Abstract
Selenium (Se) is an essential micronutrient in humans that is required for both physical and mental well-being. Low Se content in food crops is linked to Se-deficient soils globally. The aim of this study was examined the influence of sulfur (S) on the speciation and accumulation of selenium (Se) in three wheat cultivars grown in Se-deficient soils. Plants were grown in soil under glasshouse conditions with two doses of S (0 and 14 mg kg−1) as sulfate and three doses of selenium (0, 1, and 2 mg kg−1) as selenate (SeVI) in a randomized factorial design. Selenium speciation was determined using liquid chromatography inductively coupled plasma mass spectroscopy after enzymatic hydrolysis. Selenocysteine (SeCys), seleno-methyl-cysteine (SeMeCys), selenomethionine (SeMet), selenite (SeIV), and selenate (SeVI) were determined. The addition of SeVI increased the Se content in grain in all wheat cultivars compared to the control treatment. Selenium accumulated to the highest extent in leaf tissue while stem accumulated low amounts of Se. Speciation analysis in grain showed that most of the Se accumulated in wheat grain in the organic forms, SeCys and SeMeCys. Inorganic Se was below 10%, primarily as SeVI. Longsword, a multi-tillering variety, accumulated the highest proportion of SeMeCys (67%). Fertilization with S concurrently with Se resulted in decreased production of SeCys and SeMeCys in grain. The findings from this study provide new insights into the Se biofortification and speciation transformation processes in wheat as impacted by S supplementation in Se-deficient soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Selenium (Se) was first recognized in the late 1950s as an essential micronutrient in humans (Schwarz and Foltz 1957). The deficiency of Se plays a critical role in the central nervous system, male reproductive biology, the endocrine system, muscle function, cardiovascular system, and immunity (Roman et al. 2014; Rayman 2012). In addition, Se deficiency may also lead to depression and mental health disorders (Rayman 2000). The daily intake of Se varies globally, and the recommended dose is 55 µg per day for adults and children above 14 years (Bendich 2001) and the maximum dose of Se is 300 µg per day (Rayman 2017).
In nutrient-deficient soils, agronomic biofortification is an effective practice for increasing the nutrient content of the edible portion of cereal crops through fertilization practices (Broadley et al. 2010; Graham et al. 2007). This practice has been recognized as a reliable long-term approach to alleviating micronutrient (including Se) deficiency in the last decade because it is relatively easy, efficient, and affordable (Broadley et al. 2010). The Se content in food depends on the soil Se bioavailability and the ability of plants to take up and accumulate Se in edible tissues (Bañuelos et al. 2017). The Se content in cereal grain can be improved by applying a small amount of Se fertilizer in the form of sodium selenate (Na2SeO4) in soil, as has been practiced in Finland since the 1980s (Keskinen et al. 2011). Selenium occurs mainly as inorganic compounds in soil, primarily in the form of selenate (SeO42−) and selenite (SeO32−). Selenate is generally more abundant and available to plants than selenite in soils. Selenium and sulfur belong to the same group in the periodic table and have similar chemical behavior in both soil and plant systems (Wang and Becker 2013). Due to the chemical similarity to sulfate, plants absorb selenate via sulfate permeases (Schiavon et al. 2015). Sulfur (S) fertilizer exerts different regulatory effects on Se uptake in crops. Liu et al. (2017) observed that the S application along with Se fertilizer could improve the quality of Brassica napus and also reduce Se uptake significantly. Some other studies revealed that application of S fertilizer could reduce the SeIV uptake in crops such as wheat (Triticum aestivum L.), rapeseed (Brassica napus L.), ryegrass (Lolium perenne L.), and soybean (Glycine max L.). Dos Santos et al. (2022) reported that the presence of sulfate in soil reduces the SeVI adsorption during uptake progression between sulfate and SeVI at the plant root–soil solution edge, due to competition for the same membrane transporters.
Consumption of Se-biofortified plant products containing organic Se forms may lead to a higher intake of Se in humans. Moreover, organic forms of Se such as selenomethionine, selenocysteine, methyl selenocysteine, and γ-glutamyl-methyl-selenocysteine contained in some Se-enriched plant tissues may be more readily used by enzymes for promoting antioxidant activities in humans. In this regard, Ávila et al. (2014) reported that biofortification of Se in Brassica sp. showed that application of 50 μM Na2SeO4 significantly increased synthesis of a counter-carcinogenic compound, Se-methyl selenocysteine (SeMeCys). Selenium readily substitutes for S in amino acids and proteins due to the high chemical similarity of the two elements. Selenium species, selenocysteine (SeCys), and selenomethionine (SeMet) are analogs of S-containing amino acids (e.g., cysteine, methionine) (Terry et al. 2000). Hence, speciation of Se compounds in Se-biofortified grain produced via biofortification strategies is potentially linked to the production of S-enriched proteins. In addition, there are potentially antagonistic Se-S interactions in the soil environment, both at the root plasma membrane and adsorption sites in the soil matrix (Kikkert and Berkelaar 2013). We hypothesized that S would influence the total Se uptake and the incorporation of Se into desirable organic S forms in grain, such as SeMeCys.
In this study, we investigated the ability of three different wheat cultivars to accumulate Se in Se-deficient soil with the application of variable levels of Se and S. The objective of our study was to determine the chemical species of Se in the grain of wheat cultivars after being applied to various doses of selenate and sulfate.
2 Materials and Methods
2.1 Soil Collection, Processing, and Characterization
In this experiment, surface soil (0–15 cm) was collected from farmland located at Condobolin (NSW), Australia. After collection, debris and other unwanted materials were removed, air-dried, sieved to 4 mm, and homogenized. A portion of processed soil was used for physicochemical characterization using standard protocols (Table 1) (Lamb et al. 2016). The organic carbon, total N, and total S were analyzed using a CNS analyzer (LECO, TruMac CNS). Readily soluble S (measured as sulfate) was measured using a gravimetric approach (Rayment and Higginson 1992) with both water and salt (10 mM CaCl2) at a 1:5 solid-solution ratio. In this soil, sulfate solubility is not likely to be influenced by Ca from the salt extract (Lebedev and Kosorukov 2017). The adsorption behavior for SeVI was determined by following the procedure of Silva et al. (2019) with a slight modification. Briefly, 1 g soil was weighed and 20 mL of a SeVI-enriched solution containing 0.01 M MES buffer (pH 6) and 0.01 M NaCl suspension was reacted (24 h). Two concentrations of SO4 as 0 (control) and 100 µM were added to each sample as MgSO4. The SeVI concentrations were 0 (control), 1, 5, 10, 25, 50, 100, and 200 μM as sodium selenate. Reaction vessels were shaken in a rotatory shaker at 150 rpm for 24 h at 23 °C. After shaking, the samples were centrifuged for 20 min at 5000 rpm. A 10 mL aliquot was collected from each of the samples and filtered to a 0.22 μm. The concentration of dissolved Se in each sample was obtained using inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7900, Japan). The sorption isotherms were described with Langmuir’s equation using a non-linear fitting procedure in SAS (SAS, version 9.4) (Langmuir 1997).
2.2 Glasshouse Experiment
The growth experiment was conducted in a glasshouse at the University of Newcastle with five replicates. Glasshouse conditions were maintained at minimum–maximum temperature cycle of 16–27 °C and a 12-h photoperiod. Soils were amended with calcium carbonate (2 g kg−1) to increase soil pH. The amendment rate was based on a bench top trial with increasing amendment rates of calcium carbonate (Broadhurst et al. 2015). After amending with lime, the soil was incubated for 2 weeks for correction of soil acidity. The amendment was chosen which produced a final pH of 6.5 (2 g kg−1). Soils were also amended with ammonium phosphate (27 mg kg−1) and potassium nitrate (14 mg kg−1) and mixed thoroughly in a mixer. Each pot was filled with 3 kg mixed soil, and five seeds each of the three cultivars (Condo, Longsword, and Spitfire) were placed under the soil surface for germination. After germination, the plants were thinned to one plant after 2 weeks of sowing. The soils were maintained close to field capacity by watering the soil surface regularly with RO water. In order to investigate the uptake capacity of Se (selenate) and the interaction of S, two doses of sulfate (0 and 14 mg kg−1 soil) were added to the pots at 5 weeks after seeding (Both et al. 2020; Kikkert and Berkelaar 2013). After 1 week of adding sulfate, three doses of Se (control, 1, and 2 mg kg−1 soil) were used in this study. These concentrations were used to investigate the Se speciation in grains and based on previous reports in the literature (Jiang et al. 2015; Kikkert and Berkelaar 2013). Plants were grown to grain maturity. Upon harvesting, the stem, leaf, and spike were separated and rinsed with reverse osmosis water. Plant biomass was dried at 60 °C for 72 h and wheat grains were separated by hand. All plant parts were ground to a fine powder using a stainless steel grinder.
For total Se contents in plant tissue, approximately 0.25 g of plant sample was weighed into digestion tubes and 5 mL of HNO3 acid was added. Samples were cold digested overnight. The following day, the samples were heated at 70 °C for 30 min, 90 °C for 30 min, 110 °C for 30 min, and finally 140 °C (BD 50, SEAL Analytical). The digestion at 140 °C was continued until only a small residual liquid remained in each tube (approximately 1 mL) and prepared for analysis (Ming et al. 2012) by inductively coupled plasma optical emission spectrometry (ICP-OES, Avio 200, PerkinElmer Instruments, USA). Analytical accuracies of Se and S were verified using SRM 1570a (trace elements in spinach leaves) and SRM 1568a (rice flour) from NIST, USA. The SRMs were digested using a similar procedure to plant material. The total Se and S concentrations of the reference materials were within the 90–110% and 94–116% recovery of the certified values, respectively.
2.3 Chemical Speciation of Se in Wheat Grain
Selenium speciation in wheat grain samples was analyzed by high-performance liquid chromatography coupled to inductively coupled plasma mass spectrometry (HPLC-ICP-MS) after extraction of Se species with enzymatic hydrolysis following the procedure of Hart et al. (2011) with some modifications. Sample preparation for Se speciation was followed by Godin et al. (2015) with slight modification. Briefly, 100 mg of ground grain was weighed in a polypropylene tube with 10 mg protease XIV type and dissolved in 5 mL 0.1 M TRIS–HCl buffer solution (pH 7.5). The mixture was shaken in an incubator at 37 °C for 22 h, centrifuged at 3000 g for 20 min, and filtered to 0.22 µm. The solution was collected and analyzed for selenite (SeIV), selenate (SeVI), selenomethionine (SeMet), selenocysteine (SeCys), and Se-methyl selenocysteine (SeMeCys). Selenium standards were purchased from Sigma Aldrich. Chromatographic separation was achieved with a Hamilton PRP-X100 anion exchange column (Hart et al. 2011). Operating parameters are detailed in Table 2.
2.4 Statistical Analysis
Statistical data analysis was done by using SPSS (version 27) or SAS (SAS, version 9.4). Analysis of variance (three-way ANOVA) with post hoc Tukey’s HSD (p < 0.05) was used for multiple comparisons (Table 3).
3 Results
3.1 Soil Characterization and Sorption Study
The soil pH was initially lower than initially anticipated at pH 4.8. The addition of lime (CaCO3) raised the pH to 6.5, making it conducive for wheat growth (Table 1) (the addition of lime rate was based on previous benchtop trial) (Broadhurst et al. 2015). The soil texture was a clay loam with the sand, clay, and silt composition as 37.5, 24, and 38.5%, respectively. The organic carbon content, total N, and total S of the soil were 11.8, 1.11, and 0.08 g/kg, respectively. Salt (10 mM CaCl2) and water extracted 12.8 ± 1 and 19 ± 1 mg/kg, respectively, indicating that 16–24% of total S was readily soluble. The soil was also Se-deficient (3 µg kg−1). The sorption study showed that an increasing amount of SeVI was adsorbed with an increasing concentration of Se in the equilibrium solution (Fig. S1, supporting information). The presence of sulfate noticeably reduced the adsorption of Se in soil.
3.2 Response of Plant Biomass to Se and Sulfate
The effect of Se and sulfate treatments on dry matter production of the stem, leaf, and grain varied substantially between species (Fig. 1). The dry biomass of stem and leaf in the Condo cultivar did not show significant differences with the increasing Se doses compared to control (Fig. 1a). However, the dry weight of grain showed significant differences (*p < 0.05) with Se doses, while the highest grain dry weight (3.5 g) was observed in Se 1 treatment and the lowest (1.4 g) was in Se 2 treatment. Longsword did not show any significant differences in terms of stem and grain dry weight with the increased Se doses, whereas the leaf dry weight was significantly reduced (**p < 0.01) with the increase of Se doses (Fig. 1b).
Plant biomass of wheat influenced by Se and S doses. a, b, and c represent three different cultivars of wheat are Condo, Longsword, and Spitfire, respectively. Control, Se 1, and Se 2 denote the no treatment, 1 mg kg−1, and 2 mg kg−1 SeVI, respectively. S means 14 mg kg.−1 sulfur. Error bar represents the standard error (n = 5)
Sulfate addition did not show any significant differences in the dry weight of the Condo and Longsword cultivars in terms of stem, leaf, and grain dry weight. However, S enormously enhanced (***p < 0.001) (twofold) Spitfire grain production compared to control. Sulfate also significantly increased the stem (**p < 0.01) and leaf dry weight (*p < 0.05).
Low SeVI doses with S increased the dry matter content of Condo wheat. However, when the Se doses were higher than 1 mg kg−1 of soil, they led to significant decreases in the dry weight of stem (***p < 0.001) and leaf (**p < 0.01). Compared with the control, the highest dry weight of stem, leaf, and grains were obtained from 1 mg kg−1 Se with 14 mg kg−1 S (Se 1 + S) treatment. The overall plant biomass in other treatments was significantly lower than the control except for Se 1 + S.
3.3 Total Content of Selenium in Shoots and Grain
The addition of SeVI in soil increased the Se content in all the wheat cultivars compared to the control treatment (Fig. 2). The three cultivars varied in the Se contents in stem, leaf, and grain. As expected, the accumulation of Se in the leaf was the greatest while the stem accumulated low amounts of Se. However, the accumulation of SeVI in stem, leaf, and grain in all wheat cultivars showed significant differences (***p < 0.001) with the SeVI treatments. The addition of 2 mg kg−1 SeVI in soil showed the highest Se content in all the three cultivars, with the Spitfire variety showing the highest leaf Se content, about 51% of the total accumulation (Fig. 2c). Among the cultivars, Longsword accumulated the lowest Se contents in all parts (stem, leaf, and grain) (Fig. 2b). The addition of S to Se-treated soils did not show any significant difference in stem, leaf, and grain Se content for the Condo cultivar. For Longsword, only significant differences (*p < 0.05) were found between SeVI and S interactions in the grain Se contents. Finally, for Spitfire, stem and leaf Se contents showed a significant difference (**p < 0.01 and ***p < 0.001, respectively) with SeVI and S.
Se and S content of wheat influenced by different doses of Se and S. a, b, and c (left) represent the Se content, and d, e, and f (right) represent the S content of three different cultivars of wheat are Condo, Longsword, and Spitfire, respectively. Control, Se 1, and Se 2 denote the no treatment, 1 mg kg−1, and 2 mg kg−1 SeVI, respectively. S means 14 mg kg.−1 sulfur. Error bar represents the standard error (n = 5)
The different doses of SeVI and S influenced the stem, leaf, and grain S contents. In the case of Condo, there was no significant difference in stem and grain S contents between all treatments (Fig. 2d). However, the interaction of Se and S increased the leaf and grain S content significantly (*p < 0.05 and **p < 0.01, respectively). On the other hand, there were no significant differences in the stem and grain S contents for the Longsword variety, while there were substantial increases (***p < 0.001) in leaf S contents between Se only and Se + S treatments. Spitfire did not show any significant difference in grain sulfur content with any Se or S doses. The result showed that most of the Se accumulated in leaves in all of the wheat cultivars. On the other hand, Condo accumulated more S in the grain.
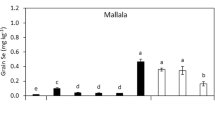
In terms of the mass of Se in grain, all of three cultivars of wheat showed a significant difference between the treatments compared to the control (Fig. 3). The mass of Se in grain increased significantly (***p < 0.001) with increasing of Se doses compared to the control in all cultivars. The addition of S to all Se treatments in Condo significantly increased the mass of Se in grain (**p < 0.01). Indeed, even in the S-control treatment, the Se mass in grain was significantly increased in both Condo and Spitfire (*p < 0.05 and ***p < 0.001, respectively). On the other hand, Longsword did not show any significant differences in the mass of Se in grain. The mass of Se in the grain of the Spitfire cultivar increased significantly (***p < 0.001) with the increasing of Se and S addition. The highest mass of Se in grain was observed in the Se 2 + S treatment for all three cultivars (0.6, 0.2, and 0.9 g, respectively).
3.4 Selenium Speciation in Wheat Grain
The percentage of each Se species in grain after enzymatic digestion of wheat grains is shown in Fig. 4. Three species were quantifiable, including SeCys, SeMeCys, and SeVI. Two unidentified large peaks were found at 12 to 14 min retention time under all treatments (Fig. 4b and c).
Chromatographic separation of Se species in standards and the grain of wheat cultivars. a Se species used in standards; b Se species present in Condo; c Se species present in Longsword; d Se species present in Spitfire when grown under two S levels, S0 (control) and S14 (S 14 mg kg−1) and SeVI dose was 2 mg kg.−1. Blue and pink lines represent the no S (S0) and added S (S14), respectively
The percentage of Se species in grain showed significant differences (***p ≤ 0.001) with Se and S amendment compared to control treatments (Fig. 5). In terms of the Condo variety, the highest percentage (79.8%) of selenocysteine (SeCys) was found in control and S only treatments. However, it was slightly decreased in the Se 1 treatment and further increased with the increased doses of Se and S. Selenate accounted for only 1 to 6% and the highest percentage (6%) of selenate was observed in the Se 1 treatments. The monomethylated form, SeMeCys, was found around 20 to 39.5% in Condo; the highest percentage (39.5%) was observed in the Se 1 treatment. The control and highest dose of Se + S showed a lower proportion of SeMeCys.
Selenium species speciation of wheat grain influenced by different doses of selenium and sulfur. a, b, and c representing three different cultivars of wheat—Condo, Longsword, and Spitfire, respectively. Control, Se 1, and Se 2 denote the no treatment, 1 mg kg−1, and 2 mg kg−1 SeVI, respectively. S means 14 mg kg.−1 sulfur
The greatest quantities of SeMeCys were present in Longsword. Exposure to S modified the Se species, specifically SeMeCys in the grain. The interaction of S and Se significantly (***p < 0.001) increased the SeMeCys content in grain (Fig. 5b). The increased presence of S reduced the total amount of SeMeCys, but also the conversion of SeVI to SeMeCys. The ratio SeVI/SeMeCys expresses the conversion of SeVI to SeMeCys in the grain. The ratio was not related to Se concentration. In Longsword, the presence of S reduced the conversion of Se to SeMeCys (***p < 0.0001).
The highest percentage of SeCys (74%) was found in the control of Longsword, followed by Se 2 and Se 2 + S treatments and the lowest percentage was observed in Se 1 + S treatment. The SeCys concentration was dependent on Se and S concentrations in addition to cultivar. In the highest Se application, S significantly reduced SeCys across the three varieties. Spitfire produced significantly higher SeCys in grain (***p < 0.0001). In this variety, SeCys represented up to 97% in the Se 2 treatment. In Spitfire, SeMeCys and SeVI were 4.5% and 7%, respectively (Fig. 5c). Only around 7% selenate was found in Se 2 + S treatment. Selenocysteine was the dominant species in all grains. Total Se was very strongly correlated to SeCys (r = 0.99, ***p < 0.0001, n = 72). The SeCys/SeVI conversion ratio was not related to cultivar but was significantly impacted by Se and S. At Se 2, there was significant enhancement in the production of SeCys relative to SeVI. The application S significantly reduced the SeVI/SeCys ratio (**p = 0.0077), similarly to SeMeCys, indicating S not only reduced the accumulation of Se in grain, but also inhibited the conversion to beneficial selenoproteins. Indeed, sorption indicated that application of S enhanced solubility in the soil environment via competition adsorption for adsorption sites. Thus, despite the enhanced solubility of SeVI, sulfate significantly reduced the conversion of SeVI to organic forms of Se in grains.
4 Discussion
Biofortification of food crops with Se is a potential method to supply adequate Se nutrition to humans (Pyrzynska 2009). In this study, we found an increasing concentration of Se in plant parts after Se fertilization in soil. Our findings from the sorption study are consistent with the previous results that demonstrated notable suppression of adsorption, thus increasing SeVI solubility in soil solution (Dhillon and Dhillon 2000). Our findings suggested that lower doses of Se (< 2 mg kg−1) decrease plant biomass, which is consistent with previous reports (Guerrero et al. 2014). Similar results have been found with a number of plant species such as wheat (Boldrin et al. 2016), lettuce (Ramos et al. 2011), rice (Boldrin et al. 2013), and ryegrass (Hartikainen et al. 2000). The addition of Se and S increased the grain biomass up to 30% compared to control in the Spitfire cultivar; this has likely occurred due to the presence of S. Boldrin et al. (2013) reported that S increased the protein content of wheat grain. Feng et al. (2013) and Kaur et al. (2014) observed that low concentrations of Se can act as an antioxidant, abiotic stress modulator, anti-senescent, and defensive molecule against pathogens, thereby promoting plant growth. In addition, Se also increased root growth and cell elongation in the root (Silva et al. 2020). Some researchers also observed increases in shoot biomass after Se supplementation in wheat crops (Muhammad et al. 2018).
SeVI application in wheat increased the Se concentration in stem, leaf, and grain (Fig. 2). The interaction of S and SeVI in wheat showed varied results upon the cultivars. The reduction of Se accumulation in the stem and leaf of Condo and Spitfire due to the antagonistic behavior of S most likely occurred at the plasma membrane. Sulfate and SeVI are not expected to interact with cell walls of plant roots, and given the shared S transporter systems, would compete at the transport across the membrane. The applied S in soil predominantly increased the S content in leaf of Longsword and Spitfire. Boldrin et al. (2018) reported that the rise in leaf S content to the improved expression of the genes related to transport proteins present in the plant roots. However, Liu et al. (2017) observed that the application of S significantly increased the available S content of soil treated with Se species and S fertilizer can influence the uptake of Se in crops through different regulatory effects. Previous research from hydroponic, pot, and field-based studies have shown contrasting results in terms of the influence of S on Se uptake in different crops (Li et al. 2008; Liu et al. 2015; Cartes et al. 2006; Golob et al. 2016; Stroud et al. 2010). Field experiments by Stroud et al. (2010) on wheat showed that S (in the form of sulfate) increased the uptake of Se in grain by 62%.
Seleno-cysteine was the dominant Se species in all cultivars, particularly the Spitfire cultivar, which disagrees with past reports (Hart et al. 2011; Cubadda et al. 2010). Seleno-cysteine was strongly correlated to total Se (r = 0.997, n = 72), representing the dominant chemical species in wheat grain. Wheat grown in India was dominated by SeMet, accounting for 72–85% of Se (Cubadda et al. 2010). Hart et al. (2011) reported that the SeMet accounted for 65–87% of total extractable Se species in wheat bread and flour. In our study, the proportion of SeCys decreased with the exposure of S. Selenium substitutes within the S position of amino acids of proteins. The fertilization of S appeared to reduce Se incorporation into selenoproteins, reducing the efficiency of selenate conversion to SeCys, which was also observed in SeMeCys (Cubadda et al. 2010; Duncan et al. 2017).
Methyl-Se-cysteine was of principal interest due to its association as an anticarcinogenic agent in humans and animals (Ip and Ganther 1992). The mixture of organic Se compounds that we have found in the wheat grain may be of added nutritional value compared to other Se-enriched food and feed supplements, mainly containing SeMeCys. The presence of SeMeCys in the grain suggests that human food products such as wheat flour or other food products and animal feed made with this Se-enriched wheat grain might have added health benefits such as cancer prevention. Literature showed two anticarcinogenic forms of Se are S-methyl cysteine and Se-methyl selenocysteine (SeMeCys). SeMeCys is reportedly one of the least toxic forms of Se and one of the two most operative anticarcinogenic forms of Se (Ip and Ganther 1992; Zayed et al. 2000). Our study also found inorganic Se species selenate (SeVI) below 10%, which agrees with the previous findings (Hart et al. 2011). These results suggest that wheat plants grown with Se and Se-enriched soils are a good source for producing SeMeCys, an effective anticarcinogenic form of Se, for use in Se-enriched biofortified foods. Sulfur fertilization showed in this study caused a significant reduction in SeMeCys production in wheat grain, particularly the multi-tillered variety. As a result of the decrease in SeMeCys and SeCys with S fertilization, caution should be taken with S fertilization during Se fertilization. There was a great impact of sulfate at higher Se fertilization rates. In soils not fertilized with SeVI, sulfate fertilization did cause any antagonistic reductions in SeMeCys and SeCys in grain.
5 Conclusion
The present study examined the influence of sulfate fertilization on the biofortification of Se in three wheat cultivars. The results showed significant differences between cultivars both in terms of Se accumulation in grain and its speciation. Similarly, sulfate fertilization influenced the quantity and speciation of Se in wheat grains. In the Condo cultivar, S uptake was highest in the grain, followed by leaf and stem. Selenium in grain, mostly present in organic forms, Spitfire cultivar grain contained 97% SeCys and Longsword cultivar grain accumulated 70% SeMeCys. Sulfate fertilization had an antagonistic impact on the conversion of SeVI to desirable organic forms, including SeCys, and importantly, SeMeCys. The results of the current study suggests that increasing the S supply negatively influences the bioavailability of Se in various plant tissues and alters the chemical species of Se in wheat grain.
References
Ávila FW, Yang Y, Faquin V, Ramos SJ, Guilherme LRG, Thannhauser TW, Li L (2014) Impact of selenium supply on Se-methylselenocysteine and glucosinolate accumulation in selenium-biofortified Brassica sprouts. Food Chem 165:578–586. https://doi.org/10.1016/j.foodchem.2014.05.134
Bañuelos GS, Lin ZQ, Broadley M (2017) Selenium biofortification. In: Pilon-Smits E, Winkel L, Lin ZQ (eds) Selenium in plants. Plant Ecophysiology, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-319-56249-0_14
Bendich A (2001) Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Institute of Medicine Washington, DC: National Academy Press. Nutrition 17:364.
Boldrin PF, Faquin V, Ramos SJ, Boldrin KVF, Ávila FW, Guilherme LRG (2013) Soil and foliar application of selenium in rice biofortification. J Food Compos Anal 31:238–244. https://doi.org/10.1016/j.jfca.2013.06.002
Boldrin PF, de Figueiredo MA, Yang Y, Luo H, Giri S, Hart JJ, Faquin V, Guilherme LR, Thannhauser TW, Li L (2016) Selenium promotes sulfur accumulation and plant growth in wheat (Triticum aestivum). Physiol Plant 158:80–91. https://doi.org/10.1111/ppl.12465
Boldrin PF, Faquin V, Clemente AdCS, de Andrade T, Guilherme LRG (2018) Genotypic variation and biofortification with selenium in Brazilian wheat cultivars. J Environ Qual 47:1371–1379. https://doi.org/10.2134/jeq2018.01.0045
Both EB, Stonehouse GC, Lima LW, Fakra SC, Aguirre B, Wangeline AL, Xiang J, Yin H, Jókai Z, Soós Á (2020) Selenium tolerance, accumulation, localization and speciation in a Cardamine hyperaccumulator and a non-hyperaccumulator. Sci Total Environ 703:135041
Broadhurst CL, Chaney RL, Davis AP, Cox A, Kumar K, Reeves RD, Green CE (2015) Growth and cadmium phytoextraction by Swiss chard, maize, rice, Noccaea caerulescens, and Alyssum murale in pH adjusted biosolids amended soils. Int J Phytorem 17:25–39. https://doi.org/10.1080/15226514.2013.828015
Broadley MR, Alcock J, Alford J, Cartwright P, Foot I, Fairweather-Tait SJ, Hart DJ, Hurst R, Knott P, McGrath SP (2010) Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 332:5–18
Cartes P, Shene C, Mora ML (2006) Selenium distribution in ryegrass and its antioxidant role as affected by sulfur fertilization. Plant Soil 285:187–195. https://doi.org/10.1007/s11104-006-9004-8
Cubadda F, Aureli F, Ciardullo S, D’Amato M, Raggi A, Acharya R, Reddy RA, Prakash NT (2010) Changes in selenium speciation associated with increasing tissue concentrations of selenium in wheat grain. J Agric Food Chem 58:2295–2301. https://doi.org/10.1021/jf903004a
Dhillon SK, Dhillon KS (2000) Selenium adsorption in soils as influenced by different anions. J Plant Nutr Soil Sci 163:577–582. https://doi.org/10.1002/1522-2624(200012)163:6%3c577::AID-JPLN577%3e3.0.CO;2-H
Dos Santos MJV, de Lima Lessa JH, de Assis MB, Raymundo JF, Ribeiro BT, Guilherme LRG, Lopes G (2022) Selenium desorption in tropical soils by sulfate and phosphate, and selenium biofortification of Mombaça grass under increasing rates of phosphate fertilisation. Crop Pasture Sci 73:56–66. https://doi.org/10.1071/CP21059
Duncan EG, Maher WA, Jagtap R, Krikowa F, Roper MM, O’Sullivan CA (2017) Selenium speciation in wheat grain varies in the presence of nitrogen and sulphur fertilisers. Environ Geochem Health 39:955–966
Feng R, Wei C, Tu S (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68
Godin S, Fontagne-Dicharry S, Bueno M, Tacon P, Prabhu PA, Kaushik S, Medale F, Bouyssiere B (2015) Influence of dietary selenium species on selenoamino acid levels in rainbow trout. J Agric Food Chem 63:6484–6492. https://doi.org/10.1021/acs.jafc.5b00768
Golob A, Gadžo D, Stibilj V, Djikić M, Gavrić T, Kreft I, Germ M (2016) Sulphur interferes with selenium accumulation in Tartary buckwheat plants. Plant Physiol Biochem 108:32–36. https://doi.org/10.1016/j.plaphy.2016.07.001
Graham RD, Welch RM, Saunders DA, Ortiz-Monasterio I, Bouis HE, Bonierbale M, De Haan S, Burgos G, Thiele G, Liria R (2007) Nutritious subsistence food systems. Adv Agron 92:1–74
Guerrero B, Llugany M, Palacios O, Valiente M (2014) Dual effects of different selenium species on wheat. Plant Physiol Biochem 83:300–307. https://doi.org/10.1016/j.plaphy.2014.08.009
Hart DJ, Fairweather-Tait SJ, Broadley MR, Dickinson SJ, Foot I, Knott P, McGrath SP, Mowat H, Norman K, Scott PR, Stroud JL, Tucker M, White PJ, Zhao FJ, Hurst R (2011) Selenium concentration and speciation in biofortified flour and bread: retention of selenium during grain biofortification, processing and production of Se-enriched food. Food Chem 126:1771–1778. https://doi.org/10.1016/j.foodchem.2010.12.079
Hartikainen H, Xue T, Piironen V (2000) Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 225:193–200
Ip C, Ganther H (1992) Comparison of selenium and sulfur analogs in cancer prevention. Carcinogenesis 13:1167–1170
Jiang C, Zu C, Shen J, Shao F, Li T (2015) Effects of selenium on the growth and photosynthetic characteristics of flue-cured tobacco (Nicotiana tabacum L.). Acta Soc Bot Pol 84:71–77. https://doi.org/10.5586/asbp.2015.006
Kaur N, Sharma S, Kaur S, Nayyar H (2014) Selenium in agriculture: a nutrient or contaminant for crops? Arch Agron Soil Sci 60:1593–1624. https://doi.org/10.1080/03650340.2014.918258
Keskinen R, Räty M, Yli-Halla M (2011) Selenium fractions in selenate-fertilized field soils of Finland. Nutr Cycl Agroecosyst 91:17–29
Kikkert J, Berkelaar E (2013) Plant uptake and translocation of inorganic and organic forms of selenium. Arch Environ Contam Toxicol 65:458–465
Lamb TD, Kader M, Ming H, Wang L, Abbasi S, Megharaj M, Naidu R (2016) Predicting plant uptake of cadmium: validated with long-term contaminated soils. Ecotoxicology 25:1563–1574. https://doi.org/10.1007/s10646-016-1712-0
Langmuir D (1997) Aqueous environmental geochemistry. Prentice-Hall, Upper Saddle River
Lebedev A, Kosorukov V (2017) Gypsum solubility in water at 25 C. Geochem Int 55:205–210
Li HF, McGrath SP, Zhao FJ (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102. https://doi.org/10.1111/j.1469-8137.2007.02343.x
Liu X, Zhao Z, Duan B, Hu C, Zhao X, Guo Z (2015) Effect of applied sulphur on the uptake by wheat of selenium applied as selenite. Plant Soil 386:35–45. https://doi.org/10.1007/s11104-014-2229-z
Liu X, Yang Y, Deng X, Li M, Zhang W, Zhao Z (2017) Effects of sulfur and sulfate on selenium uptake and quality of seeds in rapeseed (Brassica napus L.) treated with selenite and selenate. Environ Exp Bot 135:13–20
Ming H, He W, Lamb DT, Megharaj M, Naidu R (2012) Bioavailability of lead in contaminated soil depends on the nature of bioreceptor. Ecotoxicol Environ Saf 78:344–350. https://doi.org/10.1016/j.ecoenv.2011.11.045
Muhammad I, Cheema SA, Muhammad F, Abdul W (2018) Selenium nutrition for yield enhancement and grain biofortification of wheat through different application methods. Int J Agric Biol 20:1701–1709
Pyrzynska K (2009) Selenium speciation in enriched vegetables. Food Chem 114:1183–1191
Ramos SJ, Rutzke MA, Hayes RJ, Faquin V, Guilherme LRG, Li L (2011) Selenium accumulation in lettuce germplasm. Planta 233:649–660. https://doi.org/10.1007/s00425-010-1323-6
Rayman M (2000) The importance of selenium to human health. Lancet (london, England) 356:233–241
Rayman MP (2012) Selenium and human health. The Lancet 379:1256–1268
Rayman M (2017) Selenium intake and status in health & disease. Free Radical Biol Med 112:5
Rayment G, Higginson F (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press, Melbourne
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6:25–54
Schiavon M, Pilon M, Malagoli M, Pilon-Smits EA (2015) Exploring the importance of sulfate transporters and ATP sulphurylases for selenium hyperaccumulation—a comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Front Plant Sci 6:2
Schwarz K, Foltz CM (1957) Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J Am Chem Soc 79:3292–3293
Silva VM, Boleta EHM, Martins JT, Mendes dos Santos FL, da Rocha Silva AC, Alcock TD, Wilson L, de Sá ME, Young SD, Broadley MR (2019) Agronomic biofortification of cowpea with selenium: effects of selenate and selenite applications on selenium and phytate concentrations in seeds. J Sci Food Agric 99:5969–5983. https://doi.org/10.1002/jsfa.9872
Silva VM, Tavanti RFR, Gratão PL, Alcock TD, Dos Reis AR (2020) Selenate and selenite affect photosynthetic pigments and ROS scavenging through distinct mechanisms in cowpea (Vigna unguiculata (L.) Walp) plants. Ecotoxicol Environ Safety 201:110777
Stroud J, Li H, Lopez-Bellido F, Broadley M, Foot I, Fairweather-Tait S, Hart D, Hurst R, Knott P, Mowat H (2010) Impact of sulphur fertilisation on crop response to selenium fertilisation. Plant Soil 332:31–40
Terry N, Zayed A, De Souza M, Tarun A (2000) Selenium in higher plants. Annu Rev Plant Biol 51:401–432
Wang Z, Becker H (2013) Ratios of S, Se and Te in the silicate Earth require a volatile-rich late veneer. Nature 499:328–331
Zayed A, Pilon-Smits E, deSouza M, Lin Z-Q, Terry N (2000) Remediation of selenium polluted soils and waters by phytovolatilization. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. CRC Press Boca Raton
Acknowledgements
The first author is thankful to the University of Newcastle for providing UNIPRS and UNRSC central scholarship and Sher-e-Bangla Agricultural University, Dhaka, Bangladesh, for granting study leave for PhD program. The authors acknowledge the support of Tony Rothkirch from the University of Newcastle’s Analytical and Biomolecular Research Facility (ABRF) in the establishment of selenium speciation protocol. We acknowledge funding contributions from the Australian Research Council (IN190100044) and the Soil CRC. The authors are also thankful to Ms. Helen McMillian (Central West Farming Systems, Condobolin, NSW) for her support during soil collection for the experiment.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
M.Y.: conceptualization, methodology, data curation, formal analysis, writing—original draft preparation. D.L.: conceptualization, editing, resources, and supervision. G.C.: method development and optimization, writing—review and editing. M.M.R.: supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yeasmin, M., Lamb, D., Choppala, G. et al. Impact of Sulfur on Biofortification and Speciation of Selenium in Wheat Grain Grown in Selenium-Deficient Soils. J Soil Sci Plant Nutr 22, 3243–3253 (2022). https://doi.org/10.1007/s42729-022-00882-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00882-0