition of ripening and softening of peach fruits during storage. Scientia Horticulturae 256:108591. https://doi.org/10.1016/j.scienta.2019.108591
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
GSB: designed and performed the experiment, responsible of statistical analysis, and wrote and reviewed the manuscript; MSS: designed and performed the experiment, responsible of physiological analysis of the manuscript, and also wrote and reviewed the manuscript; MST: deigned and performed the experiment, responsible of the work of biotic stress, and also wrote and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bakhoum, G.S., Sadak, M.S. & Thabet, M.S. Induction of Tolerance in Groundnut Plants Against Drought Stress and Cercospora Leaf Spot Disease with Exogenous Application of Arginine and Sodium Nitroprusside Under Field Conditions. J Soil Sci Plant Nutr 23, 6612–6631 (2023). https://doi.org/10.1007/s42729-023-01514-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01514-x