Abstract
Plant essential oils (EOs) are considered a vital tool of novel natural mosquito repellents and botanical adulticides. Five plant EOs (cinnamon, cypress, lavender, lemon eucalyptus and tea tree) and their major constituents (cinnamaldehyde, citronellal, β-cymene, (R)-linalool, and α-terpinyl acetate) were investigated against adults of Culex pipiens. The efficacy of the tested compounds was manipulated as mortality and knockdown using a fumigation technique. After that, the most active compounds against adults (lemon eucalyptus oil and linalool) were investigated once more as repellents after incorporating them on a cream base against C. pipiens adults compared to their nano-cream using arm-in-cage technique. In addition, the biochemical and histological effects of dermal treatment of linalool, lemon eucalyptus,, and their nanoemulsions (NEs) were studied on male albino rats. Total protein assay, acetylcholinesterase (AChE), adenosine triphosphatase (ATPase), and liver and kidney functions were determined in blood serum. Complete blood count (CBC) was determined in whole blood. The results showed that lemon eucalyptus oil and (R)-linalool caused the highest knockdown activity against C. pipiens adults with Kt50 40.29 s and 12.73 s, respectively. The repellent effect (RC50) of nanocream formulations of lemon eucalyptus oil (10.03 mg/L) and (R)-linalool (68.11 mg/L) were higher than the original effects of these compounds with RC50 values = 100.82 mg/L and 998.54 mg/L, respectively. There are no obvious harmful side effects of the dermal topical treatments of (R)-linalool and lemon eucalyptus oil on the tested biochemical parameters of treated albino rats compared with the control. Furthermore, there are no obvious effects of the dermal topical treatments of (R)-linalool and lemon eucalyptus oil on the histological status of the treated skin of albino rats compared with untreated treatment. The tested oils and monoterpenes could be considered promising candidates for botanical adulticides against C. pipiens. Also, nano-cream of lemon eucalyptus oil and (R)-linalool could be considered promising ecofriendly repellents for C. pipiens adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mosquitoes pose the greatest threat to public health, with the disease affecting over 40% of the world’s population. By 2050, it is predicted that nearly 60% of the world’s population will be at risk of arbovirus transmission (Baz et al. 2022). Dengue fever has surged by more than 30 times in the last 50 years, and chikungunya, yellow fever, and malaria epidemics have grown and frequency since 2014 (Wang et al. 2021; Carney et al. 2023). The Assiut Governorate in Egypt announced a dengue outbreak in October 2015 with about 253 cases (Assefa 2018). In 2018, 680 dengue cases were detected as an outbreak in the Red Sea Governorate (Abozeid et al. 2018). Hundreds of thousands of people were infected with the Zika virus during the 2015–2016 pandemic in Latin America and the Caribbean, causing widespread socioeconomic upheaval (Wang et al. 2021).
The key strategy for lowering public fears about mosquito-borne diseases is vector control in larval and adult stages (Selim et al. 2021; Baz et al. 2022). Mosquito populations are controlled using residual sprays of insecticide, insecticide-treated nets, and synthetic mosquito repellents. Nevertheless, the continuous use of synthetic insecticides has adverse effects on the environment, resistance development, and respiratory health (Nakasen et al. 2021; Araújo et al. 2023; Khan et al. 2023).
The majority of commercially available insect repellents are made up of a blend of artificial and natural substances that, when applied topically, leave the skin feeling uncomfortable and unattractive to mosquitoes. IR3535, DEET, DEPA, and picaridin are examples of synthetic chemicals that are frequently utilized as active components in insect-repellent products (Najar et al. 2020; Almeida et al. 2023; Sharma et al. 2023). Many mosquito repellant sprays, creams, and lotions contain N,N-diethyl-3-methylbenzamide (DEET) because of its effectiveness against adult mosquitoes (Jaenson et al. 2006; Nguyen et al. 2023). Due to the possible negative effects of synthetic repellents, the researchers were prompted to discover botanical and ecofriendly mosquitocides. These alternatives are biodegradable, safer for non-target organisms, and available (Modise and Ashafa 2016; Kaushik et al. 2023).
Plant essential oils (EOs) are fascinating compounds with promising potential as efficient natural pesticides in the near future. They become reliable tools in vector control due to their low toxicity on non-target organisms, decreased environmental contamination, and decreased risk to the consumers (Isman et al. 2011; Pavela and Benelli 2016). Essential oils (EOs) are volatile compounds produced spontaneously in plants as secondary metabolites for many purposes include feeding and protection or attraction of pests (Bakkali et al. 2008; Devrnja et al. 2022). Plant-derived EOs can function as toxins, inhibitors of insect development (Devrnja et al. 2022), synergists and repellents (El-Wakeil 2013), and phagodeterrents (Meisner et al. 1982; Mwamburi 2022). In each, there are about 20–60 constituents in varying concentrations. Their concentrations range from 20 to 70% for two or three major components, with smaller amounts of the remaining components (Bakkali et al. 2008; Ebadollahi et al. 2020; Basha et al. 2023). Many volatile essential oils consist primarily of monoterpenes that are known to repel insects. When it comes to repelling mosquitoes and other arthropods, sesquiterpenes are also considered an active group (Dias and Moraes 2014; Taktak and Badawy 2019; Alsaraf et al. 2021).
The present study aimed to investigate the adulticidal efficacy of five plant origin of EOs (cinnamon, cypress, lavender, lemon eucalyptus, and tea tree) and their major constituents monoterpenes (cinnamaldehyde, citronellal, β-cymene, (R)-linalool, and α-terpinyl acetate) against C. pipiens. The adulticidal efficacy of the tested compounds was manipulated as mortality and knockdown using a fumigation technique. After that, the most active compounds against adults (lemon eucalyptus oil and linalool) were investigated once more as repellents after incorporating them on a cream base against C. pipiens adults compared to their nano-cream (nanoemulsions of compounds incorporated into a cream base) using arm-in-cage technique. To examine if there are any harmful effects of prepared formulations on treated skin, the biochemical and histological effects of dermal treatment of linalool, lemon eucalyptus, and their nanocream formulations were studied on albino rats. Total protein assay, acetylcholinesterase (AChE), adenosine triphosphatase (ATPase), and liver and kidney function tests were determined in blood serum. Complete blood count (CBC) was determined in whole blood. This study could be used to gather information on several areas of interest regarding the potential use of natural compounds as adulticides against Culex pipiens, as well as employing them in useful and applicable forms like nano-creams. Not only that but the possibility of whether these compounds have any harmful effects on human health will also be studied.
Materials and methods
Chemicals and reagents
Cypress oil was isolated from Monterey cypress (Cupressus macrocarpa) while lemon eucalyptus oil from Corymbia citriodora leaves according to Taktak et al. 2022b in Vector Control and Pesticide Risks Lab, High Institute of Public Health, Alexandria University. While cinnamon oil (Ceylon cinnamon tree, Cinnamomum verum), lavender oil (Lavandula spica), and tea tree oil (Melaleuca alternifolia) were obtained from LUNA Co. for Perfumes and Cosmetics Industry (6 October City, Giza, Egypt).
As previously mentioned, the tested monoterpenes were chosen based on gas chromatography-mass spectroscopy (GC-MS) examination as the main constituents of the tested EOs Taktak et al. 2022a and Taktak et al. 2022b. Sigma-Aldrich Co. (St. Louis, MO, USA) provided these compounds, which include α-terpinyl acetate (95%) and (R)-linalool (97%), citronellal (96%), β-cymene (99%), and cinnamaldehyde (≥ 95%).
Fumigant bioassay
At the High Institute of Public Health insectary at Alexandria University in Alexandria, Egypt, a susceptible strain of a C. pipiens culture was reared (Taktak et al. 2021). The toxicity of EOs and monoterpenes vapors was examined against 3–4 day-old C. pipiens adults according to Zahran and Abdelgaleil 2011. Glass jars (0.7 L) with screw caps were used as exposure chambers. The tested EOs and monoterpenes were applied on Whatman no. 1 filter paper pieces (3 × 3 cm2) attached to the lower surface of the screw caps. While liquid monoterpenes and EOs were applied directly to the filter paper, solid monoterpenes were first dissolved in acetone before being applied to the filter paper pieces. After evaporation of acetone for 2 min, the caps were screwed tightly onto the jars containing 10 mosquito adults. The adult knockdown and mortality were observed at 10 mg/L (air) after different preliminary tests. The adult knockdown was observed up to 2 h of exposure, while the mortality percentage was determined after 24. Then the knockdown values as expressed as KT50 were calculated by the probit method (Finney 1971a)
Repellent activity bioassay
The more active compounds resulting from previousfumigant tests were studied as repellent candidates. Therepellent activity of these compounds and their nano-emulsions(NEs) were examined after incorporating them into the cream base to facilitate the topical application on the skin, then called these forms a nanocream. The NE formulations were prepared and characterized in our previously published studyTaktak et al. 2022b. This repellent assay test was investigated against adult females of C. pipiens by arm-in-cage technique (El-Sheikh et al. 2016; Koundal et al. 2018) with some modifications. The tested concentrations were prepared in a cream base and then applied to volunteer arms. The tested concentrations of linalool were 50–5000 mg/L (technical form) and 50–200 mg/L (NE form). While the tested concentrations of lemon eucalyptus oil were 10–300 mg/L (pure oil), and 1–30 mg/L (NE form). DEET (0.1, 0.3, 0.5, 0.7, and 1 mg/L) was used as a standard repellent for comparison as a positive control.
For acclimatization, fifty female mosquitoes (3–4 days old) were released in an experimental cage (30 × 30 × 30 cm) before 24 h of the experiment. The volunteer’s hands were washed with scent-free soap and dried before the experiment. The volunteer wore gloves on both hands that covered the entire hand and arm except for an area of 25 cm2 on the dorsal side of the hands. A cream base (0.1 g) containing the tested concentration of each tested compound was applied to the exposed area of the hand. A cream base with ethanol solvent was applied on the hand as a negative control and the solvent was allowed to evaporate for 3 min before the experiment. An experimental cage was set up to expose a hand to mosquitoes for five minutes and count the number of mosquitoes landing on the negative control hand and treated hand. A negative control sample and a test sample were both repeated randomly five times. For each replicate, a new mosquito population was used.
The percentage of repellency was calculated by using the following formula:
Were, C: control T: treatment.
Median repellence concentrations (RC50) values were calculated by the probit method (Finney 1971a).
Biochemical and histological effects of dermal topical treatment of linalool, lemon eucalyptus, and their NEs on albino rat
Tested animals
Forty healthy male adults (Rattus norvegicus) albino rats of Wistar strain (100 ± 5 g) were purchased from the animal facility of the Institute of Graduate Studies and Research, Alexandria University. An animal facility with 15% air circulation per hour housed the animals in individual polyethylene cages. The animals were provided with food and water adlibitum. In a 12 h/12 h light and dark cycle, rats were maintained at 25 ± 2ºC and 50 ± 5% relative humidity. Prior to the beginning of the experiment, rats were acclimatized for 14 days to the laboratory conditions. At the baseline (1st day) rats were shaved in the dorsal regions (5 cm2) to prevent licking the topical area. All procedures were carried out under national animal care regulations, the eighth edition of the NIH guidelines for the care and use of laboratory animals, and the European Community Directive (86/609/EEC).
Experimental design and treatments
Animals were randomly divided into four experimental groups and four control groups: 5 rats in each group. Rats were classified as follows; control group: rats did not take any treatment. Ethanol control group: rats were daily treated topically with 100 µL ethanol. DMSO-Tween 80 control group: rats daily treated topically with 100 µL of DMSO (5%) and Tween 80 (2.5%). Tween control group: rats were daily treated topically with 100 µL Tween 80 (10%). The experimental groups were divided as follows: Linalool technical group: rats daily treated topically with 100 µL technical linalool (2%) dissolved in ethanol. Linalool NE group: rats daily treated topically with 100 µL of linalool NE (2%) diluted from previously prepared linalool NE (linalool 2.5%, DMSO 5%, tween 2.5%, and water 90%) according to Taktak et al. 2022b method. Lemon eucalyptus group: rats daily treated topically with 100 µL pure lemon eucalyptus oil (0.3%) dissolved in ethanol. Lemon eucalyptus NE group: rats daily treated topically with 100 µL of lemon eucalyptus oil NE (0.3%) which was diluted from previously prepared lemon eucalyptus oil NE (lemon eucalyptus oil 10%, tween 10%, and water 80%) according to Taktak et al. 2022b method. The experiment continued for five days (Fig. 1A). The applied concentrations are considered 100 times from recommended doses (which cause 100% repellent of C. pipiens adults).
Sampling and biochemical analysis
At the end of the study protocol (five days) rats were decapitated using decapitation equipment and blood samples were collected and divided into two tubes (EDTA and serum). The following parameters were analyzed: Total protein assay in blood serum was investigated according to the Lowry method (Lowry et al. 1951). Acetylcholinesterase (AChE) (Ellman et al. 1961) and ATPase (Koch 1969) enzymes were also determined in blood serum. In addition, complete blood count (CBC) including [haemoglobin (Hb), hematocrit (HCT), red blood cell count (RBCs), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), white blood cells (WBCs), and Platelets] were also determined in whole blood (EDTA) (Tentori and Salvati 1981). Liver function tests including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) (Schumann et al. 2002), and kidney function tests (Urea and creatinine) were also investigated in serum (Tobias et al. 1962; Chou 1984).
Histopathological examination
Paraffin sections were stained with hematoxylin and eosin (H&E) for studying the histological changes of skin tissues under the light microscope at the unit of histopathology in the Medical Technology Center, Medical Research Institute, Alexandria University, Alexandria, Egypt. Parts of skin tissues from rats of each group were processed as follows and stained by conventional hematoxylin and eosin (H&E) stains (Perini et al. 2015). The tissue was fixed at formaldehyde (10%). Then dehydrated in ascending grades of alcohol (70–100%) and cleared in xylene. After that, it is impregnated in molten paraffin at 600ºC for two hours to produce a paraffin block. A microtome was used to cut sections of these prepared blocks into 5 mm thickness. In a water bath, the sections were floated, picked up on clean glass slides, and then dried at 400oC for fixed sections. A deparaffinizing solution of xylene was applied to the paraffin sections, and then they were rehydrated in alcohols ranging from 100 to 70%. The slides were rinsed in hematoxylin stain for one minute and washed in tap water to differentiate the violet color and remove excess color, then rinsed in eosin stain for 5 min. After dehydrating the slides in alcohol, they were cleaned with xylene and then dehydrated again in alcohol. Light microscopy at X100 and X400 magnifying powers was used to mount the slides by DPX technique (a mixture of distyrene, a plasticizer such as dibutyl phthalate, and xylene).
Statistical analysis
Statistical analysis was performed using the IBM SPSS software version 25.0 (SPSS, Chicago, IL, USA) (IBM) IPM 2017. The mean of mortality percentages were calculated for each treatment and corrected using Abbott’s equation (Abbott 1925). Means and standard error (SE) were obtained from three independent replications performed for each treatment. According to the probit analysis, the log dose-response lines (LdP line) were used in the determination of the KT50 and RC50 values for the mosquito’s bioassay (Finney 1971a). The least-square regression analysis was used to determine the 95% confidence limits. Analysis of variance (ANOVA) of the biochemical data was conducted and means property values were separated (p ≤ 0.05) with Student-Newman-Keuls (SNK).
Results and discussion
Mortality and knockdown activities of EOs against C. pipiens adults
The tested oils were evaluated against adult females of C. pipiens using the fumigation technique at 10 mg/L (Table 1). The results were expressed as KT50 (sec) and mortality (%). The results show that lemon eucalyptus oil had the highest significant activity (KT50 = 40.29 s). Followed by cypress oil caused KT50 of 53.03 s followed by lavender oil, cinnamon oil, and tea tree oil with KT50 values = 84.72, 152.03, and 266.13, respectively. All the tested oils caused 100% mortality after 24 h of exposure at 10 mg/L except lavender oil which caused 70% mortality.
The combination of different compounds derived from EOs could increase the insecticidal activities compared to that of single compounds. Adulticidal properties of EOs have been investigated against mosquitoes in various studies such as Yang et al. 2005 who investigated the adulticidal activity of five EOs against C. pipiens L. using a fumigation technique. They proved that Rutaceae oil was the most toxic oil. As well as citral oil showed marked adulticidal activity in a short period of exposure. Furthermore, Zahran et al. 2017 revealed that the flowering plant artemisia (Artemisia monosperma), peppertree (S. terebinthifolius), and oregano (Origanum vulgare) oils had remarkable toxicity based on their LC50 values which ranged between 0.06 and 12.84 mg/L against the C. pipiens adults as fumigants. Manimaran et al. 2012 found that 10% of cinnamon (C. veerum) and eucalyptus (E. globulus) oils caused KT50 values of 33.92 and 33.38 min, respectively. Moreover, cinnamon, eucalyptus, and lavender (L. Angustifolia) oils caused 100% knockdown at 10% formulation for 1 h against C. quinquefasciatus (Ramar et al. 2014). In addition, the mixture of citronella, mint, and clove oils (5%) caused 50% mortality against Ae. Aegypti adults through 25 min of exposure (Alavez-Rosas et al. 2022).
Mortality and knockdown activities of monoterpenes against C. pipiens adults
The main constituents of tested EOs (monoterpenes) were also investigated against C. pipiens adults at 10 mg/L using a fumigation technique (Table 2). The results were expressed as mortality and knockdown. Linalool had the highest effect with the lowest KT50 value of 12.73 s. The effect of citronellal was slightly higher than terpinyl acetate with KT50 values of 26.40 and 30.41 s, respectively. The two compounds with the lowest effects, cymene and cinnamaldehyde, had KT50 values of 65.19 and 43.29 s, respectively. At the same concentration, all tested substances showed 100% mortality after 24 h of exposure.
Various main components of EOs have been evaluated as highly effective against mosquito adults (Songkro et al. 2012). Mixtures of monoterpenes, terpenoids, and related aromatic compounds are secondary plant metabolites and main components of EOs (Maia and Moore 2011). These constituents of EOs are responsible for their mosquitocidal effects (Lupi et al. 2013). The current findings are in agreement with Kim et al. 2020 who proved that linalool induced substantial mortality of Ae. albopictus adult.
Repellent activity of lemon eucalyptus oil and linalool
Based on previous results from fumigation techniques, the most active compounds of EO and monoterpenes have been investigated as repellent candidates against C. pipiens adults. The repellent activity of these compounds (Lemon eucalyptus oil and Linalool) and their nano-emulsions (NEs) were examined after incorporating them into the cream base.
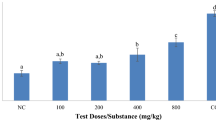
The repellent activity of these compounds against adults is presented as RC50 (mg/L) and shown in Table 3; Fig. 2. The data show that lemon eucalyptus oil was stronger than linalool with RC50 values of 100.82 and 998.84 mg/L, respectively. While the RC50 values were 68.11 and 10.03 mg/L for their NE formulations, respectively. It’s really obvious, the effect of converting the crude oils to NEs form, increased the activity of compounds by 10 times of EOs and 70 times of linalool. DEET as a commercially recommended synthetic repellent showed the highest repellent activity causing RC50 value of 0.17.
Repellents are compounds that cause local dissociation, preventing insects from settling on or biting human or animal skin (Nerio et al. 2010). So, personal protection by mosquito repellents is one of the most important measures used for the control of several life-threatening vector-borne diseases. In developing countries, EOs have already been employed as effective repellents against mosquito bites by applying them to the skin or clothing of rural communities, due to their diverse structures and potent phytotoxic effects (Verdeguer et al. 2020). These factors make EOs potential candidates for the production of new repellents.
Several studies are reporting the bioactivities of EOs as repellents and their main components against mosquitoes (Haris et al. 2023). The repellency bioassay of cinnamon oil against C. quinquefasciatus adults proved that the 50 mg/L caused 100% repellency for up to 180 min (Nakasen et al. 2021). While 20% oil solutions of lavender (L. angustifolia), eucalyptus (E. globulus), and cinnamon (C. zeylanicum) oils, compared with DEET caused 24.3, 56.7, 70.3, and 45.9% repellency, respectively against Ae. Aegypti adults, 80.9, 28.6, 100, and 100% repellency, respectively against An. stephensi adults, and 85.7, 100, 100, and 100% repellency, respectively against C. quinquefasciatus adults (Amer and Mehlhorn 2006). The mixture of citronella, mint, and clove oils (5%) had a repellent effect similar to DEET against Ae. aegypti adults (Alavez-Rosas et al. 2022).
Monoterpenes are one of the most important constituents of EOs which may be responsible for their mosquito repellency (Pohlit et al. 2011). Caryophyllene and its derivatives (present in clove oil) possess repellent activity against Ae. aegypti (Nararak et al. 2019). Additionally, studies on the effectiveness of menthol and menthol acetate (the main components of the mint oil) as insect repellents showed promising results (Kamatou et al. 2013; Lim et al. 2019). Components of rosemary oil are effective insect repellents (Lim et al. 2019; Sun et al. 2020). Some EO compounds, like thujone, eucalyptol, and linalool, also react with the same odorant receptors that are sensitive to DEET (Syed and Leal 2008). Since all chemicals can be hazardous if exposed above a safe concentration, it is necessary to estimate EO toxicity levels, just as synthetic chemicals are, to ensure that humans and water are treated safely (Nerio et al. 2010). In previous attempts, researchers failed to find adverse effects of EOs in repellent assays (Trumble 2002).
Effect of dermal topical treatment of linalool, lemon eucalyptus oil, and their NEs
After 5 days of the dermal topical treatment of the tested compounds on albino rat skin, there were no harmful effects or differences in the appearance of the treated skin (Fig. 1, B).
Complete blood count of albino rat
The effect of dermal topical application of linalool (2%), lemon eucalyptus oil (0.3%), and their NEs on albino rat complete blood count (CBC) was investigated after 5 days of treatment and presented in Table 4. The haematological alteration was assessed by comparing it with the haematological parameters of untreated rats (control). Pure lemon eucalyptus oil (11.70 thousand) and technical linalool (9.78 thousand) caused a noticeable increase in WBCs of treated rats compared to untreated rats (7.01 thousand). Treated with lemon eucalyptus oil NE showed a very close count of WBCs (6.47 thousand) compared to control. While NE of linalool caused a slight increase of WBCs (8.36 thousand) in treated rats. The pure compounds caused a slight increase in RBCs count more than their NEs. There is no obvious effect of tested treatments on RBCs and Hb. There were no significant effects of tested compounds on HCT and MCHC in the blood of treated rats compared to untreated rats (43.87% and 27.63 g/dl, respectively). NEs of lemon eucalyptus oil and linalool did not show significant effects on MCH (19.04 and 18.54 pg, respectively), while their pure compounds caused a slight decrease (17 and 17.32 pg, respectively) compared with control (18.33 pg). NEs of lemon eucalyptus oil and linalool did not cause clear effects on MCV (67.40 and 66.86 fl., respectively) compared with control (66.47 fl.), while their pure compounds caused slightly decreasing (61.30 and 63.02 fl., respectively). The conversion of pure compounds to NEs decreases their significant effects on these blood parameters.
The blood parameters are used to monitor feeding experiments and the general state of health of the organism (Gultepe et al. 2017). The number of red blood cells (RBCs) is a value that is directly affected by the metabolic rate and also has a right proportional to Hb (Gültepe 2020). All treatments caused a slightly non-significant decrease in blood platelet count. In agreement with our results, the data obtained by (Macedo et al. 2010; Ribeiro et al. 2015) prepared E. staigeriana NE and performed the toxicological tests on rodents. They revealed that the hematological parameters were not altered after treatment.
Kidney and liver enzymes of albino rat blood serum
The effect of dermal topical treatment of linalool, lemon eucalyptus oil, and their NEs on kidney and liver enzymes of albino rat blood serum was also studied. Urea (mg/dl), Creatinine (mg/dl), ALT (U/L), AST (U/L), and ALP (U/L) concentrations were determined as indicators for kidney and liver enzymes and presented in Table 5. The results showed that different treatments significantly decreased the urea levels in blood serum. NE of linalool caused the greatest effect on urea level (30.40 mg/dl) compared with its level in the control sample (38.00 mg/dl). While there is not any significant difference between other treatments. On the other hand, different treatments elevated creatinine levels in blood serum. The NE of lemon eucalyptus oil significantly increased creatinine level (0.48 mg/dl) in blood serum compared to the control sample (0.35 mg/dl). All treatments caused a significant increase in ALT concentrations. Technical linalool showed the highest activity that caused a significant increase in ALT (61.60 U/L compared to 44.67 U/L in control). While NE of linalool caused an increase in ALT concentration to 46.20 U/L. Lemon eucalyptus oil caused a slight increase in ALT levels (46.00 U/L) while its NE caused a significant increase (50.80 U/L) compared to the control. All treatments elevated AST concentrations in blood serum. NE of linalool caused the highest effect on the AST enzyme (317.60 U/L) compared with its concentration in the control sample (258.33 U/L). There are no significant differences between the effects of technical linalool (281.60 U/L) and NE of lemon eucalyptus oil (276.20 U/L) on AST in blood serum. While pure lemon eucalyptus oil showed the lowest effect (270.60 U/L) compared with control (258.33 U/L). These results agree with the results obtained by Beier et al. 2014 who proved that, linalool activated AST enzyme from 189.20 IU/L in the untreated sample to 236.93 IU/L in the treated sample. In the case of the ALP assay, the results showed that all treatments significantly decreased the activity of the ALP enzyme except the NE of linalool which caused an increase in enzyme activity. There are no significant differences between ALP concentrations in treated samples with pure and NE of lemon eucalyptus oil and technical linalool (216.20, 236.60, and 228.80 U/L, respectively). Linalool (NE) caused an increase in enzyme activity from 275 U/L in the control sample to 320.80 U/L in the treatment. Transaminases (AST and ALT) are enzymes that show the health status of the body, especially the liver.
Biochemical evaluation is considered the important step that has been reported following the use of phyto-repellent products (El Hilaly et al. 2004; Isnard et al. 2004; Saad et al. 2006). The results were obtained by Macedo et al. 2010 who formulated E. staigeriana NE and performed the toxicological tests on rodents. They found that it was not observed significant alterations in the levels of AST, ALT, urea, and creatinine caused by E. staigeriana administration. This result indicates that kidney and hepatic functions were preserved (Ribeiro et al. 2015).
AChE and ATPase of albino rat blood serum
The effects of topical application of linalool, lemon eucalyptus oil, and their NEs on AChE and ATPase of albino rat blood serum are presented in Table 6. All treatments increased AChE activity. Technical linalool was the most active compound which increased the activity with 40.21%. The NE form of lemon eucalyptus oil increased its activity from 3.01% for pure oil to 27.86%. While the NE form of linalool decreased AChE activity from 40.21% for the technical form to 19.83%. The activation effect of linalool on ATPase was higher than lemon eucalyptus oil. Linalool caused enzyme activation with 30.44% for technical form and 26.67% for NE. While pure lemon eucalyptus oil had a slight activation effect (1.69%) against ATPase. In contrast, Bianchini et al. 2017 found that S-(+)-linalool did not alter the AChE activity muscle and brain of silver catfish (Rhamdia quelen).
Histological effects of linalool, lemon eucalyptus, and their NEs on treated skin
The observed effects of topical application of linalool, lemon eucalyptus oil, and their NEs on the histological status of albino rat skin were presented in Fig. 3 at X100 magnify and Fig. 4 at X400 magnify. A light microscopy study of histopathological changes on normal control and experimental rat skin section stained with H&E stains as follows: Paraffin section photomicrograph of control (untreated rat skin) (Fig. 3A) shows that an epidermal layer (E) with well-formed basement membrane (BM) infolded in the dermal layer and keratinocytes (K) upper the epithelial cells (EC). A few infiltrations of many lymphocytes (IF), blood vessels (BV), and blood capillaries (BC) mild disorganized collagen fibrin (C) recovering the dermis layer (D), and A migrated hairs follicles (HF) and the sebaceous gland (SC) to the upper epidermal was seen. The Fig. 4A, at X400 magnify shows the thick keratinocytes (K) upper the epithelial cells (EC) have a well-formed basement membrane (BM). Besides the foci of pyknotic one of the epidermal layers (E), many migrated dark pigmented (Pg) and many congested dilated blood vessels (BV) and blood capillaries (BC). The dermis layer has cellular infiltration lymphocytes (IF), mildly disorganized collagen fibrin (C) in different orientations and few edemas (ED).
Photomicrograph of untreated rat skin (A) and treated skin with ethanol (B), Tween 80 (C), Tween 80 + DMSO (D), pure lemon eucalyptus oil (E), NE of lemon eucalyptus oil (F), linalool (G), and NE of linalool (H) at X100 magnify. Blood vessels (BV), blood capillaries (BC), collagen fibrin (C), dermis layer (D), epidermal layers (E), epithelial cells (EC), edema (ED), fibrotic cell (F), keratinocytes (K), infiltration of cells (IF), hairs follicles (HF), sebaceous gland (SG).
Photomicrograph of untreated rat skin (A) and treated skin with ethanol (B), Tween 80 (C), Tween 80 + DMSO (D), pure lemon eucalyptus oil (E), NE of lemon eucalyptus oil (F), linalool (G), and NE of linalool (H) at X400 magnify. Blood vessels (BV), blood capillaries (BC), collagen fibrin (C), dermis layer (D), epidermal layers (E), epithelial cells (EC), edema (ED), fibrotic cell (F), keratinocytes (K), infiltration of cells (IF), hairs follicles (HF), sebaceous gland (SG).
Paraffin section photomicrograph of rat skin treated with ethanol (Fig. 3B) shows proliferating keratinocytes (K) and migrated upper the thin epidermal layers. The dermis layer has few cellular infiltration lymphocytes with marked increased and migrated dilated blood vessels (BV) and capillaries (BC), hair follicles, and sebaceous gland to the upper epidermal layer, and mild destructive of collagen fibrin and edema was seen. Figure 4B, at X400 magnify shows migrated and separated keratinocytes (K) from thin epidermal layers have squamous epithelial cells (Pc) of the basement membrane, few cellular infiltrating lymphocytes (IF), migration of marked congested and dilated blood vessels (BV) and blood capillaries (BC) in epidermal layer mild destructive of collagen fibrin (c), hair follicles sebaceous gland (SG).
Paraffin section photomicrograph of rat skin treated with Tween 80 (Fig. 3C) shows separated keratinocytes (K) and migrated upper thin epidermal layers (E), marked increased dilated and congested blood vessels (BV) and blood capillaries (BC), marked destructive and disorganized collagen fibrin distribution (C) and edema ED. Figure 4C, at X400 magnify, notes a thin epidermal layer (E) separated from the keratinize layer the epithelial cells (EC) covered by pigmented granules. A dermis layer has marked disorganized rearrangement of collagen fibrin (C) with few inflammations of the reactive fibrotic cell (F) and lymphocytes blood capillaries (BC) and vessels (BV) and marked edema (ED) was seen.
Paraffin section photomicrograph of treated skin rat with Tween 80 and DMSO (Fig. 3D), shows an area of organized architecture of the skin epidermal layer covered by thin keratinized cells (K). The dermis (D) is made by collagen fibrin in different directions (C), sebaceous glands (SG), hair follicle and edema (ED), and many congested blood capillaries (BC) and few blood vessels. High power of the previous Fig. (Fig. 4D) shows the skin epidermal layer covered by thin keratinized cells (K) and a thin layer of pigmented granules and have stratified squamous epithelium cells (EC) lying basement membrane (BM) which folded with hair follicles (HF) in dermis layer (D), which has mildly disorganized collagen fibrin (C), sebaceous glands (SG), hair follicle, edema (ED), and marked congested and dilated blood capillaries (BC) and blood vessel.
Paraffin section photomicrograph of treated skin rat with pure lemon eucalyptus oil (Fig. 3E), shows organized architecture of skin epidermal layer with separated keratinized layer (K). The dermis (D) is made by collagen fibrin in different directions (C). Which has sebaceous glands (SG), hair follicles (HF), and many congested and dilated blood capillaries (BC) and vessels (BV). Figure 4E, at X400 magnify, shows the area of the organized architecture of the skin epidermal layer covered by thick keratinized layer (K) and dark pigmented granules. Stratified squamous epithelium cells (EC) lying in basement membrane (E) folded in the dermis layer. The dermis (D) has collagen fibrin in a different direction (C), sebaceous glands (SG), the hair follicle (HF) and edema (ED) and marked dilation and congested blood capillaries (BC) and blood vessel (BV) was seen.
Paraffin section photomicrograph of treated skin rat with lemon eucalyptus (NE), shows mild disorganized architecture of skin epidermal layer has many folded basement membranes (BM) with hair follicles and congested blood vessels (BV) and the upper covered by proliferating keratinized cells (K) form many keratinized layers. The dermis (D) layer has collagen, sebaceous glands (SG), and many hair follicles (HF) migrated to the epidermal layer and edema (ED), marked dilated and congested blood vessel and capillaries (BC) and infiltrating lymphocytes (IF) was seen (Fig. 3F). The Fig. 4F, at X400 magnify shows the area of the organized architecture of the skin epidermal layer (E) covered by a thick keratinized layer (K) and separated from epithelial cells by dense pigmented granules. Stratified squamous epithelium cells (EC) lying basement membrane (BM) which folded with hair follicles in the dermis layer (HF). The dermis (D) has spread collagen fibrin, hair follicle, and edema (ED), and marked dilated and congested blood vessel (BV)and capillaries and infiltrating lymphocytes and plasma cells (IF)was seen.
Paraffin section photomicrograph of treated rat skin with technical linalool (Fig. 3G), shows a thin keratin layer (K) upper the epithelial cells (EC), which formed a smooth basement membrane of epidermal layers(EP), many cellular infiltration lymphocytes (IF), marked dilated blood vessels (BV) and blood capillaries(BC) perpendicular to the epidermal layer, the dermis has well-formed and condensed collagen fibrin(C), migrated the sebaceous gland (SG) and hair follicles to the upper epidermal was seen. High power of the previous figure, note thin smooth epidermal layer (EP) covered with keratinized layer followed by dense pigment granules and thin squamous epithelial cells (E). The dermis layer is covered by thick collagen fibrin (C) arranged along the epidermal layer, many inflammatory cells (IF), moderate dilation of blood capillaries (BC)and vessels (BV), moderate edema (ED) and migrated sebaceous gland (SG) and hair follicles to the upper epidermal was seen (Fig. 4G).
Paraffin section photomicrograph of treated rat skin with linalool NE (Fig. 3H) shows an organized skin structure with a keratinized layer (K) covered by the upper epithelial cells (EC) which formed basement membrane of the epidermal layers (E), the dermis layer has mild cellular infiltration of many lymphocytes (IF), marked dilated blood vessels (BV) and blood capillaries(BC) perpendicular to the epidermal layer, destructive collagen fibrin (C), and migrated the sebaceous gland (SG) to the upper epidermal was seen. Figure 4H, at X400 magnify shows less thickness of the epidermal layer (EP) covered with a keratinized proliferating layer followed by a dense pigment granulated layer and stratified squamous epithelial cells (EC). The dermis layer has spread collagen fibrin (C), few inflammatory cells (IF), moderate dilation of blood capillaries (BC), and edema (ED) were seen. All previous results did not show any obvious irritating or sensitizing effects.
These results agree with those obtained by Bickers et al. 2003 who proved that linalool was not irritating, phototoxic, or sensitizing. Sköld et al. 2004 proved that linalool (T) did not show sensitizing potential in female mice. Also, Jeong et al. 2010 indicated that lemon eucalyptus oil had no skin or eye irritation. As reported by Macedo et al. 2010 for E. staigeriana EO, no histopathological tissue changes and body weight gain in the animals were observed.
In accordance with the chemical structure of EOs and the pathophysiological setting of the skin, their topical applications may have various effects on the skin. Triglycerides, phospholipids, FFAs, phenolic compounds, and antioxidants found in plant oils may work together synergistically when applied topically through several processes, including antioxidant activities, anti-inflammatory qualities, direct and indirect anti-microbial characteristics (upregulation of antimicrobial peptides), wound healing capabilities, maintaining skin barrier homeostasis, anti-carcinogenic properties, and antioxidant activities are just a few Lin et al 2017.
Conclusion
Personal protection by mosquito repellents is one of the most important measures used for the control of mosquitoes. Considering the structural variety and potent phytotoxic activity of EOs, novel repellents may be developed using them. Lemon eucalyptus oil and linalool have the highest activities against C. pipiens adults. The repellent activity of lemon eucalyptus nano-cream was higher than (R)-linalool against C. pipiens adults. Converting lemon eucalyptus oil and (R)-linalool to NE forms increased their repellent effect, many times the original effect of these compounds. As a repellent, nano-cream of lemon eucalyptus oil and (R)-linalool could be considered promising eco-friendly alternatives against C. pipiens adults.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18(2):265–267
Abozeid S, Elsayed AK, Schaffner F, Samy AM (2018) Re-emergence of Aedes aegypti in Egypt. Lancet Infect Dis 18(2):142–143. https://doi.org/10.1016/S1473-3099(18)30018-5
Alavez-Rosas D, Socorro-Benitez C, Cruz-Esteban S (2022) Repellent and adulticidal effect of essential oils mixtures on Aedes aegypti females. Int J Trop Insect Sci 42(2):1885–1892. https://doi.org/10.1007/s42690-021-00716-z
Almeida AR, Oliveira ND, Pinheiro FASD, de Morais WA, Ferreira LDS (2023) Challenges encountered by natural repellents: since obtaining until the final product. Pestic Biochem Physiol 195:105538. https://doi.org/10.1016/j.pestbp.2023.105538
Alsaraf S, Hadi Z, Akhtar MJ, Khan SA (2021) Chemical profiling, cytotoxic and antioxidant activity of volatile oil isolated from the mint (Mentha spicata L,) grown in Oman. Biocatal Agric Biotechnol 43:102034. https://doi.org/10.1016/j.bcab.2021.102034
Amer A, Mehlhorn H (2006) Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol Res 99(4):478–490. https://doi.org/10.1007/s00436-006-0184-1
Araújo MF, Castanheira EM, Sousa SF (2023) The buzz on insecticides: a review of uses, molecular structures, targets, adverse effects, and alternatives. J Mol 28(8):3641. https://doi.org/10.3390/molecules28083641
Assefa Y (2018) Expansion of the WHO public health approach to HIV. Lancet Infect Dis 18(2):143–144. https://doi.org/10.1016/S1473-3099(18)30019-7
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils–a review. Food Chem Toxicol 46(2):446–475. https://doi.org/10.1016/j.fct.2007.09.106
Basha AN, Subramanian R, Chithan K, Vincent GG, Murugesan K, Ramachandran A et al (2023) Properties and mechanism of Antimicrobial agents from Plant-Derived essential oils. Bioprospecting of Tropical Medicinal Plants, Springer, pp 1347–1363
Baz MM, Selim A, Radwan IT, Alkhaibari AM, Khater HF (2022) Larvicidal and adulticidal effects of some Egyptian oils against Culex pipiens. " Sci Rep 12(1):1–18. https://doi.org/10.1038/s41598-022-08223-y
Beier RC, Byrd JA, Kubena IILF, Hume ME, McReynolds JL, Anderson RC (2014) Evaluation of linalool, a natural antimicrobial and insecticidal essential oil from basil: effects on poultry. Poult Sci 93(2):267–272. https://doi.org/10.3382/ps.2013-03254
Bianchini A, Garlet Q, Cunha JD, Bandeira G, Brusque I, Salbego J et al (2017) Monoterpenoids (thymol, carvacrol and S-(+)-linalool) with anesthetic activity in silver catfish (Rhamdia quelen): evaluation of acetylcholinesterase and GABAergic activity. Braz J Med Biol 50. https://doi.org/10.1590/1414-431X20176346
Bickers D, Calow P, Greim H, Hanifin JM, Rogers AE, Saurat JH et al (2003) A toxicologic and dermatologic assessment of linalool and related esters when used as fragrance ingredients. Food Chem Toxicol 41(7):919–942. https://doi.org/10.1016/S0278-6915(03)00016-4
Carney RM, Long A, Low RD, Zohdy S, Palmer JR, Elias P et al (2023) Citizen science as an approach for responding to the threat of anopheles stephensi in Africa. Citiz Sci: Theory Pract 8:60. https://doi.org/10.5334/cstp.616
Chou D (1984) Clinical guide to laboratory tests. JAMA 251(19):2587–2588. https://doi.org/10.1001/jama.1984.03340430079046
Devrnja N, Milutinović M, Savić J (2022) When scent becomes a weapon—plant essential oils as potent bioinsecticides. Sustain Sci 14(11):6847. https://doi.org/10.3390/su14116847
Dias CN, Moraes DFC (2014) Essential oils and their compounds as Aedes aegypti L.(Diptera: Culicidae) larvicides. Parasitol Res 113(2):565–592. https://doi.org/10.1007/s00436-013-3687-6
Ebadollahi A, Ziaee M, Palla F (2020) Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Mol 25(7):1556. https://doi.org/10.3390/molecules25071556
El Hilaly J, Israili ZH, Lyoussi B (2004) Acute and chronic toxicological studies of Ajuga Iva in experimental animals. " J Ethnopharmacol 91(1):43–50. https://doi.org/10.1016/j.jep.2003.11.009
El-Sheikh TM, Al-Fifi ZI, Alabboud MA (2016) Larvicidal and repellent effect of some Tribulus terrestris L.,(Zygophyllaceae) extracts against the dengue Fever mosquito. Aedes aegypti (Diptera: Culicidae) " J Saudi Chem Soc 20(1):13–19. https://doi.org/10.1016/j.jscs.2012.05.009
El-Wakeil NE (2013) Retracted Article: Botanical Pesticides and Their Mode of Action. Gesunde Pflanzen 65(4):125–149. https://doi.org/10.1007/s10343-013-0308-3
Ellman GL, Courtney KD, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Finney DJ (1971a) Probit Analysis. Cambridge University Press
Gultepe N, Sazykina M, Sazykin I, Sonmez AY, Khmelectsova LE, Khammami M (2017) Comparative study of haematological parameters of three sturgeon species in recirculating aquaculture system. Indian J Fish 64(1):87–90. https://doi.org/10.21077/ijf.2017.64.1.62577-15
Gültepe N (2020) Protective effect of d-limonene derived from orange peel essential oil against Yersinia ruckeri in rainbow trout. Aquaculture Rep 18:100417. https://doi.org/10.1016/j.aqrep.2020.100417
Haris A, Azeem M, Abbas MG, Mumtaz M, R Mozūratis and, Binyameen M (2023) Prolonged repellent activity of plant essential oils against Dengue Vector. Aedes aegypti " Molecules 28(3):1351
IBM Corp. Released 2017. IBM SPSS statistics for Windows, Version 25.0. Armonk, NY: IBM Corp
Isman MB, Miresmailli S, Machial C (2011) Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem Rev 10(2):197–204. https://doi.org/10.1007/s11101-010-9170-4
Isnard BC, Deray G, Baumelou A, Quintrec ML, Vanherweghem J (2004) Herbs and the kidney. Am J Kidney Dis 44(1):1–11. https://doi.org/10.1053/j.ajkd.2004.02.009
Jaenson TG, Pålsson K, Borg-Karlson A-K (2006) Evaluation of extracts and oils of mosquito (Diptera: Culicidae) repellent plants from Sweden and Guinea-Bissau. J Med Entomol 43(1):113–119. https://doi.org/10.1093/jmedent/43.1.113
Jeong MH, Kwon MJ, Park SJ, Hong SS, Park KH, Park JE et al (2010) Evaluation of acute toxicity of plant extracts, lavender, lemon eucalyptus and cassia essential oil. Korean J Pestic Sci 14(4):339–346
Kamatou GP, Vermaak I, Viljoen AM, Lawrence BMJP (2013) Menthol: a simple monoterpene with remarkable biological properties. Phytochem. 96:15–25. https://doi.org/10.1016/j.phytochem.2013.08.005
Kaushik M, Yadav J, Singh A, Dubey MK (2023) A systematic review of plant-based mosquito repellents and their activity. " NIScPR-CSIR, India
Khan BA, Nadeem MA, Nawaz H, Amin MM, Abbasi GH, Nadeem M et al (2023) Pesticides: impacts on agriculture productivity, environment, and management strategies. Emerging Contaminants and Plants: Interactions, Adaptations and Remediation Technologies, Springer: 109–134
Kim HK, Seo J-W, Kim G-H (2020) Various effects of volatile constituents from Magnolia kobus flowers against Aedes albopictus. (Diptera: Culicidae) " Ind Crops Prod 145:112109. https://doi.org/10.1016/j.indcrop.2020.112109
Koch R (1969) Chlorinated hydrocarbon insecticides: inhibition of rabbit brain ATPase activities. J Neurochem 16(2):269–271. https://doi.org/10.1111/j.1471-4159.1969.tb05944.x
Koundal R, Dolma SK, Chand G, Agnihotri VK, Reddy SE (2018) Chemical composition and insecticidal properties of essential oils against diamondback moth (Plutella Xylostella L.). Toxin rev. https://doi.org/10.1080/15569543.2018.1536668
Lim V, Mohd Narawi M, Chiu HI, Tung WH, Tan JJ, Lee CK (2019) Selected essential oils as repellents against Aedes aegypti: validation of the bioconstituents using gas chromatography. J Essent Oil Bear Plants 22(4):1058–1073. https://doi.org/10.1080/0972060X.2019.1661796
Lin T-K, L Zhong andJLJIjoms Santiago (2017). Anti-inflammatory and skin barrier repaireffects of topical application of some plant oils. International journalof molecular sciences 19(1): 70.https://doi.org/10.3390/ijms19010070
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J biol Chem 193(1):265–275
Lupi E, Hatz C, Schlagenhauf P (2013) The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp.–A literature review. Travel Med Infect Dis 11(6):374–411. https://doi.org/10.1016/j.tmaid.2013.10.005
Macedo IT, Bevilaqua CM, de Oliveira LM, Camurça-Vasconcelos AL, Vieira LS, Oliveira FR et al (2010) Anthelmintic effect of Eucalyptus Staigeriana essential oil against goat gastrointestinal nematodes. Vet Parasitol 173(1–2):93–98. https://doi.org/10.1016/j.vetpar.2010.06.004
Maia MF, Moore SJ (2011) Plant-based insect repellents: a review of their efficacy, development and testing. Malar J 10(1):1–15. https://doi.org/10.1186/1475-2875-10-S1-S11
Manimaran A, Cruz MMJJ, Muthu C, Vincent S, Ignacimuthu S (2012) Larvicidal and knockdown effects of some essential oils against Culex quinquefasciatus Say, Aedes aegypti (L.) and Anopheles Stephensi (Liston). Adv biosci biotechnol. 3:8. https://doi.org/10.4236/abb.2012.37106
Meisner J, Fleischer A, Eizick C (1982) Phagodeterrency induced by (–)-carvone in the larva of Spodoptera Littoralis (Lepidoptera: Noctuidae). J Econ Entomol 75(3):462–466. https://doi.org/10.1093/jee/75.3.462
Modise SA, Ashafa AOT (2016) “Larvicidal, pupicidal and insecticidal activities of Cosmos bipinnatus, Foeniculum vulgare and Tagetes minuta against Culex quinquefasciatus mosquitoes.” Trop J Pharm Res 15(5): 965–972. https://doi.org/10.4314/tjpr.v15i5.10
Mwamburi LA (2022) Role of plant essential oils in Pest Management. New and Future Development in Biopesticide Research: Biotechnological Exploration. Springer, pp 157–185
Najar B, Pistelli L, Venturi F, Ferroni G, Giovanelli S, Cervelli C et al (2020) Salvia Spp. Essential oils against the arboviruses Vector Aedes albopictus (Diptera: Culicidae): Bioactivity, Composition, and Sensorial Profile—Stage.” J Biol: 341
Nakasen K, Wongsrila A, Prathumtet J, Sriraj P, Boonmars T, Promsrisuk T et al (2021) Bio efficacy of Cinnamaldehyde from Cinnamomum verum essential oil against Culex quinquefasciatus (Diptera: Culicidae). J Entomol Acarol Res 53(1). https://doi.org/10.4081/jear.2021.9400
Nararak J, Sathantriphop S, Kongmee M, Mahiou-Leddet V, Ollivier E, Manguin S et al (2019) Excito-repellent activity of β-caryophyllene oxide against Aedes aegypti and Anopheles Minimus. Acta Trop 197:105030. https://doi.org/10.1016/j.actatropica.2019.05.021
Nerio LS, Olivero-Verbel J, Stashenko E (2010) Repellent activity of essential oils: a review. Bioresour Technol 101(1):372–378. https://doi.org/10.1016/j.biortech.2009.07.048
Nguyen Q-BD, Vu M-AN, Hebert AA (2023) “Insect repellents: an updated review for the clinician.” JAAD 88(1): 123–130. https://doi.org/10.1016/j.jaad.2018.10.053
Pavela R, Benelli G (2016) Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci 21(12):1000–1007. https://doi.org/10.1016/j.tplants.2016.10.005
Perini JA, Angeli-Gamba T, Alessandra-Perini J, Ferreira LC, Nasciutti LE, Machado DE (2015) Topical application of Acheflan on rat skin injury accelerates wound healing: a histopathological, immunohistochemical and biochemical study. BMC Complement Altern Med 15(1):1–8. https://doi.org/10.1186/s12906-015-0745-x
Pohlit AM, Lopes NP, Gama RA, Tadei WP, VF de Andrade Neto (2011) Patent literature on mosquito repellent inventions which contain plant essential oils–a review. Planta Med 77(06):598–617. https://doi.org/10.1055/s-0030-1270723
Ramar M, Ignacimuthu S, Paulraj MG (2014) Mosquito knock-down and adulticidal activities of essential oils by vaporizer, impregnated filter paper and aerosol methods. Int J Mosq Res 1(3):26–32
Ribeiro WLC, Camurça-Vasconcelos ALF, Macedo ITF, dos Santos JML, de Araújo-Filho JV, de Carvalho J, Ribeiro et al (2015) In vitro effects of Eucalyptus staigeriana nanoemulsion on Haemonchus contortus and toxicity in rodents. Vet Parasitol 212(3–4):444–447. https://doi.org/10.1016/j.vetpar.2015.07.019
Saad B, Azaizeh H, Abu-Hijleh G, Said O (2006) Safety of traditional arab herbal medicine. Evid Based Complement Alternat Med 3(4):433–439. https://doi.org/10.1093/ecam/nel058
Schumann G, Bonora R, Ceriotti F, Férard G, Ferrero CA, Franck PF et al (2002) IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 C. Part 4. Reference procedure for the measurement of catalytic concentration of alanine aminotransferase. CCLM. https://doi.org/10.1515/CCLM.2002.124
Selim A, Shoulah S, Abdelhady A, Alouffi A, Alraey Y, Al-Salem WS (2021) Seroprevalence and risk factors associated with canine Leishmaniasis in Egypt. " Vet Sci 8(10):236. https://doi.org/10.3390/vetsci8100236
Sharma M, Nagori K, Jain V, Balan NS (2023) Herbal, safe and effective mosquito repellents: recent development and opportunity. RJPT. 16(5):2557–2564. https://doi.org/10.52711/0974-360X.2023.00420
Sköld M, Börje A, Harambasic E, Karlberg A-T (2004) Contact allergens formed on air exposure of linalool. Identification and quantification of primary and secondary oxidation products and the effect on skin sensitization. Chem Res Toxicol 17(12):1697–1705. https://doi.org/10.1021/tx049831z
Songkro S, Hayook N, Jaisawang J, Maneenuan D, Chuchome T, Kaewnopparat N (2012) Investigation of inclusion complexes of citronella oil, citronellal and citronellol with β-cyclodextrin for mosquito repellent. J Incl Phenom Macrocycl Chem 72(3):339–355. https://doi.org/10.1007/s10847-011-9985-7
Sun JS, Feng Y, Wang Y, Li J, Zou K, Liu H et al (2020) α-pinene, caryophyllene and β-myrcene from Peucedanum terebinthaceum essential oil: Insecticidal and repellent effects on three stored-product insects. Rec Nat Prod 14(3):189. https://doi.org/10.25135/rnp.149.19.05.1287
Syed Z, Leal WS (2008) Mosquitoes smell and avoid the insect repellent DEET. PNAS 105(36):13598–13603. https://doi.org/10.1073/pnas.0805312105
Taktak NE, Badawy ME (2019) Potential of hydrocarbon and oxygenated monoterpenes against Culex pipiens larvae: toxicity, biochemical, pharmacophore modeling and molecular docking studies. Pestic Biochem Physiol 158:156–165
Taktak NE, Badawy ME, Awad OM, Abou El-Ela NE, Abdallah SM (2021) “Enhanced mosquitocidal efficacy of pyrethroid insecticides by nanometric emulsion preparation towards Culex pipiens larvae with biochemical and molecular docking studies.” JEPHA 96(1): 1–19. https://doi.org/10.1186/s42506-021-00082-1
Taktak N, Badawy M, Osama A, and N ABOU EL-ELA (2022a) “Comparative toxicity of cinnamon oil, cinnamaldehydetheir nano-emulsions against Culex pipiens (L.) larvae with biochemicaldocking studies.” IJPBP 2(1): 51–63
Taktak NE, Badawy ME, Awad OM, Abou El-Ela NE (2022b) Nanoemulsions containing some plant essential oils as promising formulations against Culex pipiens (L.) larvae and their biochemical studies. Pestic Biochem Phys 105151. https://doi.org/10.1016/j.pestbp.2022.105151
Tentori L, Salvati A (1981) Hemoglobinometry in human blood. Methods in enzymology. B. F. Eichman. Elsevier 76:707–715
Tobias GJ, McLaughlin RF Jr, Hopper J Jr (1962) “Endogenous creatinine clearance: a valuable clinical test of glomerular filtration and a prognostic guide in chronic renal disease.” NEJM 266(7): 317–323
Trumble JT (2002) Caveat emptor: safety considerations for natural products used. In Arthropod Control " Am Entomol 48(1):7–13. https://doi.org/10.1093/ae/48.1.7
Verdeguer M, Sánchez-Moreiras AM, Araniti F (2020) Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 9(11):1571. https://doi.org/10.3390/plants9111571
Wang G-H, Gamez S, Raban RR, Marshall JM, Alphey L, Li M et al (2021) Combating mosquito-borne Diseases using genetic control technologies. Nat Commun 12(1):1–12. https://doi.org/10.1038/s41467-021-24654-z
Yang P, Ma Y, Zheng S (2005) Adulticidal activity of five essential oils against Culex pipiens Quinquefasciatus. Pestic Sci 30(2):84–89. https://doi.org/10.1584/jpestics.30.84
Zahran HM, Abdelgaleil SA (2011) Insecticidal and developmental inhibitory properties of monoterpenes on Culex pipiens L.(Diptera: Culicidae). J Asia Pac Entomol 14(1):46–51. https://doi.org/10.1016/j.aspen.2010.11.013
Zahran HE-DM, Abou-Taleb HK, Abdelgaleil SA (2017) Adulticidal, larvicidal and biochemical properties of essential oils against Culex pipiens L. J Asia Pac Entomol 20(1):133–139. https://doi.org/10.1016/j.aspen.2016.12.006
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taktak, N.E.M., Badawy, M.E.I., Awad, O.M. et al. Preparation and evaluation of ecofriendly nanocreams containing some plant essential oils and monoterpenes against adults of Culex pipiens L. with some biochemical and histological studies on albino rat. Int J Trop Insect Sci 44, 189–203 (2024). https://doi.org/10.1007/s42690-023-01145-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-023-01145-w