Abstract
Background
Computer keyboards and mice are potential reservoirs of nosocomial pathogens, but routine disinfection for non-water-proof computer devices is a problem. With better hand hygiene compliance of health-care workers (HCWs), the impact of these potential sources of contamination on clinical infection needs to be clarified.
Methods
This study was conducted in a 1600-bed medical center of southern Taiwan with 47 wards and 282 computers. With education and monitoring program of hand hygiene for HCWs, the average compliance rate was 74% before our surveillance. We investigated the association of methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa and Acinetobacter baumannii, three leading hospital-acquired pathogens, from ward computer keyboards, mice and from clinical isolates in non-outbreak period by pulsed field gel electrophoresis and antibiogram.
Results
Our results revealed a 17.4% (49/282) contamination rate of these computer devices by S. aureus, Acinetobacter spp. or Pseudomonas spp. The contamination rates of MRSA and A. baumannii in the ward computers were 1.1% and 4.3%, respectively. No P. aeruginosa was isolated. All isolates from computers and clinical specimens at the same ward showed different pulsotypes. However, A. baumannii isolates on two ward computers had the same pulsotype.
Conclusion
With good hand hygiene compliance, we found relatively low contamination rates of MRSA, P. aeruginosa and A. baumannii on ward computer interface, and without further contribution to nosocomial infection. Our results suggested no necessity of routine culture surveillance in non-outbreak situation.
Similar content being viewed by others
Background
In developed countries, computers are used in the bedside area for multiple functions, including ordering, checking laboratory and image results, recording patients' conditions, and accounting. Moreover, most computer devices, such as keyboards and mice, in many countries are not water-proof and not specially designed for hospital disinfection needs. Therefore, there is a good possibility that computer interface surfaces may serve as reservoirs for nosocomial pathogens. Besides, the rate of hand washing compliance in healthcare institutions is low (~40%), which is presumably related to the contamination of inanimate surfaces of medical equipments and hospital environment with nosocomial pathogens [1]. Studies have shown that the hands or gloves of healthcare workers (HCWs) can be contaminated after touching inanimate objects in patient rooms or after touching environmental surfaces near patients [2–4].
One study reported that microbial contamination of computer interface surfaces was so prevalent that various microorganisms were isolated from more than 50% of the keyboards of hospital computers [5]. The levels of contamination varied with the proximity to the patients, the texture of inanimate surfaces and the frequency of contact. The hospital ward computer is found being less likely to be contaminated than bedside computers [6]. Schultz et al. have reported that 95% of keyboards in close proximity to patient sites had bacterial contamination. However, only 5% of these were pathogens known to be associated with nosocomial transmission [7]. Most previous studies have reported the contamination of computer interface surfaces by potential pathogens such as Methicillin-resistant Staphylococcus aureus (MRSA) [3, 8]and Acinetobacter baumannii [9], but few have studied the relationship between contamination of the ward computers and clinical isolates in hospitals with improved hand hygiene compliance and during a non-outbreak period. Clinically, A. baumannii, P. aeruginosa, and MRSA cause the most common nosocomial infections and their presence correlates with environmental surface contamination [10–12]. We conducted a hospital-based surveillance study of these three important pathogens on computer interface surfaces in different ward settings and then examined the relationship of contaminated computer interface surfaces with the presence of clinical isolates in these wards during a non-outbreak period.
Methods
We conducted a cross-sectional surveillance for S. aureus, Pseudomonas species and Acinetobacter species on the keyboards and mice of computers in all ward stations of Kaohsiung Medical University Hospital, a 1600-bed tertiary referral hospital that contained various speciality departments, in July 2006. The three organisms are among the most common causes of nosocomial infection in the study site where Vancomycin resistant enterococcus (VRE) accounted for less than 1% enterococcus clinical isolates. Clinical isolates of MRSA, P. aeruginosa, and A. baumannii were recovered two weeks before or after the day of the cross-sectional surveillance, when computer-associated bacteria were collected. We selected clinical and computer isolates of the same species in the same ward for comparison by antibiograms and pulsed field gel electrophoresis (PFGE) typing.
All medical records and ordering systems were computerized in this hospital. No routine disinfection protocol had been established for computer equipment. The keyboards were not covered. No routine cleaning for the surfaces of computer interfaces was performed. Hand hygiene compliance has been continuously educated and monitored every three months with method as previously described [13] by members in infection control room and the department of nursing. Every week, HCWs' hand hygiene compliance was monitored for 30 minutes in each ward. The mean of the rates from four times of monitoring was regarded as the hand hygiene compliance rate of every ward.
A sterile swab (CultureSwab Transport System, Difco, Detroit, MI) moistened with sterile saline solution was moved over the keys of keyboards and the buttons of computer mice. Then the swabs were added to brain heart infusion broth medium for 48 h at 37°C. The inoculated broth was subcultured onto blood agar plates (BBL, Cockeysville, MD, USA) and Mac-Conkey agar plates (BBL, Cockeysville, MD, USA). Organisms were identified using standard methods and the API Identification System (bioMe'rieux, Marcy l'Etoile, France). Isolates of Pseudomonas and Acinetobacter were identified to species level. We used the coagulase test (Coagulase Plasma System; Difco) to identify S. aureus strains. MRSA was preliminarily detected by its characteristic growth on mannitol salt agar supplemented with oxacillin (4 μg/mL). All suspected MRSA isolates were inoculated onto Mueller-Hinton agar (Becton Dickinson Microbiology Systems) supplemented with 6 μg/ml oxacillin and 4% NaCl. Isolates identified as P. aeruginosa, A. baumannii, and MRSA were further tested for antimicrobial susceptibility and for PFGE typing.
Antimicrobial susceptibility was determined by the agar diffusion method, according to the CLSI guideline [14]. Pulsed field gel electrophoresis (PFGE) was performed as described previously [15, 16]. Restriction enzymes were used for identification, SmaI for MRSA and ApaI for Acinetobacter spp. The band patterns were visually compared and classified as indistinguishable (no difference), closely related (clonal variants, one to three band differences), possibly related (four to six band differences), and unrelated (more than six band differences), according to the criteria previously described [17]. To identify PFGE polymorphisms, each sample was analyzed by Molecular Analyst Fingerprinting, Fingerprinting Plus, and Fingerprinting DST Software (Bio-Rad Laboratories, Richmond, CA, USA). After calculation of similarities for every pair of organisms using Pearson correlation coefficients, we used the grouping method to deduce a dendrogram from the matrix via the Unweighted Pair Group Method using Arithmetic Average (UPGMA) clustering technique.
Statistics were run with SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA). P values were calculated by the Chi-Square test for categorical variables. All tests were two-tailed, and p < 0.05 was considered significant.
Results
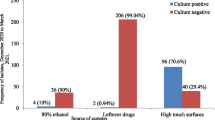
We screened 282 computer samples that each had a keyboards and a mouse device in 47 ward stations for S. aureus, Pseudomonas spp. and Acinetobacter spp. Twelve of the ward stations were from intensive care units (ICUs) and 35 were from Non-ICU wards One hundred and forty-four samples were from ICU computers and 420 from ward computers. All nurse stations had six desktop computers, one for accounting, one for radiological images, and four for ordering and laboratory data checking. Although the computers for accounting were mainly operated by accountants without direct contact with patients, the other HCWs accessed these computers occasionally when the accountants were off duty. The average compliance rate was 74% in the month that the cross-sectional surveillance on computer underwent. There was no significant difference on compliance rates among wards.
Before final species identification, we found 18 isolates of S. aureus, 17 isolates of Pseudomonas spp., and 22 isolates of Acinetobacter spp. from 49 computer interface samples. Three computers had positive isolation from both keyboard and mouse device. Five computer interfaces had two different species identified. The overall computer contamination rate of the above three organisms is 17.4% (49/282).
For Acinetobacter isolates, 12 were A. baumannii, 7 were A. lwoffii, and 3 were A. junii. For S. aureus isolates, 3 were MRSA and 15 were MSSA. For Pseudomonas isolates, there were 12 P. putida isolates, 1 P. alcaligenes, 4 P. stutzeri, but none was P. aeruginosa. The computer contamination rate for A. baumannii was 4.3% (12/282) and the rate for MRSA was 1.1% (3/282) (Table 1). The combined contamination rate of either MRSA or A. baumannii is 5.3% (15/282).
There was no significant difference about contamination rate by any of S. aureus, Pseudomonas spp. and Acinetobacter spp. between non-ICU (16.7%, 35/210) and ICU (19.4%, 14/72) computers (p = 0.591) and between accounting, radiology and order computers (p = 0.699). When the radiology and order computers were grouped together into the "clinical use" category, there was no significant difference in the contamination rate between accounting (10/47) and "clinical use" (39/235) computers (p = 0. 439). No significant higher rate of contamination of any of the above three between keyboard and mouse (p = 0.474) but there is a trend that the occurrence of keyboard contamination is associated with the occurrence of mouse device contamination (p = 0.054) by McNemar test.
When only considering MRSA, P. aeruginosa, and A. baumannii, all the differences between non-ICU and ICU computers (p = 0.476), keyboard and mouse specimens (p = 0.191), and accounting and "clinical use" computers (p = 0.722), were not significant (Table 2).
Twenty isolates of A. baumannii, 34 isolates of MRSA, and 97 isolates of P. aeruginosa were identified from clinical specimens in the same hospital two weeks before and after the computer surveillance day. One MRSA isolate and two A. baumannii were isolated from patients of the wards where computer devices were also contaminated with MRSA and A. baumannii. We compared the clinical and computer isolates by antibiograms and PFGE typing. The antibiogram results of the A. baumannii isolated from clinical and computer specimens in ward 18EN were different on fewer than three classes of antimicrobial agents. For the other groups of MRSA and A. baumannii isolates, the antibiograms differed in susceptibility by more than three classes of antimicrobial agents. The PFGE patterns of computer and clinical isolates in the same ward (MRSA isolates from ward 8B and A. baumannii isolates from ward 11CI and 18 EN) were different that had similarity less than 70% (Fig. 1 and Fig. 2). However, interestingly, two A. baumannii computer isolates from ward 7C and one A. baumannii computer isolate from ward 15ESI2 had the same pulsotype (Figure 1).
Similarity of PFGE patterns of A. baumannii isolated in hospital wards, calculated by the unweighted pair group method using arithmetic averages (UPGMA). Similarities >70% represent the clonal spread of strains. Isolate number, source of specimens and the ward number are listed in the right side of the figure.
Discussion
This hospital-based surveillance study indicated the rate of MRSA and A. baumannii contamination of ward computers was 1.1% and 4.3%, respectively. No computer device was contaminated by P. aeruginosa. The MRSA contamination rate in our study was lower than that reported in a UK referral centre (1%) [18], but very different from that reported for a UK acute district general hospital (24%) [19]. This difference might be related to differences in hospital size, extent of computer use, and hand hygiene compliance. We supposed the relatively good hand hygiene compliance among our HCWs contributed to the lower contamination rate, although the two previous studies did not provide hand hygiene compliance data. The significant difference in the level of contamination of ward computers at different hospitals implies that computers can have very different roles as reservoirs of nosocomial pathogens. Compared with a high rate (37%) of environmental surfaces in patients' rooms harbor MRSA sampled from studies during outbreak and non-outbreak situations [20], ward computer interface surfaces seem to play a minor role as pathogen reservoirs.
Studies in ICUs indicate a more important role of computers there as pathogen reservoirs than do the computers of Non-ICU wards. Two previous studies have shown that contamination rate of computers in ICU workstations was as prevalent as that of the computers in patient rooms [8, 21]. Computer interface surfaces in an ICU station were contaminated with potentially pathogenic microorganisms at a higher rate (6.3%) than the other surfaces [21]. However, for computer interfaces at ward stations, our results did not reveal a higher contamination rate in ICUs than that in non-ICUs.
Although contamination of inanimate surfaces by microorganisms has long been recognized, its impact on patients' infections is not clear [22]. The fact that forty-two percent of personnel had MRSA contamination on their gloves without entering the rooms of patients with MRSA infections suggests that contaminated environmental surfaces outside infected patients' rooms may increase the risk of MRSA transmission [3]. The association of clinical isolates and environmental contamination isolates was demonstrated for a 9-bed ICU, in which the researchers used PFGE typing of sequential isolates from clinical and environmental specimens [23]. An investigation of computer interface devices in an ICU indicated indistinguishable strains of MRSA from computers of patients' rooms, doctors' station, and clinical specimens [8]. Our hospital-based surveillance of MRSA by PFGE typing did not identify an association of MRSA isolates from computer interface devices and from patients. It is noteworthy that our study was different for a significantly lower rate of MRSA contamination (1.1%) than this previous study (11.1%) [8]. However, the identity of indistinguishable strains of A. baumannii from computer devices in two different wards (Figure 1) suggested the computer as a potential route of clonal spread in a hospital, although no subsequent clinical isolate was of this strain in our study.
Compared with the relatively low MRSA contamination rate (1.1%) on ward computer interface surfaces in our study, the 53% MRSA hand contamination rate among HCWs after contacting environmental surfaces near hospitalized patients in Bhalla A. et al study, showed that HCW's role as pathogen reservoirs and suggested hand hygiene to be important [2]. Beside to improve hand-hygiene compliance, improvement of cleaning services could be administered as an effective infection control measure [20]. Disinfectants including chlorine, alcohol, phenol and quaternary ammonium are all effective against MRSA, P. aeruginosa, and vancomycin-resistant Enterococcus species on keyboards of computers; and even sterile water is effective to remove more than 95% bacteria [5]. Flat keyboard with an alarm was suggested for being easy to clean and associated with better cleaning compliance [24]. Although keyboards can be safely and successfully disinfected and the need to clean computer interface surfaces as a routine practice is generally accepted, no specific cleaning and disinfection frequency and procedure for computer accessories has been defined [6, 25]. Domestic cleaning has been reported useful to control MRSA [26]. As daily cleaning and hygiene regulation for using computer were demonstrated to be useful interventions to reduce keyboard contamination [9], several recommendations were gradually adopted, including that computers should be disinfected daily and when visibly soiled and HCW should not touch computer keyboards with contaminated hands [5]. Our result of the trend for the association of contamination rate of any of S. aureus, Pseudomonas spp. and Acinetobacter spp on the keyboards and mouse devices suggested both interface surfaces required attention when conducting cleaning service.
In contrast to previous studies on the role of environmental colonization that were performed during nosocomial pathogen outbreaks [27], our study was conducted when there was no outbreak. We did not investigate other factors in the transmission route, such as HCWs' hand carriage and colonization of patients. As sporadic MRSA strains differed in the shorter survival duration on environmental surface than outbreak strains [28], our study of a non-outbreak setting might be associated with the lower chance to find the similar genotype strains between computer and clinical isolates. Our study result is also limited by the cross sectional design that no measurement of the contamination levels was performed at two time periods with different hand hygiene compliance. Therefore it is difficult to make a conclusion about hand hygiene could help to prevent contamination of hospital ward computers, though inanimate environment surfaces in hospitals played a role among many steps for the transmission of pathogen to patients and hand hygiene promotion may be beneficial to reduce the risk of cross contamination [29].
Conclusion
Our report documents that the contamination rates of computer interface surfaces by MRSA, P. aeruginosa, and A. baumannii in a hospital-wide surveillance were low when a relatively good hand hygiene compliance of HCWs was observed. Furthermore, no clinical correlation of contamination of these computer devices to clinical isolates was found. Routine disinfection and even surveillance of these computer devices may not be mandatory in non-outbreak settings.
References
Boyce JM, Pittet D: Guideline for Hand Hygiene in Health-Care Settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect Control Hosp Epidemiol. 2002, 23 (12 Suppl): S3-40. 10.1086/503164.
Bhalla A, Pultz NJ, Gries DM, Ray AJ, Eckstein EC, Aron DC, et al: Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol. 2004, 25 (2): 164-167. 10.1086/502369.
Boyce JM, Potter-Bynoe G, Chenevert C, King T: Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997, 18 (9): 622-627. 10.1086/647686.
Hartstein AI, Rashad AL, Liebler JM, Actis LA, Freeman J, Rourke JW, et al: Multiple intensive care unit outbreak of Acinetobacter calcoaceticus subspecies anitratus respiratory infection and colonization associated with contaminated, reusable ventilator circuits and resuscitation bags. Am J Med. 1988, 85 (5): 624-631. 10.1016/S0002-9343(88)80233-X.
Rutala WA, White MS, Gergen MF, Weber DJ: Bacterial contamination of keyboards: efficacy and functional impact of disinfectants. Infect Control Hosp Epidemiol. 2006, 27 (4): 372-377. 10.1086/503340.
Neely AN, Weber JM, Daviau P, MacGregor A, Miranda C, Nell M, et al: Computer equipment used in patient care within a multihospital system: recommendations for cleaning and disinfection. Am J Infect Control. 2005, 33 (4): 233-237. 10.1016/j.ajic.2005.03.002.
Schultz M, Gill J, Zubairi S, Huber R, Gordin F: Bacterial contamination of computer keyboards in a teaching hospital. Infect Control Hosp Epidemiol. 2003, 24 (4): 302-303. 10.1086/502200.
Bures S, Fishbain JT, Uyehara CF, Parker JM, Berg BW: Computer keyboards and faucet handles as reservoirs of nosocomial pathogens in the intensive care unit. Am J Infect Control. 2000, 28 (6): 465-471. 10.1067/mic.2000.107267.
Neely AN, Maley MP, Warden GD: Computer keyboards as reservoirs for Acinetobacter baumannii in a burn hospital. Clin Infect Dis. 1999, 29 (5): 1358-1360. 10.1086/313463.
Engelhart S, Krizek L, Glasmacher A, Fischnaller E, Marklein G, Exner M: Pseudomonas aeruginosa outbreak in a haematology-oncology unit associated with contaminated surface cleaning equipment. J Hosp Infect. 2002, 52 (2): 93-98. 10.1053/jhin.2002.1279.
Getchell-White SI, Donowitz LG, Groschel DH: The inanimate environment of an intensive care unit as a potential source of nosocomial bacteria: evidence for long survival of Acinetobacter calcoaceticus. Infect Control Hosp Epidemiol. 1989, 10 (9): 402-407. 10.1086/646061.
Sekiguchi J, Teruya K, Horii K, Kuroda E, Konosaki H, Mizuguchi Y, et al: Molecular epidemiology of outbreaks and containment of drug-resistant Pseudomonas aeruginosa in a Tokyo hospital. J Infect Chemother. 2007, 13 (6): 418-422. 10.1007/s10156-007-0560-5.
Rosenthal VD, McCormick RD, Guzman S, Villamayor C, Orellano PW: Effect of education and performance feedback on handwashing: the benefit of administrative support in Argentinean hospitals. Am J Infect Control. 2003, 31 (2): 85-92. 10.1067/mic.2003.63.
Clinical and Laboratory Standards Institute: Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement (M100-S17). 2007, Wayne, Pennsylvania, USA: Clinical and Laboratory Standards Institute
Macfarlane L, Walker J, Borrow R, Oppenheim BA, Fox AJ: Improved recognition of MRSA case clusters by the application of molecular subtyping using pulsed-field gel electrophoresis. J Hosp Infect. 1999, 41 (1): 29-37. 10.1016/S0195-6701(99)90034-8.
Takahashi A, Yomoda S, Kobayashi I, Okubo T, Tsunoda M, Iyobe S: Detection of carbapenemase-producing Acinetobacter baumannii in a hospital. J Clin Microbiol. 2000, 38 (2): 526-529.
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al: Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995, 33 (9): 2233-2239.
Man GS, Olapoju M, Chadwick MV, Vuddamalay P, Hall AV, Edwards A, et al: Bacterial contamination of ward-based computer terminals. J Hosp Infect. 2002, 52 (4): 314-315. 10.1053/jhin.2002.1302.
Devine J, Cooke RP, Wright EP: Is methicillin-resistant Staphylococcus aureus (MRSA) contamination of ward-based computer terminals a surrogate marker for nosocomial MRSA transmission and handwashing compliance?. J Hosp Infect. 2001, 48 (1): 72-75. 10.1053/jhin.2001.0955.
Dancer SJ: Importance of the environment in methicillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet Infect Dis. 2008, 8 (2): 101-113. 10.1016/S1473-3099(07)70241-4.
Hartmann B, Benson M, Junger A, Quinzio L, Rohrig R, Fengler B, et al: Computer keyboard and mouse as a reservoir of pathogens in an intensive care unit. J Clin Monit Comput. 2004, 18 (1): 7-12. 10.1023/B:JOCM.0000025279.27084.39.
Hota B: Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection?. Clin Infect Dis. 2004, 39 (8): 1182-1189. 10.1086/424667.
Hardy KJ, Oppenheim BA, Gossain S, Gao F, Hawkey PM: A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients' acquisition of MRSA. Infect Control Hosp Epidemiol. 2006, 27 (2): 127-132. 10.1086/500622.
Wilson AP, Ostro P, Magnussen M, Cooper B: Laboratory and in-use assessment of methicillin-resistant Staphylococcus aureus contamination of ergonomic computer keyboards for ward use. Am J Infect Control. 2008, 36 (10): e19-25. 10.1016/j.ajic.2008.09.001.
Sehulster L, Chinn RY: Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003, 6;52 (RR-10): 1-42.
Rampling A, Wiseman S, Davis L, Hyett AP, Walbridge AN, Payne GC, et al: Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2001, 49 (2): 109-116. 10.1053/jhin.2001.1013.
Harris AD: How important is the environment in the emergence of nosocomial antimicrobial-resistant bacteria?. Clin Infect Dis. 2008, 46 (5): 686-688. 10.1086/527395.
Wagenvoort JH, Sluijsmans W, Penders RJ: Better environmental survival of outbreak vs. sporadic MRSA isolates. J Hosp Infect. 2000, 45 (3): 231-234. 10.1053/jhin.2000.0757.
Pittet D, Allegranzi B, Sax H, Dharan S, Pessoa-Silva CL, Donaldson L, Boyce JM: Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006, 6 (10): 641-652. 10.1016/S1473-3099(06)70600-4.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/9/164/prepub
Acknowledgements
This work was supported by grants from the National Science Council (NSC 95-2314-B-037-076 to Lu PL, Chen YH) and National Health Research Institutes (Siu LK), Taiwan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PLL and TPC designed the study. YHC and SFL advised on the clinical aspects of the study. LM and WGC participated in the laboratory work. PLL, LKS, YHC and CTC prepared the manuscript with major contributions from other authors. All authors read and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Lu, PL., Siu, L., Chen, TC. et al. Methicillin-resistant Staphylococcus aureus and Acinetobacter baumanniion computer interface surfaces of hospital wards and association with clinical isolates. BMC Infect Dis 9, 164 (2009). https://doi.org/10.1186/1471-2334-9-164
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2334-9-164