Abstract
Schistosomiasis is a vector-borne water-based disease caused by Schistosoma blood flukes. It mostly affects people in low-income regions, 90% of reported cases being in developing countries. Schistosoma has a complex lifecycle, alternately infecting mammalian hosts and snails. The snails hosting the parasite are the most viable targets. Selective preparations for reducing the parasite pool in snails and infected water are required as current molluscicides are also nontoxic to other organisms, including fish, and thus affect food supplies in infected areas. Plants (e.g. Annona crassiflora Mart., A. muricata L., and A. montana Macfad.) are attractive potential sources as alternative molluscicides and novel entity to treat the disease owned to their diverse biologically potent compounds including; saponins, alkaloids, terpenoids, and tannins. Additionally, they can be locally cultivated, providing income for farmers and reducing treatment costs. Here, we review plants, plant extracts and isolated compounds that have shown activities against the host snails or Schistosoma in various parts of its life cycle. Plants have a lot of potential and will continue to contribute feasible, effective medicines and/or pesticides; more research is warranted to fully explore their future applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis, a vector-borne water-based disease caused by Schistosoma blood flukes, is one of the most devastating parasitic diseases in tropical and subtropical regions (Walz et al. 2015). It is regarded by World Health Organization (WHO) as the third most common tropical disease, following malaria and intestinal helminthiasis (Murungi et al. 2021). It is also recognized as one of the neglected tropical diseases with high morbidity and mortality rates, affecting a billion of the world’s poorest people, mainly in the developing countries (Pereira et al. 2011). The disease is an underappreciated global burden and linked geographically to poverty, and poor health services (Payne and Fitchett 2010). It mostly affects young adults in their productive years, and children younger than 10 years. Untreated infections in young people impair their immunity, increasing their vulnerability to growth retardation, malnutrition and cognitive disorders (Dabo et al. 2011). More than 500,000 people die every year from this parasitic disease (Botelho et al. 2009).
Schistosoma has a complex lifecycle, alternately infecting mammalian hosts and snails especially S. japonicum which spreads via all kinds of mammals (Nelwan 2019). One in 30 of the total human population surveyed in endemic areas were apparently infected by Schistosoma flukes via contaminated water or direct contact with mammals hosting the parasite (Sheir et al. 2001). Molluscicides have a long history of both failures and successes in the control of schistosomiasis (Fenwick et al. 2006). Their application is now considered a key element that has significantly contributed to the decline in schistosomiasis infection and morbidity rates in a number of African countries during the last decades (Brackenbury and Appleton 1997). Chemotherapy and chemoprophylaxis with, praziquantel (PZQ), currently play a crucial role in curing, controlling and preventing the disease (Yousif et al. 2007). PZQ is highly valuable, due to its broad activity spectrum, for instance, it has a potent effect against S. mansoni especially at 6–7 weeks old infections. PZQ is thought to target the β subunits of voltage-gated Ca2+ channels in Schistosoma (Doenhoff et al. 2008). Despite the success, resistance to PZQ is apparently emerging and exacerbating challenges in the management of schistosomiasis globally (Melman et al. 2009). Pinto-Almeida and his colleagues analyze the proteome of S. mansoni PZQ-resistant adult worms and compare it with its parental fully PZQ-susceptible strain, using a high throughput LC–MS/MS identification. The results revealed that different proteins of the S. mansoni proteome in worms, were downregulated after the exposure to PZQ (Pinto-Almeida et al. 2018). Cotton and Doyle recently identified a gene responsible for PZQ resistance in experimentally selected resistant S. mansoni. This study shows that variation at or near Sm. (S. mansoni) TRPMPZQ is associated with the resistance (Cotton and Doyle 2022).
Nature has always been a valuable source of drugs and continues to deliver important drug leads (Tulp and Bohlin 2004). WHO estimates that 65–80% of the developing world’s population relies on traditional medicine to meet primary health care needs (Kumar et al. 2021) and traditional medicinal plants have supplied numerous pharmacologically active compounds (Yousif et al. 2007). Thus, considerable efforts have been made to identify plants products that are environmentally safe, non-toxic and selectively active for the integrated control of schistosomiasis, and several of them have shown promising results. For example, we have recently shown that the Egyptian medicinal plant Asparagus stipularis Forssk. has anti-schistosomal activity (El-Seedi et al. 2012). Recent reports documented the potential of some natural products such as Pulsatilla chinensis (Bunge) Regel extracts (Table 1) and its molluscicidal activity against O. hupensis. They are less harmful to non-target aquatic organisms than the reference molluscicide niclosamide (Chen et al. 2012). In addition, Agave attenuata Salm-Dyck is toxic to the target snail Bulinus africanus but has fewer (or no) adverse effects on fish and mammals (Brackenbury and Appleton 1997).
Both synthetic molluscicides and molluscicides derived from plants have made progress in the field of host snails eradication. The rising expense and toxicity to non-target organisms of synthetic molluscicides has rekindled interest in plant-based molluscicides (Clark et al. 1997; Zheng et al. 2021).
This review highlights the use of medicinal plants as new alternatives that could either complement or replace conventional control approaches. Potential molluscicidal plant species and their bioactive constituent based on traditional uses (Tables 2 and 3), are listed, in accordance to the literature survey done on the biological control of Schistosoma in the field using natural products, at all life stages.
Life cycle of Schistosoma species
There are three main species of human helminth parasites of the genus Schistosoma: S. haematobium, found in Africa and Asia; S. mansoni, present in Africa, the Middle-East and South America; and S. japonicum, endemic in China and Southwest Asia (Colley et al. 2014). There are also two minor species, S. intercalatum and S. mekongi, found in West and central Africa (Colley et al. 2014). The adult worms are live in mesenteric vessels (S. mansoni and S. japonicum) or the vessels surrounding the bladder (S. haematobium). The females produce large numbers of eggs that migrate through the blood vessel wall or are excreted with urine or feces (Nation et al. 2020).
The lifecycles of all Schistosoma species are quite similar, requiring freshwater snails as intermediate hosts, and mammalian hosts infected via water-borne contact with the free-swimming larvae released from the snails, as illustrated in Fig. 1. (Selbach et al. 2016). The lifecycle involves sexual maturation of adult schistosomes in man and an asexual multiplicative stage in the molluscan host (Nanes Sarfati et al. 2021). Differences between the species lie mainly in the type of intermediate host snails and the infective sites in the mammalian host, although there are also differences (inter alia) in egg morphology, and rates of both egg production and infection of mammalian hosts (Salvador-Recatalà and Greenberg 2012).
Once eggs reach fresh water they hatch and release swimming miracidia (larvae) that infect specific aquatic snails. The genera Biomphalaria, Oncomelania and Bullinus host S. mansoni, S. japonicum, and S. haematobium, respectively (Fig. 1) (Ross et al. 2002). In the snails, the miracidia asexually multiply after a few weeks and form thousands of cercariae that leave the snail and penetrate the skin of humans (e.g. children, fishermen, farmers, and women doing daily domestic tasks in infected water) or other mammals. If the cercariae penetrate the human body, they develop into schistosomules, then mate and migrate to the perivascular or mesenteric veins (S. mansoni and S. japonicum) or urinary bladder plexus (S. haematobium), where they produce eggs and the cycle starts again (Fig. 1) (Gryseels et al. 2006). Schistosoma often live 5–10 years, and even lifespans up to 40 years have been reported (Hokke and Deelder 2001).
History of schistosomiasis
Schistosomiasis has a history in Egypt of more than 5000 years (Barakat 2013; Abou-El-Naga 2018), with reports of S. haematobium eggs in ancient mummies (Barakat 2013). The disease was described by the ancient Egyptians on medical papyri as “â-a-â” (Abou-El-Naga 2018) and Avicenna (Ibn-Sina) in his famous book “The Canon of Medicine” (Othman and Soliman 2015), Key scientific discoveries regarding the disease have also been made in Egypt. Theodor Bilharz, a German surgeon who became Chief of the Surgical Services in Kasr El-Ain Hospital and Medical School, Cairo, discovered Schistosoma worms in 1851. Their lifecycle was subsequently described by Robert Leiper, while working in Cairo, in 1915 (Di Bella et al. 2018). The Delta Nile was become a favorite habitat for breeding the host snails of both urinary and intestinal schistosomiasis (Malek 1975).
S. japonicum eggs were discovered in two Chinese ancient bodies dating back roughly to 2180 years ago. Symptoms similar to schistosomiasis were described in the earliest Chinese ancient literature of around 4700 years ago. In the mid-1950s, the first nationwide assessment of the disease indicated that schistosomiasis was endemic in 433 cities across 12 provinces globally, affecting about 11.6 million people (Zhou et al. 2021).
Distribution of schistosomiasis
Schistosomiasis is endemic in 77 countries, and most prevalent in tropical and subtropical regions (Tefera et al. 2020). It is estimated that the severe cases are located in Sub-Saharan Africa; other highly endemic areas are Yemen and the Philippine Island of Mindanao. It is also still endemic, but at lower levels, in North Africa and several Middle East countries. In Asia, it is endemic in parts of the Yangtze River Basin in China, and middle reaches of the Mekong River in Laos and Cambodia (Bergquist and Tanner 2010). In China, a national survey in 2007 revealed that the infection rate among residents in the Fork Beach endemic area was 1.87% (Chen et al. 2012). At least 3 million people in Brazil are thought to be infected by Schistosoma, and around 25 million are at risk (Chitsulo et al. 2000). It is also endemic in several Venezuelan states, and several foci in Suriname (Zoni et al. 2016).
Economic implications
Calculating economic effects of schistosomiasis is far from straightforward, but it has been estimated to cause annual losses due to disability (complete or partial) amounting to US$ 445, 16, 118, and 60 million in Africa, South-West Asia, South-East Asia and the USA, respectively (at least US$ 641 million in total). These sums do not include all costs of related public health programs, medical care or compensation for illness, which may also be substantial (Wright 1972). For instance, estimated costs for controlling schistosomiasis morbidity among school-age children by selective and mass chemotherapy are US$ 0.67 and 0.59 per infected child, respectively (Talaat and Evans 2000). Thus, reviving large-scale school-based health programs to reduce morbidity rates of school-aged children, and to improve their physical growth and cognitive development, would be very costly (Husein et al. 1996).
Globally, the number of people treated for schistosomiasis rose from 12.4 million in 2006 to 33.5 million in 2010 (Carod Artal 2012). US$150 million have been spent on controlling neglected tropical diseases, including schistosomiasis, in sub-Saharan Africa (Gray et al. 2010). In 2008, a presidential initiative was announced calling for a commitment of US$ 350 million funding by the USA over five years to combat neglected tropical diseases, and an increase in the number of targeted countries (Liese and Schubert 2009). It has been estimated that 1.2 billion PZQ tablets will be needed annually to treat 400 million people in Africa for at least five years, at an annual cost of US$100 million (Utzinger et al. 2009). This sum could not be afforded easily within the endemic areas of the world without help from the developed countries. However, much less money would be required if alternative medicine(s) could be produced locally in appropriate financial and ecological frameworks.
Geographical implications
In many areas with high rates of schistosomiasis the local health systems are under-resourced (Amazigo et al. 2012) and subject to diverse disturbances that disrupt ecological equilibria and contexts, within which disease hosts or vectors and parasites breed, develop, and transmit the disease (Cable et al. 2017). Thus, they may strongly influence the emergence and proliferation of parasitic diseases, including schistosomiasis. It has been estimated that 97% of the infections are on the African continent (Steinmann et al. 2006), at least partly due to the lack (or paucity) of health systems, poverty and general neglect (Utzinger et al. 2011). Furthermore, 95% of all parasitic infections occur in the developing world, since it has the most conducive combinations of human behavior, anthropogenic disturbance and both climatic and physical conditions (Sattenspiel 2000). Notably, in endemic parts of the world schistosomiasis is intimately connected to the construction of infrastructure such as small, multipurpose dams and large hydroelectric dams for power production and irrigation systems (Utzinger et al. 2011). In the modern era, for example, the Aswan High Dam caused ecological changes that stimulated disease transmission (Malek 1975). Schistosomiasis remains the most important water-borne disease, and it was promoted by prevailing socio-ecological systems, since transmission is governed by unsafe human behavior (e.g. unprotected direct surface water contacts and open defecation) (Acka et al. 2010).
In Egypt, recent projects have been carried out in many regions to reclaim land from the desert for agriculture using water of the Nile River. This, together with the increased human activities in the reclaimed areas, has stimulated transmission of both S. mansoni and S. haematobium, manifested by the presence of infected B. truncatus and B. alexandrina, the intermediate host snails for the respective parasitic species, in these areas. Parts of the desert utilizing Nile water has wider spread of schistosomiasis (El-Kady et al. 2000). Schistosomiasis became a national burden and emerging as a major public health problem in the Egyptian reclaimed areas unless adequate control measures are taken (Abou-El-Naga 2018). There are also cases of human infection in American and European metropolitan areas, due to immigrations (Fuertes et al. 2010; Roure et al. 2017).
Medical implications
Hepatitis has been detected in 70% of surveyed schistosomiasis patients, and S. mansoni is one of the two major risk factors, together with viral hepatitis, for chronic liver disease and liver cirrhosis. Thus, millions of the world´s population live under constant risk of both schistosomiasis and hepatitis (Halim et al. 1999). Similarly, clinical history of urinary schistosomiasis is associated with significantly increased risk of bladder cancer (Bedwani et al. 1998). Furthermore, schistosomiasis can infect the children in endemic populations, and disease symptoms in children include anemia, liver fibrosis, immunity impairment, and severe physical and mental disorders (El Baz et al. 2003).
The free-swimming Schistosoma larvae penetrate the skin, causing rashes, erythema and itchy skin prior to fever, chills, coughm and muscle aches. As the parasite matures in the host veins (mesenteric or vesical), the symptoms change dramatically, and blood becomes visibly present in patients’ urine and stool (Kolářová et al. 2013; Nation et al. 2020). Under chronic conditions and if parasite eggs damage the organ in which they were deposited, the carrier can suffer from liver, kidney, and bladder complications. Advanced intestinal schistosomiasis is manifested with enlarged liver and spleen, fibrosis, and portal hypertension while symptoms of advanced uro-genital schistosomiasis include hydronephrosis and calcification of the bladder (Andrade 2009; Salas-Coronas et al. 2020).
Social implications
Advances in understanding Schistosoma epidemiology, and the greater availability of effective diagnosis and new tools have improved both current management and prospects for refining control of schistosomiasis. Nevertheless, infection and morbidity rates of the disease are still high, particularly in poor and otherwise disadvantaged populations. This disease of poverty has proved to be difficult to control for centuries (Utzinger et al. 2011). Despite prolonged mass antiparasitic drug therapy programs and other control measures, it has not been eradicated and continues to spread to new geographical areas (Siddiqui et al. 2011). Globally, WHO has estimated that about (600–779) million people are at risk of infection due to their exposure to contaminated water and 200–209 million people are infected with these parasites (Steinmann et al. 2006; WHO 2012). Schistosome infections require direct contact in water between the skin and the infective cercariae, thus sociocultural factors are strongly linked to infection rates, as illustrated in Fig. 2. In addition, recording and reporting of incidences, prevalence and death rates remain a challenge, and further efforts are needed to improve tracking the disease both nationally and globally. Accordingly, schistosomiasis is still considered one of the major healths, socio-economic and developmental challenges facing many of the world´s poorest countries.
Strategies to eliminate schistosomiasis
There is an urgent need to increase global awareness and support endemic countries’ endeavors to develop appropriate methods to control the disease (Rollinson et al. 2013). As already mentioned, it is generally agreed that no single method will be sufficient to eliminate schistosomiasis; integrated approaches will be required due to its complexities (Mo et al. 2014). Application of molluscicides to reduce intermediate host snails populations is one of the most efficient methods for controlling the disease (Rapado et al. 2011). However, there are four major strategies for eradication: (1) treating infected individuals to reduce morbidity and mortality, and preventing the spread of Schistosoma parasite eggs, (2) providing communities with adequate, appropriate sanitation and accessible safe water to reduce environmental contamination and hence minimize the chances of miracidia transmission, (3) snail control to block the lifecycle of the parasite, and (4) health education (Fig. 3) (Chimbari 2012). To accelerate progress, a wider application of existing interventions combined with implementation of new methods in a manner tailored to the socio-ecological setting is required. In China, a comprehensive control strategy to reduce rates of S. japonicum transmission from humans and cattle to snails was evaluated from 2005 to 2007. The strategy included removing cattle from snail-infested areas, providing farmers with mechanical equipment, improving sanitation and implementing health-education programs in the endemic areas. This integrated strategy reportedly reduced S. japonicum infection in humans to less than 1% in an endemic area (Wang et al. 2009).
Medical control of schistosomiasis by treating infected humans
A systematic search for chemotherapeutic drugs has been ongoing for several decades. The synthesis of PZQ in 1970 revolutionized the treatment and led to dramatic reductions in morbidity and mortality rates. PZQ is the only drug being used to treat human schistosomiasis on a large scale (Doenhoff et al. 2008; Mordvinov and Furman 2010), and is highly recommended in disease-control programs (Salvador-Recatalà and Greenberg 2012). The scope of therapeutic programs, as treatment with PZQ has largely been restricted to children attending school due to the lack of infrastructure and other logistical problems in many areas of the developing world (Siddiqui et al. 2011).
Although treatment of schistosomiasis in patients worldwide relies heavily on PZQ and the drug has thoroughly recognized efficacy against schistosomes (Keiser et al. 2010), evidence is now accumulating that PZQ cannot prevent re-infection, and may sometimes even exacerbate it (Chandiwana et al. 1991; Doenhoff et al. 2008). Moreover, PZQ resistance is emerging in endemic areas due to repeated use (Wang et al. 2012). Obviously, there is a need for new alternatives for treating schistosomiasis (Cioli et al. 2014; Bergquist et al. 2017). Important potential sources are plants, and already many have been tested and some showed relevant activities, especially members of the medicinal plants. Many of them have been already tested and some showed relevant activities, especially members of the plant families Euphorbiaceae, Annonaceae, Fabaceae Asteraceae, Asphodelaceae, and Asparagaceae (Tables 1, 2, 3, 4 and 5).
Strategic controls of schistosomiasis by eliminating intermediate host-snails
Schistosoma parasites, like other parasitic helminths, can survive in their hosts for a long time, but the intermediate host is the weakest link in the transmission cycle. Thus, molluscicides are widely used as experimental models in programs to investigate the disease by killing the intermediate host snails (mollusks) (Wang et al. 2018), thereby disrupting the parasite’s lifecycle and stopping transmission to people in contact with water in high-risk areas (Birley 1991).
Synthetic molluscicides
The major synthetic molluscicides thatare currently used to control the snail vectors are metaldehyde, niclosamide, carbamate, organophosphate, and synthetic pyrethroids (Singh et al. 2010). Niclosamide is one of the most widely used synthetic molluscicides at present. However, it is highly toxic towards non-target organisms, including fish, and it is also ecologically destructive (He et al. 2017). Nevertheless, it is one of the most important molluscicides approved by WHO due to the lack of robust alternatives (He et al. 2017). It is most active against O. hupensis snails with an LC50 of 0.12 µg/ml and LC90 of 0.98 µg/ml after 24 h (Chen et al. 2007) but it kills amphibians and fish if used in effective concentrations. There are also several chemical molluscicides (e.g. copper sulfate, calcium cyanamide, chlorinated lime and carbamate derivatives). However, they are environmentally hazardous, and snails develop resistance to them (Bilia et al. 2000b).
Available molluscicides are also costly, which poses major problem for low-income countries where schistosomiasis is widely distributed. Despite all recent research and development current synthetic molluscicides also raise serious environmental concerns and threats to both human health and income (particularly in areas where fishing is a major source of food and income) due to their lack of specificity (Andrews et al. 1982; He et al. 2017). Thus, identifying or developing more selective molluscicidal herbal preparations that do not have adverse effects (or at least acceptably weak effects) in non-target aquatic organisms and are biodegradable remains a high priority (El-Sherbini et al. 2009).
Plant molluscicides
Several thousand synthetic compounds have been tested for their ability to control host snails but none of of them was proven to be as entirely satisfactory as yet. Therefore, natural sources of novel agents came into play an are considered now as attractive alterantives to seek for novel drug leads (Ribeiro et al. 2021; Xing et al. 2021). Primary target sources are medicinal plants because they are generally a rich source of renewable bioactive organic chemicals, and they may grow well in endemic areas, providing income for local farmers. Furthermore, plant derived molluscicides may have several advantages like low costs, high target specificity, water solubility, high biodegradability, and low toxicity towards normal organs in the human hots (Singh et al. 2010).
Since 1982, the potential utility of more than 150 plant species for controlling freshwater snails has been tested. Numerous groups of compounds plant-derived compounds were toxic to target organisms at acceptable doses, ranging from < 1 to 100 µg/ml (Singh et al. 2010). The molluscicidal activity of plant extracts and often their active compounds have been reported (Tables 1 and 2). Commonly identified active compounds belong to saponins, alkaloids, terpenoids and tannins (Fig. 4). The plant extracts evaluated against the intermediate host (snails and larvae of Schistosoma) are listed in Table 4. These include, for instance, methanol and aqueous extracts of Jatropha curcas L. (Euphorbiaceae) on snails transmitting S. mansoni and S. haematobium (Rug and Ruppel 2000). Different parts were traditionally used as antimicrobial agents. The plant had traditional uses as antimicrobial agents. Plant species known to be rich in bioactive molluscidal compounds are presented in Tables 2 and 5. Tannins are particularly suitable for snail control because tannin-containing plants are not only widely distributed but tannins can be also relatively easily isolated (Al-Sayed et al. 2014). The highest contents of molluscicidal agents have been found in members of Euphorbiaceae followed by Annonaceae, Mimosaceae, Polygonaceae, Verbenaceae and Caesalpinaceae families, the active compounds of a number of plants are presented in (Fig. 4). More extensive efforts are warranted to isolate and identify the remaining unknown compounds from other plants, that could meet all the desirable criteria of molluscicides.
Classes of compounds used as plant molluscicides
Saponins
Saponins comprise a structurally diverse class of triterpenes, based on triterpenoids often in the form of glycosides. They occur in plant species and have been also isolated from marine organisms (Challinor and De Voss 2013). Saponins derive their name from the soapwort plant, genus Saponaria (Caryophyllaceae). The triterpene skeletons of saponins are formed from the C30 precursor oxidosqualene. They are diverse, and decorated with different functional groups in different plant species (Challinor and De Voss 2013). They have hemolytic properties, toxic effects on most cold-blooded animals and proven molluscicidal activity (Francis et al. 2002; Sparg et al. 2004).
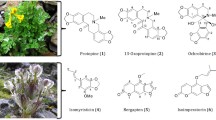
Triterpenoids and saponins are widespread in nature. The most active molluscicidal saponin compounds have an oleanolic acid-based aglycone and trisaccharide sugar moiety (Mølgaard et al. 2000). This is consistent with our recent finding that asparagalin A (Fig. 5) (1), a triterpene saponin from the Egyptian species Asparagus stipularis Forssk., suppresses S. mansoni egg-laying. Shoots and roots of this plant used in folk medicine as diuretic for curing jaundice, liver ailments, against bilharzias (El-Seedi et al. 2012). Sometimes molluscicidal compounds are formed by an enzymatic reaction during extraction of plants, for instance if Phytolacca dodecandra L'Hér. berries are crushed with water (Parkhurst et al. 1989).
The desert tree Balanites aegyptiaca (L.) Delile (common name Higleeg) (Suleiman 2015) has a well-known molluscicidal activity. It was the first plant reported for the control of schistosomiasis owing to its saponins content (Marston and Hostettmann 1985). The fruits of this plant were used as remedy to eradicate intestinal parasites. This plant species is traditionally (Table 3) used in treatment of malaria and is apparently suitable for vector control (Chothani and Vaghasiya 2011).
Pulsatilla chinensis (Bunge) Regel displayed potent molluscicidal activity against O. hupensis, attributable to the presence of the active triterpenoid saponins. The plant roots are widely used in traditional Chinese medicine as remedies for amebiasis, malaria, vaginal trichomoniasis and bacterial infections. Treatment with a sub-lethal concentration reportedly caused significant inhibition of acetyl cholinesterase (AHE), alanine transaminase (ALT), and alkaline phosphatase (ALP) activities in the liver and the cephalopodium of O. hupensis (Chen et al. 2012).
Extracts of Calendula officinalis L. and Ammi majus L. (common name, Khilla sheitani) have molluscicidal activity against Bullinus truncates and B. Alexandria (less strongly). The recorded LC50 and LC90 values indicated that C. officinalis L. is more toxic to both snails where A. majus, and Bullinus. truncatus snails are more sensitive to the extracts of both plants than B. alexandrina. Prolonged exposure to sub-lethal concentrations of A. majus L. affected the egg-laying and survival of both snails. In addition, treatment with sub-lethal doses of extracts of both plants clearly inhibited transaminase activity, diminished the total protein content, and markedly increased total lipid contents in both snails hemolymph (Rawi et al. 1996). The activity of A. majus L. is thought to be due to saponins, and furocoumarins, although the effect seems to be highly snail species-dependent (Marston and Hostettmann 1985; Rawi et al. 1996).
Phytolacca dodecandra L'Hér. is one of the most active molluscicidal plants, and thus has been widely studied. Its activity was initially reported by Lemma`s group (Lemma 1965, 1970) and the presence of an active saponin named lemmatoxin was demonstrated (Parkhurst et al. 1974). Aqueous extracts of the dried berries contain up to 25% saponins (Treyvaud et al. 2000) which were stable in water for two days at room temperature (Mølgaard et al. 2000). Some isolated saponins are lethal to snails at very low concentrations. However, a major disadvantage of using saponins as molluscicides is that they are also generally lethal to fish compared to flavonoids and other phenolics that are generally less toxic to non-target organisms (Wei et al. 2002).
Glycoalkaloids
Several glycoalkaloids (Fig. 6) identified from Solanum species are molluscicidal. Extracts of S. elaegnifolium Cav. berries, and S. sodomaeum Dunal leaves and seeds reveal molluscicidal activity against Bu. truncates, the intermediate host of S. haematobium (Table 2) (Bekkouche et al. 2000). In addition, S. sisymbriifolium Lam. fruits are active against the snails, the active fractions contain the steroidal alkaloids solamargine (Fig. 7) (2) and β-solamarine (Bagalwa et al. 2010), but solamargine is the most effective of these compounds, due to its specific sugar substitution (Miranda et al. 2012). Solamargine (containing a chacotriose sugar chain moiety) is also more active than solasonine, (containing a solatriose sugar chain moiety) against adult S. mansoni worms (Miranda et al. 2012).
Phorbol esters
Phorbol (Fig. 8) (3) was initially isolated in 1934 as a hydrolysis product of Croton tiglium L. vegetable oil (Flaschenträger, v. Wolffersdorff 1934; Abegaz and Kinfe 2020), and its structure was subsequently elucidated by Hecker and colleagues (Hecker 1967). Phorbol esters are diterpenes, which are, among others, major constituents of Jatropha curcas L. oil. They exhibit molluscicidal activity against aquatic snails (Liu et al. 1997). Some of the phorbol esters are mutagenic and thus not suited as a molluscicidal medicine (Liu et al. 1997).
Phorbol esters (and anthraquinone) are also the most bioactive compounds tested against miracidia and cercaria (Fig. 9). There have been few attempts to isolate bioactive water larvicidal compounds; however, more extensive investigations are warranted.
Neolignins
Neolignans are a group of dimeric phenylpropanoids that are formed in Myristicaceae and other primitive plant families (e.g. Piperaceae, Eucommiaceae and Lauraceae) by oxidative coupling of allyl and phenyl propanoids (Alves et al. 2002). The biological activities of neolignan derivatives have been investigated against fungi such as Microsporum canis, M. gypseum, Tricophyton mentagrophytes, T. rubrum, and Epidermophyton floccosum (Zacchino et al. 1997), bacteria (e.g. Staphylococcus aureus and Bacillus subtilis) (Pessini et al. 2003), and a panel of cancer cell lines (Siripong et al. 2006). A number of these compounds displayed anti-schistosomiasis activity (Mengarda et al. 2021), Structure–activity relationships of 18 synthetic neolignan derivatives have also been studied (Alves et al. 2002). Two neolignans isolated from the leaves of Virola surinamensis (Rol. ex Rottb.) Warb., virolin (Fig. 10) (4) and surinamensin have been active against S. mansoni (Alves et al. 1998).
Latex with molluscicidal activity
Latex from Euphorbiaceae species has strong molluscicidal activity, particularly against aquatic snails (Bah et al. 2006). The crude latex of Euphorbia milii var. hislopii, the most powerful molluscicidal agent, has activity against the schistosomiasis-transmitting snails, B. glabrata and B. tenagophila, (Yadav and Jagannadham 2008). This could be due to the presence of triterpenes, flavonoids, macrolides, ingenol and a phorbol ester (Zani et al. 1993). The latex had stronger effects than the molluscicide niclosamide and was less less harmful to nontarget aquatic organisms (Oliveira‐Filho et al. 1999; dos Santos et al. 2007). It also affects the cercaeia and larval stage of Schistosoma species (De-Carvalho et al. 1998).
Latex of E. splendens Bojer ex Hook. is also a potent and specific molluscicide (LC90 < 1.5 µg/ml) against the vector snails (Schall et al. 1998). It did not show acute toxicity or mutagenic activity towards non-target species at the concentrations of 10–12 µg/ml (Schall et al. 1991). Attempts to identify the components responsible for the effects on snails showed that normal, processed, non-proteinaceous and proteinaceous fractions had molluscicidal activity, but the proteinaceous fraction containing alkaloids had the strongest physiological and lethal effects on fresh water snails (B. glabarata) (Yadav and Singh 2011).
Clinical trials for uses medicinal plants against schistosomiasis
70 schistosomiasis haematobium patients both sexes (aged > 15–60 years old) were treated with Mirazid at10 mg/Kg. The cure rate reached 91.9% after two months and 95.2% on the 3rd post-Mirazid treatment month (El Baz et al. 2003).
In total, 268 school children infected with S. mansoni were divided into three groups: PZQ (87), arachidonic acid (ARA, 91), and PZQ plus ARA (90). Over the course of three weeks, and for 15 days, PZQ 40 mg/kg/day, ARA 10 mg/kg/day, or PZQ combined with ARA (40 mg/kg on the first day of treatment, then 15 doses of ARA 10 mg/kg per day for 5 doses/week) were administrated. In children with light and heavy infections, PZQ and ARA together evoked cure rates of 83% and 78%, respectively. ARA, like PZQ, induced moderate cure rates (50% and 60%, respectively) in school children with light infection and modest cure rates (21% and 20%, respectively) in school children with high infection. Taken together, combination of PZQ and ARA might be useful for treatment of children with schistosomiasis in high-endemicity regions (Barakat et al. 2015).
Conclusion
Schistosomiasis is a major neglected tropical disease with high public health impact. It is difficult to control the disease due to the complex life cycle of the parasite and its wide distribution in tropical and subtropical areas where health and sanitation services are poorly developed. Field control currently relies mostly on some synthetic compounds, which are costly, especially for the low-income regions where the disease is endemic. Since 1981 more than 150 plant species have been tested for molluscicidal activity, and more than 60 natural molluscicidal compounds have been isolated. Preliminary results are encouraging but further investigations are needed to bring more significant progress to a to a large-scale application in the field, including rigorous pharmaceutical validation, particularly to assess candidate compounds’ specificity. More screening of natural sources is also needed, with particular emphasis on structure–activity relationships and action mechanisms. Saponins, for example, specifically block homeostatic circuits.
More research is also needed to discover and identify new environmentally friendly molluscicides and larvicides of plant origin that are not toxic to non-target aquatic organisms. Establishment of a global database of the distribution of schistosomiasis vector snails is also necessary for large-scale application of plant molluscicides and optimization of control programs.
The present review here shows that nature offers a plethora of possibilities for improving schistosomiasis control. Production of locally growing plants with molluscicidal and/or anti-schistosome activities would be an attractive alternative, since they could provide abundant agents to interfere with multiple stages of the parasite’s lifecycle without imposing excessive financial burdens. Natural products are likely to be the strongest defense; myriads remain to be discovered.
References
Abdelgaleil SAM, El-Aswad AF, Nakatani M (2002) Molluscicidal and anti-feedant activities of diterpenes from Euphorbia paralias L. Pest Manag Sci 58:479–482. https://doi.org/10.1002/ps.487
Abdel-Gawad MM, El-Amin SM, Ohigashi H et al (2000) Molluscicidal saponins from Anagallis arvensis against schistosome intermediate hosts. Jpn J Infect Dis 53:17–19
Abdel-Hamid HF (2003) Molluscicidal and in-vitro schistosomicidal activities of the latex and some extracts of some plants belonging to Euphorbiacea. J Egypt Soc Parasitol 33:947–954
Abegaz BM, Kinfe HH (2020) 1. Secondary metabolites, their structural diversity, bioactivity, and ecological functions: an overview. In: Ntie-Kang F (ed) Fundam, vol 1. Bost. De Gruyter, Berlin, pp 7–54
Abou Basha LM, el Sayad MH, Allam AF, Osman MM (1994) The effect of Ambrosia maritima (Damsissa) on the viability of Lymnaea cailliaudi; An experimental study. J Egypt Soc Parasitol 24:513–517
Abou-El-Naga IF (2018) Towards elimination of schistosomiasis after 5000 years of endemicity in Egypt. Acta Trop 181:112–121. https://doi.org/10.1016/j.actatropica.2018.02.005
Acka CA, Raso G, N’Goran EK et al (2010) Parasitic worms: knowledge, attitudes, and practices in western Côte d’Ivoire with implications for integrated control. PLoS Negl Trop Dis 4:e910
Adenusi AA, Odaibo A (2009) Effects of varying concentrations of the crude aqueous and ethanolic extracts of Dalbergia sissoo plant parts on Biomphalaria pfeifferi egg masses. Afr J Tradit Complement Altern Med 6:139–149
Adetunji VO, Salawu OT (2010) Efficacy of ethanolic leaf extracts of Carica papaya and Terminalia catappa as molluscicides against the snail intermediate hosts of schistosomiasis. J Med Plants Res 4:2348–2352
Ahmed AH, Ramzy RMR (1997) Laboratory assessment of the molluscicidal and cercaricidal activities of the Egyptian weed, Solanum nigrum L. Ann Trop Med Parasitol 91:931–937. https://doi.org/10.1080/00034983.1997.11813221
Ahmed AH, Rifaat MMA (2004) Molluscicidal and cercaricidal efficacy of Acanthus mollis and its binary and tertiary combinations with Solanum nigrum and Iris pseudacorus against Biomphalaria alexandrina. J Egypt Soc Parasitol 34:1041–1050
Allam AF, El Sayad MH, Khalil SS (2001) Laboratory assessment of the molluscicidal activity of Commiphora molmol [myrrh] on Biomphalaria alexandria, Bulinus truncatus and lymnaea calilliaudi. J Egypt Soc Parasitol 31:683–690
Al-Sayed E, Hamid HA, Abu El Einin HM (2014) Molluscicidal and antischistosomal activities of methanol extracts and isolated compounds from Eucalyptus globulus and Melaleuca styphelioides. Pharm Biol 52:698–705. https://doi.org/10.3109/13880209.2013.865240
Alves CN, Barroso LP, Santos LS, Jardim IN (1998) Structure-activity relationship of compounds which are anti-schistosomiasis active. J Braz Chem Soc 9:577–582
Alves CN, de Macedo LGM, Honório KM et al (2002) A structure-activity relationship (SAR) study of neolignan compounds with anti-schistosomiasis activity. J Braz Chem Soc 13:300–307
Al-Zanbagi NA (2005) Two molluscicides from saudi arabian euphorbia lesagainst bulinus wrighti. J King Abdel Aziz Univ Sci 17:11–19
Al-Zanbagi NA, Banaja A-EA, Barrett J (2000) Molluscicidal activity of some Saudi Arabian Euphorbiales against the snail Biomphalaria pfeifferi. J Ethnopharmacol 70:119–125. https://doi.org/10.1016/S0378-8741(99)00155-5
Alzérreca A, Hart G (1982) Molluscicidal steroid glycoalkaloids possessing stereoisomeric spirosolane structures. Toxicol Lett 12:151–155. https://doi.org/10.1016/0378-4274(82)90178-3
Amazigo UV, Leak SGA, Zoure HGM et al (2012) Community-driven interventions can revolutionise control of neglected tropical diseases. Trends Parasitol 28:231–238. https://doi.org/10.1016/j.pt.2012.03.002
Andrade ZA (2009) Schistosomiasis and liver fibrosis: review article. Parasite Immunol 31:656–663. https://doi.org/10.1111/j.1365-3024.2009.01157.x
Andrews P, Thyssen J, Lorke D (1982) The biology and toxicology of molluscicides, bayluscide. Pharmacol Ther 19:245–295. https://doi.org/10.1016/0163-7258(82)90064-X
Apers S, Barónikova S, Sindambiwe JB et al (2001) Antiviral, haemolytic and molluscicidal effects of triterpenoid saponins from Maesa lanceolata: establishment of structure-activity relationships. Planta Med 67:528–532
Aziz IZA, El-bady A, El-Gayed SH (2011) In vitro anti-schistosomal activity of “Plectranthus tenuiflorus” on miracidium, cercaria and schistosomula stages of Schistosoma mansoni. Res J Parasitol 6:74–82
Bagalwa J-JM, Voutquenne-Nazabadioko L, Sayagh C, Bashwira AS (2010) Evaluation of the biological activity of the molluscicidal fraction of Solanum sisymbriifolium against non target organisms. Fitoterapia 81:767–771. https://doi.org/10.1016/j.fitote.2010.04.003
Bah S, Diallo D, Dembélé S, Paulsen BS (2006) Ethnopharmacological survey of plants used for the treatment of schistosomiasis in Niono District, Mali. J Ethnopharmacol 105:387–399. https://doi.org/10.1016/j.jep.2005.11.026
Bakry FA (2009) Use of some plant extracts to control Biomphalaria alexandrina snails with emphasis on some biological effects. Pestic Biochem Physiol 95:159–165. https://doi.org/10.1016/j.pestbp.2009.08.007
Bakry FA, Mohamed RT (2011) Impact of Euphorbia milii latex on infectivity of Schistosoma mansoni larval stages to their hosts. J Evol Biol Res 3:101–107
Barakat RMR (2013) Epidemiology of schistosomiasis in Egypt: travel through time: review. J Adv Res 4:425–432. https://doi.org/10.1016/j.jare.2012.07.003
Barakat R, Abou El-Ela NE, Sharaf S et al (2015) Efficacy and safety of arachidonic acid for treatment of school-age children in Schistosoma mansoni high-endemicity regions. Am J Trop Med Hyg 92:797–804. https://doi.org/10.4269/ajtmh.14-0675
Bedwani R, Renganathan E, El Kwhsky F et al (1998) Schistosomiasis and the risk of bladder cancer in Alexandria. Egypt Br J Cancer 77:1186–1189. https://doi.org/10.1038/bjc.1998.197
Bekkouche K, Markouk M, Larhsini M et al (2000) Molluscicidal properties of glycoalkaloid extracts from Moroccan Solanum species. Phyther Res 14:366–367. https://doi.org/10.1002/1099-1573(200008)14:5%3c366::AID-PTR640%3e3.0.CO;2-E
Benson O (2012) Efficacy of Chromolaena odorata leaf extracts on Biomphalaria pfeifferi eggs in control of schistosomiasis. Zool Ecol 22:236–239. https://doi.org/10.1080/21658005.2012.745269
Bergquist R, Utzinger J, Keiser J (2017) Controlling schistosomiasis with praziquantel: how much longer without a viable alternative? Infect Dis Poverty 6:74. https://doi.org/10.1186/s40249-017-0286-2
Bergquist R, Tanner M (2010) Chapter 5: Controlling schistosomiasis in Southeast Asia: a tale of two countries. In: Zhou X-N, Bergquist R, Olveda R, Utzinger JBT-A in P (eds) Important helminth infect. Southeast Asia divers. Potential control elimin. Part A. Academic Press, pp 109–144
Bezerra JCB, Silva IA, Ferreira HD et al (2002) Molluscicidal activity against Biomphalaria glabrata of Brazilian Cerrado medicinal plants. Fitoterapia 73:428–430. https://doi.org/10.1016/S0367-326X(02)00121-1
Bilia AR, Braca A, Mendez J, Morelli I (2000a) Molluscicidal and piscicidal activities of Venezuelan Chrysobalanaceae plants. Life Sci 66:PL53–PL59. https://doi.org/10.1016/S0024-3205(99)00600-1
Bilia AR, Nieri E, Braca A, Morelli I (2000b) Screening of mediterranean Rosaceae plants for their molluscicidal and piscicidal activities. Phyther Res 14:126–129. https://doi.org/10.1002/(SICI)1099-1573(200003)14:2%3c126::AID-PTR553%3e3.0.CO;2-V
Birley MH (1991) Guidelines for forecasting the vector-borne disease implications of water resources development. World Heal Organ Retrieved
Botelho M, Ferreira AC, Oliveira MJ et al (2009) Schistosoma haematobium total antigen induces increased proliferation, migration and invasion, and decreases apoptosis of normal epithelial cells. Int J Parasitol 39:1083–1091. https://doi.org/10.1016/j.ijpara.2009.02.016
Brackenbury TD, Appleton CC (1997) A comprehensive evaluation of Agave attenuata, a candidate plant molluscicide in South Africa. Acta Trop 68:201–213. https://doi.org/10.1016/S0001-706X(97)00095-8
Cable J, Barber I, Boag B et al (2017) Global change, parasite transmission and disease control: lessons from ecology. Philos Trans R Soc B Biol Sci 372:20160088
Carod Artal FJ (2012) Cerebral and spinal schistosomiasis. Curr Neurol Neurosci Rep 12:666–674. https://doi.org/10.1007/s11910-012-0305-4
Challinor VL, De Voss JJ (2013) Open-chain steroidal glycosides, a diverse class of plant saponins. Nat Prod Rep 30:429–454. https://doi.org/10.1039/C3NP20105H
Chandiwana SK, Woolhouse MEJ, Bradley M (1991) Factors affecting the intensity of reinfection with Schistosoma haematobium following treatment with praziquantel. Parasitology 102:73–83. https://doi.org/10.1017/S0031182000060364
Chen S-X, Wu L, Yang X-M et al (2007) Comparative molluscicidal action of extract of Ginko biloba sarcotesta, arecoline and niclosamide on snail hosts of Schistosoma japonicum. Pestic Biochem Physiol 89:237–241. https://doi.org/10.1016/j.pestbp.2007.07.010
Chen YQ, Xu QM, Liu YL et al (2012) Laboratory evaluation of the molluscicidal activity of Pulsatilla chinensis (Bunge) Regel saponins against the snail Oncomelania hupensis. Biomed Environ Sci 25:224–229. https://doi.org/10.3967/0895-3988.2012.02.015
Chifundera K, Baluku B, Mashimango B (1993) Phytochemical screening and molluscicidal potency of some Zairean medicinal plants. Pharmacol Res 28:333–340. https://doi.org/10.1006/phrs.1993.1135
Chimbari MJ (2012) Enhancing schistosomiasis control strategy for Zimbabwe: building on past experiences. J Parasitol Res 2012:353768. https://doi.org/10.1155/2012/353768
Chitsulo L, Engels D, Montresor A, Savioli L (2000) The global status of schistosomiasis and its control. Acta Trop 77:41–51. https://doi.org/10.1016/s0001-706x(00)00122-4
Chothani DL, Vaghasiya HU (2011) A review on Balanites aegyptiaca Del (desert date): phytochemical constituents, traditional uses, and pharmacological activity. Pharmacogn Rev 5:55–62. https://doi.org/10.4103/0973-7847.79100
Cichewicz RH, Lim K, Mckerrow H, Nair MG (2002) Kwanzoquinones A–G and other constituents of Hemerocallis fulva ‘Kwanzo’ roots and their activity against the human pathogenic trematode Schistosoma mansoni. Tetrahedron 58:8597–8606
Cioli D, Pica-Mattoccia L, Basso A, Guidi A (2014) Schistosomiasis control: praziquantel forever? Mol Biochem Parasitol 195:23–29. https://doi.org/10.1016/j.molbiopara.2014.06.002
Clark TE, Appleton CC (1997) The molluscicidal activity of Apodytes dimidiata E. Meyer ex Arn (Icacinaceae), Gardenia thunbergia L.f. (Rubiaceae) and Warburgia salutaris (Bertol. F.) Chiov. (Cannelaceae), three South African plants. J Ethnopharmacol 56:15–30. https://doi.org/10.1016/S0378-8741(96)01496-1
Clark TE, Appleton CC, Drewes SE (1997) A semi-quantitative approach to the selection of appropriate candidate plant molluscicides: a South African application. J Ethnopharmacol 56:1–13. https://doi.org/10.1016/S0378-8741(96)01495-X
Colley DG, Bustinduy AL, Secor WE, King CH (2014) Human schistosomiasis. Lancet (London, England) 383:2253–2264. https://doi.org/10.1016/S0140-6736(13)61949-2
Cotton JA, Doyle SR (2022) A genetic TRP down the channel to praziquantel resistance. Trends Parasitol 38:351–352. https://doi.org/10.1016/j.pt.2022.02.006
Cui T-Y, Zheng Q-A, Mi L-X et al (1999) Screening for molluscicidal activity in medicinal plant. Stud Plant Sci 6:230–232. https://doi.org/10.1016/S0928-3420(99)80031-7
Dabo A, Badawi HM, Bary B, Doumbo OK (2011) Urinary schistosomiasis among preschool-aged children in Sahelian rural communities in Mali. Parasit Vectors 4:21. https://doi.org/10.1186/1756-3305-4-21
de Vasconcellos MC, de Amorim A (2003) Molluscicidal action of the latex of Euphorbia splendens var. hislopii NEB (“ Christ’s Crown”)(Euphorbiaceae) against Lymnaea columella (Say, 1817)(Pulmonata: Lymnaeidae), intermediate host of Fasciola hepatica Linnaeus, 1758 (Trematode: Fasciolidae): 1-t. Mem Inst Oswaldo Cruz 98:557–563
de S. Luna J, dos Santos AF, de Lima MRF et al (2005) A study of the larvicidal and molluscicidal activities of some medicinal plants from northeast Brazil. J Ethnopharmacol 97:199–206. https://doi.org/10.1016/j.jep.2004.10.004
De-Carvalho RR, Maldonado A Jr, de Oliveira-Filho EC et al (1998) Effects of Euphorbia milii latex on Schistosoma mansoni eggs, miracidia and cercariae. Mem Inst Oswaldo Cruz 93:235–237
Di Bella S, Riccardi N, Giacobbe DR, Luzzati R (2018) History of schistosomiasis (bilharziasis) in humans: from Egyptian medical papyri to molecular biology on mummies. Pathog Glob Health 112:268–273. https://doi.org/10.1080/20477724.2018.1495357
Doenhoff MJ, Cioli D, Utzinger J (2008) Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis 21:659–667
dos Santos AF, Sant’Ana AEG (2001) Molluscicidal properties of some species of Annona. Phytomedicine 8:115–120. https://doi.org/10.1078/0944-7113-00008
dos Santos JAA, Tomassini TCB, Xavier DCD et al (2003) Molluscicidal activity of Physalis angulata L. extracts and fractions on Biomphalaria tenagophila (d’Orbigny, 1835) under laboratory conditions. Mem Inst Oswaldo Cruz 98:425–428
dos Santos AF, de Azevedo DPL, dos Santos Mata R da C et al (2007) The lethality of Euphorbia conspicua to adults of Biomphalaria glabrata, cercaria of Schistosoma mansoni and larvae of Artemia salina. Bioresour Technol 98:135–139. https://doi.org/10.1016/j.biortech.2005.11.020
dos Santos AF, Cavada BS, da Rocha BAM et al (2010) Toxicity of some glucose/mannose-binding lectins to Biomphalaria glabrata and Artemia salina. Bioresour Technol 101:794–798. https://doi.org/10.1016/j.biortech.2009.07.062
dos Santos EA, de Carvalho CM, Costa ALS et al (2012) Bioactivity evaluation of plant extracts used in indigenous medicine against the snail, Biomphalaria glabrata, and the larvae of Aedes aegypti. Evid Based Complement Altern Med 2012:846583. https://doi.org/10.1155/2012/846583
Drewes SE, Kayonga L, Clark TE et al (1996) Iridoid molluscicidal compounds from Apodytes dimidiata. J Nat Prod 59:1169–1170. https://doi.org/10.1021/np960404y
Ekabo OA, Farnsworth NR, Henderson TO et al (1996) Antifungal and molluscicidal saponins from Serjania salzmanniana. J Nat Prod 59:431–435. https://doi.org/10.1021/np960208r
El Babili FE, Moulis C, Bon M et al (1998) Three furano-diterpenes from the bark of Croton campestris. Phytochemistry 48:165–169. https://doi.org/10.1016/S0031-9422(97)00701-2
El Babili F, Fabre N, Moulis C, Fouraste I (2006) Molluscicidal activity against Bulinus truncatus of Croton campestris. Fitoterapia 77:384–387. https://doi.org/10.1016/j.fitote.2006.03.003
El Baz MA, Morsy TA, El Bandary MM, Motawea SM (2003) Clinical and parasitological studies on the efficacy of Mirazid in treatment of Schistosomiasis haematobium in Tatoon, Etsa Center, El Fayoum Governorate. J Egypt Soc Parasitol 33:761–776
El-Kady GA, Shoukry A, Reda LA, El-Badri YS (2000) Survey and population dynamics of freshwater snails in newly settled areas of the Sinai Peninsula. Egypt J Biol 2:42–48
El-Nahas HA, Abdel-Hameed ES, Sabra AA, El-Wakil EA (2005) Steroidal glycosides of Furcraea selloa and their biological properties against different Schistosoma mansoni stages. Bull Pharm Sci Assiut 28:169–183. https://doi.org/10.21608/bfsa.2005.65283
El-Seedi HR, El-Shabasy R, Sakr H et al (2012) Anti-schistosomiasis triterpene glycoside from the Egyptian medicinal plant Asparagus stipularis. Rev Bras Farm 22:314–318
El-Sheikh YWA, Eltamny HM, Soliman HA et al (2012) Molluscicidal activity of eco-friendly natural compound (Rutin) gained from ethanolic flowers extract of Calendula officinalis on B. alexandrina, B. truncatus and Lymanea snails. New York Sci J 5:19–27
El-Sherbini GT, Zayed RA, El-Sherbini ET (2009) Molluscicidal activity of some Solanum species extracts against the snail Biomphalaria alexandrina. J Parasitol Res 2009:474360. https://doi.org/10.1155/2009/474360
Esser KB, Semagn K, Wolde-Yohannes L (2003) Medicinal use and social status of the soap berry endod (Phytolacca dodecandra) in Ethiopia. J Ethnopharmacol 85:269–277. https://doi.org/10.1016/S0378-8741(03)00007-2
Fenwick A, Rollinson D, Southgate V (2006) Implementation of human schistosomiasis control: challenges and prospects. In: Molyneux DHBT-A in P (ed) Control Hum. Parasit. Dis. Academic Press, pp 567–622
Flaschenträger B, v. Wolffersdorff R (1934) Über den Giftstoff des Crotonöles. 1. Die Säuren des Crotonöles. Helv Chim Acta 17:1444–1452. https://doi.org/10.1002/hlca.193401701179
Francis G, Kerem Z, Makkar HPS, Becker K (2002) The biological action of saponins in animal systems: a review. Br J Nutr 88:587–605. https://doi.org/10.1079/BJN2002725
Fronczek FR, Vargas D, Fischer NH, Hostettmann K (1984) The molecular structure of 7α-hydroxy-3-desoxyzaluzanin C, a molluscicidal sesquiterpene lactone. J Nat Prod 47:1036–1039. https://doi.org/10.1021/np50036a026
Fuertes PZ, Pérez-Ayala A, Pérez Molina JA et al (2010) Clinical and epidemiological characteristics of imported infectious diseases in Spanish travelers. J Travel Med 17:303–309. https://doi.org/10.1111/j.1708-8305.2010.00433.x
Gaudani R, Patel J, Prajapati H et al (2010) Peristrophe bicalyculata: a review. Pharmacogn J 2:39–45. https://doi.org/10.1016/s0975-3575(10)80070-7
Gopalsamy N, Gueho J, Julien HR et al (1990) Molluscicidal saponins of Polyscias dichroostachya. Phytochemistry 29:793–795. https://doi.org/10.1016/0031-9422(90)80020-H
Gray DJ, McManus DP, Li Y et al (2010) Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis 10:733–736. https://doi.org/10.1016/S1473-3099(10)70099-2
Gryseels B, Polman K, Clerinx J, Kestens L (2006) Human schistosomiasis. Lancet 368:1106–1118. https://doi.org/10.1016/S0140-6736(06)69440-3
Halim A-B, Garry RF, Dash S, Gerber MA (1999) Effect of schistosomiasis and hepatitis on liver disease. Am J Trop Med Hyg 60:915–920
Hamburger MO, Cordell GA, Ruangrungsi N (1991) Traditional medicinal plants of Thailand XVII biologically active constituents of Plumeria rubra. J Ethnopharmacol 33:289–292. https://doi.org/10.1016/0378-8741(91)90091-Q
Han B, Chen J, Yang X et al (2010) Molluscicidal activities of medicinal plants from eastern China against Oncomelania hupensis, the intermediate host of Schistosoma japonicum. Rev Bras Farm 20:712–718
Hannedouche S, Souchard JP, Jacquemond-Collet I, Moulis C (2002) Molluscicidal and radical scavenging activity of quinones from the root bark of Caryopteris x clandonensis. Fitoterapia 73:520–522. https://doi.org/10.1016/S0367-326X(02)00163-6
Hasheesh WS, Mohamed RT, Abd El-Monem S (2011) Biological and physiological parameters of Bulinus truncatus snails exposed to methanol extract of the plant Sesbania sesban plant. Adv Biol Chem 1:65
Hassan SE, Rahman EHA, El-Monem ARA (2010) Molluscicidal activity of butanol fraction of Meryta denhamii flowers against Lymnaea natalensis and Biomphalaria alexandrina. Glob Vet 4:15–21
Hassan AA, Mahmoud AE, Attia RAH, Huseein EAM (2012) Evaluation of the ethanolic extracts of three plants for their molluscicidal activities against snails intermediate hosts of Schistosoma mansoni and Fasciola. Int J Basic Appl Sci 1:235–249
He W, Van Puyvelde L, Bosselaers J et al (2002) Activity of 6-pentadecylsalicylic acid from Ozoroa insignis against marine crustaceans. Pharm Biol 40:74–76. https://doi.org/10.1076/phbi.40.1.74.5862
He P, Wang W, Sanogo B et al (2017) Molluscicidal activity and mechanism of toxicity of a novel salicylanilide ester derivative against Biomphalaria species. Parasit Vectors 10:383. https://doi.org/10.1186/s13071-017-2313-3
Hecker E (1967) Phorbol esters from croton oil chemical nature and biological activities. Sci Nat 54:282–284. https://doi.org/10.1007/BF00620887
Hmamouchi M, Lahlou M, Agoumi A (2000) Molluscicidal activity of some Moroccan medicinal plants. Fitoterapia 71:308–314. https://doi.org/10.1016/S0367-326X(99)00152-5
Hokke CH, Deelder AM (2001) Schistosome glycoconjugates in host-parasite interplay. Glycoconj J 18:573–587. https://doi.org/10.1023/A:1020634602161
Husein MH, Talaat M, El-Sayed MK et al (1996) Who misses out with school-based health programmes? A study of schistosomiasis control in Egypt. Trans R Soc Trop Med Hyg 90:362–365. https://doi.org/10.1016/S0035-9203(96)90506-4
Hymete A, Iversen T-H, Rohloff J, Erko B (2005) Screening of Echinops ellenbeckii and Echinops longisetus for biological activities and chemical constituents. Phytomedicine 12:675–679. https://doi.org/10.1016/j.phymed.2004.01.013
Jaiswal P, Singh DK (2008) Molluscicidal activity of Carica papaya and Areca catechu against the freshwater snail Lymnaea acuminata. Vet Parasitol 152:264–270. https://doi.org/10.1016/j.vetpar.2007.12.033
Keiser J, N’Guessan NA, Adoubryn KD et al (2010) Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin Infect Dis 50:1205–1213. https://doi.org/10.1086/651682
Kishor N, Sati OP (1990) A new molluscicidal spirostanol glycoside of Yucca aloifolia. J Nat Prod 53:1557–1559. https://doi.org/10.1021/np50072a025
Kolářová L, Horák P, Skírnisson K et al (2013) Cercarial dermatitis, a neglected allergic disease. Clin Rev Allergy Immunol 45:63–74. https://doi.org/10.1007/s12016-012-8334-y
Kumar M, Rawat S, Nagar B et al (2021) Implementation of the use of ethnomedicinal plants for curing diseases in the Indian Himalayas and its role in sustainability of livelihoods and socioeconomic development. Int J Environ Res Public Health 18:1509
Larhsini M, Sebbane R, Kchakech H et al (2010) Screening of some Moroccan plant extracts for molluscicidal activity. Asian J Exp Biol Sci 1:964–967
Lemma A (1965) A preliminary report on the molluscicidal property of endod (Phytolacca dodecandrd). Ethiop Med J 3:187–190
Lemma A (1970) Laboratory and field evaluation of the molluscicidal properties of Phytolacca dodecandra. Bull World Health Org 42:597–612
Lemmich E, Cornett C, Furu P et al (1995) Molluscicidal saponins from Catunaregam nilotica. Phytochemistry 39:63–68. https://doi.org/10.1016/0031-9422(94)00866-R
Lemmich E, Adewunmi CO, Furu P et al (1996) 5-deoxyflavones from Parkia clappertoniana. Phytochemistry 42:1011–1013. https://doi.org/10.1016/0031-9422(96)00101-X
Liese BH, Schubert L (2009) Official development assistance for health–how neglected are neglected tropical diseases? An analysis of health financing. Int Health 1:141–147. https://doi.org/10.1016/j.inhe.2009.08.004
Liu SY, Sporer F, Wink M et al (1997) Anthraquinones in Rheum palmatum and Rumex dentatus(Polygonaceae), and phorbol esters in Jatropha curcas(Euphorbiaceae) with molluscicidal activity against the schistosome vector snails Oncomelania, Biomphalaria, and Bulinus. Trop Med Int Health 2:179–188. https://doi.org/10.1046/j.1365-3156.1997.d01-242.x
Mahato SB, Sahu NP, Ganguly AN et al (1980) Steroidal alkaloids from Solanum khasian um: application of 13C NMR spectroscopy to their structural elucidation. Phytochemistry 19:2017–2020. https://doi.org/10.1016/0031-9422(80)83026-3
Mahmoud MB, Ibrahim WL, Abou-El-Nour BM et al (2011) Biological and biochemical parameters of Biomphalaria alexandrina snails exposed to the plants Datura stramonium and Sesbania sesban as water suspensions of their dry powder. Pestic Biochem Physiol 99:96–104. https://doi.org/10.1016/j.pestbp.2010.11.005
Malek EA (1975) Effect of the Aswan High Dam on prevalence of schistosomiasis in Egypt. Trop Geogr Med 27:359–364
Mantawy MM, Aly HF, Zayed N, Fahmy ZH (2012) Antioxidant and schistosomicidal effect of Allium sativum and Allium cepa against Schistosoma mansoni different stages. Eur Rev Med Pharmacol Sci 16(Suppl 3):69–80
Marston A, Hostettmann K (1985) Review article number 6: plant molluscicides. Phytochemistry 24:639–652. https://doi.org/10.1016/S0031-9422(00)84870-0
Masoud AM, Fawzy SM, Salama OM (2000) Laboratory studies on the molluscicidal and cercaricidal activities of Commiphora molmol. Egypt J Aquat Biol Fish 4:251–266. https://doi.org/10.21608/ejabf.2000.1671
Melman SD, Steinauer ML, Cunningham C et al (2009) Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl Trop Dis 3:e504
Mendes NM, Queiroz RO, Grandi TSM et al (1999) Screening of Asteraceae (Compositae) plant extracts for molluscicidal activity. Mem Inst Oswaldo Cruz 94:411–412
Mengarda AC, Silva MP, Cirino ME et al (2021) Licarin A, a neolignan isolated from Nectandra oppositifolia Nees & Mart. (Lauraceae), exhibited moderate preclinical efficacy against Schistosoma mansoni infection. Phyther Res 35:5154–5162. https://doi.org/10.1002/ptr.7184
Ming Z, Gui-Yin LI, Jian-Guo Z et al (2011) Evaluation of molluscicidal activities of benzo [c] phenanthridine alkaloids from Macleaya cordata (Willd) R. Br. on snail hosts of Schistosoma japonicum. J Med Plants Res 5:521–526
Miranda MA, Magalhães LG, Tiossi RFJ et al (2012) Evaluation of the schistosomicidal activity of the steroidal alkaloids from Solanum lycocarpum fruits. Parasitol Res 111:257–262. https://doi.org/10.1007/s00436-012-2827-8
Mo AX, Agosti JM, Walson JL et al (2014) Schistosomiasis elimination strategies and potential role of a vaccine in achieving global health goals. Am J Trop Med Hyg 90:54–60. https://doi.org/10.4269/ajtmh.13-0467
Mølgaard P, Chihaka A, Lemmich E et al (2000) Biodegradability of the molluscicidal saponins of Phytolacca dodecandra. Regul Toxicol Pharmacol 32:248–255. https://doi.org/10.1006/rtph.2000.1390
Mølgaard P, Nielsen SB, Rasmussen DE et al (2001) Anthelmintic screening of Zimbabwean plants traditionally used against schistosomiasis. J Ethnopharmacol 74:257–264. https://doi.org/10.1016/S0378-8741(00)00377-9
Mordvinov VA, Furman DP (2010) The digenea parasite Opisthorchis felineus: a target for the discovery and development of novel drugs. Infect Disord Drug Targets Disord 10:385–401
Murungi JN, Karanja S, Wanjau P (2021) A deterministic analysis of the effectiveness of non-clinical approaches in the control of transimission of schistosomiasis: case study of Mwea irrigation scheme, Kenya. Eur J Math Stat. https://doi.org/10.24018/ejmath.2021.2.6.70
Nanes Sarfati D, Li P, Tarashansky AJ, Wang B (2021) Single-cell deconstruction of stem-cell-driven schistosome development. Trends Parasitol 37:790–802. https://doi.org/10.1016/j.pt.2021.03.005
Nation CS, Da’dara AA, Marchant JK, Skelly PJ (2020) Schistosome migration in the definitive host. PLoS Negl Trop Dis 14:e0007951
Nelwan ML (2019) Schistosomiasis: life cycle, diagnosis, and control. Curr Ther Res Clin Exp 91:5–9. https://doi.org/10.1016/j.curtheres.2019.06.001
Nihei K, Ying B, Murakami T et al (2005) Pachyelasides A−D, novel molluscicidal triterpene saponins from Pachyelasma tessmannii. J Agric Food Chem 53:608–613. https://doi.org/10.1021/jf048570w
Obied HK, Bedgood DR, Prenzler PD, Robards K (2007) Bioscreening of Australian olive mill waste extracts: biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem Toxicol 45:1238–1248. https://doi.org/10.1016/j.fct.2007.01.004
Ojewole JAO (2004) Indigenous plants and schistosomiasis control in South Africa: molluscicidal activity of some Zulu medicinal plants. Bol Latinoam Caribe Plantas Med Aromat 3:8–22
Oliveira-Filho EC, De-Carvalho RR, Paumgartten FJR (1999) The influence of environmental factors on the molluscicidal activity of Euphorbia milii latex. J Environ Sci Health Part B 34:289–303. https://doi.org/10.1080/03601239909373198
Othman AA, Soliman RH (2015) Schistosomiasis in Egypt: A never-ending story? Acta Trop 148:179–190. https://doi.org/10.1016/j.actatropica.2015.04.016
Parkhurst RM, Thomas DW, Skinner WA, Cary LW (1974) Molluscicidal saponins of Phytolaccadodecandra: Lemmatoxin. Can J Chem 52:702–705. https://doi.org/10.1139/v74-110
Parkhurst RM, Mthupha BM, Liang Y-S et al (1989) The molluscicidal activity of Phytolacca dodecandra I. Location of the activating esterase. Biochem Biophys Res Commun 158:436–439. https://doi.org/10.1016/S0006-291X(89)80066-X
Pasi S, Aligiannis N, Pratsinis H et al (2009) Biologically active triterpenoids from Cephalaria ambrosioides. Planta Med 75:163–167
Payne L, Fitchett JR (2010) Bringing neglected tropical diseases into the spotlight. Trends Parasitol 26:421–423. https://doi.org/10.1016/j.pt.2010.06.002
Pereira AR, Etzbach L, Engene N et al (2011) Molluscicidal metabolites from an assemblage of Palmyra Atoll cyanobacteria. J Nat Prod 74:1175–1181. https://doi.org/10.1021/np200106b
Pessini GL, Dias Filho BP, Nakamura CV, Cortez DAG (2003) Antibacterial activity of extracts and neolignans from Piper regnellii (Miq.) C. DC. var. pallescens (C. DC.) Yunck. Mem Inst Oswaldo Cruz 98:1115–1120
Pinto-Almeida A, Mendes T, Ferreira P et al (2018) Comparative proteomics reveals characteristic proteins on praziquantel-resistance in Schistosoma mansoni. bioRxiv. https://doi.org/10.1101/314724
Puri R, Wong TC, Puri RK (1994) 1H- and 13C-Nmr assignments and structural determination of a novel glycoalkaloid from Solanum platanifolium. J Nat Prod 57:587–596. https://doi.org/10.1021/np50107a004
Rapado LN, Nakano E, Ohlweiler FP et al (2011) Molluscicidal and ovicidal activities of plant extracts of the Piperaceae on Biomphalaria glabrata (Say, 1818). J Helminthol 85:66–72. https://doi.org/10.1017/S0022149X10000258
Rawi SM, El-Gindy H, Abd-El-Kader A (1996) New possible molluscicides from Calendula micrantha officinalis and Ammi majus: II. Molluscicidal, physiological, and egg-laying effects against Biomphalaria alexandrina and Bulinus truncatus. Ecotoxicol Environ Saf 35:261–267. https://doi.org/10.1006/eesa.1996.0109
Rawi SM, Al-Hazmi M, Al Nassr FS (2011) Comparative study of the molluscicidal activity of some plant extracts on the snail vector of Schistosoma monsoni, Biomphalaria alexandrina. Int J Zool Res 7:169–189
Ribeiro ECG, Leite JAC, Luz TRSA et al (2021) Molluscicidal activity of monoterpenes and their effects on inhibition of acetylcholinesterase activity on Biomphalaria glabrata, an intermediate host of Schistosoma mansoni. Acta Trop. https://doi.org/10.1016/j.actatropica.2021.106089
Rollinson D, Knopp S, Levitz S et al (2013) Time to set the agenda for schistosomiasis elimination. Acta Trop 128:423–440. https://doi.org/10.1016/j.actatropica.2012.04.013
Ross AG, Bartley PB, Sleigh AC et al (2002) Current concepts. N Engl J Med 346:1212–1220
Roure S, Valerio L, Pérez-Quílez O et al (2017) Epidemiological, clinical, diagnostic and economic features of an immigrant population of chronic schistosomiasis sufferers with long-term residence in a non-endemic country (North Metropolitan area of Barcelona, 2002–2016). PLoS ONE 12:e0185245
Rug M, Ruppel A (2000) Toxic activities of the plant Jatropha curcas against intermediate snail hosts and larvae of schistosomes. Trop Med Int Health 5:423–430. https://doi.org/10.1046/j.1365-3156.2000.00573.x
Salama MM, Taher EE, El-Bahy MM (2012) Molluscicidal and mosquitocidal activities of the essential oils of Thymus capitatus Hoff. et Link. and Marrubium vulgare L. Rev Inst Med Trop Sao Paulo 54:281–286
Salas-Coronas J, Vázquez-Villegas J, Lozano-Serrano AB et al (2020) Severe complications of imported schistosomiasis, Spain: a retrospective observational study. Travel Med Infect Dis 35:101508. https://doi.org/10.1016/j.tmaid.2019.101508
Salvador-Recatalà V, Greenberg RM (2012) Calcium channels of schistosomes: unresolved questions and unexpected answers. Wiley Interdiscip Rev Membr Transp Signal 1:85–93. https://doi.org/10.1002/wmts.19
Sattenspiel L (2000) Tropical environments, human activities, and the transmission of infectious diseases. Am J Phys Anthr 113:3–31. https://doi.org/10.1002/1096-8644(2000)43:31+%3c3::AID-AJPA2%3e3.0.CO;2-Z
Schall VT, Vasconcellos MC, Valent GU et al (1991) Evaluation of the genotoxic activity and acute toxicity of Euphorbia splendens latex, a molluscicide for the control of schistosomiasis. Braz J Med Biol Res Rev = Bras Pesqui Med Biol 24:573–582
Schall VT, Vasconcellos MC, Rocha RS et al (2001) The control of the schistosome-transmitting snail Biomphalaria glabrata by the plant Molluscicide Euphorbia splendens var. hislopii (syn milli Des. Moul): a longitudinal field study in an endemic area in Brazil. Acta Trop 79:165–170. https://doi.org/10.1016/S0001-706X(01)00126-7
Schall VT, De Vasconcellos MC, De Souza CP, Baptista DF (1998) The molluscicidal activity of Crown of Christ (Euphorbia splendens var. hislopii) latex on snails acting as intermediate hosts of Schistosoma mansoni and Schistosoma haematobium. Am J Trop Med Hyg 58(1):7–10
Selbach C, Soldánová M, Sures B (2016) Estimating the risk of swimmer’s itch in surface waters: a case study from Lake Baldeney, River Ruhr. Int J Hyg Environ Health 219:693–699. https://doi.org/10.1016/j.ijheh.2015.03.012
Sharma S, Singh T, Vijayvergia R (2009) Molluscicidal activity of some medicinal plants. J Herb Med Toxicol 3:155–157
Sheir Z, Nasr AA, Massoud A et al (2001) A safe, effective, herbal antischistosomal therapy derived from myrrh. Am J Trop Med Hyg 65:700–704
Siddiqui AA, Siddiqui BA, Ganley-Leal L (2011) Schistosomiasis vaccines. Hum Vaccin 7:1192–1197. https://doi.org/10.4161/hv.7.11.17017
Silva TMS, Câmara CA, de Fátima Agra M et al (2006) Molluscicidal activity of Solanum species of the Northeast of Brazil on Biomphalaria glabrata. Fitoterapia 77:449–452. https://doi.org/10.1016/j.fitote.2006.05.007
Silva TMS, Camara CA, Freire KRL et al (2008) Steroidal glycoalkaloids and molluscicidal activity of Solanum asperum Rich. Fruits. J Braz Chem Soc 19:1048–1052
Silvério MS, Del-Vechio-Vieira G, Pinto MAO et al (2013) Chemical composition and biological activities of essential oils of Eremanthus erythropappus (DC) McLeisch (Asteraceae). Molecules 18:9785–9796. https://doi.org/10.3390/molecules18089785
Singh SK, Yadav RP, Singh A (2010) Molluscicides from some common medicinal plants of eastern Uttar Pradesh, India. J Appl Toxicol 30:1–7. https://doi.org/10.1002/jat.1498
Singh KL, Singh DK, Singh VK (2012) Characterization of the molluscicidal activity of Bauhinia variegata and Mimusops elengi plant extracts against the fasciola vector Lymnaea acuminata. Rev Inst Med Trop Sao Paulo 54:135–140
Siripong P, Kanokmedakul K, Piyaviriyagul S et al (2006) Antiproliferative naphthoquinone esters from Rhinacanthus nasutus Kurz. roots on various cancer cells. J Tradit Med 23:166–172. https://doi.org/10.11339/jtm.23.166
Slacanin I, Vargas D, Marston A, Hostettmann K (1988) Determination of molluscicidal sesquiterpene lactones from Ambrosia maritima (compositae). J Chromatogr A 457:325–331. https://doi.org/10.1016/S0021-9673(01)82080-X
Socolsky C, Borkosky SA, Asakawa Y, Bardón A (2009) Molluscicidal phloroglucinols from the fern Elaphoglossum piloselloides. J Nat Prod 72:787–790. https://doi.org/10.1021/np800724h
Sparg SG, van Staden J, Jäger AK (2000) Efficiency of traditionally used South African plants against schistosomiasis. J Ethnopharmacol 73:209–214. https://doi.org/10.1016/S0378-8741(00)00310-X
Sparg SG, Light ME, van Staden J (2004) Biological activities and distribution of plant saponins. J Ethnopharmacol 94:219–243. https://doi.org/10.1016/j.jep.2004.05.016
Steinmann P, Keiser J, Bos R et al (2006) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis 6:411–425. https://doi.org/10.1016/S1473-3099(06)70521-7
Suleiman MHA (2015) An ethnobotanical survey of medicinal plants used by communities of Northern Kordofan region, Sudan. J Ethnopharmacol 176:232–242. https://doi.org/10.1016/j.jep.2015.10.039
Talaat M, Evans DB (2000) The costs and coverage of a strategy to control schistosomiasis morbidity in nonenrolled school-age children in Egypt. Trans R Soc Trop Med Hyg 94:449–454. https://doi.org/10.1016/S0035-9203(00)90137-8
Tefera A, Belay T, Bajiro M (2020) Epidemiology of Schistosoma mansoni infection and associated risk factors among school children attending primary schools nearby rivers in Jimma town, an urban setting, Southwest Ethiopia. Plos One 15:e0228007
Thiilborg ST, Christensen SB, Cornett C et al (1993) Molluscicidal saponins from Phytolacca dodecandra. Phytochemistry 32:1167–1171. https://doi.org/10.1016/S0031-9422(00)95085-4
Thiilborg ST, Brøgger Christensen S, Cornett C et al (1994) Molluscicidal saponins from a zimbabwean strain of Phytolacca dodecandra. Phytochemistry 36:753–759. https://doi.org/10.1016/S0031-9422(00)89811-8
Treyvaud V, Marston A, Dyatmiko W, Hostettmann K (2000) Molluscicidal saponins from Phytolacca icosandra. Phytochemistry 55:603–609. https://doi.org/10.1016/S0031-9422(00)00233-8
Truiti MCT, Ferreira ICP, Zamuner MLM et al (2005) Antiprotozoal and molluscicidal activities of five Brazilian plants. Brazilian J Med Biol Res 38:1873–1878
Tulp M, Bohlin L (2004) Unconventional natural sources for future drug discovery. Drug Discov Today 9:450–458. https://doi.org/10.1016/S1359-6446(04)03066-1
Utzinger J, Raso G, Brooker S et al (2009) Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology 136:1859–1874. https://doi.org/10.1017/S0031182009991600
Utzinger J, N’Goran EK, Caffrey CR, Keiser J (2011) From innovation to application: social–ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop 120:S121–S137. https://doi.org/10.1016/j.actatropica.2010.08.020
Vieira PC, Kubo I (1990) Molluscicidal quinoline alkaloids from Galipea bracteata. Phytochemistry 29:813–815. https://doi.org/10.1016/0031-9422(90)80024-B
Vieira PC, Kubo I, Kujime H et al (1992) Molluscicidal acridone alkaloids from Angostura paniculata: isolation, structures, and synthesis. J Nat Prod 55:1112–1117. https://doi.org/10.1021/np50086a012
Walz Y, Wegmann M, Dech S et al (2015) Risk profiling of schistosomiasis using remote sensing: approaches, challenges and outlook. Parasit Vectors 8:163. https://doi.org/10.1186/s13071-015-0732-6
Wang L-D, Chen H-G, Guo J-G et al (2009) A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med 360:121–128. https://doi.org/10.1056/NEJMoa0800135
Wang W, Wang L, Liang Y-S (2012) Susceptibility or resistance of praziquantel in human schistosomiasis: a review. Parasitol Res 111:1871–1877. https://doi.org/10.1007/s00436-012-3151-z
Wang W, Mao Q, Yao J et al (2018) Discovery of the pyridylphenylureas as novel molluscicides against the invasive snail Biomphalaria straminea, intermediate host of Schistosoma mansoni. Parasit Vectors 11:291. https://doi.org/10.1186/s13071-018-2868-7
Wanyonyi AW, Chhabra SC, Mkoji G et al (2002) Bioactive steroidal alkaloid glycosides from Solanum aculeastrum. Phytochemistry 59:79–84. https://doi.org/10.1016/S0031-9422(01)00424-1
Wanyonyi AW, Chhabra SC, Mkoji G et al (2003) Molluscicidal and antimicrobial activity of Solanum aculeastrum. Fitoterapia 74:298–301. https://doi.org/10.1016/S0367-326X(03)00030-3
Wei FH, Xu XJ, Liu JB et al (2002) Toxicology of a potential molluscicide derived from the plant Solanum xanthocarpum: a preliminary study. Ann Trop Med Parasitol 96:325–331. https://doi.org/10.1179/000349802125000727
WHO (2012) Schistosomiasis: population requiring preventive chemotherapy and number of people treated in 2010. Wkly Epidemiol Rec Relev Épidémiologique Hebd 87:37–44
Wright WH (1972) A consideration of economic impact of schistosomiasis. Bull World Health Org 47:559–565
Xing Y, Yao J, Qu G et al (2021) Evaluation of the molluscicidal activities of arylpyrrole on Oncomelania hupensis, the intermediate host of Schistosoma japonicum. PeerJ 9:1–17. https://doi.org/10.7717/peerj.12209
Yadav SC, Jagannadham MV (2008) Physiological changes and molluscicidal effects of crude latex and Milin on Biomphalaria glabrata. Chemosphere 71:1295–1300. https://doi.org/10.1016/j.chemosphere.2007.11.068
Yadav RP, Singh A (2011) Efficacy of Euphorbia hirta latex as plant derived molluscicides against freshwater snails. Rev Inst Med Trop Sao Paulo 53:101–106
Yang X, Chen S, Zhang R et al (2006) Study on molluscicidal active components of petrol ether extract of Ginkgo sarcotestas. Chin J Zoonoses 22:961
Yang X, Chen S, Xia L, Chen J (2008) Molluscicidal activity against Oncomelania hupensis of Ginkgo biloba. Fitoterapia 79:250–254. https://doi.org/10.1016/j.fitote.2007.11.030
Yousif F, Hifnawy MS, Soliman G et al (2007) Large-scale in vitro. Screening of Egyptian native and cultivated plants for schistosomicidal activity. Pharm Biol 45:501–510. https://doi.org/10.1080/13880200701389425
Zacchino S, Rodríguez G, Pezzenati G et al (1997) In vitro evaluation of antifungal properties of 8.O.4‘-neolignans. J Nat Prod 60:659–662. https://doi.org/10.1021/np9605504
Zani CL, Marston A, Hamburger M, Hostettmann K (1993) Molluscicidal milliamines from Euphorbia milii var. hislopii. Phytochemistry 34:89–95. https://doi.org/10.1016/S0031-9422(00)90788-X
Zheng L, Deng L, Zhong Y et al (2021) Molluscicides against the snail-intermediate host of Schistosoma: a review. Springer, Berlin Heidelberg
Zhou Y, Chen Y, Jiang Q (2021) History of human schistosomiasis (bilharziasis) in China: from discovery to elimination. Acta Parasitol 66:760–769. https://doi.org/10.1007/s11686-021-00357-9
Zoni AC, Catalá L, Ault SK (2016) Schistosomiasis prevalence and intensity of infection in Latin America and the Caribbean countries, 1942–2014: a systematic review in the context of a regional elimination goal. PLoS Negl Trop Dis 10:e0004493
Acknowledgements
The authors did not receive support from any organization for the submitted work
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Dedicated with great pleasure and honor to Prof. Lars Bohlin, Uppsala University, Sweden and Prof. Rob Verpoorte, Leiden University, Netherland on the occasion of their 75th birthday.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Seedi, H.R., Khalifa, S.A.M., Mohamed, A.H. et al. Plant extracts and compounds for combating schistosomiasis. Phytochem Rev 22, 1691–1806 (2023). https://doi.org/10.1007/s11101-022-09836-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-022-09836-x