Abstract
Premature failure of armoured face conveyor (AFC) chains due to corrosion is a significant proportion of the unplanned downtime experienced on longwall equipment. The AFC chains are constantly in contact with water and wet coal. The premature failure issue of AFC chains has become more prominent since the introduction of longwall top-coal caving with its additional AFC. Reverse osmosis (RO) is a popular water treatment method for reducing salinity and dissolved solids, but its impact on the corrosion of the AFC chains is unclear. This study has been commissioned to investigate the direct effects of RO water on the corrosion of AFC chains. An immersion test was carried out using AFC chain steel submerged in two water samples: untreated dam water and treated water from an RO treatment plant. Elemental analysis was conducted for both water samples, and four corrosion indices were measured for both water samples. The RO water more vigorously dissolves calcium carbonate scales leading to increased corrosion of the AFC chains although the dam water has much higher levels of calcium, chloride, sulphate, sodium, and magnesium. Bicarbonate ions are the main alkaline factor of water that provides the buffering capacity to acids. Decreased alkalinity without balancing other ions in water causes high corrosivity and decreased scaling tendencies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Longwall mining has been one of the primary underground coal mining methods worldwide due to its high productivity [1]. The mechanised longwall mining system consists of three major parts, namely hydraulic shields, shearers, and an armoured face conveyor (AFC). All these parts are crucial and systematically linked; therefore, failure of one component leads to interruption to the operation of the entire system. Despite its high potential, substantial downtime of a longwall system has been a significant drawback; typical downtime of longwalls is around 60% of the calendar time in Australia [2]. A more recent study reported a slightly improved downtime figure of 40% for longwall mining systems at coal mines in Poland [3]. Apart from the planned downtime with extensive maintenance practices, a large portion of this downtime is attributed to unplanned downtime related to face equipment failures. Four major equipment failures have been reported to account for 30% of the total unscheduled equipment downtime [2], two of which are related to the premature failure of AFC chains. The premature failure of AFC chains has become a prominent issue since the introduction of longwall top-coal caving to cover thick coal seams with an additional AFC to the rear of the hydraulic system [4].
Overloading and corrosion of AFC chains have been known as the two major causes of premature failure of AFC chains. Several studies have tackled the overloading issues of AFC chains with the advancement of sensor and monitoring systems [5, 6], and simulation techniques [7, 8]. A wireless monitoring system was developed by Nicolay et al. [5] to track traction forces on the AFC chain links. Multiple minor causes for the premature failure of AFC chains were identified in this work, including unbalanced voltage inputs to the motor and uneven loadings on the AFC. In the work by Hua et al. [6], sensors using ultrawideband were attached to two ends of flight bars to monitor the angle of the flight bars (also known as scraper bars). If changes in this angle over the threshold are observed, the longwall system is paused and inspected for loose and damaged AFC chains to avoid unexpected failures. Multibody dynamic simulation with finite element analysis has been the most popular method to model the AFC system for characterising its dynamic behaviour. A set of AFC sprockets and chains were modelled and simulated in the work of Baiyan et al. [7], which proposed the optimised loading conditions. Vibration characteristics of AFC chains under different loading conditions were investigated by Jiang et al. [8] using multibody dynamics simulation. The optimum velocity of the chain speed with minimum vibrations and critical points within the chain assembly was suggested in their work.
On the contrary, not much work has been done to investigate the prevention of corrosion on AFC chains. At the same time, the environment of underground mines promotes highly corrosive conditions with high relative humidity, temperature, and airborne dust [9]. There have been several attempts to suppress corrosion on AFC chains using conventional methods, such as heat treatments, alloying, and hot-dip galvanising. High-strength steel used for AFC chains must have well-balanced mechanical properties with high toughness, resistance to wear and fatigue, and strength. It is a great challenge to increase resistance to corrosion of these materials without compromising other essential properties [10]. Hot-dip galvanising is also known to degrade the mechanical properties of high-strength steels by nullifying heat treatment during its galvanising process [11]. Furthermore, the highly abrasive conditions in which AFC chains are used lead to the galvanised coating on the chain surface being ineffective due to the peeling and flaking of the coating [12].
Water is one of the critical elements in longwall mining used for dust suppression [13]. Water is infused in the coal seam before shearing and sprayed while shearing and transporting coal. Therefore, the AFC and its chains are in direct contact with water and wet coal during the operation. Groundwater is another major factor in steel corrosion rates, with a high level of dissolved solids, biological species, and conductivity [9]. The characteristics and contaminants of groundwater are highly site-specific and widely varying in different regions [14]. Therefore, various types of water treatment have been developed to reduce corrosion and scale formations before use and meet environmental regulations before discharge. Among these different water treatment methods, reverse osmosis (RO) has been the most popular method, taking an 80% share in the total number of desalination treatment plants worldwide [15]. This trend is similar to the coal mining industry owing to RO being efficient in power consumption, desalination, and land use with the prefiltered process [16, 17]. However, the direct impacts of treated water concerning corrosion on metals have not been investigated thoroughly, while corrosion-related failures have been observed in the mining industry [18]. A coal mine in Australia has reported more corrosion damages on their mining equipment after using RO-treated water for over 5 years. Hence, this study has been commissioned to investigate the direct effects of RO-treated water on the corrosion rate of AFC chains used at this underground coal mine.
2 Experimental Method
An immersion test was carried out at 25 °C using steel samples cut from intact AFC chains submerged in two water samples: dam water and treated water from an RO treatment plant. The detailed procedures for the sample preparation and experimental set-up are described in this section.
2.1 Reverse Osmosis Plant and Water Sampling
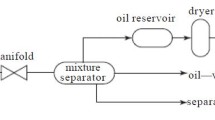
The water samples used for this investigation were prepared from a coal mine in the Bowen Basin region in Queensland, Australia. This coal mine site involves two underground operations with the longwall mining method and produces 16 million tonnes of metallurgical coal annually. The surface operations comprise the Coal Handling and Preparation Plant (CHPP) with associated coal handling and stockpile areas, as shown in Fig. 1. The predicted volume of pumped groundwater at this mine site between 2015 and 2018 is 267 ML. This coal mine procures its water from a nearby creek, and mine water is pumped from underground workings. Two types of water were sampled from a terminal dam and the RO water treatment plant, respectively (Fig. 1). An onsite RO plant in this coal mine consists of three stages for its water treatment. The first stage is pre-treatment using hollow-fibre ultrafiltration membranes to enable 6 ML/day permeate production, achieving a total dissolved substance (TDS) of less than 450 mg/L. The second stage is RO with DuPont FilmTec membranes under 22 bar operating pressure. The RO water is post-treated at the final stage with calcite filters and sodium hydroxide dosing to control the alkalinity and pH of the RO-treated water.
2.2 Steel Specimen Preparation
The AFC chain links used in this study were manufactured following a German standard, DIN 22252, with a nominal size of 48 × 144/160 in millimetres, weighing 48.2 kg per metre. The steel product used for AFC chains is alloyed to increase its abrasion resistance and wear life. This steel product’s detailed chemical composition and manufacturing process is commercial-in-confidence, while its base compositions and minimum mechanical properties are disclosed in Table 1.
Immersion testing specimens were prepared in accordance with ASTM G 01-03. All testing specimens were cut and milled to a size of 70 mm (length) × 40 mm (width) × 20 mm (height) from new AFC chain links. To ensure the precise and consistent dimensions of the specimens, the measurements at six locations of each sample were continuously monitored down to three decimal places during the milling process. Edges of the specimens were chamfered to prevent possible fractures from cold working. The oily residue from the milling process was removed and degreased using acetone to prepare for the immersion test. The cleaned and rinsed specimens were dried and stored in a desiccator until the ten immersion testing billets were ready. Five were submerged into each water sample. Each steel billet was weighed to two decimals places and tagged before the experiment, as presented in Table 2.
2.3 Immersion Test
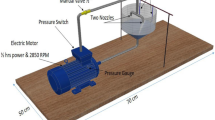
The prepared steel billets were placed on a specially fabricated support that provides small three contact points (<1 mm2) to maximise specimens’ exposure and contact with the sampled water. The steel specimens and the supports were placed in an aquarium tank (300 mm × 600 mm × 300 mm) equipped with a water-recirculating pump, immersion heating element, and removable carbon filter, as shown in Fig. 2. Two aquarium tanks were prepared for two water samples, which were poured slowly and simultaneously. The temperature on the heating element was set at 25 °C, continuously monitored by a digital temperature gauge every 6 h during the planned immersion test of 240 h.
2.4 Analysis of Water Samples and Corrosion Indices
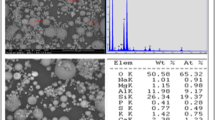
Elemental analysis was conducted for the water samples collected from the terminal dam and RO water treatment plant before the immersion test. Total dissolved solids and critical elements for corrosion indices, including calcium hardness as calcium carbonate (CaCO3), bicarbonate as CaCO3, chloride, and sulphate, were measured following ISO 11885:2007 using Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES). Water samples were first filtered through a membrane filter with a pore size of 0.45 μm to remove microorganisms. A concentric nebuliser was used to atomise each water sample which was then ionised by the heat from the argon plasma at approximately 8000 K. With the removal of the heat, the excited electrons returned to a lower energy level during which the wavelengths of the analytical lines were obtained using an optical spectrometer, thereby determining the concentration of dissolved solids.
A nephelometer measured the turbidity of the water samples, and their pH and conductivity were determined following ISO 10523:2008 and ISO 7888:1985, respectively. The ability of water to deposit CaCO3 is often used in connection with the corrosion control and scale formation of water in conjunction with various corrosion indices. These corrosion indices have been widely used to determine whether water inside pipes causes corrosion on the pipe wall or form a scale that can cause blockages. The elemental analysis results, pH, and conductivity measurements were used to determine the corrosivity of the water samples using corrosion indices listed in Table 3.
Indices using calcium carbonate (CaCO3) saturation have commonly been used and standardised to evaluate scale-forming and scale-dissolving tendencies of water in distribution systems [19]. The Langelier Saturation Index (LSI) has been one of the most popular corrosion indices to monitor surface water. The formation of a carbonate film is obstructed due to a lack of calcium and carbonate ions [20] by comparing the measured pH with the pH at calcite saturation (pHS). When no protective scale is formed, water is considered to be corrosive. Corrosive effects of water are expected if the LSI is less than −0.4 at 20 °C, equivalent to 0.0 at 70 °C. The Ryznar Stability Index (RSI) is another conventional but typical empirical method using the saturation of CaCO3 [21]. The RSI was developed to improve the LSI’s accuracy in predicting water scaling and corrosion tendencies by providing quantitative significance.
The Larson-Skold index (LS) was developed to investigate the corrosive effect of water on steel and cast iron by correlating the corrosive agents (chloride and sulphate ions) with inhibitive agents (carbonate and bicarbonate) [22]. Their experimental analysis confirmed that the corrosion rate of steel accelerated with the chloride/alkalinity ratios of 0.4 to 0.7 regardless of the concentration of free chlorine in the water. The Puckorius scaling index (PSI), also known as the practical scaling index, was developed to overcome the limitations of the LSI and RSI indices that do not consider modern chemical water treatment. The PSI accounts for the buffering capacity of water and the maximum quantity of precipitate [23]. These two parameters indicate a scale-forming tendency by incorporating the saturation state of water into the index. The PSI index was developed similarly to the RSI but used an equilibrium pH instead of the actual pH value.
2.5 Surface Micromorphology
The effect of two water samples on the surface micromorphology of the steel billets after immersion was explored using a scanning electron microscope (SEM) and atomic force microscope (AFM). The corrosion products of each steel specimen were cleaned after the immersion test. The specimens were dried in an oven at 40 °C for 24 h to remove residual moisture. Samples were then cut and milled on the opposite side of the observed surface to fit in the scopes. Low accelerating voltage SEM imaging was carried out using JEOL JSM-6360 LV, equipped with a single-tilt specimen holder (−10°~90°) that can rotate continuously. The most damaged portions of the specimens were observed using SEM with a magnification ranging from ×50 to ×550. Surface roughness was obtained for the parts observed by SEM using the AFM images to quantify the corrosion damages on each sample. Nanosurf FlexAFM integrated with an inverted microscope system was used to measure the depth of pitting corrosion.
3 Results and Discussion
The weight measurements of each steel sample after the immersion are shown in Table 2 along with reductions in percentage compared to those prior to the test. Corrosion is a deteriorating process, and it is a standardised method to measure the change in weight and exposed surface area to evaluate corrosion rates. However, the trend in the weight loss of the steel specimens is inconsistent regardless of the water type. All steel samples show a subtle change in weight of less than ±0.5%. The immersion period of 240 h is insufficient to create a significantly detrimental effect on the steel samples. Furthermore, the cleaning and removal process of the corrosion products after the immersion test for the surface morphology analysis and residual corrosion products have resulted in irregular weight changes.
In contrast, the surface morphology of the steel samples submerged in the two water samples demonstrates distinctive differences. Figure 3a depicts the intact steel specimen’s microscopic image, showing no visible damages other than the trace of machining with milling. The surface morphology of the steel sample immersed in the terminal dam water at ×50 and ×500 magnifications is shown in Fig. 3b, c, respectively. Sparse localised damages can be seen from the lower magnification image (b), and a cluster of shallow pitting corrosion is evidenced from the higher magnification image (c). Similar corrosion attacks can be seen from the steel immersion specimens from the RO water sample but with deeper and more extensive damages. The size of localised pitting corrosion shown in Fig. 3d is more significant than those in Fig. 3b at the same magnification with more residual corrosion products (black areas). The higher magnification image at ×500 of the steel taken from the RO water sample is shown in Fig. 3e, with more intensive and deeper corrosion damage compared to Fig. 3c. It should be noted that slightly lower beam energy was used to improve the quality and contrast of the image by removing noise caused by the remaining corrosion products.
To investigate and further visualise the intensity of the corrosion damage by observing surface roughness, an area of the steel surface from each water sample of 50 μm × 50 μm was observed using an AFM, as shown in Fig. 4. The SEM was implemented to provide qualitative analysis of corrosion damage whereas AFM focused more on quantitative analyses to provide the depth of the pits. The most damaged areas were focussed using a microscope attached to the AFM. Distinctive differences in surface roughness of the dam water and RO water samples cannot be viewed from the 2D AFM images shown in Fig. 4a, c, respectively. However, their 3D images with the deflection profiles measured by the AFM cantilever depict more severe corrosion damage on the steel surface immersed in the RO water sample, as shown in Fig. 4b, d. Apart from milling traces on both samples, the maximum depth of pits on the RO water sample is measured at 3.9 μm whereas that of the dam water sample is at 2.32 μm. The irregular surface finish observed by the AFM at the nanoscale caused by milling could contribute to non-uniform localised damage through pitting corrosion [24].
The partial cause of pitting and localised corrosion damage evidenced on the surface micromorphology analysis results is attributed to the breakdown or interference of naturally formed protective films and passive films on metals [25, 26]. The results of the elemental analysis of the two water samples and the corrosion indices introduced earlier are presented in Table 4 and further discussed to investigate the root cause of more aggressive corrosion damage of the RO water sample. The RO plant dropped the pH level of the dam water from 7.7 to the neutral level of 7 and successfully reduced the total dissolved solids from 526 to 224 mg/L. These reduced total dissolved solids include minerals that increase hardness, including calcium and magnesium, reducing more than two-thirds of the total hardness in the dam water. At the same time, the RO treatment decreased the concentration of chloride and sulphate, which are known to have adverse corrosive effects on steel in conjunction with their mass ratio [27, 28]. Significant removal of the total dissolved solids due to the RO process led to improved clarity of the water samples, its turbidity changing from 12.2 to 2.8 NTU, as shown in Figures 1(a) and (e).
A reduction in charged particles, including metallic and non-metallic ions, reduced electrical conductivity by more than 50% through the RO process. It is well known that there is a strong tendency for corrosion rates to increase with increasing water conductivity due to the electrolyte becoming more effective [29]. The reduced concentration of calcium and magnesium in the RO water sample, as shown in Table 4, led to a more than 70% reduction in the total hardness of the water. A decrease in the hardness of water from the reduced minerals is intended to suppress the deposition of scale in the water distribution system and equipment at mines. All parameters and concentrations from the water analysis ostensibly indicate that the RO process removed or reduced unfavourable substances that potentially caused scale and corrosion. However, these measurements do not closely agree with the surface topology of the steel samples observed earlier by SEM and AFM.
Therefore, the four most common corrosion indices were used to investigate the discrepancy between the elemental analysis results on the water samples and the observations at micro- and macroscopic levels. These micro- and macroscopic observations include the SEM and AFM images and the excessive corrosion product on the steel and water samples after the immersion test. As shown in Table 4, the corrosion indices of the dam and RO water samples indicated by the LSI were 0.26 and −1.13, respectively. While the RO treatment process neutralised the dam water by reducing the pH level to 7.0 from 7.7, the LSI indicates that RO water is expected to have higher corrosivity than untreated water. The RSI result suggests that the dam and RO water samples are corrosive. The RSI of the dam water barely exceeds the equilibrium state, and that of the RO water implies excessive corrosivity. The higher corrosivity of the RO water anticipated by the LSI and RSI is due to a significant difference between the measured pH and the pH at calcite saturation. These indices evaluate calcium carbonate precipitation, forming calcium carbonate films to suppress corrosion. The indices are a relatively simple equilibrium index to assess the thermodynamic driving force of the CaCO3 saturation only when specific redox reactions happen simultaneously.
The LS and PSI were then implemented to evaluate the corrosivity of the water samples using different parameters from the previous two, namely the LSI and RSI. The LS and PSI evaluation results show a similar trend to those discussed earlier, as shown in Table 4. Despite a significant reduction in chloride and sulphate ions through the RO process by 52.6% and 53.8%, respectively, the LS of the RO water is greater than that of the dam water sample with a further reduced concentration of bicarbonate ions by 63.3%. Although the LS of the RO water indicates that the contents of chloride and sulphate ions within the water are insufficient to inhibit the formation of natural films, the LS of the RO water is 28.5% greater than that of the dam water. The index of 0.66 is closer to the boundary of the following indication with an increased corrosion rate. The results of the PSI demonstrate a different effect to the LS but still indicate that the RO water is more corrosive. Based on the indices evaluated by the PSI, both water samples are likely to dissolve protective films as both indices exceed 7.0.
Nonetheless, the PSI of the dam water sample hardly crosses the borderline to be classified as corrosive. However, the PSI of the RO water is 9.13, which is significantly greater than the neutral 7.0. This large gap indicates that the RO water has a strong tendency to dissolve calcium carbonate scales, leading to increasing corrosion tendencies [23].
4 The Significance of Parameters in the Corrosion Indices
The corrosive scale indices of water discussed in the previous sections have various parameters, including pH, total dissolved solids, calcium, alkalinity, chloride, and sulphate. The influence and significance of these parameters are investigated in this section. These parameters affect each other, and other substances directly influence the pH level and total dissolved solids. For example, the pH scale is determined by the concentration of hydrogen ions that can be neutralised by alkalinity. Therefore, pH itself has been excluded from this investigation which has left three indicators, namely pHS, Larson-Skold Index, and Puckorius Scaling Index. Each axis represents an essential parameter or index in the 3D domain. Indices are presented with a colour corresponding to the bar shown on the right of each 3D scatter plot. The 3D scatter plots of each index are presented with two viewing angles to visualise the entire three-dimensional contour, as shown in Fig. 5.
3D scatter plots of pHS with azimuth and elevation angles at a −37.5° and 30° and at b 37.5° and −30° respectively, Larson-Skold Index with azimuth and elevation angles at c −37.5° and 30° and at d 37.5° and −30° respectively, and Puckorius Scaling Index with azimuth and elevation angles at e −37.5° and 30° and at f 37.5° and −30° respectively
LSI and RSI utilise the pH scale with pHS. pHS involves the total dissolved solids, water temperature, calcium ions, and bicarbonate alkalinity. The water samples for this study were sampled and tested at the same temperature. The effect of temperature on the corrosivity of water is not a variable in this study. The concentration of total dissolved solids demonstrates nil effect on pHS regardless of the other two variables, as shown in Fig. 5a, b. When the concentration of bicarbonate alkalinity is less than 60 mg/L, calcium is the critical factor in determining pHS. On the other hand, with a low calcium level (less than 25 mg/L), pHS will be calculated as excessively high, resulting in high corrosive tendencies indicated by LSI and RSI. It is evident that the concentrations of bicarbonate and calcium need to be maintained in inverse proportions if a neutral level of saturation pH is pursued.
As shown in Fig. 5c, d, all three variables in the Larson-Skold Index are highly influential. Sulphate and chloride ions known to cause corrosion [27] are the significant variables affecting the index. More than 50 mg/L of sulphate and chloride without alkalinity is expected to increase severe corrosion, as indicated by LS. This tendency decreases linearly with an increase in the total alkalinity level. The range of ideal indices set by LS is 0.75~0.8 since water with LS less than 0.75 is predicted to initiate the formation of scale on metal surfaces [22]. Therefore, water treatment based on the Larson-Skold Index requires a sophisticated process to control the concentration of all three parameters. The study by Nguyen et al. [28] observed that water samples with a ratio of bicarbonate ions to chloride and sulphate ions less than one showed high galvanic corrosion. They concluded that corrosion increased due to low alkalinity, causing a lower current-carrying capacity of bicarbonate ions than sulphate and chloride ions. The same trend has been reported in the experimental work of Lytle et al. [30]. Bicarbonate helped reduce iron release from cast iron, while the iron release was observed with excessive amounts of sulphate and chloride ions.
Similar to LSI and RSI, PSI utilises the pH at which water is saturated with CaCO3 (pHS). In contrast, an equilibrium pH is used in PSI instead of the actual pH level in LSI and RSI to evaluate water’s corrosion and scaling potential. The ideal PSI range to prevent scale formation and corrosion is between 6 and 7, as shown in Table 3. Again, the amount of the total dissolved solids in water does not influence the PSI. As shown in Fig. 5e, f, a good balance between calcium and total alkalinity concentrations is required. With a high concentration of calcium ions (>150 mg/L), the total alkalinity level needs to be maintained at 50~100 mg/L for the non-corrosive and non-scaling condition, which increases exponentially with calcium ions. Water with a low amount of calcium ions (<50 mg/L) requires an excessive amount of bicarbonate ions (>150 mg/L) to achieve higher alkalinity and to avoid adverse effects on steel. With relatively higher concentrations of calcium and bicarbonate ions (>100~175 mg/L and >100~200 mg/L, respectively), the water is expected to have a scaling tendency as estimated by PSI. Consequently, there is a higher corrosion tendency after the RO process has successfully reduced the concentrations of all contaminants. The RO process reduced more bicarbonate ions from 160.7 to 59.0 mg/L (−63.3%) and calcium ions from 31.5 to 16.0 mg/L (−49.2%). Bicarbonate ions are the main alkaline factor of water that provides the buffering capacity to acids. Decreased alkalinity without balancing other ions in water causes an increased corrosion tendency and decreased scaling [31, 32].
5 Conclusions
AFC chains are one of the critical components in a longwall system that have been reported to suffer from corrosion issues despite using treated water using RO. The elemental analysis of two water samples indicated the RO process successfully reduced contaminants, including corrosive substances such as chloride and sulphate. However, macroscopic and microscopic observations in this study using SEM and AFM on the steel samples from the immersion test demonstrated higher corrosivity of RO water than that of raw terminal dam water. Four well-received corrosion indices were implemented further to investigate the source of corrosive behaviour due to the RO process. Three of the four indices indicated a higher corrosion tendency of the RO water, except for LS, which predicted the likelihood of natural film formation for both water samples. According to the LS index, however, the LS of the RO water sample is higher than that of the raw water sample, which suggests the greater index of the RO water is closer to the next indication with increasing corrosivity.
This analytical investigation with the experimental results indicates that no one parameter nor acid neutralisation will minimise the corrosivity of water. RO treatment could accelerate corrosion if the balance of bicarbonate, chloride, calcium, and sulphate ions is not appropriately maintained at the same time. Several additional treatments can be considered; (a) corrosion inhibitors can be added to RO-treated water to slow down the rate of corrosion. Since the efficiency of a collodion inhibitor can be greatly affected by the chemical properties of water and physical conditions such as flow rate and temperature, it is important to investigate the properties and condition of water beforehand; (b) the process of inducing a chemical reaction to create a protective film layer or creating table anodic passivation layers can also effectively reduce the corrosion rate. However, since it is a site-specific issue as investigated in this study, compatibility with available water treatment systems must be carefully considered.
Data availability
All authors confirm that data and materials used to derive the conclusions of the manuscript are presented in the manuscript.
References
Zhao W, Qin C, Xiao Z, Chen W (2022) Characteristics and contributing factors of major coal bursts in longwall mines. Energy Sci Eng 10:1314–1327. https://doi.org/10.1002/ese3.1102
Guan Z, Gurgenci H (2004) Reliability improvement through smart longwalls project. CRCMining Conference, Noosa Head, Queensland, Australia
Stecula K, Tutak M (2017) Knowledge management and the costs of exploitations of machines. European scientific conference PEFnet. Brno, Czech Republic
Jangara H, Ozturk CA (2021) Longwall top coal caving design for thick coal seam in very poor strength surrounding strata. Int J Coal Sci Technol 8:641–658. https://doi.org/10.1007/s40789-020-00397-y
Nicolay T, Schmitt M, Blum A (2004) Telemetric chain link force measurement in coal mining armored face conveyors. IEEE SENSORS, Vienna, Austria
Hua Y, Zhu Z, Zhou G, Shen G (2022) Chain state monitoring for a heavy scraper conveyor using UWB-based extended kalman filter technique with range constraint selection method. IEEE Trans Instrum Meas 71:1–9. https://doi.org/10.1109/TIM.2021.3136254
Baiyan H, Guoping L, Hui S, Hang L (2009) Dynamic behaviour modelling and simulation of the chain transmission system for an armoured face conveyor. IEEE 10th International Conference on Computer-Aided Industrial Design & Conceptual Design, Wenzhou, China
Jiang S, Zhang X, Gao K, Gao J, Wang Q, Hidenori K (2017) Multi-body dynamics and vibration analysis of chain assembly in armoured face conveyor. Int J Simul Model 16:458–470. https://doi.org/10.2507/IJSIMM16(3)8.391
Wu S, Northover M, Craig P, Canbulat I, Hagan PC, Saydam S (2018) Environmental influence on mesh corrosion in underground coal mines. Int J Min, Reclam Environ 32:519–535. https://doi.org/10.1080/17480930.2017.1299604
Newton M (2010) An AFC chain management strategy extends life and prevents unplanned downtime. Mining Media International https://www.coalage.com/features/an-afc-chain-management-strategy-extends-life-and-prevents-unplanned-downtime/. Accessed 22 February 2023
Liu H, Li F, Shi W, Swaminathan S, He Y, Rohwerder M et al (2012) Challenges in hot-dip galvanizing of high strength dual phase steel: surface selective oxidation and mechanical property degradation. Surf Coat Technol 206:3428–3436. https://doi.org/10.1016/j.surfcoat.2012.02.001
Scott CL, Metcalfe RG (2019) Reliability of AFC chain through galvanizing. Solid State Phenom 295:29–34. https://doi.org/10.4028/www.scientific.net/SSP.295.29
Ji Y, Ren T, Wynne P, Wan Z, Ma Z, Wang Z (2016) A comparative study of dust control practices in Chinese and Australian longwall coal mines. Int J Min Sci Technol 26:199–208. https://doi.org/10.1016/j.ijmst.2015.12.004
Thiruvenkatachari R, Younes M, Su S (2011) Coal mine site investigation of wastewater quality in Australia. Desalin Water Treat 32:357–364. https://doi.org/10.5004/dwt.2011.2722
Fortunato L, Alshahri AH, Farinha ASF, Zakzouk I, Jeong S, Leiknes T (2020) Fouling investigation of a full-scale seawater reverse osmosis desalination (SWRO) plant on the Red Sea: membrane autopsy and pretreatment efficiency. Desalination 496:114536. https://doi.org/10.1016/j.desal.2020.114536
Jeong S, Naidu G, Vollprecht R, Leiknes T, Vigneswaran S (2016) In-depth analyses of organic matters in a full-scale seawater desalination plant and an autopsy of reverse osmosis membrane. Sep Purif Technol 162:171–179. https://doi.org/10.1016/j.seppur.2016.02.029
Qi J, Lv J, Bian W, Li J, Liu S (2021) Experimental study on the membrane distillation of highly mineralized mine water. Int J Coal Sci Technol 8:1025–1033. https://doi.org/10.1007/s40789-021-00432-6
Craig B (2020) Some corrosion and metallurgy issues in coal mines. Association for Materials Protection and Performance https://www.materialsperformance.com/articles/material-selection-design/2018/06/some-corrosion-and-metallurgy-issues-in-coal-mines. Accessed 23 November 2022
American Public Health Association (2018) 2330 Calcium carbonate saturation; standard methods for the examination of water and wastewater, 23rd. https://doi.org/10.2105/SMWW.2882.024
Langelier WF (1936) The analytical control of anti-corrosion water treatment. J Am Water Works Assoc 28:1500–1521. https://doi.org/10.1002/j.1551-8833.1936.tb13785.x
Ryznar JW (1944) A new index for determining amount of calcium carbonate scale formed by a water. J Am Water Works Assoc 36:472–483. https://doi.org/10.1002/j.1551-8833.1944.tb20016.x
Larson TE, Skold RV (1958) Laboratory studies relating mineral quality of water to corrosion of steel and cast iron. Corrosion 14:43–46. https://doi.org/10.5006/0010-9312-14.6.43
Puckorius PR, Brooke JM (1991) A new practical index for calcium carbonate scale prediction in cooling tower systems. Corrosion 47:280–284. https://doi.org/10.5006/1.3585256
Akpanyung KV, Loto RT (2019) Pitting corrosion evaluation: a review. J Phys: Conf Ser 1378:022088. https://doi.org/10.1088/1742-6596/1378/2/022088
Loto RT (2019) Comparative study of the pitting corrosion resistance, passivation behavior and metastable pitting activity of NO7718, NO7208 and 439L super alloys in chloride/sulphate media. J Mater Res Technol 8:623–629. https://doi.org/10.1016/j.jmrt.2018.05.012
Chen Z, Zhang G, Bobaru F (2016) The influence of passive film damage on pitting corrosion. J Electrochem Soc 163:C19. https://doi.org/10.1149/2.0521602jes
Li J, Chen J, Song J, Zhang X, Li Z, Luo H et al (2022) Research on corrosion behavior of truck body steel in chlorine-containing sulfuric acid environment. J Mater Res Technol 21:1878–1889. https://doi.org/10.1016/j.jmrt.2022.10.005
Nguyen CK, Clark BN, Stone KR, Ma Edwards (2011) Role of chloride, sulfate, and alkalinity on galvanic lead corrosion. J Sci Eng: Corrosion 67:065005-1–065005-9. https://doi.org/10.5006/1.3600449
Zakowski K, Narozny M, Szocinski M, Darowicki K (2014) Influence of water salinity on corrosion risk-the case of the southern Baltic Sea coast. Environ Monit Assess 186:4871–4879. https://doi.org/10.1007/s10661-014-3744-3
Lytle DA, Tang M, Francis AT, O’Donnell AJ, Newton JL (2020) The effect of chloride, sulfate and dissolved inorganic carbon on iron release from cast iron. Water Res 183:116037. https://doi.org/10.1016/j.watres.2020.116037
Macadam J, Parsons SA (2004) Calcium carbonate scale formation and control. Rev Environ Sci Bio/Technol 3:159–169. https://doi.org/10.1007/s11157-004-3849-1
Gryta M (2008) Alkaline scaling in the membrane distillation process. Desalination 228:128–134. https://doi.org/10.1016/j.desal.2007.10.004
Acknowledgements
The authors wish to express their sincere gratitude to Vicky Carrol and Tania Collins at Central Queensland Innovation and Research Precinct for their invaluable help with SEM and AFM images.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Conceptualisation: Jung-Hoon Sul and Benjamin Probyn; data collection: Benjamin Probyn; data analysis: Jung-Hoon Sul and Brendan Donnelley; writing: Jung-Hoon Sul, Benjamin Probyn, and Brendan Donnelly; review: Lasitha Piyathilaka and Sanghyun Jeong; revision: Lasitha Piyathilaka; editing: Sanghyun Jeong
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sul, JH., Probyn, B., Donnelly, B. et al. Investigating Effects of Reverse Osmosis-Treated Water on the Corrosion Rate of Chains in Armoured Face Conveyor for Longwall Mining. Mining, Metallurgy & Exploration 40, 2193–2204 (2023). https://doi.org/10.1007/s42461-023-00862-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-023-00862-7