Abstract
Fenugreek is a globally important legume that is widely cultivated for its therapeutic benefits in most parts of the world. Seeds on the other hand have a poor germination and growth rate when exposed to salinity. The effect of ultrasonic exposure period on germination and early seedling behaviors of fenugreek seeds under salt stress was investigated in a laboratory experiment. During germination and early seedling stages, all tests were conducted at 40 kHz in a water bath ultrasonic device with two durations (10 and 20 min) under salinity stress using different concentrations of NaCl (0, 1000, 3000, and 5000 mg/l). The results revealed a substantial decrease in germination percentage, all growth criteria, with increasing NaCl concentration and a significant increase in biomass produced by the Fenugreek (total soluble protein, total soluble carbohydrate, and proline), all of which are thought to be mechanisms for salinity resistance. Ultrasonication of fenugreek seeds for 10 and 20 min has a significant impact on seed germination, early seedling development and biochemical constituents under normal and stress conditions. The genetic stability of fenugreek DNA content was affected by these different treatments. This variation was estimated by RAPD-PCR molecular marker, and resulted in a total polymorphism percentage of 49.72% from all the primers. All these different treatments caused variation in the physiological responses and DNA content. This variation enhanced with more ultrasonic and salt treatments. Hence, these stresses can be used for enhancing the variable metabolic processes in fenugreek plant and stimulate its medicinal properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fenugreek (Trigonella foenum-graecum L.) is an annual herb in the Fabaceae family whose leaves and seeds are used as spices and condiments due to their strong flavor and scent. Fenugreek has also gained popularity as an ingredient in traditional remedies (Shrivastava 2012). Fenugreek is well-known for its therapeutic properties, including anti-diabetic, anti-carcinogenic, hypocholesterolemia, antioxidant, and immunological effects. Besides its therapeutic use, it is also utilized as a food stabilizer, glue, and emulsifying agent in the manufacture of numerous food products (Wani and Kumar 2016). Fenugreek is a spice, vegetable, and significant medicinal crop that is grown all over the world (Olfa et al. 2018). Many medicinal plants must be professionally farmed in order to meet rising demand for indigenous systems of medicine as well as the pharmaceutical industry, but soil salinity poses major challenges to plant cultivation (Qureshi et al. 2005). Most plant species' seed germination and seedling growth phases are known to be particularly sensitive to salt stress (Cuartero et al. 2006). Drought stress, or salinity, has a devastating secondary effect on cells. The impacts can be direct, such as decreased CO2 availability (Chaves et al. 2009), or indirect, such as changes in photosynthetic metabolism (Pinheiro and Chaves 2011), or secondary, such as oxidative stress. Saberali and Moradi (2019) investigated the effect of salinity on germination and seedling growth of fenugreek, dragonhead, savory, and dill, and found that as the NaCl concentration increased, germination and seedling growth decreased, with the greatest reduction occurring at the highest salt level. All of the species studied experienced a linear reduction in germination, germination velocity, and seedling growth as a result of salinity stress, but the losses differed. The effects of salinity on seedling germination and growth are mainly linked to osmotic effects, which can cause water uptake inhibition, and ion toxicity, which can prevent cell growth and division (Munns and Tester 2008; Morais et al. 2018). Olfa et al. (2018) investigated the effect of different NaCl concentrations (0, 50, 100, 150, and 200 mM) on Fenugreek plant growth and determined that the salt effect reduced the biomass generated by the Fenugreek, as measured by leaf, stem, and root dry weight, at 150 and 200 mM.
Utilizing inaudible sound frequencies, ultrasonic interaction with materials is a ground-breaking physical technique (20–100 kHz). It has been shown that treating substances with ultrasound can change their state and even expedite processes (Aladjadjiyan 2007). This approach is unique among seed preparation techniques because it is straightforward, affordable, environmentally friendly, and multifunctional (Goussous et al. 2010). Many seed varieties have been treated with ultrasound to encourage germination, including maize, barley, rice, and sunflower (Aladjadjiyan 2002; Florez et al. 2007; Yaldagard et al. 2008). Ultrasound has a significant impact on plant growth and development when used as a kind of stress. The growth of various plant organs can be affected by ultrasonic treatment. When seeds are subjected to ultrasonic process, researchers discovered that a small amount of ultrasound can encourage cell division, a medium dose of ultrasound can inhibit cell division, and a higher amount of ultrasound can kill cells. Ultrasonic processing boosted the rate of germination of spinach and cabbage seeds, according to the study. Mild ultrasonic can cause plant root cells to actively proliferate, improve plant development ability, and encourage the plant to strike roots, which can reduce yearly plant respiration intensity while increasing plant respiration intensity for two years (Zhang et al. 2008). Seeds from two rice cultivars, Guangyan1 and Huahang 31, were treated to ultrasonic vibration for 30 min before being transplanted into lead contaminated soil, according to Rao et al. (2018) the Pb content in roots, stems, leaves, panicles (at heading), and brown rice (at maturity) was lower in the ultrasonic treatment than in the control, indicating that seed treatment with ultrasonic waves could improve rice performance and minimize brown rice lead accumulation in lead polluted soils. Previous research has ascribed the improvement to a variety of factors, including: Ultrasound improves water uptake and oxygen availability by increasing the porosity of the seed through acoustic cavitation. Ultrasound speeds up mass transfer by allowing extra-absorbed water to react freely and readily with the cell embryo, releasing gibberellic acid and speeding up metabolic activities in aleurone cells. By disrupting cell membranes, ultrasound aids in the mobilization of endosperm nutrients. The same results were also showed by Wang et al. (2020) and Carrillo-Lopez et al. (2021).
With salt stress brought on by NaCl, this study sought to ascertain the effects of ultrasonic wave duration on specific seedling growth, metabolic components, and genetic stability in fenugreek.
Material and methods
The present study was conducted at the Laboratory of Plant Physiology of Helwan University, Faculty of Science, Ain Helwan, Cairo. Healthy and uniform seeds of fenugreek (Trigonella foenum-graecum L.) were selected and used for the ultrasonication that were carried out at 40 kHz on the digital ultrasonic cleaner (MFUC-80A). Ultrasound treatments were performed in a water bath at constant temperature (25 °C) and for two time periods (10 and 20 min). After exposure to ultrasonic waves equal number (25 seeds) of fenugreek seeds were transferred to sterile Petri dishes with one filter paper Whatman No. 1. Four replicates were prepared for each treatment. The seeds were germinated under different osmotic stress values of NaCl (0.0, 1000, 3000, 5000 mg/l). A seed was considered to be germinated at the emergence of both radicle and plumule up to 2 mm length (Chartzoulakis and Klapaki 2000).
Germination percentage and growth parameters
The experiment was terminated on at the 7th day, data on germination percentage, plumule and radicle length (cm), fresh and dry weight of plumule, radicle (mg), plumule to radicle length ratio and seedling vigour index were recorded. The data on plumule and radicle dry weight was recorded after drying in hot air oven at 65 °C for 48 h.
The germination percentage was determined by using the following formula (Aniat-ul-Haq and Agnihotri 2010).
The plumule to radicle length ratio of seedling was calculated by the following formula (Kökten et al. 2009):
The seedling vigour index was determined by multiplying the sum total of mean length of plumule and radicle of a seedling with germination percentage of the respective seedling by the following formula (Iqbal and Rahmati 1992):
where, RL = Mean radicle length, PL = Mean plumule length, GP = Germination percentage.
The germinating seeds were used for measuring the total soluble sugars, total soluble proteins and proline.
Chemical analysis
A known weight of germinated seeds was extracted in 70% ethanol and completed to a known volume with distilled water and used for estimation of total soluble sugars using anthrone reagent (Umbreit et al. 1959) and the total soluble proteins by the procedure of Lowry et al. (1951). Approximately 0.5 g leaves were homogenized in 10 ml of 3% aqueous sulfosalicylic acid, and then the homogenate filtered through Whatman filter paper no.2. and used for estimation of proline according to Bates et al. (1973).
DNA isolation and RAPD-PCR bioassay
The total genomic DNA was extracted by CTAB method, according to Doyle and Doyle (1990). Half gram of leaves was mixed with 800 µl of 2% CTAB buffer, then incubated 45 min at 65 °C (vortex each 10 min). The tubes were centrifuged at 12,000 rpm for 12 min, then the supernatant transferred into new tubes with addition of equal volume of chloroform: isoamyl alcohol (24:1) and set for 3 min at room temperature. After that, tubes were centrifuged (12,000 rpm/10 min/4 °C). Then the upper aqueous layer was transferred to new Eppendorf tubes with addition of 800 µl of absolute ice-cold ethanol and left overnight at − 20 °C. Tubes were centrifuged to precipitate DNA pellets then washed them with ice-cold 70% ethanol. Finally, resuspend the pellets in 50 µl of TE buffer and kept at − 20 °C till applying RAPD-PCR.
Seven decamers were applied in this study, however only four of them yielded scorable and reproducible bands (Table 2). The RAPD-PCR reaction was carried out in Biometra thermocycler with a total volume of 25 µl of 12.5 µl Taq master mix (COSMO PCR RED M. Mix, W1020300x), 3 µl of genomic DNA, 1.5 µl for each primer (Willowfort) and 8 µl ddH2O. The reaction program was designed for 40 cycles as follow: Denaturation for 30 s at 94 °C, annealing 30 s at different degrees and extension for 1 min at 72 °C; followed by one step of final extension at 72 °C for 10 min then cooling at 4 °C. The amplified PCR product was run on 1.4% agarose gel compared to (New England Biolab, #N3232S) ladder.
Statistical analysis
The significance of the results was determined using Least Significant Difference (LSD) values at p = 0.05 according to Snedecor and Cochran's method of complete randomized blocks design (CCRBSE. Bas) utilizing analysis of variance (1980). The analysis of gel electrophoresis resulted in images that were analyzed by band scoring (1, 0). These computations were carried out using Bio-Rad Quantity one (4.6.2).
Results and discussion
Effect of salt stress on germination and growth parameters
According to the findings, there was a general drop in germination percentage with increasing salt content, with a maximum loss of 74% at concentration 5000 mg/l NaCl (Fig. 1). The use of the seed's stored food supply is necessary for seed germination. Water absorption by seedlings is hampered by salinity. As a result, the hydrolysis of seed reserves is inhibited, causing seed germination to be delayed and reduced (Begum et al. 2010). Furthermore, due to the concentration of soluble solutes around the seeds, which increases the osmatic pressure, the seeds require more water intake during germination when exposed to salt. As a result of the excessive uptake of ions, the plant becomes poisonous. Furthermore, due to the concentration of soluble solutes around the seeds, which increases the osmatic pressure, the seeds require more water intake during germination when exposed to salt. As a result, the uptake is enormous.
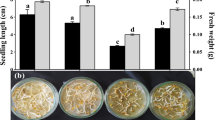
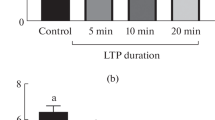
Effect of ultrasonic exposure time (10 and 20 min) and salt stress on germination percentage of fenugreek at early seedlings stage. Values represent the mean of three replicates. Different letters (a, b, c, d and e) indicate statistical differences at 5% probability according to Duncan’s test. Error bars are standard errors of the mean (LSD least significant difference)
Toxicity in the plant is caused by a buildup of ions. Furthermore, there is a difference in water potential between the external environment and the seeds, (lower water availability) suppresses the development of main roots (Delachiave and DePinho 2003).
Many authors, including Saberali and Moradi (2019), Olfa et al. (2018); and Behairy et al. (2017), have documented salt damage on the seed germination of fenugreek (Trigonella foenum-graecum). However, after exposing seedlings to ultrasonic waves for 10 or 20 min, the detrimental effects of salt stress were significantly decreased, and the germination percentage in both stressed and unstressed circumstances improved. There was a notable increase in germination percentage of unstressed seeds from 84 to 96% with exposure of seeds to ultrasonic waves for 20 min, while under salinity stress it was observed that the germination values at 20 min were consistently greater than those at 10 min. Goussous et al. (2010) on wheat, Yaldagard et al. (2008) on barley, and Aladjadjiyan (2002) on Zea mays all observed Improved germination performance following ultrasonic application. The main effects of ultrasonic are revealed to be mechanical effects (acoustic cavitation) and rupture of plant cell walls, resulting in increased water uptake. Cavitation during the sonication process was hypothesized as another possible reason that induced micro fissures on the seed coat, which may have improved the imbibition of moisture for enhanced germination speed (Yaldagard et al. 2008).
As shown in Table 1, there was a negative relationship between salinity stress and all morphological traits of growth parameters such as (plumule and radicle length (cm), fresh and dry weight of plumule, radicle (mg), seedling vigor index, and plumule to radicle length ratio of seedling) that were optimal in the absence of salt and reduced by increasing salt concentrations. The lowest values for all growth parameters were obtained at 5000 mg/l of salt. Our findings corroborate previously published findings. Many authors (Ratnakar and Raib 2013) showed that salt stress hindered the growth of fenugreek seedlings. Olfa et al. (2018) investigated the growth responses of fenugreek to salt stress and found that dry weights of leaves, stems, and roots were reduced. These alterations were linked to a decrease in water content, K+, and Ca2+ concentrations, as well as a significant increase in Na+ and Cl− concentrations in many organs. Salinity stress Inhibited seedling development by reducing assimilate supply and turgor pressure (Munns and Tester 2008). Salt's direct impacts on plant growth include (1) a drop in the osmotic potential of the soil solution, which limits plant accessible water, and (2) toxicity of excessive sodium or chloride towards the plasma membrane (Oktem et al. 2006). Seeds' ability to keep harmful substances out for salt tolerance in Suaeda salsa, a medicinal halophyte (Wang et al. 2015a), was dependent on the ability of seeds to remove harmful Na+ from the developing embryo.
Although the plumule to radicle length ratio is a derived property, it was also discovered that the general trend of salinity stress indicated a steady decrease in plumule to radicle length ratio of seedlings as salt concentration rises, when compared to control. Such observation was reported earlier in fenugreek (Kapoor and Pande 2015; Ratnakar and Raib 2013), in spinach (Keshavarzi et al. 2011) and in oat (Chauhan et al. 2016).
The seed vigor Index, also known as seed power or seed viability, is a measurement of a seed's capacity to emerge under stressful condition and seeds with a higher seed vigor index is more vigorous. Table 1 shows that all growth metrics had a negative and substantial relationship with seedling vigor index that dropped as salinity concentrations rose. Despite the deleterious effects of salt stress on seedling growth, ultrasonic treatment had a significant impact on seedling growth, with all growth parameters after 10 or 20 min of exposure. According to research, a small amount of ultrasound can accelerate cell division in some plant organs whereas a substantial dose of ultrasound can inhibit cell division (Zhang 2008). According to Machikowa et al. (2013) germination, Seed vigor index, and the root and shoot lengths of seedlings treated with ultrasonic were different depending on the ultrasound's intensity, from the control the highest level at 10 min of ultrasonic treatment, the seedling's Seed vigor index was discovered therapy at intensities of 40–60% and the treatments, which lasted between 5 and 20 min, yielded the best results.
The effects of ultrasonic assisted seeds priming (20, 40, 80, and 160 s) with a frequency of 24 kHz on seed enhancement of aged Milk thistle seeds were investigated by (Moosavi 2018) who showed that root growth was significantly improved by using ultrasonic energy for 20 s, and root length was 10.39 cm, compared to 5.48 cm without ultrasonic energy. The effects of salinity and ultrasonic pretreatment on the development of clover sprouts were investigated by Tahany et al. (2021) after being processed with ultrasound at 20, 28, and 40 kHz for 30 min at 30 °C, the clover seeds were steeped in deionized water with 1000 and 2000 ppm of sodium chloride for 9 h, and then sprouted for 3 days in the dark. Clover sprout length dramatically decreased as salinity concentration was increased to 2000 ppm NaCl and increased for samples that had previously had ultrasonic treatment, indicating that the growth of clover sprouts was less negatively impacted by salinity. Aladjadjiyan (2012) found comparable findings with lentils, Fateh et al. (2012) with fennel, and Shekari (2015) with sesame.
Effect of salt stress on various physiological parameters
Fenugreek had a higher concentration of total soluble proteins and total soluble carbohydrate and the greatest value was reported at 5000 mg/L NaCl concentration as compared to the control value as shown from (Figs. 2, 3) This coincides with the findings of Tort and Turkyilmaz (2004), who found a significant increase in protein content in barley plants (Hordeum vulgare L.) after treating them with 120 mM sodium chloride. The findings of a recent study on Vigna mungo (L.) by Kapoor and Srivastava (2010) back up the earlier findings. When the salt concentration was increased, they saw a rise in protein content. Under salinity stress, amylase and protease enzymes become more active, which could explain this response.
Effect of ultrasonic exposure time (10 and 20 min) and salt stress on soluble proteins (mg g−1d.m) content of fenugreek at early seedlings stage. Values represent the mean of three replicates. Different letters (a, b, c, d, e, f, g, h, i and j) indicate statistical differences at 5% probability according to Duncan’s test. Error bars are standard errors of the mean, dm-dry matter (LSD least significant difference)
Effect of ultrasonic exposure time (10 and 20 min) and salt stress on soluble sugars (mg g−1d.m) content of fenugreek at early seedlings stage. Values represent the mean of three replicates. Different letters (a, b, c, d, e, f, g and h) indicate statistical differences at 5% probability according to Duncan’s test. Error bars are standard errors of the mean, dm-dry matter (LSD least significant difference)
As a process of osmoregulation under salt stress, an increase in cellular content of organic solutes, such as soluble carbohydrates and soluble proteins, reduces the cellular water potential. The increased activity of the amylase enzyme, according to Dhingra and Varghese (1986) was also responsible for the reduction in polysaccharide levels that was linked to a rise in total soluble sugars. Furthermore, Yasseen et al. (2018) speculated that soluble sugars in polysaccharide formation might be underutilized. Zayed and Zeid (1998) on mung bean and Santos and Andrade (2005) on sunflower both came up with similar results. Plants accumulate osmo-protective solutes like proline and soluble carbohydrates in response to salt stress (Chelli-Chaabouni et al. 2010). One of the most prevalent responses of plants to changes in the external osmotic potential is the formation of metabolites that act as compatible solutes (Munns and Tester 2008). Plants' defensive mechanisms against ROS-induced damage frequently entail the accumulation of soluble protein, which aids osmoregulation and cell turgor maintenance (Anjum et al. 2011). The soluble protein in fenugreek seedlings was significantly increased after treatment with ultrasonic waves when comparing untreated controls to 20 min of ultrasonic treatment, the greatest values for soluble protein and soluble carbohydrates of fenugreek seedlings were reported as showed from (Figs. 2, 3). Sound waves of particular intensities (100 dB) and frequencies (1000 Hz) raised the quantities of soluble sugar, protein, and amylase activity in Chrysanthemum, indicating that sound stimulation could boost the metabolism of roots and the growth of Chrysanthemum (Yi 2003). Sound waves of various frequencies and intensities have been found to change the secondary structure of tobacco cell wall proteins by altering Amide I and Amide II links (Ziwei et al. 1999). Sound at specific frequencies and intensities also increased the content of soluble proteins and carbohydrates in the cytoplasm of Dendranthema morifolium callus (Zhao et al. 2003). The ideal intensity and frequency sound field stimulation at the right strength and frequency significantly increased soluble protein content and chrysanthemum growth (Bochu et al. 2004; Yiyao et al. 2002; Yi et al. 2003).
Salt stress led to accumulation of proline while it was reduced by ultrasonication for short periods especially at exposure time 10 min (Fig. 4). In pistachios, proline accumulation under salinity stress can be used as a biochemical indicator of salt stress levels (Shamshiri and Fattahi 2014). Proline has been shown to have several functions during stress, including osmoregulation, osmo-protection, free radical scavenger and antioxidant, protection of macromolecules from denaturation, regulation of cytosolic acidity, carbon and nitrogen reserve after stress relief, reducing lipid membrane oxidation, and stabilization and protection of membranes, proteins, and enzymes from salt damage (Verbruggen and Hermans 2008). Proline accomplishes these goals by safeguarding the photosynthetic apparatus (Ashraf and Foolad 2007). Exhibiting antioxidant activity (Okuma et al. 2004) and acting as an oxygen radical scavenger (Heuer 2003). Proline accumulation is responsible for the hydration of biopolymers, allowing them to survive as a readily usable energy source and a nitrogen source during periods of slowed growth (Kala and Godara 2011). Many writers have obtained similar results, including Chen et al. (2006) on common bean, Ahmad et al. (2009); Nazarli et al. (2011) and Nounjan et al. (2012) on rice seedlings, and Rahneshan et al. (2018) on pistachio seedlings.
Effect of ultrasonic exposure time (10 and 20 min.) and salt stress on proline (mg g−1f.m) (milli gram/gram of fresh matter) content of fenugreek at early seedlings stage. Values represent the mean of three replicates. Different letters (a, b and c) indicate statistical differences at 5% probability according to Duncan’s test. Error bars are standard errors of the mean, dm-dry matter (LSD least significant difference)
The quantities of proline and soluble protein in the seeds of two rice cultivars, Guangyan1 and Huahang31, were significantly raised after ultrasonic wave treatment for both 10 and 20 min, according to Rao et al. (2018).
Molecular analysis
RAPD is a PCR-based molecular technique, simple and require only small quantities of DNA samples. In this study, the reproducible 4 RAPD primers were: OPA-05, OPA-12, OPA-13 and OPD-15. These primers gave a total number of 26 bands for fenugreek. The total polymorphism percentage was 49.72%. These primers' data in detail were illustrated in the (Table 2; Fig. 5).
The polymorphism percentage and variation in the propagated plants' genetic stability could be explained for many reasons. Such reasons are explained by Sundaram and Purwar (2011) and Mamatha et al. (2017) who applied RAPD-PCR to characterize the different fenugreek genotypes and accessions. Also, Hanafy and Akladious (2018) applied RAPD-PCR to estimate the effect of gamma radiation on fenugreek plant. In general, it is essential to confirm that the regenerants in genetic fidelity are genetically true-to-type of their donor plants. The RAPD marker system has been used for this target to know where there is an aberration in the regenerated plants. This scheme has been shown to be a potential marker for the distinction between genetic variation and genetic fidelity of different plants (Goda et al. 2017).
In this study, there were variation reflected in the morphological and physiological behavior of fenugreek plant. This could be due to variation in DNA content which is translated into altered proteins. This was confirmed by other studies as follow: it was claimed that certain sound waves could speed up plant growth and alter the transition temperature of cell walls, which was closely related to plant cell division. It was discovered that sound waves sped up (Xiujuan et al. 2003). At the molecular level, ultrasound can have positive or negative effects on the environment, enzymes, substrates, and interactions between them. Ultrasound produces the effects of cavitation, magnetostrictive force, and mechanical oscillation under the right frequency and low intensity conditions. To hasten the contact between the enzyme and substrate, it modifies the conformation of the enzyme. Enzyme biological activity is thereby encouraged (Wang et al. 2015b).
Conclusion
Osmotic and ionic effects on fenugreek seeds cause salinity to suppress germination and plant growth. At 5000 mg/l NaCl, the largest decrease in germination percentage was observed. At the early seedling stage, salinity stress also decreased all growth characteristics. As sodium chloride concentrations rose, levels of total soluble carbohydrates, total soluble protein, and proline rose sharply, indicating that this accumulation was necessary for osmotic correction as salinity levels rose. Fenugreek germination and seedling growth were increased and improved by ultrasonically treating seeds at 40 kHz for 10 and 20 min under control and salinity conditions. By increasing the seed's porosity, ultrasound increases its ability to absorb water and oxygen. It also speeds up metabolic processes, which helps the endosperm's ability to mobilize nutrients through the cell membrane. Due to modifications in the ultrasonic and salt stress treatments, the polymorphism percentage is high and could reach 49.72%. This demonstrates how sensitive plants are to changes in salt and ultrasonic.
Data availability
All data of this work are available in this paper.
References
Ahmad A, Rafatullah M, Sulaiman O, Ibrahim MH, Hashim R (2009) Scavenging behaviour of meranti sawdust in the removal of methylene blue from aqueous solution. J Hazard Mater 170:357–365
Aladjadjiyan A (2002) Study of the influence of magnetic field on some biological characteristics of Zea mays. J Central Euro Agric 3(2):89–94
Aladjadjiyan A (2007) The use of physical methods for plant growing stimulation in Bulgaria. J Cent Eur Agric 3:369–380
Aladjadjiyan A (2012) Physical factors for plant growth stimulation improve food quality. In: Aladjadjiyan A (ed) Food Production: Approaches, Challenges and Tasks, Intech Open 145–168
Aniat-ul-Haq RV, Agnihotri RK (2010) Effect of osmotic stress (PEG) on germination and seedling survival of lentil (Lens culinaris Medik.). Res. J. Agric. Sci 1(3):201–204
Anjum SA, Wang LC, Farooq M, Hussain M, Xue LL, Zou CM (2011) Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci 197(3):177–185. https://doi.org/10.1111/j.1439-037X.2010.00459.x
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59(2):206–216
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Begum F, Ahmed IM, Nessa A, Sultana W (2010) The effect of salinity on seed quality of wheat. J Bangladesh Agric Univ 8:19–22
Behairy RT, El-Hamamsy SMA, El-khamissi HAZ (2017) Alleviation of salinity stress on Fenugreek seedling growth using salicylic acid, citric acid and proline. Middle East J Agric 474–483
Bochu W, Jiping S, Biao L, Jie L, Chuanren D (2004) Soundwave stimulation triggers the content change of the endogenous hormone of the Chrysanthemum mature callus. Colloids Surf B Biointerfaces 37(3–4):107–112. https://doi.org/10.1016/j.colsurfb.2004.03.004
Carrillo-Lopez LM, Garcia-Galicia IA, Tiradogallegos JM (2021) Recent advances in the application of ultrasound in dairy products: effect on functional, physical, chemical, microbiological and sensory properties. J Ultrasonics Sonochemistry 73:105467
Chartzoulakis KS, Klapaki G (2000) Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci Hortic 86:247–260. https://doi.org/10.1016/S0304-4238(00)00151-5
Chauhan A, AtulKumar BA, Verma JS, Ghramh HA, AliKhan K, Ansari MJ (2016) Influence of gibberellic acid and different salt concentrations on germination percentage and physiological parameters of oat cultivars. Saudi J Biol Sci 26(6):1298–1304
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. https://doi.org/10.1093/aob/mcn125
Chelli-Chaabouni A, Ben Mosbah A, Maalej M, Gargouri K, GargouriBouzid R, Drira N (2010) In vitro salinity tolerance of two pistachio rootstocks: Pistacia vera L. and P. atlantica Desf. Environ Exp Bot 69:302–312
Chen JY, Wen PF, Kong WF, Pan QH, Zhan JC, Li JM, Wan SB, Huang WD (2006) Effect of salicylic acid on phenylpropanoids and phenylalanine ammonialyase in harvested grape berries. Postharvest Biol Technol 40:64–72
Cuartero J, Bolarin MC, Asins MJ, Moreno V (2006) Increasing salt tolerance in the tomato. J Exp Bot 57(5):1045–1058. https://doi.org/10.1093/jxb/erj102
Delachiave MEA, De Pinho SZ (2003) Germination of Senna occidentalis link: seed at different osmotic potential levels. Brazlian Arch Tech 46:163–166
Dhingra HR, Varghese TM (1986) Effect of NaCl salinity on the activities of amylase and invertase in Zea mays L. Pollen Ann Bot 57:101–104
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Fateh E, Sohrabi SS, Gerami F (2012) Evaluation the allelopathic effect of bindweed (Convolvulus arvensis L.) on germination and seedling growth of millet and basil. Adv Environ Biol 6(3):940–950
Florez M, Carbonell V, Martínez E (2007) Exposure of maize seeds to stationary magnetic fields: Effects of germination and early growth. Environ Exp Bot 59:68–75. https://doi.org/10.1016/j.envexpbot.2005.10.006
Goda SM, Ahmed SA, El Sherif F, Ibrahim HHA, AK, (2017) Genetically stable plants with boosted flavonoids content after in vitro regeneration of the endangered Capparis spinosa L. Global Drugs Ther 2(4):1–7
Goussous SJ, Samarah NH, Alqudah AM, Othman MO (2010) Enhancing seed germination of four crops species using an ultrasonic technique. Exp Agric 46:231–242
Hanafy RS, Akladious SA (2018) Physiological and molecular studies on the effect of gamma radiation in fenugreek (Trigonella foenum-graecum L.) plants. Journal of Genetic Eng Biotechnol 16(2):683–692. https://doi.org/10.1016/j.jgeb.2018.02.012
Heuer B (2003) Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Sci 165(4):693–699
Iqbal MZ, Rahmati K (1992) Tolerance of Albizia lebbeck to Cu and Fe application. Ekologia (CSFR) 11:427–430
Kala S, Godarara AK (2011) Effect of moisture stress on leaf total proteins, proline and free amino acid content in commercial cultivars of Ziziphus mauritiana. J Sci Res 55:65–69
Kapoor K, Srivastava A (2010) Assessment of Salinity Tolerance of Vigna mungo Var. Pu-19 using ex vitro and in vitro methods. Asian J Biotechnol 2:73–85
Kapoor N, Pande V (2015) Effect of salt stress on growth parameters, moisture content, relative water content and photosynthetic pigments of fenugreek variety RMt-1. J Plant Sci 10(6):210–221
Keshavarzi B, Rafsanjani MSO, Moussavinik M, Lak AP (2011) Effect of salt (NaCl) stress on germination and early seedling growth of Spinach (Spinacia oleracea). Ann Biol Res 2:490–497
Kökten K, Karaköy T, Bakoğlu A, Akçura M (2009) Determination of salinity tolerance of some lentil (Lens culinaris M.) varieties. J Food Agric Environ 81:140–143
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with Folin phenol reagent. J Biol Chem 193:25–275
Machikowa T, Kulrattanarak T, Wonprasaid S (2013) Effects of ultrasonic treatment on germination of synthetic sunflower seeds. Int J Agr Biol Eng 7(1):1–3
Mamatha NC, Tehlan SK, Srikanth M, Shivaprasad MK, Karthik RP (2017) Molecular characterization of fenugreek (Trigonella foenum-graecum L.) genotypes using rapd markers. Int J Curr Microbiol Appl Sci 6(6):2573–2581. https://doi.org/10.20546/ijcmas.2017.606.306
Moosavi SA, Siadat SA, Poshtdar A, Direkvand F (2018) Ultrasonic assisted seed priming to alleviate aging damages to milk thistle (Silybum marianum) Seeds. Not Sci Biol 10(2):275–281
Morsi MM, Abdelmigid HM, Aljoudi NGS (2018) Exogenous salicylic acid ameliorates the adverse effects of salt stress on antioxidant system in Rosmarinus officinalis L. Egypt J Bot 58:249–263. https://doi.org/10.21608/ejbo.2018.1772.1124
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Nazarli H, Faraji F, Zardashti MR (2011) Effect of drought stress and polymer on osmotic adjustment and photosynthetic pigments of sunflower. Cercetari Agronomice in Moldova 44(1):35–41. https://doi.org/10.2478/v10298-012-0022-9
Nounjan N, Nghia PT, Theerakulpisut P (2012) Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J Plant Physiol 169(6):596–604. https://doi.org/10.1016/j.jplph.2012.01.004
Oktem HA, Eyidogan F, Selcuk F, Silva JATD, Yucel M (2006) Osmotic stress tolerance in plants: transgenic strategies. Floricult Ornament Plant Biotechnol 194–208
Okuma E, Murakami Y, Shimoishi Y, Tada M, Murata Y (2004) Effects of exogenous application of proline and betaine on the growth of tobacco cultured cells under saline conditions. Soil Sci Plant Nutr 50(8):1301–1305. https://doi.org/10.1080/00380768.2004.10408608
Olfa B, Maha Z, Nada B, Zeineb OA (2018) Effects of Nacl on plant growth and antioxidant activities in Fenugreek (Trigonella foenum graecum L.). Biosci J Uberlândia 34(3):683–696
Pinheiro C, Chaves MM (2011) Photosynthesis and drought: can we make metabolic connections from available data? J Exp Bot 62:869–882. https://doi.org/10.1093/jxb/erq340
Qureshi MI, Israr M, Abdin MZ, Iqbal M (2005) Responses of Artemisia annua L. to lead and salt-induced oxidative stress. Environ Exp Bot 53:185–193
Rahneshan Z, Nasibi F, Lakehal A, Bellini C (2018) Unravelling salt stress responses in two pistachio (Pistacia vera L.) genotypes. Acta Physiologiae Plantarum 40:172. https://doi.org/10.1007/s11738-018-2745-1
Rao G, Ashraf U, Huang S, Cheng S, Abrar M, Mo Z, Pan S, Tang X (2018) Ultrasonic seed treatment improved physiological and yield traits of rice under lead toxicity. Environ Sci Pollut Res 25:33637–33644
Ratnakar A, Raib A (2013) Effect of sodium chloride salinity on seed germination and early seedling growth of Trigonella foenum-graecum L. Var PEB Octa J Environ Res 1:304–309
Saberali SF, Moradi M (2019) Effect of salinity on germination and seedling growth of Trigonella foenum-graecum, Dracocephalum moldavica, Satureja hortensis and Anethum graveolens. J Saudi Soc Agric Sci 18:316–323. https://doi.org/10.1016/j.jssas.2017.09.004
Santos C, Andrade S (2005) Comparison of carbohydrate metabolism in Na2SO4-and naturally-induced senescent sunflower leaves. Agrochimica Pisa 49(5):252–259
Shamshiri MH, Fattahi M (2014) Evaluation of two biochemical markers for salt stress in three pistachio rootstocks inoculated with arbuscular mycorrhiza (Glomus mosseae). J Stress Physiol Biochem 10(1):335–346
Shekari F, Mustafavi SH, Abbasi A (2015) Sonication of seeds increase germination performance of sesame under low temperature stress. Acta Agric Slovenica 105:203–212
Snedecor GW, Cochran WG (1980) Statistical methods, 6th edn. Iowa State University Press, Ames
Srivastava D, Rajiv J, Naidu MM, Puranaik J, Srinivas P (2012) Effect of fenugreek seed husk on the rheology and quality characteristics of muffins. Food Nutr Sci 3(11):1473–1479. https://doi.org/10.4236/fns.2012.311191
Sundaram S, Purwar S (2011) Assessment of genetic diversity among fenugreek (Trigonella foenum-graecum L.), using RAPD molecular markers. J Med Plants Res 5(9):1543–1548
Tahany AAA, Abdullateef TM, Lei Z, Xiaojie Y, Abu Elgasim AY, Haile M, Li C, Cunshan Z (2021) Interaction effects of salinity and ultrasound pretreatment on the phytochemical compounds of clover sprouts. Acta Sci Nutr Health 5(3):90–101. https://doi.org/10.31080/ASNH.2020.05.0838
Tort N, Turkyilmaz BA (2004) physiological investigation on the mechanisms of salinity tolerance in some barley culture forms. J.F.S 27:1–16
Umbreit WW, Burris RH, Stauffer JF, Cohen PP, Johanse WJ, Lee PGA, Potter VR, Schneider WC (1959) Manometric Techniques. Minnieap. Burgess Publ. Co. a manual description method, applicable to study of desiring metabolism. Burgess Publishing Company. c.f. Razak, A.A. 1979. 239
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Wang J, Wang J, Vanga SK, Raghavan V (2020) High-intensity ultrasound processing of kiwifruit juice: effects on the microstructure, pectin, carbohydrates and rheological properties. Food Chem 313:126121. https://doi.org/10.1016/j.foodchem.2019.126121
Wang S, Liu P, Chen D, Yin L, Li H, Deng X (2015a) Silicon enhanced salt tolerance by improving the root water uptake and decreasing the ion toxicity in cucumber. Front Plant Sci 17(6):759. https://doi.org/10.3389/fpls.2015.00759
Wang WW, Meng TT, Guo DZ, Hai-Le MA, Cao Y, Wang WX (2015b) Research progress on ultrasonic biological effect of food processing, Sci. Technol. Food Ind.
Wani SA, Kumar P (2016) Fenugreek enriched extruded product: optimization of ingredients using response surface methodology. Int Food Res J 23(1):18–25
Xiujuan W, Bochu W, Yi J, Chuanren D, Sakanishi A (2003) Effect of sound wave on the synthesis of nucleic acid and protein in chrysanthemum. Colloids Surf, B 29(2003):99–102
Yaldagard M, Mortazavi SA, Tabatabaie F (2008) Influence of ultrasonic stimulation on the germination of barley seed and its alpha-amylase activity. Afr J Biotech 7:2465–2471
Yasseen BT, Al-Thani RF, Alhadi FA, Abbas RAA (2018) Soluble sugars in plants under stress at the Arabian gulf region: possible roles of microorganisms. J Plant Biochem Physiol 6:224. https://doi.org/10.4172/2329-9029.1000224
Yi J, Bochu W, Xiujuan W, Daohong W, Chuanren D, Toyama Y, Sakanishi A (2003) Effect of sound wave on the metabolism of chrysanthemum roots. Colloids Surfaces b: Biointerfaces 29(2):115–118. https://doi.org/10.1016/S0927-7765(02)00155-8
Yiyao L, Wang B, Xuefeng L, Chuanren D, Sakanishi A (2002) Effects of sound field on the growth of Chrysanthemum callus. Colloid Surface B 24:321–326
Zayed MA, Zeid IM (1998) Effect of water and salt stresses on growth, chlorophyll, mineral ions and organic solutes contents, and enzymes activity in mung bean seedlings. Biol Plant 40:351–356
Zhang YQ, Fu EH, Liang JH (2008) Effect of ultrasonic waves on the saccharification processes of lignocellulose. Chem Eng Technol 31(10):1510–1515
Zhao H, Wu J, Zheng L, Zhu T, Xi B, Wang B, Cai S, Younian W (2003) Effect of sound stimulation on Dendranthema morifolium callus growth. Colloids Surf B Biointerfaces 29(2–3):143–147. https://doi.org/10.1016/S0927-7765(02)00184-4
Ziwei S, Keli S, Jun Y, Guoyuo C, Baoshu X (1999) The secondary structure changes of plant cell-wall proteins aroused by strong sound waves using FT-IR. Acta Photon Sin 28(7):600–602
Acknowledgements
The authors would like to thank Botany and Microbiology Department, Faculty of Science, Helwan University, Egypt for support with chemicals and instruments required for this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sattar, A.M.A., Tawfik, E. Effects of ultrasonic waves on seedling growth, biochemical constituents, genetic stability of fenugreek (Trigonella foenum-graecum) under salinity stress. Vegetos 36, 1427–1436 (2023). https://doi.org/10.1007/s42535-022-00545-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-022-00545-6