Abstract
Purpose
The walls, ceiling, and floor of a surgical environment, as well as the surfaces used in this place, must be submitted to a disinfection protocol to minimize nosocomial infections. Health regulations recommend two stages; the first is characterized by cleaning procedures, mainly using an enzymatic detergent, and the second is use of a disinfection agent. Ozone is a natural substance that has a relevant oxidative property for inactivating microorganisms and has emerged as an interesting agent in the hospital environment. Compared with conventional chemical products for disinfection, ozonated water has advantages such as a lack of storage control, disposal, and handling safety. The objective of this study was to use ozonated water as a disinfectant agent on a hospital metal surface, in comparison with 70% alcohol.
Methods
The degree of disinfection of the metal surface was quantitatively analyzed with use of an instrument by bioluminescence for a disinfection test.
Results
Qualitative terms indicated gram-positive cocci microorganisms and yeasts, suggesting that bacteria and fungi from the environment were identified. After the use of ozonated water as a disinfectant, the quantitative analysis indicated values below 100 RLU, showing evidence of a surface suitable for use in surgical procedures.
Conclusion
The use of ozonated water as a disinfectant agent for a metal surface in a hospital environment showed more effectiveness than 70% alcohol. Thus, ozonated water is a promising agent for disinfecting surfaces in surgical environments.
Similar content being viewed by others
Introduction
The association of surgical procedures with microbial infection is a severe risk to patients and health professionals (Flanagan et al. 2011). The hospital environment has been the main feature responsible for the occurrence of nosocomial infections. Joint contact surfaces in the surgical sector may be responsible for nosocomial infection, including the proliferation of multi-resistant microorganisms (Otter et al. 2011). These organisms can survive for an extended period, and can even remain viable for months (Lu et al. 2009).
The ceilings, walls, floors, and surfaces used in surgical sectors have shown microbiological contamination (Carducci et al. 2011; Hooker et al. 2011; Morgan et al. 2011). Microbiological transmission can occur due to the movement of personnel (patients and health professionals) between sectors and via the air-flow dynamics through the hospital (Borkow and Gabbay 2008; O’Connor et al. 2014).
The organic materials in general, including microorganisms and the media that nourish them, are characterized by having significant capacity for adhesion to surfaces. The surface decontamination procedure generally involves two steps. In the first, routine use is made of a water solution with enzymatic detergent, due to its properties for removing organic material adhered onto surfaces. The second stage demands an agent for disinfection, which means inactivation of the majority of microorganism present (Barker et al. 2004; Gebel et al. 2013; Barker et al. 2004; Rutala and Weber 2001). In this sense, the use of traditional chemical products, such as peracetic acid and hydrogen peroxide, has shown good effectiveness in terms of surface disinfection. However, some disadvantages have been verified such as the rigid protocol of use, transport and storage control, and pollution problems relative to discarding these products (Panouillères et al. 2007).
Products commonly used as disinfecting agents, such as peracetic acid, glutaraldehyde, among others are microbicidal by means of chemical action. In this sense, their use demands procedures such as production control, storage, and technical training for use. It is common in surgical environments to use 70% alcohol as a disinfectant, as it denatures organic substances and consequently produces the inactivation of microorganisms (Parikh and Parikh 2021).
Ozone (O3) is a natural substance produced by the presence of oxygen (O2) in the earth’s atmosphere, due to natural ultraviolet light from the sun (Andersen et al. 2013). Ozone has recently emerged as an interesting agent for inactivating microorganisms in healthcare (Botelho-Almeida et al. 2019; Nomura et al. 2021). Ozonated water has relevant microbicidal properties (Fonseca et al. 2015, 2020; Marson et al. 2016; Moreira et al. 2022; Carvalho et al. 2023), including the inactivation of resistant microorganisms (Nascente et al. 2021).
In assessing the degree of surface cleanliness, there is a technique for performing this process using bioluminescence, by collecting organic material—adenosine Triphosphate (ATP)—from the surface with a specific sterile swab. This allows the bioluminescence to indicate the degree of cleanliness/disinfection in real time, correlated to the presence of viable microorganisms. The equipment (luminometer) indicates a scale of values in relative light units (RLU), and values below 100 RLU indicate properly cleaned surfaces (Willis et al. 2007). Some studies involving the characterization of surface cleanliness in a hospital environment by the bioluminescence method also used the traditional microbiological assessment approach in quantitative terms (Willis et al. 2007). The aim of the present study was to verify the disinfection capacity of ozonated water on the hospital instrument table.
Materials and methods
Study location
This research was conducted in a hospital surgical center in the Vale do Paraíba, São Paulo, Brazil.
Cleanliness analysis
The degree of surface disinfection was evaluated using a commercial luminometer (SystemSure Plus, Hygiena LLC, Camarillo, CA, USA). A commercially available swab (snap valve—Ultrasnap Total, Hygiena LLC, Camarillo, CA, USA) was also used by friction to collect the organic material sample from a surgical metal surface area of 100 cm2, for the purpose of analyzing it.
After each cesarean procedure, a stainless-steel metal hospital surface belonging to a table for surgical procedures was analyzed. The degree of cleanliness was evaluated by the bioluminescence method before and after the cleanliness protocol involving cleaning (enzymatic detergent) and disinfection (70% alcohol).
The cleaning procedure was performed using an enzymatic detergent properly diluted in tap water. For this purpose, the solution was sprayed on the delimited place (100 cm2) until it formed a homogeneous liquid film. A time of 1 min has waited, and then the metal surface was cleaned using a sterile gauze. With regard to the disinfection procedure, the ozonated water was used by following the same protocol as that previously mentioned for use with the enzymatic detergent. A second cleanliness protocol was performed using the same detergent, but using 70% alcohol as a control agent. At the center where the study was conducted, 70% alcohol is adopted as a standard for surface disinfection. Considering each protocol, one area of 100 cm2 was delimited to assess the degree of disinfection by bioluminescence. For this purpose, a sterile paper with two openings, each with an area of 10 × 10 cm was used, one relative to the before-condition and the other area, relative to the after-condition.
In the specific case of ozonated water, the water container was weighed before and after spraying the surface, to quantify the volume of ozonated water sprayed. Thus, knowing the ozone concentration in water, we could estimate the amount of ozone mass transferred onto the metal surface.
The ozone protocol was performed twenty times (n = 20), and this was also performed with 70% alcohol. The effectiveness of each disinfection procedure was analyzed by the percentage (%) of reduction, considering the bioluminescence values (RLU) before and after each cleaning procedure.
Qualitative microbiological analysis
A qualitative microbiological analysis was made with a sterile cotton probe, by performing the smear at the site for evaluating disinfection, and then the probe was packed and sealed for further microbial laboratory analysis. The samples collected from the metal surfaces were also seeded on culture media for bacteria and fungi, TSA (Trypitc Soy Agar), and Sabouraud Agar, respectively. After this step, the samples were stained with Gram staining Gram (Coico 2005).
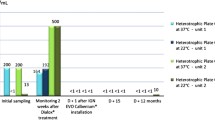
The water ozonation procedure was performed as shown in Fig. 1. We used a volume of 500 mL of distilled water in a glass reservoir and a stainless-steel bubble diffuser that was submerged. The diffuser was connected by a silicone tube to a photometrically calibrated corona discharge ozone generator (MS3G, Medical Systems Ltda, Brazil) that supplied the mixture of O2 + O3 gas at an ozone concentration of 44 mg/L, and an oxygen flow rate of 0.25 L/min.
The water temperature was kept low (8.2 ± 0.1 °C) with the insertion of some distilled ice cubes obtained from freezing some of the same liquid fluid mentioned. A sensor (TIZ-OEM, Anseros Klaus Nonnenmacher, Germany) was coupled to the water to allow the ozone dissolved to be monitored in a hydrodynamic manner, by using a magnetic impeller (stirrer). The procedure was carried out inside a bench with an air exhaust system to collect the excess ozone and dispose of it into the external environment. The sensor also allowed measurement of the temperature in the liquid, and this procedure was also performed in triplicate. Afterward, the ozonated water was transferred to another glass reservoir duly coated with thermally insulating material to keep the temperature constant.
Statistical analysis
The sample size (n = 20/group) was calculated using G*Power software (v. 3.1, Faul F., Germany) with a point biserial model with an alpha (α) error of 0.05 (95%), effect size (ρ) of 0.61, and a power of 0.95 (95%). Statistical analysis was performed using the Instat software (v. 3.05, Graphpad Software, San Diego, CA, USA). The data were submitted to a normality test (Kolmogorov–Smirnov, p < 0.1). In the RLU values, the data showed a non-normal distribution and was performed a Mann–Whitney U test. In the comparative analysis of disinfection protocols effectiveness (% RLU), the data showed normal distribution and thus was performed an unpaired t-test with Welch correction. The tests used a significance level of 0.05 and a confidence interval of 95% for the mean or the mean difference was also applied to compare the groups.
Results and discussion
The water ozonation procedure related to the ozone concentration measurement, shown in Fig. 2, showed that after 10 min of ozone mass transfer, a mean water ozone concentration stability of 2.7 ± 0.1 mg/L was obtained. The insertion of ice resulted in a more significant mass transfer of ozone; the total volume reached was established at the value of 750 mL, and the mean temperature measured during the ozonation process was 8.2 ± 0.1 °C. The ozone concentration in the water continued to be monitored, and after approximately 1 h, when the mean temperature rose to and stabilized at 19.2 ± 0.1 °C, it was found that the sensor indicated a mean concentration of 2.3 ± 0.1 mg/L.
We transferred a mass of 2.5 g of water to the metal surface using the sprayer method. Hence, the volume applied resulted in a value of 2.5 mL. Considering the ozone concentration of 2.7 mg/L in water, the mass quantity of ozone applied was 6.8 µg. The thickness of the water film transferred onto the surface of 100 cm2, considering the transfer volume, was calculated to be 0.25 mm. The total volume of ozonized water produced was 750 mL, capable of covering an area of 3 m2. The procedure with 70% alcohol used the same volume of alcohol.
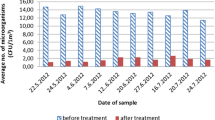
Table 1 shows the residual organic material before and after the disinfection protocols, measured by the ATP bioluminescence swab testing. Twenty tests were performed, using ozonated water, and all values indicated were shown to be lower than 100 RLU. This suggested that all these surfaces were properly clean; after checking all tests carried out with 70% alcohol, four of them were shown to have exceeded the limit value (100 RLU). The comparative statistical analysis of the two disinfection agents showed a significant difference between them (p = 0.0002), thus indicating greater disinfecting effectiveness of ozonated water. It should be noted that before the procedure, surfaces that were subsequently disinfected with ozonated water had shown a higher degree of contamination (p = 0.0005) than those that were disinfected with 70% alcohol.
Table 2 shows the mean values of percentage effectiveness (%) in the disinfection procedures using alcohol and ozonated water; the data refer to percentage (%) reduction in the RLU values. From the statistical analysis (t-test) of the data obtained relative to the percentage of effectiveness, there was a significant difference (p < 0.0001) between the agents used. Moreover, ozonated water was more efficient in terms of disinfection of surfaces than 70% alcohol, 98.1% and 86.5%, respectively.
Before the cleaning and disinfection procedures, the qualitative microbiological swab of the surfaces indicated the presence of gram-positive cocci and yeasts. After the disinfection protocols with 70% alcohol and ozonated water, no microbial growth was detected.
Our study showed that ozonated water produced better cleanliness in terms of disinfection compared with 70% alcohol (gold standard). Indeed, ozone is a highly oxidative agent with the potential to inactivate microorganisms. In this context, using ozonated water as a disinfectant has an advantage over chemical agents in terms of not being a toxic element. Indeed, in the scientific literature, there are reports of its use in treating wounds and even for human consumption as a drink (Leon et al. 2022; Hayakumo et al. 2013).
Breidablik et al. (2019) showed that ozonated tap water (0.8 or 4 ppm O3) had antimicrobial properties and could be an alternative to traditional alcohol-based hand disinfectants for nursing students, especially valuable for persons with contact dermatitis. In a short report (Breidablik et al. 2020), the same researchers showed that ozonated water together with a regular soap-and-water hand wash may be more effective than alcohol for the removal of bacteria from artificially contaminated hands.
Piletić et al. (2022) also use an ATP luminometer to determine the efficiency of ozone, in the form of gas, on ceramic plates (in vitro) contaminated with Klebsiella pneumoniae and showed that ozone caused a significant reduction in biofilm. Recently, in the COVID-19 pandemic context, Mascarenhas et al. (2022) used an ozonated water spray (0.7–0.9 ppm O3) to disinfect the garments and accessories of a hundred volunteers. The results showed a higher efficacy in microbial reduction and perception of acceptance of its use.
Another aspect to emphasize is that ozonated water is non-waste and pollutant in the environmental context; in this sense, an item of equipment adapted to a hospital condition could be developed to produce ozonated water for disinfecting surfaces. Apart from the costs of electrical energy and medical oxygen, the hospital staff would be able to insert purified water into the device by the distillation or reverse osmosis method. The device would be responsible for carrying out the ozonation at low temperature, and after a few minutes, the same professionals would collect the ozonated water, and put it into the container already fitted with the spray device.
As regards the methodology and the results of this investigation, some limitations warrant discussion. Further experiments on ozone kinetics, the use of resistant microorganisms, and penetration into biofilms need to be studied. A protocol applied to other hospital metal surfaces and various surgical environments must be elaborated to establish ozone disinfection.
Conclusion
The use of ozonated water as a disinfectant agent for a metal surface in a hospital environment showed more effectiveness than 70% alcohol. Thus, ozonated water is a promising agent for disinfecting surfaces in surgical environments.
References
Andersen SO, Halberstadt ML, Parnell NB. Stratospheric ozone, global warming, and the principle of unintended consequences and are ongoing science and policy success stories. J Air Waste Manag Assoc. 2013;63(6):607–47. https://doi.org/10.1080/10962247.2013.791349.
Barker J, Vipond IB, Bloomfield SF. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via environmental surfaces. J Hosp Infect. 2004;58(1):42–9. https://doi.org/10.1016/j.jhin.2004.04.021.
Borkow G, Gabbay J. Biocidal textiles can help fight nosocomial infections. Med Hypotheses. 2008;70(5):990–4. https://doi.org/10.1016/j.mehy.2007.08.025.
Botelho-Almeida TS, Lourenço FR, Kikuchi IS, et al. Evaluating the potential, applicability, and effectiveness of ozone sterilization process for medical devices. J Pharm Innov. 2019;13(2):87–9. https://doi.org/10.1007/s12247-017-9308-7.
Breidablik HJ, Lysebo DE, Johannessen L, Skare Å, Andersen JR, Kleiven OT. Ozonized water as an alternative to alcohol-based hand disinfection. J Hosp Infect. 2019;102(4):419–24. https://doi.org/10.1016/j.jhin.2019.01.026.
Breidablik HJ, Lysebo DE, Johannessen L, Skare Å, Andersen JR, Kleiven O. Effects of hand disinfection with alcohol hand rub, ozonized water, or soap and water: time for reconsideration? J Hosp Infect. 2020;105(2):213–5. https://doi.org/10.1016/j.jhin.2020.03.014.
Carducci A, Verani M, Lombardi R, Casini B, Privitera G. Environmental survey to assess viral contamination o fair and surfaces in hospital settings. J Hosp Infect. 2011;77(3):242–7. https://doi.org/10.1016/j.jhin.2010.10.010.
Carvalho MCO, Fernandes AB, Carvalho HC, Zângaro RA, Lima CJ. Preliminary study: Disinfection of colonoscope using a reprocessing system based on a hydrodynamic model with ozonated water. Ozone Sci Eng. 2023. https://doi.org/10.1080/01919512.2022.2164251
Coico R. Gram staining. Curr Protoc Microbiology. 2006;00(1). https://doi.org/10.1002/9780471729259.mca03cs00
Flanagan ME, Welsh CA, Kiess C, et al. A national collaborative for reducing healthcare-associated infections: current initiatives, challenges, and opportunities. Am J Infect Control. 2011;39(8):685–9. https://doi.org/10.1016/j.ajic.2010.12.013.
Fonseca PMM, Feitosa L, Fernandes AB, Zângaro RA, Lima CJ. Disinfection of dental instruments contaminated with Streptococcus mutants using ozonated water alone or combined with ultrasound. Ozone Sci Eng. 2015;37(1):85–9. https://doi.org/10.1080/01919512.2014.904740.
Fonseca PMM, Palacios DAB, Júnior PLS, et al. Preliminary study: a comparative analysis of the effects of ozone and ultrasound on Streptococcus mutans. Ozone Sci Eng. 2020;43(3):263–75. https://doi.org/10.1080/01919512.2020.1796581.
Gebel J, Exner M, French G, et al. The role of surface disinfection in infection prevention. GMS Hyg Infec Control. 2013;8(1):1–12. https://doi.org/10.3205/dgkh000210.
Hayakumo S, Arakawa S, Mano Y, Izumi Y. Clinical and microbiological effects of ozone nano-bubble water irrigation as an adjunct to mechanical subgingival debridement in periodontitis patients in a randomized controlled trial. Clin Oral Invest. 2013;17:379–88. https://doi.org/10.1007/s00784-012-0711-7.
Hooker EA, Allen SD, Gray LD. Terminal cleaning of hospital bed mattresses and bed decks does not eliminate bacterial contamination. J Hosp Infect. 2011;39(5):E23–4. https://doi.org/10.1016/j.ajic.2011.04.067.
Leon BR, Romary DJ, Landsberger SA, Bradner KN, Ramirez M, Lubitz RM. Risks of ozonated oil and ozonated water on human skin: a systematic review. Int Wound J. 2022;19(7):1901–10. https://doi.org/10.1111/iwj.13760.
Lu P-L, Siu LK, Chen T-C, et al. Methicillin-resistant Staphylococcus aureus and Acinetobacter baumanni on computer interfaces of hospital wards and association with clinical isolates. BMC Infect Dis. 2009;9:164. https://doi.org/10.1186/1471-2334-9-164.
Marson RF, Melo LHMS, Zângaro RA, de Lima CJ, Fernandes AB. Use of ozonated water for disinfection of gastrointestinal endoscopes. Ozone Sci Eng. 2016;38(5):345–51. https://doi.org/10.1080/01919512.2016.1192455.
Mascarenhas LAB, Dos Santos LMC, Oliveira FO, et al. Evaluation of the microbial reduction efficacy and perception of use of an ozonized water spray disinfection technology. Sci Rep. 2022;12(1):13019. https://doi.org/10.1038/s41598-022-16953-2.
Moreira LH, Salomão MK, Carvalho HC, et al. Ozonation of bovine peritoneal membrane for preservation: preliminary investigation. Ozone Sci Eng. 2022;44(6):587–92. https://doi.org/10.1080/01919512.2021.1984205.
Morgan DJ, Liang SY, Smith CL, et al. Frequent multidrug-resistant Acinetobacter baumanni contamination of gloves, gowns, and hands of healthcare workers. Infect Control Hosp Epidemiol. 2011;31(7):716–21. https://doi.org/10.1086/653201.
Nascente EP, Rauecker UN, Teles AV, et al. Inactivation of multidrug-resistant Salmonella Heidelberg isolated under different conditions. Ozone Sci Eng. 2021;44(4):363–71. https://doi.org/10.1080/01919512.2021.1960147.
Nomura Y, Yamamura J, Fukui C, et al. Performance evaluation of bactericidal effect and endotoxin inactivation by low-temperature ozone/hydrogen peroxide mixed gas exposure. J Biomed Mater Res B Appl Biomater. 2021;109(11):1807–16. https://doi.org/10.1002/jbm.b.34840.
O’Connor N, Cahill O, Daniels S, GalvanHumphreys S. Cold atmospheric pressure plasma and decontamination Can it contribute to preventing hospital-acquired infections? J Hosp Infect. 2014;88(2):59–65. https://doi.org/10.1016/j.jhin.2014.06.015.
Otter JA, Yezli S, French GL. A role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32(7):687–99. https://doi.org/10.1086/660363.
Panouillères M, Boillot C, Perrodin Y. Study of the combined effects of a peracetic acid-based disinfectant and surfactants contained in hospital effluents on Daphnia magna. Ecotoxicol. 2007;16(3):327–40. https://doi.org/10.1007/s10646-007-0136-2.
Parikh SR, Parikh RS. Chemical disinfectants in ophthalmic practice. Indian J Ophthalmol. 2021;69(3):510–6. https://doi.org/10.4103/ijo.IJO_1549_20.
Piletić K, Kovač B, Perčić M, et al. Disinfecting action of gaseous ozone on OXA-48-producing Klebsiella pneumoniae biofilm in vitro. Int J Environ Res Public Health. 2022;19(10):6177. https://doi.org/10.3390/ijerph19106177.
Rutala WA, Weber DJ. Surface disinfection: Should we do it? J Hosp Infect. 2001;48(1):S64–8. https://doi.org/10.1016/s0195-6701(01)90017-9.
Willis C, Morley R, Westbury J, Greenwood M, Pallett A. Evaluation of ATP bioluminescence swabbing as a monitoring and training tool for effective hospital cleaning. British J Infect Control. 2007;8(5):17–21. https://doi.org/10.1177/1469044607083604.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira, C.R., de Oliveira Carvalho, M.C., Schmitz, G.V. et al. Ozonated water in disinfection of hospital instrument table. Res. Biomed. Eng. 39, 329–334 (2023). https://doi.org/10.1007/s42600-023-00272-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42600-023-00272-0