Abstract
Purpose
Boron (B) is an essential micronutrient required throughout the growth cycle of plants so effectively supplying crops with B using fertilizers is challenging. The purpose of this study was to assess the agronomic effectiveness of mechanochemically synthesized zinc borate as a slow release B source and compare it to commonly used B sources after incorporation with different macronutrient carriers.
Methods
Zinc borate synthesized using a green mechanochemical method as well as commercial B sources (borax, colemanite, and commercial zinc borate) were incorporated with various macronutrient fertilizers (monoammonium phosphate – MAP, muriate of potash – MOP and urea). The fertilizers were evaluated by a) assessing the solubility behaviour of these products; and b) comparing potential leaching losses, plant growth, and plant uptake through a greenhouse study.
Results
The mechanochemically synthesized zinc borate, commercial zinc borate, and colemanite had similar dissolution rates when MAP was the carrier, but both zinc borates dissolved more B than colemanite when MOP and urea were the carriers. In the pot trial, high losses of B in leachates resulted in low B uptake by plants fertilized with soluble sodium tetraborate. All the slow-release B sources showed less B leaching and greater B uptake compared to the soluble B treatment, but more B was leached for the mechanochemically synthesized than for the commercial zinc borate.
Conclusions
Our study indicates that mechanochemically synthesized zinc borate could be effective in matching plant demand for B and reducing leaching losses in high rainfall environments, particularly with urea as the carrier, while providing the benefit of lower waste stream production compared to commercial zinc borate sources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Boron (B) is an essential micronutrient for crop growth. Boron fertilization can be challenging due to the narrow window between deficiency and toxicity and the high mobility of B in soil (Mengel et al. 2001). Both B toxicity and deficiency will harm plant growth (Camacho-Cristóbal et al. 2008; Gupta 1983; Wimmer and Eichert 2013) and thus decrease crop yield (Findeklee et al. 1997; Pregno and Armour 1992). Boron deficiency generally occurs in highly weathered, coarse textured, low organic matter accumulated, and frequently cultivated soils, because B is easily removed from the soil through leaching, runoff, and plant uptake (Shorrocks 1997). Zinc (Zn) is present in the soil as a cation and is commonly deficient in calcareous soils due to strong sorption and fixation (Alloway 2009). Until now, Zn deficiency remains a global health problem that affects more than 17% of the world’s population (Myers et al. 2015).
The most commonly applied B sources are soluble compounds, such as boric acid and sodium borates. However, these soluble sources can cause toxicity and are easily leached from soil. Slow-release sources of B could contribute to a more efficient and precise B application. Existing slow-release B sources are mainly colemanite, ulexite and other sodium/calcium borates (Broschat 2008). More recently, new slow-release B compounds have been proposed such as boron phosphates (BPO4) (Abat et al. 2015a, c, d), layered double hydroxides with B incorporated (Songkhum et al. 2018), and organic (Svishcheva et al. 2022; Thombare et al. 2021) or hybrid matrices incorporated with B (Xie et al. 2011).
Generally, micronutrients required by plants in small amounts are combined with macronutrients nitrogen (N), phosphorus (P), and/or potassium (K), or applied as foliar liquids to decrease the costs of application and promote the uniform distribution of micronutrients in the field (Gupta, 1993). However, combining slow-release micronutrients with macronutrients through coating or co-granulation could change their release behaviour due to new chemical reactions or physical aggregation (Abat et al. 2015d; Milani et al. 2012). It is important to find new slow-release fertilizers that retain their slow-release character when combined with macronutrients, and have potential to achieve better plant growth and agronomic and environmental efficiency. Only a few attempts have been made to address this issue when assessing new slow-release B sources (Abat et al. 2015c, d; da Silva et al. 2018; Samreen et al. 2021).
Mechanochemistry has been proposed as a more sustainable synthesis method than wet chemistry because it relies solely on mechanical forces to decrease particle size, increase particle contact area, accumulate defects and promote reactions without waste streams being produced (Ardila-Fierro and Hernández 2021; Boldyrev 2006). Mechanochemical methods such as ball milling, crushing, and shearing are existing processes in the fertilizer industry for phosphate rock and other mineral processing and have been widely applied in other industries such as alloy and pharmaceutical production (Baláž, 2008; Baláž et al. 2013). Several studies have assessed the possibility of using mechanochemistry to synthesize slow-release fertilizers. The focus of previous mechanochemically synthesized fertilizer studies was mainly the macronutrients N, P and K. For instance, metal (hydr)oxides (Honer et al. 2018; Julien et al. 2020), LDH (Lei et al. 2018; Solihin et al. 2010), and ionic co-crystals (Barčauskaitė et al. 2020; Honer et al. 2017; Sharma et al. 2019) have been used to incorporate N, clay minerals (Solihin et al. 2011), LDH (Buates and Imai 2021), metal (hydr)oxides (Zhang and Saito 2009) to incorporate P, and struvite (Solihin et al. 2010), phlogopite (Said et al. 2018), clay minerals (Borges et al. 2017; Solihin et al. 2011), SiO4 (Yuan et al. 2014) and LDH (Lei et al. 2018) to incorporate K. Most of these proposed slow-release fertilizers had low water solubility in static or batch solubility tests. However, very few agronomic comparisons were made to compare mechanochemically synthesized products with commercial products.
Hydrated zinc borates are low-solubility B compounds that are used as flame retardants and preservatives for bio-composite/ wood (Schubert, 2019) and could potentially be used as slow-release B and Zn fertilizer sources, with the addition of zinc, an essential plant nutrient, being an added benefit. Different approaches have been used to synthesize zinc borate, such as hydrothermal methods which produce large amounts of waste streams during manufacturing (Gao and Liu 2009; Gönen et al. 2009). In a previous study, we used mechanochemistry to synthesize a zinc borate using a ball mill and water-assisted reaction (Zheng et al. 2021). Minor amount of water was used for the synthesis (1.7 mL water per g product) compared to other conventional methods (77.5–250 g water per g product). The B release behaviour of the mechanochemically synthesized zinc borate with water assisted reaction was compared to commercial colemanite and sodium borate in a column dissolution study in which only 26% of B was released from zinc borate compared to 95% of B from sodium borate over 72 h.
This study aimed to compare the agronomic effectiveness of the mechanochemically synthesized zinc borate with other B sources when coated onto or co-granulated with macronutrient carriers. We hypothesize that a) zinc borate could be synthesized in situ during the coating process using water atomization to promote water-assisted reaction; b) some macronutrients may affect the slow-release characters while others would be more suited as carrier for B source; and c) the mechanochemical synthesized zinc borate could be used as a slow-release B source in high rainfall areas for plant growth.
2 Material and methods
2.1 Mechanochemical synthesis of zinc borate
Zinc borate was prepared using a mechanochemical method described in the previous study with or without further water-assisted reaction (Zheng et al. 2021). In short, 1.35 g of ZnO and 1.65 g of B2O3 were added in 50 mL zirconia jars with six zirconia balls (15 mm diameter, 10 g per ball) to achieve a ball to solid (reactants) mass ratio around 20. A minor volume of water (510 µL) was added for liquid assisted grinding (LAG) to promote homogeneity. In the previous study, we used a Retsch MM400 mixer mill (Haan, Germany), but in this study a planetary mill (PM200, Retsch, Haan, Germany) was used to confirm the repeatability of the synthesis method. The milling procedure was performed for 8 h with a change of direction every 1 h and a 5-min break after 1 h of milling to avoid overheating. A control sample was subsampled 1 min after the milling procedure started. Further water-assisted reaction (WAR) was performed in the same manner as described in the previous study for both subsampled control and sample milled for 8 h. The WAR was achieved by adding a small amount of water (1.5 mL water per g sample) to the products to make a paste-like sample and the sample was sealed for 72 h before drying under room temperature for the reaction to complete. The synthesized zinc borate was confirmed with X-ray diffraction and field emission scanning electron microscopy (FE-SEM, Quanta 450, FEI, USA).
2.2 Manufacturing of fertilizer treatments
Macronutrient fertilizers were coated or compacted (for the pot trial) with different sources of B and Zn (Table 1) to assess the solubility and plant response of the formulations. The B/Zn sources used were control samples with essentially no milling either without (C) or with water-assisted reaction (WC) and the samples that had been milled for 8 h without (ZB) or with water-assisted reaction (WZB). For comparison, a commercial zinc borate (CZB) from Turkey (Etimine USA. Inc.) was included, as well as a commercial fast-release B source (sodium tetraborate, TB) (U.S. Borax Inc,) and a commercial slow-release source (colemanite (Co) from Turkey (Etimine USA Inc). The formula of the synthesized zinc borate is 2ZnO·3B2O3·7H2O based on the XRD results and the commercial zinc borate is 2ZnO·3B2O3·3.5H2O according to the chemical label. The digestion results (Table 1) indicated that the B content in the synthesized zinc borate was 12% compared to 10% for the commercial zinc borate. The zinc sources of ZnO and ZnSO4·7H2O were chosen due to the differences in solubility of Zn. In addition, both of them are cheap and commonly used as zinc fertilizers in agricultural practice. All slow-release B treatments contained slow-release Zn sources (zinc borate or ZnO), while ZnSO4·7H2O was compacted with TB to produce a fast-release B and Zn treatment in the pot experiment. The macronutrient granules, urea, MOP (muriate of potash) and MAP (monoammonium phosphate), were either sieved to achieve a similar size (2.0–3.35 mm) before being coated or were ground, sieved (< 250 μm) and co-compacted with the B sources. The target B and Zn concentrations in the fertilizers were 5 g kg−1 and 10 g kg−1, respectively.
For the coating procedure, 50 g of sieved macronutrient granules and coating material were added to a Petri dish with cover lid. The weight of powdered coating material added was around 2.1 g for WC, ZB and WZB, 1.7 g for C, 2.3 g for Co and 2.8 g for TB. The powders were mixed to achieve the target concentrations of 5 g B kg−1 and 10 g Zn kg−1. The powders and granules were manually shaken before spraying a total of 750 µL deionized (DI) water in three pulses (each 5 s with a flow rate of 3 mL per min) to allow physical and chemical reactions at the granule surface. The coated granules were dried at room temperature for two days and sieved for further study. The coated granules were digested overnight at room temperature using aqua regia (HNO3: HCl = 1:3 v/v), followed by 45 min of heating at 80° C and 165 min of heating at 125° C in a digestion block. The digests were analyzed by inductively-coupled plasma optical emission spectroscopy (ICP-OES, Avio 200, Perkin Elmer, Waltham, USA) to determine the total content of Zn and B coated on the granules. The amounts of Zn and B coated onto the granules varied between treatments because a) relatively large amounts of ZnSO4·7H2O were required for the treatment with soluble sources, making it hard to achieve full adherence; and b) ZnO and zinc borate tend to agglomerate rather than evenly distribute on the surface of the granules as a result of hydrophobicity. Therefore, for the pot experiment, we used compacted fertilizers to achieve more precise B and Zn contents. The compaction was carried out by mixing the micronutrient and macronutrient powders and compacting the powders using a pellet press. The powders were mixed to achieve the same target concentrations as before (5 g B kg−1 and 10 g Zn kg−1) and 7 g of the powder was mixed with 0.3 mL of DI water to form a paste. The paste was placed into a 4 cm cylinder and 9.8 MPa pressure was applied to form a large pellet. The pellet was air-dried and cut into smaller pellets of similar size (2–3 mm) and weight (around 40 mg).
2.3 Surface morphology and component of coated granules
A previous study indicated that Zn-containing coating material could react with MAP and primarily form ZnNH4PO4 when using water to assist with coating (Milani et al. 2012). To confirm the crystal structure of the treatments coated onto MAP, the granules were abraded by rotated 10 g of granules placed in a 75 mL plastic container at 30 rpm for 30 s. A fraction of the abraded coating material was sieved through a 1.0-mm sieve and analyzed by XRD to verify the composition of the coating material on the surface of the granules. The surface and cross-sectional morphology of the sample coated with Co, CZB and WZB were evaluated using field emission scanning electron microscopy (FE-SEM, Quanta 450, FEI, USA).
2.4 Batch solubility
The solubility of the samples was assessed by weighing 0.1 g of the sample in a 50 mL digestion tube with 10 mL of DI water. The samples were shaken in an end-over-end shaker for 1 h followed by 10 min centrifugation at 4685 g. A subsample of supernatant was taken for further analysis and 5 mL of DI water was added to the original 50 mL tube and shaken for 23 h. The suspensions were filtered through a 0.2 µm syringe filter prior to analysis using ICP-OES). To check the mass balance, the sediment remaining in the centrifuge tubes was digested overnight at room temperature using aqua regia (HNO3: HCl = 1:3 v/v), followed by 45 min heating at 80° C and 165 min heating at 125° C in a digestion hot block. Concentrations of B and Zn in the digest were analyzed by ICP-OES.
2.5 Soil leaching and pot experiment
In the soil leaching and pot experiment, only the treatments produced by the co-compaction with urea and MOP were used. To assess the plant response under leaching conditions, a pot experiment was performed in a growth chamber (20°C/15°C day/night temperature and relative humidity of 90–100%) for the first eight weeks and in a glasshouse for the remaining four weeks. Canola was chosen as the test crop because the sensitivity to B deficiency is high (Salisbury 1999). The soil used in this study was Mt Compass (Entisol), an acidic sandy soil selected for its low B concentration (< 0.3 mg B kg−1 soil) and prone to B losses through leaching (Table 2). The soil was collected from the topsoil (0–10 cm) at Mount Compass, South Australia and air dried before being sieved to less than 2 mm. Plastic pots with a diameter of 12 cm were used and filled with 1 kg of soil. The soil was wetted and mixed in a plastic bag to reach 50% field capacity 2 days before transfer into the pots.
The fertilizer used in the pot experiment was prepared by compacting 5 g B kg−1 (CZB, WZB, Co and TB) and 10 g Zn kg−1 (ZnO in Co and ZnSO4 in TB) with MOP and urea. The compacted fertilizer granules had an average weight of around 40 mg per granule. To each pot, 5 granules (totaling 0.2 g fertilizer containing approximately 1 mg B and 2 mg Zn) were equally distributed 3.5 cm below the soil surface 2 days before imposition of the leaching treatment. Treatments were randomized with three replicates for each treatment using a complete block design generated by GenStat 19th edition. The leaching procedure was carried out with artificial rain water (ARW, 5 × 10–4 M CaCl2, Ca(NO3)2, MgCl2 and 10–4 M Na2SO4, KCl) with a pH of 5.9. Each pot was slowly leached four times with 350 mL (or a total of four pore volumes) of ARW to mimic a heavy rainfall event and remove the available B from the soil. The leachates of each pot were collected and filtered for measurement of pH and ICP-OES analysis for B concentrations. After the leaching event, the pots were left to dry under ambient conditions for 3 days prior to planting. Canola (Brassica napus L.) seeds (antifungal treated) supplied by Seed Service Australia were geminated in an incubator for 3 days. The geminated seeds (five seeds per pot) were transplanted 1.5 cm below the soil surface and thinned to three seedlings after 1 week, two after 2 weeks and one after 3 weeks. All pots received 10 mL basal nutrient solution 5 days after planting and every week during the growth period (11 times in total). In total, all pots received 347 mg N kg−1, 136 mg P kg−1, 124 mg Ca kg−1, 80.6 mg S kg−1, 20.6 mg Mg kg−1, 9.4 mg Fe kg−1, 6.9 mg Mn kg−1, 0.7 mg Cu kg−1 and 0.07 mg Mo kg−1. All pots were maintained at 50% field capacity by checking the weight every day and adding deionized water as needed. Harvesting was carried out 12 weeks after planting. The plant shoots were cut 1 cm above the soil surface and dried in an oven at 60° C. The weight was recorded, and the samples further ground and digested for ICP-OES analysis of the B and Zn concentrations.
2.6 Statistical analysis
Differences in the amounts of B and Zn leached, B uptake and concentration in the plant and plant yield were assessed using one-way ANOVA (analysis of variance), where needed, the data were log-transformed to homogenize variance, followed by a post-hoc Duncan's Multiple Range Test at P ≤ 0.05 using GenStat 19th.
3 Results
3.1 Micronutrient concentrations and crystal morphology
The crystal phase of the mechanochemically synthesized zinc borate was confirmed by XRD and its morphology was confirmed by SEM (Figure S1). Consistent with the previous study, the 8 h milling followed by water-assisted reaction successfully synthesized an octahedral crystal of zinc borate with a high conversion rate (Zheng et al. 2021). The Zn and B concentrations of coated fertilizers (Table S1) were generally close to the target concentrations. However, in some treatments such as TB, WC and Co there was less than half of the expected concentrations in the granules.
3.2 Analysis of abraded material
The formation of NH4ZnPO4 occurred during the coating procedure with minor amounts of water applied. The XRD results collected from sieving coated MAP showed large amounts of commercial zinc borate were transformed to NH4ZnPO4 (Figure S2b). In line with the previous results (Zheng et al. 2021), the morphology of the CZB coating changed to a different crystal morphology (plate like) when coated to MAP compared to MOP and urea (Figure S3d, e, f). Although WZB was largely maintained as zinc borate during the coating process (Figure S2a, S3g, h, i), it still largely dissolved in the batch dissolution test of the MAP granules.
3.3 Batch solubility
The batch dissolution test revealed that the B solubility in all treatments was significantly influenced by the macronutrient carrier (Fig. 1). The lowest B dissolution was observed in the WZB, CZB and Co treatments for both urea and MOP carriers. However, the B solubility of these treatments with MOP as the carrier was more than 2 times higher than with urea. When urea was the carrier, WZB, CZB, Co and ZB released most extractable B within the first hour and a minor amount of B was released in the following 23 h, while they continued to dissolve after 1 h when MOP was the carrier. Significantly higher amounts of dissolved Zn (20–30%) were observed for all MAP treatments compared to the MOP and urea treatments (Fig. 1b, d and f). The Zn release was higher with MOP than with urea, although the magnitude of dissolution was very small in both cases.
The B and Zn release of coated (a, b) urea, (c, d) MOP (muriate of potash), and (e, f) MAP (monoammonium phosphate) with C (ZnO + B2O3), WC (ZnO + B2O3 and water-assisted reaction), ZB (ZnO + B2O3, 8 h milling), WZB (ZnO + B2O3, 8 h milling and water-assisted reaction), CZB (commercial zinc borate), Co (Colemanite + ZnO) and TB (tetraborate + ZnO) after 1 h and 24 h shaking in a batch water-solubility test. The error bars represent the standard deviation of three replicates
3.4 Leaching of boron and zinc
For the TB treatments with highly soluble B and Zn, more than 60% of the added B was found in the leachates regardless of the macronutrient carrier (Fig. 2a). Approximately 40–50% of total B leached was in the first leaching event. For the low-solubility B treatments, at most 38% of B leached from the soil, mostly in the first leaching event. Total leached B was higher with MOP than with urea as the carrier. The amount of B in the leachate was higher for WZB than for CZB and Co. The total B leached from WZB was 38% of total B added when incorporated with MOP and 10% with urea.
a B and b Zn recovered in leachates from four successive leaching events of pots (L1 to L4) for MOP (muriate of potash) and urea treatments alone (N) or co-compacted with borax + ZnSO4 (TB), colemanite + ZnO (Co), commercial zinc borate (CZB), or mechanochemically synthesized zinc borate (WZB). The error bars represent standard deviation of three replicates and different letters indicate significant differences (P ≤ 0.05, Duncan’s Multiple Range Test). The red line indicates the amount of B and Zn added per pot
The concentration of Zn in leachates was highest in the MOP + TB, but still accounted for only 3% of added Zn (Fig. 2b). Slightly higher Zn release was observed in the MOP treatments compared to the urea treatments. This is likely because the pH of the MOP leachates were all around 4.4, while the urea leachate started off around 6.5 then decreased to around 4.4 for the last two leaching events (Table S3). However, even though the difference was significant in some treatments, Zn leaching was very limited overall.
3.5 Plant growth and boron and zinc uptake
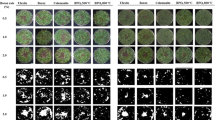
Treatments with B added had more vigorous growth and advanced phenology compared to the urea and MOP treatments without any added B (Fig. 3). Both treatments without added B did not grow above the elongation or rosette stage (BBCH growth stage < 34, BBCH-scale was introduced by Meier (1997) to describe the phenological development of canola plants) and some replicates almost died in the final 4 weeks, indicating severe B deficiency in this leached acid sandy soil. Symptoms of B deficiency were observed in both vegetative and reproductive growth in most replicates of the TB treatments. Two replicates of the urea + TB treatment did not reach the elongation stage after 12 weeks of growth.
All treatments with added B had significantly higher fresh and dry weight yields than the treatments without B (Fig. 4). Additionally, no visual signs of B deficiency were observed in the low solubility B treatments. The B concentration of the whole plant in the treatments followed Co = CZB = WZB > TB > N for MOP and Co = CZB > WZB > TB > N for urea. The B uptake of the canola followed the same order as the B concentrations in shoots. Concentrations of Zn in plants were similar in all treatments, while Zn uptake was significantly higher in all B added treatments compare to N due to the higher yield.
The (a) fresh and (b) dry weight; (c) B and (d) Zn concentration of shoots; and (e) B and (f) Zn uptake and (e) fresh and (f) dry weight for canola grown for 12 weeks after leaching, in response to the application of different B (+ Zn) sources co-compacted with MOP (muriate of potash) or urea (N: no B; TB: sodium tetraborate; Co: colemanite, CZB: commercial zinc borate; WZB: mechanochemically synthesized zinc borate). The error bars represent the standard deviation of three replicates and different letters indicate significant differences between treatments for a given macronutrient carrier—capital letter assigned to MOP and small letter assigned to urea (P ≤ 0.05, Duncan’s Multiple Range Test)
4 Discussion
Fertilizers are commonly produced with micronutrients incorporated into or onto macronutrients (N, P, K), as applying micronutrients separately can lead to segregation (in bulk blends) and poor spatial distribution of nutrient in the field. This is especially important for B due to the possibility of both deficiency and seedling toxicity occurring (da Silva et al. 2018). To achieve successful and homogenous incorporation of micronutrients, several methods can be used such as coating, compaction or granulation. The two methods assessed in this study, coating and compaction, indicated that more precise micronutrient incorporation could be achieved through the co-compaction method on a laboratory scale. The lower recovery rate from the coating method was likely caused by poor coating adhesion due to: (a) the coating material having different particle sizes and hydrophobicity; (b) more coating material required for compounds with low Zn/B content resulting in higher losses; and (c) variation in the porosity, moisture content and shape of the macronutrient granules (Goldszal and Bousquet, 2001; Iley, 1991). However, concentrations of B and Zn in compacted products were on average 89% of the target concentration for B and 103% for Zn (data not shown), values which are closer to the target compared to those produced by coating (Table S1).
Our previous results showed that a WAR was essential to obtain a zinc borate with a low solubility associated with an octahedral crystal morphology. We hypothesized that it might be possible to incorporate the WAR as part of the coating process, allowing for a more efficient production method. However, when coated onto MOP and urea, the mechanochemically synthesized sample without WAR (ZB) was more soluble than the one synthesized using WAR (WZB), indicating that the water atomization during coating was not as effective in reducing solubility as the separate WAR prior to coating. This lower effectiveness of the WAR during coating might be due to the presence of the macronutrient carrier potentially absorbing some of the water or reacting with the reagents.
When coating or co-compacting micronutrients with macronutrient fertilizers, it is important to consider potential chemical interactions, as the macronutrients have different pH and ionic strength which could affect the release character of the incorporated micronutrient compounds (da Silva et al. 2018). We observed that all low-solubility B treatments became much more soluble when coated onto MAP and almost all B was released within the first hour. Generally, the dissolution of MAP acidified the solution thus promoting the release of B. Similar results were observed in a previous study using colemanite, which was found to be more soluble when incorporated with MAP than with MOP (Abat et al. 2015d). Furthermore, the high concentration of the phosphate and ammonium released from MAP likely drove the dissolution of colemanite and zinc borate further by removing Ca and Zn from the solution through precipitation of CaHPO4, Zn3(PO4)2 and/or NH4ZnPO4. This may also apply to other P macronutrient carriers such as superphosphates. The solubility of the slow-release B compounds (zinc borate and colemanite) was lower when incorporated into urea and MOP than when incorporated into MAP, with the lowest solubility for urea. Therefore, MOP and urea are preferred over MAP as macronutrient carriers for low solubility B sources.
The management of B-containing fertilizers requires careful consideration as plants generally require small amounts of B over the whole growing season for structural development (Brown and Hu 1997). Highly soluble B sources may result in considerable leaching losses in high-rainfall conditions causing deficiency, or may cause toxicity to plants due to excessive B concentrations close to the seedling (da Silva et al. 2018). We found the losses of B in leachates were higher with MOP than with urea for all treatments, indicating that urea is less likely to increase the dissolution of the low solubility B sources. The highest losses of B were found with the most soluble B source (TB), were lower in WZB treatments and lowest in CZB and Co treatments, which is in line with the results of the batch solubility test. The TB treatments had a residual soil concentration of less than 0.5 mg B kg−1, which is the minimum concentration suggested by other studies for healthy canola growth (Bullock and Sawyer, 1991; Shorrocks 1997). We indeed observed impairment of reproductive organs (flower buds or flowers) and incomplete or damaged embryo formation in the MOP + TB treatment (Fig. 3b), indicting severe B deficiency (Dell and Huang, 1997). Except for one replicate of the urea + Co treatment, all replicates of the slow-release B treatments successfully reached the flowering and reproduction stage.
Even though deficiency symptoms were observed in the TB treatments, the fresh and dry weight of canola were very similar between the treatments with added B. When considering canola yields in the field, the most important part is the development of seeds as these are used for oil extraction. However, the dry weights of canola were largely made up of the stem and petioles before the development of other photosynthetically active organs after flowering (Al-barzinjy et al. 2003), and the growth stage varied between treatments at harvest. Therefore, it is more appropriate to compare B concentrations in plants and B uptake as an indicator of B availability in the pot trial. Notably, the B concentrations in urea + Co and CZB treatments were double that of the urea + WZB treatment. This could be explained by two possible reasons: a) the greater B leaching for the WZB treatments (Fig. 2), and/or b) the B in the WZB with urea treatment having a slower rate of B release – the former is the most likely. Several studies have concluded or suggested that the critical plant B concentration for deficiency is around 10 mg kg−1 (Asad et al. 1997; Bell, 1997; Huang et al. 1996). Indeed, deficiency symptoms were observed during harvest for the plants with B concentrations less than 10 mg kg−1, namely the N and TB treatments (Fig. 4a). In the slow-release B treatments, shoot concentrations were above 10 mg kg−1 and no deficiency was observed. Therefore, after heavy leaching, the B supply from the slow-release B source was adequate for canola growth.
We did not consider toxicity of B in our experiment, as the simulated rainfall prior to the growth resulted in removal of highly soluble B. However, under low-rainfall conditions, application of soluble B fertilizers poses a potential toxicity risk (Degryse 2017). The slow-release B fertilizers not only reduce leaching losses which may cause deficiency later in the season, but also reduce the risk of toxicity to seedlings (Abat et al. 2015c).
Compared to other low solubility B sources such as colemanite, zinc borates have an advantage as Zn is also supplied as part of the low-solubility B compound. There was no difference in Zn concentration or uptake between the different B treatments. The Zn uptake was higher for all treatments with B compared to the control, due to the enhanced plant growth in presence of B (Grewal et al. 1998). Thus, applying zinc borate as a slow-release B fertilizer could also assure adequate zinc uptake and benefit plant growth.
The zinc borate synthesized mechanochemically was slightly more soluble than the commercial zinc borate, which led to slightly greater leaching losses from soil, and slightly lower B uptake by plants (after leaching). However, the mechanochemical synthesis used has a much lower environmental footprint than hydrothermal methods for synthesis of this compound (Zheng et al. 2021), and further refinement of the synthesis conditions should be investigated to lower B solubility further.
5 Conclusions
In this study, we concluded that mechanochemically synthesized zinc borate co-compacted with urea or muriate of potash was a more effective B fertilizer than commonly used soluble B sources under leaching conditions. This study highlights the importance of agronomic comparisons of fertilizer formulations, as the behavior of the fertilizers will change when incorporated with other nutrients or applied in soil for crop growth. Mechanochemistry offers an opportunity to transform fertilizer manufacturing methods from conventional to sustainable with less waste stream produced, but the resultant products need to be evaluated not only for their manufacturing environmental footprint, but also for their agronomic and environmental performance and their compatibility with current nutrient delivery systems. Field studies are required to further confirm the effectiveness of this mechanochemically synthesized zinc borate as a B and Zn fertilizer.
Data Availability
Data available on request from the authors.
References
Abat M, Degryse F, Baird R, McLaughlin MJ (2015a) Boron Phosphates (BPO4) as a Seedling-safe Boron Fertilizer Source. Plant Soil 391:153–160. https://doi.org/10.1007/s11104-015-2424-6
Abat M, Degryse F, Baird R, McLaughlin MJ (2015b) Responses of Canola to the Application of Slow-Release Boron Fertilizers and Their Residual Effect. Soil Sci Soc Am J 79:97–103. https://doi.org/10.2136/sssaj2014.07.0280
Abat M, Degryse F, Baird R, McLaughlin MJ (2015c) Slow-release Boron Fertilisers: Co-granulation of Boron Sources with Mono-ammonium Phosphate (MAP). Soil Res 53:505–511. https://doi.org/10.1071/SR14128
Alloway BJ (2009) Soil Factors Associated with Zinc Deficiency in Crops and Humans. Environ Geochem Health 31:537–548. https://doi.org/10.1007/s10653-009-9255-4
Ardila-Fierro KJ, Hernández JG (2021) Sustainability Assessment of Mechanochemistry by Using the Twelve Principles of Green Chemistry. Chemsuschem 14:2145–2162. https://doi.org/10.1002/cssc.202100478
Baláž P, Achimovičová M, Baláž M, Billik P, Cherkezova-Zheleva Z, Criado JM, Delogu F, Dutková E, Gaffet E, Gotor FJ, Kumar R, Mitov I, Rojac T, Senna M, Streletskii A, Wieczorek-Ciurowa K (2013) Hallmarks of Mechanochemistry: from Nanoparticles to Technology. Chem Soc Rev 42:7571–7637. https://doi.org/10.1039/C3CS35468G
Baláž, P, (2008) Applied Mechanochemistry, in: Baláž, P. (Ed.) Mechanochemistry in Nanoscience and Minerals Engineering. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 297–405. https://doi.org/10.1007/978-3-540-74855-7_6
Barčauskaitė K, Brazienė Z, Avižienytė D, Silva M, Drapanauskaite D, Honer K, Gvildienė K, Slinksiene R, Jancaitiene K, Mazeika R, Staugaitis G, Dambrauskas T, Baltakys K, Baltrusaitis J (2020) Mechanochemically Synthesized Gypsum and Gypsum Drywall Waste Cocrystals with Urea for Enhanced Environmental Sustainability Fertilizers. J Environ Chem Eng 8:103965. https://doi.org/10.1016/j.jece.2020.103965
Boldyrev VV (2006) Mechanochemistry and Mechanical Activation of Solids. Russ Chem Rev 75:177–189. https://doi.org/10.1070/rc2006v075n03abeh001205
Borges R, Prevot V, Forano C, Wypych F (2017) Design and Kinetic Study of Sustainable Potential Slow-Release Fertilizer Obtained by Mechanochemical Activation of Clay Minerals and Potassium Monohydrogen Phosphate. Ind Eng Chem Res 56:708–716. https://doi.org/10.1021/acs.iecr.6b04378
Broschat TK (2008) Release Rates of Soluble and Controlled-release Boron Fertilizers. HortTechnol Hortte 18:471–474. https://doi.org/10.21273/HORTTECH.18.3.471
Brown, PH, Hu, H (1997) Does boron play only a structural role in the growing tissues of higher plants?, in: Ando, T., Fujita, K., Mae, T., Matsumoto, H., Mori, S., Sekiya, J. (Eds.), Plant Nutrition for Sustainable Food Production and Environment: Proceedings of the XIII International Plant Nutrition Colloquium, 13–19 September 1997, Tokyo, Japan. Springer Netherlands, Dordrecht, pp. 63–67. https://doi.org/10.1007/978-94-009-0047-9_6
Buates J, Imai T (2021) Assessment of Plant Growth Performance and Nutrient Release for Application of Phosphorus-loaded Layered Double Hydroxides as Fertilizer. Environ Technol Innov 22:101505. https://doi.org/10.1016/j.eti.2021.101505
Camacho-Cristóbal JJ, Rexach J, González-Fontes A (2008) Boron in Plants: Deficiency and Toxicity. J Integr Plant Biol 50:1247–1255. https://doi.org/10.1111/j.1744-7909.2008.00742.x
da Silva RC, Baird R, Degryse F, McLaughlin MJ (2018) Slow and Fast-Release Boron Sources in Potash Fertilizers: Spatial Variability, Nutrient Dissolution and Plant Uptake. Soil Sci Soc Am J 82:1437–1448. https://doi.org/10.2136/sssaj2018.02.0065
Degryse F (2017) Boron fertilizers: use, challenges and the benefit of slow-release sources–a review. J of Boron 2:111–122
Findeklee, P, Wimmer, M, Goldbach, HE, (1997) Early Effects of Boron Deficiency on Physical Cell Wall Parameters, Hydraulic Conductivity and Plasmalemma-bound Reductase Activities in Young C. pepo and V. faba Roots, in: Bell, R.W., Rerkasem, B. (Eds.), Boron in Soils and Plants: Proceedings of the International Symposium on Boron in Soils and Plants held at Chiang Mai, Thailand, 7–11 September, 1997. Springer Netherlands, Dordrecht, pp 221–227. https://doi.org/10.1007/978-94-011-5564-9_43
Gao Y-H, Liu Z-H (2009) Synthesis and Thermochemistry of Two Zinc Borates, Zn2B6O11·7H2O and Zn3B10O18·14H2O. Thermochim Acta 484(1):27–31. https://doi.org/10.1016/j.tca.2008.11.013
Gönen M, Balköse D, Gupta RB, Ülkü S (2009) Supercritical Carbon Dioxide Drying of Methanol−Zinc Borate Mixtures. Ind Eng Chem Res 48(14):6869–6876. https://doi.org/10.1021/ie9003046
Gupta UC (1983) Boron Deficiency and Toxicity Symptoms for Several Crops as Related to Tissue Boron Levels. J of Plant Nutrition 6:387–395. https://doi.org/10.1080/01904168309363098
Honer K, Kalfaoglu E, Pico C, McCann J, Baltrusaitis J (2017) Mechanosynthesis of Magnesium and Calcium Salt-Urea Ionic Cocrystal Fertilizer Materials for Improved Nitrogen Management. ACS Sustain Chem Eng 5:8546–8550. https://doi.org/10.1021/acssuschemeng.7b02621
Honer K, Pico C, Baltrusaitis J (2018) Reactive Mechanosynthesis of Urea Ionic Cocrystal Fertilizer Materials from Abundant Low Solubility Magnesium- and Calcium-Containing Minerals. ACS Sustain Chem Eng 6:4680–4687. https://doi.org/10.1021/acssuschemeng.7b03766
Julien PA, Germann LS, Titi HM, Etter M, Dinnebier RE, Sharma L, Baltrusaitis J, Friščić T (2020) In Situ Monitoring of Mechanochemical Synthesis of Calcium Urea Phosphate Fertilizer Cocrystal Reveals Highly Effective Water-based Autocatalysis. Chem Sci 11:2350–2355. https://doi.org/10.1039/C9SC06224F
Lei Z, Cagnetta G, Li X, Qu J, Li Z, Zhang Q, Huang J (2018) Enhanced Adsorption of Potassium Nitrate with Potassium Cation on H3PO4 Modified Kaolinite and Nitrate Anion into Mg-Al Layered Double Hydroxide. Appl Clay Science 154:10–16. https://doi.org/10.1016/j.clay.2017.12.040
Meier, U (1997) Growth Stages of Mono-and Dicotyledonous Plants, Blackwell Wissenschafts-Verlag
Mengel, K, Kirkby, EA, Kosegarten, H, Appel, T (2001) Boron, in: Mengel, K., Kirkby, E.A., Kosegarten, H., Appel, T. (Eds.), Principles of Plant Nutrition. Springer Netherlands, Dordrecht, pp 621–638. https://doi.org/10.1007/978-94-010-1009-2_18
Milani N, McLaughlin MJ, Stacey SP, Kirby JK, Hettiarachchi GM, Beak DG, Cornelis G (2012) Dissolution Kinetics of Macronutrient Fertilizers Coated with Manufactured Zinc Oxide Nanoparticles. J Agric Food Chem 60:3991–3998. https://doi.org/10.1021/jf205191y
Myers SS, Wessells KR, Kloog I, Zanobetti A, Schwartz J (2015) Effect of Increased Concentrations of Atmospheric Carbon Dioxide on the Global Threat of Zinc Deficiency: a Modelling Study. Lancet Glob Health 3:639–645. https://doi.org/10.1016/s2214-109x(15)00093-5
Pregno LM, Armour JD (1992) Boron Deficiency and Toxicity in Potato cv. Sebago on an Oxisol of the Atherton Tablelands North Queensland. Aust J Exp Agric 32(2):251–253
Said A, Zhang Q, Qu J, Liu Y, Lei Z, Hu H, Xu Z (2018) Mechanochemical Activation of Phlogopite to Directly Produce Slow-release Potassium Fertilizer. Appl Clay Sci 165:77–81. https://doi.org/10.1016/j.clay.2018.08.006
Salisbury, PA (1999) Canola in Australia: The First Thirty Years. Organising Committee of the 10th International Rapeseed Congress
Samreen T, Degryse F, Baird R, da Silva RC, Zahir ZA, Nazir MZ, Wakeel A, Sidra Tul M, McLaughlin M (2021) Development and Testing of Improved Efficiency Boron-Enriched Diammonium Phosphate Fertilizers. J Soil Sci Plant Nutr 21(2):1134–1143. https://doi.org/10.1007/s42729-021-00428-w
Sharma L, Kiani D, Honer K, Baltrusaitis J (2019) Mechanochemical Synthesis of Ca- and Mg-Double Salt Crystalline Materials Using Insoluble Alkaline Earth Metal Bearing Minerals. ACS Sustain Chem Eng 7(7):6802–6812. https://doi.org/10.1021/acssuschemeng.8b06129
Shorrocks VM (1997) The Occurrence and Correction of Boron Deficiency. Plant Soil 193(1):121–148. https://doi.org/10.1023/A:1004216126069
Solihin ZQ, Tongamp W, Saito F (2010) Mechanochemical Route for Synthesizing KMgPO4 and NH4MgPO4 for Application as Slow-Release Fertilziers. Ind Eng Chem Res 49(5):2213–2216. https://doi.org/10.1021/ie901780v
Solihin ZQ, Tongamp W, Saito F (2011) Mechanochemical Synthesis of Kaolin–KH2PO4 and kaolin–NH4H2PO4 Complexes for Application as Slow Release Fertilizer. Powder Technol 212(2):354–358. https://doi.org/10.1016/j.powtec.2011.06.012
Songkhum P, Wuttikhun T, Chanlek N, Khemthong P, Laohhasurayotin K (2018) Controlled Release Studies of Boron and Zinc from Layered Double Hydroxides as the Micronutrient Hosts for Agricultural Application. Appl Clay Sci 152:311–322. https://doi.org/10.1016/j.clay.2017.11.028
Svishcheva NB, Uspenskii SA, Sedush NG, Khaptakhanova PA, Kasatova AI, Buzin AI, Dmitryakov PV, Piskarev MS, Aleksandrov AI, Taskaev SY (2022) Biodegradable Boron-Containing Poly(Lactic Acid) for Fertilizers with Prolonged Action. Mater Today Commun 33:104514. https://doi.org/10.1016/j.mtcomm.2022.104514
Thombare N, Mishra S, Shinde R, Siddiqui MZ, Jha U (2021) Guar Gum Based Hydrogel as Controlled Micronutrient Delivery System: Mechanism and Kinetics of Boron Release for Agricultural Applications. Biopolymers 112(3):e23418. https://doi.org/10.1002/bip.23418
Wimmer MA, Eichert T (2013) Review: Mechanisms for Boron Deficiency-mediated Changes in Plant water Relations. Plant Sci 203–204:25–32. https://doi.org/10.1016/j.plantsci.2012.12.012
Xie L, Liu M, Ni B, Zhang X, Wang Y (2011) Slow-release Nitrogen and Boron Fertilizer from a Functional Superabsorbent Formulation Based on Wheat Straw and Attapulgite. Chem Eng J 167(1):342–348. https://doi.org/10.1016/j.cej.2010.12.082
Yuan W, Solihin Z Q, Kano J, Saito F (2014) Mechanochemical Formation of K-Si–Ca–O Compound as a Slow-release Fertilizer. Powder Technol 260:22–26. https://doi.org/10.1016/j.powtec.2014.03.072
Zhang Q, Saito F (2009) Mechanochemical Synthesis of Slow-Release Fertilizers through Incorporation of Alumina Composition into Potassium/Ammonium Phosphates. J Am Ceram Soc 92(12):3070–3073. https://doi.org/10.1111/j.1551-2916.2009.03291.x
Zheng B, Kabiri S, Andelkovic IB, Degryse F, da Silva R, Baird R, Self P, McLaughlin MJ (2021) Mechanochemical Synthesis of Zinc Borate for Use as a Dual-Release B Fertilizer. ACS Sustain Chem Eng 9(47):15995–16004. https://doi.org/10.1021/acssuschemeng.1c07111
Acknowledgements
Bo Zheng is grateful for the scholarship and support from The University of Adelaide, School of Agriculture, Food and Wine. We thank the help and support from Adelaide Microscopy and Ken Neubauer for SEM analysis. The authors acknowledge the support of The Mosaic Company LLC. The authors thank Bogumila Tomczak, Ashleigh Broadbent and Andrea Paparella for their advice and technical support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, B., Degryse, F., Andelkovic, I.B. et al. Agronomic Comparison of Mechanochemically Synthesized Zinc Borate and Other Boron Sources Granulated with Macronutrient Fertilizers. J Soil Sci Plant Nutr 23, 6407–6417 (2023). https://doi.org/10.1007/s42729-023-01495-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01495-x