Abstract
Preservation of perishable fruits and vegetables through edible coatings having functional roles, has gained importance now a days. The present study was designed to investigate the effect of water chestnut powder-based edible coatings on the quality and shelf life of apples (Kala kullu). Two treatments, T1 and T2, were developed by utilizing two different concentrations of heated water chestnut powder (2% and 2.5%, respectively). Whereas, 2% guar gum (T0+) was accustomed as positive control, and uncoated apples as negative control (T0−). Before developing emulsions, water chestnut powder was subjected to chemical, functional and structural analysis, after that emulsions developed were used to coat apples, which were investigated for physicochemical and sensory parameters, during 80 days storage at 20 °C. The findings indicated that emulsion capacity, emulsion stability, foaming capacity and viscosity of the coatings was significantly high (p ≤ 0.05) for T2, as compared to T1. During the storage of apples, significant (p ≤ 0.05) decrease in the weight loss and decay percentage was observed in T2. Increased pH and decrease in titratable acidity were also noted in T2. Coated apples (both T2 and T1) maintained the firmness of apples during storage duration, as compared to the uncoated ones. Significantly high (p ≤ 0.05) total phenolic contents and antioxidant capacity was shown by T2 as compared to other treatments, as during storage a comparatively lesser decrease in the values of these parameters was observed for T2. Sensory analysis results showed that T2 was given high scores for color, texture, taste, flavor and overall acceptability, as compared to other treatments. It was noted that T2 showed better outcomes in terms of sensory and physiochemical attributes, thus edible coatings having 2.5% water chestnut powder could be used to increase the shelf life of fruits.
Graphical abstract

Article Highlights
-
Water chestnut powder based edible coating could be an option for increasing shelf life of fruits.
-
Heating improved the functional (emulsifying, water and oil holding) properties of water chestnut powder.
-
Decreased weight loss and decay in apples, coated with 2.5% water chestnut powder emulsions was observed.
-
Coated apples were high in total phenolic contents and antioxidant capacity even during 80 days of storage.
-
Coated apples showed high sensory scores during storage duration, as compared to the uncoated ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Research on fresh food preservation technology has gained prominence in the realm of food quality and safety, as the consumers demand for fresh food has increased dramatically in recent years. Today, deterioration of perishable foods is a big problem for both fresh fruits and foods prepared from fresh fruits. The global food industry is focused on prolonging the shelf life of fruits, while maintaining their freshness and quality [1, 2].
Apples (Malus domestica) are most popular health promoting fruits in the world. Apple is a nutritious fruit, which is rich in many essential food components including proteins, carbohydrates and fats. It also provides soluble and insoluble fiber to the consumer along with energy [3]. They are rich in phytochemicals including triterpenes and phenolic compounds, which aids in decreasing the chances of various disease like cardiovascular diseases, asthma, diabetes and certain types of cancers. The bioactive compounds, which are present in apples prevents the body cells from oxidative damage that can be caused by free radicals, because of their antioxidant properties [4, 5]. However, high perishability, fast decay and lower shelf life of apples are main concern for their nutritive and bioactives loss during long-term storage.
An edible coating is made from edible material in the form of thin film, which helps to protect and preserve the coated food, and can be eaten along the food item. It can be applied on the surface of food by brushing, spraying or dipping to form a thin layer [6]. The main objective of edible coating is to enhance the food appearance, furthermore through its environment friendly nature it provides safety to the food. The material for coating can be obtained from both vegetable and animal sources [7, 8]. Edible coating improves the storage life and quality of fruit by minimizing the moisture and weight loss, while enhancing the barrier properties of fruits against gas, oil, aroma and flavor. The appearance, color, preservation of rheological and mechanical properties of fruit are also enhanced by edible coating [9].
Non-traditional food sources have drawn a lot of attention lately since they are utilized as useful ingredients in the creation of new foods and food compositions. Physiochemical characteristics of water chestnut (Trapa natans), such as mineral content, growth period, cultivar, grain size, environmental factors and amylase/ amylopectin ratio depends on the properties of its starch. Water chestnut starch is used in many food dishes to enhance the textural properties of foods, such as for stabilizing sauces and soups [10,11,12]. There is a unique property of water chestnut starch, that is when it is heated during canning or cooking, it achieves very dense and crunchy texture. This property discriminates it from other starches like potato and rice starch [8, 13] Water chestnut starch has been used to develop edible coatings for the fruits [14]. According to Mei et al. [15], water chestnut starch films have potential to increase the shelf life of fruits through their antimicrobial properties. Yadav et al. [16] claimed that heating water chestnut starches can modify them to make them appropriate for certain starch-based edible coating applications. Water chestnut is very beneficial for heart diseases, blood pressure, cancer prevention, weight loss and digestive health [12, 17] Whereas, the antioxidants present in water chestnut decreases the risk of developing cancer, because the antioxidants slow down the growth of cancerous cells. Water chestnuts are also low in calories due to low fat and high starch contents [18, 19]. Although water chestnut has lot of applications in food industry but its use in edible coatings is very limited, whereas functional properties of its powder are attractive enough to be utilized to coat fresh commodities. Some previous studies as mentioned earlier, were found to be using water chestnut starch to develop coatings, whereas, rare studies used heated water chestnut powder with fine particle size, to develop edible coatings. Therefore, this present study has been aimed to develop water chestnut powder-based edible coating to process and preserve apples, and to study the effect of these edible coatings on the physicochemical quality and shelf life of the apples. For this purpose, pretreated water chestnuts were dried to develop powder, which was heated and used in two different concentrations to develop coatings, which were further compared with guar gum coated and uncoated apples, during 80 days of storage.
2 Material and methods
2.1 Raw materials
Raw Indian water chestnuts were procured from the local market of Sargodha, Punjab, Pakistan. Fresh apples (Kala kullu) were procured from the local fruit and vegetable market of Sargodha, for coating purpose. The fully ripened apples of uniform color, size and shape were carefully chosen along with the stem, which were free from blemishes, cuts and bruises, and fungal and pathogenic infections. Manual sorting and grading of apples were done in the microbiology laboratory. Other raw materials like glycerol, guar gum, oil and plastic bottles were procured from the local super market, while all the chemicals were collected from Sigma Aldrich, Germany.
2.2 Preparation of water chestnut powder
Raw water chestnuts were first thoroughly washed with distilled water, and then were peeled manually. Then they were cut in to thin slices, and were dipped in solution made by using 0.5% of KMS and 1% citric acid, for 30 min to destroy any microbes present, and to avoid the browning during drying process. Then they were dried in a hot air oven (HAT120- Biobase, China) for 48 h at 90 °C. After that water chestnuts were grinded in to fine powder, sieved through U.S. Standard sieves (Endecotts, London, UK) to obtain powder with 80 µm particle size, which was finally stored in zip lock bags. The water chestnut powder was divided in to two portions and coded as W1 and W2, where W1 was kept without heating, while W2 was heated in an electric oven at 130 °C for 2 h to find the variations in the functional properties as discussed in the later sections. Throughout the preparation of powder, guidelines were taken from the earlier study of Singh et al. [20].
2.3 Proximate analysis of water chestnut powder
Proximate analysis of water chestnut powder was done according to the standard methods of Association of Official Analytical Chemistry (AOAC). For determination of moisture, ash, crude fiber, protein and fat content of water chestnut powder, their respective AOAC, [21] methods were followed. While, nitrogen free extract (NFE) was calculated through subtracting sum of other parameters from 100 as given below;
2.4 Structural analysis of water chestnut powder through FTIR
FTIR research was performed on the water chestnut powder to look for any potential functional groups in the 400–4000 cm−1 frequency range. The protocol used by Gorsi et al. [22] was modified in a few ways. In other words, 0.01 g of powder and 0.01 g of KBr anhydrous were homogenized using mortar and pestle. A transparent pellet was produced by pressing the sample and KBr combination at 1.2 psi using a vacuum hydraulic press (Graseby Specac). The scanned sample was subjected to an infrared beam, which was picked up by a computer-connected detector that gave a description of the sample's spectrum. Samples were typically scanned in the absorbance region between 400 and 4000 cm−1. The chemical structure, molecular binding form, and particular functional group of the samples under investigation were determined by the study and served as the basic building blocks of the spectrum type.
2.5 Functional analysis of water chestnut powder
2.5.1 Water holding capacity
Water holding capacities (WHC) of water chestnut powder without heating (W1) and with heating (W2), were determined by the method given by Hussain et al. [23], with a little modification. For water holding capacity, 1 g of water chestnut powder was taken in two separate beakers and 10 mL of distilled water was added in each beaker. One beaker was heated on hot plate along with stirring at 150 °C for almost 15 min. After that, the contents of both beakers were poured in already weighed centrifuge tubes and was vortexed for 10 min each. Then these tubes were centrifugated (Hettich Zentrifugen, ROTINA 380R, Germany) for 15 min at 1000 rpm. The excess water from the tube was discarded and the wet weight of leftover was measured along with the centrifuge tube. The WHC was calculated by the formula given below;
\({{Water\; holding\; capacity\; (\% ) = }}\frac{{weight\;of\;wet\;residue\;\left( g \right) - weight\;of\;dry\;residue\;(g)}}{{weight\;of\;dry\;residue\;(g)}} \times 100\)
2.5.2 Oil holding capacity
By following the practice used by Hussain et al. [23], oil holding capacity (OHC) of water chestnut powder, without heating (W1), and with heating (W2), was determined. Briefly, 1 g of water chestnut powder was taken in 2 separate beakers and 10 mL oil was added in each beaker. One beaker was subjected to heating on hot plate for almost 15 min at 150 °C, while stirring. Then these oil solutions were taken in test tubes and were vortexed for 10 min. Then centrifugation was done for 15 min at 1000 rpm speed. The oil holding capacity was measured by the formula given below;
\({{Oil}}\;{{holding}}\;{{capacity}}\;(\% ){{ = }}\frac{{\left[ {weight\;of\;content\;of\;tube\;after\;draining\;\left( g \right) - weight\;of\;dried\;powder\;(g)} \right]}}{{weight\;of\;dried\;powder}} \times 100\)
2.5.3 Emulsifying properties
Emulsion capacity (EC) and emulsion stability (ES) of water chestnut powder added emulsions were estimated by the procedure described by Huang et al. [24], with few modifications. Two different emulsions of water chestnut powder were made by using different concentrations of heated water chestnut powder, oil and glycerin. First of all, 2 g (T1) and 2.5 g (T2) of water chestnut powder was added in 100 mL distilled water separately, and heated at 150 °C for about 15 min. When the solution started to thicken, 2 mL glycerin and 3 mL oil was added in each and the solution was vortexed for 30 min to form emulsion. Emulsions were transferred into graduated tubes to measure the emulsion capacity.
2.5.4 Foaming capacity
Foaming capacity of water chestnut powder-based tow different emulsions (T1 and T2) was measured by the method of Akhter et al. [25]. Briefly, 2 g of water chestnut powder was added in 100 mL distilled water, and was mixed vigorously for 3 min, in food mixer at high speed. Volume of the mixture was noted before and after mixing. Foaming capacity was calculated using the given formula;
where, M1 = volume of solution before mixing and M2 = volume of solution after mixing.
2.5.5 Viscosity of emulsion
The viscosity of the developed emulsions was determined after the samples were conditioned at 4 °C. A rotator-type viscometer from Lamy Rheology Instruments was used to measure the samples' viscosity. Approximate viscosity of the material was measured, with some slight adjustments, using the process described by Siddique et al. [26]. The viscosity was measured using spindle number 5, and the speed of instrument was set to 50 rpm.
2.6 Development of edible coating
Edible coatings were made following the method of Thakur et al. [27], with few modifications. Water chestnut powder (2 and 2.5 g, separately) were taken and dissolved in distilled water separately. Then stirring was done by using a hot plate magnetic stirrer for 1 h at 40 °C and heating at 150 °C for 7 min for each solution to gelatinize the starch. After gelatinization, 2 mL glycerol and 3 mL oil were added in each treatment, and then was vortexed this solution for 30 min to obtain uniform solution. These emulsions were filled in bottles and stored at refrigerated temperature. Table 1 presents the treatment plan of edible coatings developed from water chestnut powder.
2.7 Application of edible coating
Kala kullu apples designated for experiments were first rinsed with distilled water and then dried with paper towel to remove any impurity from the surface of apples. To allow the smooth application of coating on the apples, the dipping method was used. The clean and dried apples were dipped in the emulsion for 30 s, twisted to remove any excess coating, placed in baskets and allowed to dry at room temperature (25 ± 5 °C). All the coated and uncoated apples were kept at room temperature for 80 days for further analysis.
2.8 Physiochemical analysis
2.8.1 Weight loss
As previously done by Zakriya et al. [28], the weight of each coated and untreated apple was determined using an electronic weighing balance at 20-day intervals throughout the course of 80 days. The weight loss percentage was then computed using the formula below;
2.8.2 Decay percentage
Out of each treatment, apples were taken to calculate the proportion of apples that had decayed. According to Bashir et al. [8], the percentage of fruits with visible microbial infection and rotten was used to determine the decay percentage. By using the below given formula, decay percentage was calculated.
2.8.3 Firmness
Using the procedures of Thakur et al. [29], a texture analyzer (TA-XT2., Stable Micro systems, UK) with a 35 mm diameter stainless steel cylindrical aluminium probe was used to test the firmness of whole apple fruit treatments throughout varied storage periods. The pre and post-test speeds of 5 mm/s, the compression degree of 3.0 g, and the trigger force of 10 s time were the operational parameters employed for the firmness measurement. From each group, precisely six fruits were chosen, and each fruit's measurements were recorded independently. While the coatings from two opposite sides of each coated apples were removed manually, to determine their firmness.
2.8.4 Total soluble solids (TSS)
TSS of different apple samples was determined by following the method recently used by Zakriya et al. [28], by using digital refractometer (RHB-32 ATC model, China), at room temperature by expressing the values in the form of brix. First, distilled water was used to complete the blank run. Following that, two to three drops of apple puree samples were added to the refractometer's brix reading container. The sample brix reading was then taken by clicking the "Start" button. The values were obtained and all computations were performed in triplicate.
2.8.5 pH
A digital pH metre (Model: Elico, LI 120, Hungary) was used to measure the pH of apples in accordance with AOAC [21] method 981.12. The pH metre rod was first cleaned with distilled water, dried with tissue paper and then submerged in a sample of apple puree for 30–40 s. To get the mean values, each calculation was run through three times.
2.8.6 Titratable acidity
Titratable acidity of apples was determined by following the standard method no 942.15 of AOAC [21], by diluting the apple juice sample and then titrating it against 0.1 N NaOH solution along with few drops of phenolphthalein indicator until it turns pink, and NaOH readings were noted. Titratable acidity was determined using the following formula, with each trial conducted three times and the results expressed as means;
Titratable acidity (%) = mL. of titrant used × N. of titrant × 0.064 / sample wt. × 100.
2.8.7 Antioxidant capacity
The method of measuring the antioxidant capacity of various apple treatments was adapted from Cheng et al. [30], with necessary modifications. To put it briefly, 20 mL of ethanol was combined with 5 mL of apple juice sample from each treatment in a flask. After shaking the combination in an orbital shaker (Biosan ES-20 Japan), at 200 rpm for a time period of 120 h at 25 °C, filter paper (Whatman No. 1) was used to filter the mixture. Next, 2.5 mL of DPPH was added to 0.5 mL of filtrate in a test tube, which was then left in the dark for 2 h at room temperature. At 517 nm, the UV absorption was measured using spectrophotometer (Hitachi U-2900 Japan). Trolox was used as standard. Following computations, antioxidant capacity was reported as µg trolox equivalent/g.
2.8.8 Total phenolic content (TPC)
Total phenolic content of apples was determined by following the technique used by Hussain et al. [31], through preparing the extracts from each apple treatment and then taking each 0.5 mL of prepared extract in test tubes. Then 1 mL of Folin-Ciocalteu reagent was added in it and left for 5 min. After 5 min, 2 mL of sodium carbonate reagent was disintegrated in each test tube and all test tubes were placed in incubator for 60 min at 30 °C. Then the samples were run through spectrophotometer (Hitachi U-2900 Japan) at 760 nm wavelength and readings were noted to calculate the TPC as µg GAE/g.
2.9 Sensory analysis
Using a nine-point hedonic scale, the sensory parameters scores of various apple treatments were ascertained using the methodology utilized by Rafique et al. [32]. First, the trained taste panel consisting of 40 members of both gender having an average age of 35 years, were briefed on the project in its entirety, after having their informed consent. The judges graded each apple preparation according to a nine-point hedonic scale: 1 was extremely poor, 2 was very poor, 3 was poor, 4 was below fair and above fair, 5 was fair, 6 was below fair and below poor, 7 was good, 8 was very good, and 9 was excellent. Under standard laboratory conditions, the sensory analyses were performed, while giving the treatments secret codes and providing the judges distilled water bottles for rinsing purposes.
2.10 Statistical analysis
Data gathered during the analyses of the coatings and coated apples were subjected to statistical analysis using complete randomized design (CRD) in order to ascertain the effects of various treatments, storage durations, and their interactions on the apples. Following the methodology described by Steel et al. [33], the data were also subjected to the Duncan's Multiple Range Test (DMRT) and analysis of variance (ANOVA) procedures, with p ≤ 0.05 level of significance, using the MSTAT-C software.
3 Results and discussion
3.1 Proximate analysis of water chestnut powder
Proximate analyses are done to determine the quantity of components in the food. These are done to calculate moisture, fat, protein, ash, NFE and fiber of the food. Data given in Table 2 indicates that the quantity of moisture in water chestnut powder was found to be 7.26%, fat 0.21%, protein 0.24%, fiber 0.26%, ash 1.72% and NFE 90.28%, respectively. Similar results were also reported by Kaur et al. [14], when the starch from water chestnut was extracted and proximate composition of the starch was determined. Results of another study from Kaur et al. [14] showed that water chestnut powder had the moisture content 7–8%, ash 0.04% and protein content 0.21–0.22%, respectively. In another similar study by Beigh et al. [34], the outcomes revealed similar results for proximate composition of water chestnut flour. The estimated percentage for moisture was 10.20%, fat 0.23% and ash 2.36%, respectively. Present findings were also in line with a more recent work carried out by Din et al. [10], as they witnessed water chestnut as good source of ash, fiber and carbohydrates.
3.2 Structural analysis of water chestnut powder
The functional and structural features of water chestnut powder are of great interest for the product development, but the qualities and suitability of this powder are left unexplored. The interaction of water chestnut starch by biochemical reactions with minor and major ingredients results in overall acceptability of the final product [35]. FTIR analysis of water chestnut powder was done for the characterization of chemical bonds confederate with structural arrangement of starch molecules, and outcomes have been presented in Fig. 1. To retrieve the swap in morphology of starch granules that is caused by preconditioning process, spectral quiver of different functional groups was used. As shown in (Fig. 1) the absorption peak of the sample was depicted in range of 3500–500 cm−1 as in starches. The peak at 3477.66 cm−1 represents the presence of bonded stretching of amines and amides. The peaks at 3263.56, 2924.097 and 1651.07 cm−1 in sample correspond to carving quivering of poly C-H, -OH and bending quivers of water bonds. Peaks 3151.69 and 3105.39 cm−1 denotes the alkynes in the sample. The absorbance peaks at 2347.37 and 2140.99 cm−1 corresponds to C–N and C–C bonds. The peak at 1535.34 cm−1 denotes H–CH, 1423.47 and 1334.74 cm−1 corresponds to C–H bending vibrations. The absorbance peak displayed at 1134.14 and 1012.63 cm−1 linked with stretching of C–O–H functional groups. The bands displayed at 925.83 and 854.47 cm−1 corresponds to vibrations of α1, 4 glycosidic bands in glucopyranosyl rings. The end 3 absorbance peaks 754.17, 642.30 and 569.00 cm−1 represent the presence of halogens in the sample [35]. These findings were also supported from the FTIR analysis performed by Gorsi et al. [22]. Present findings regarding structural analysis of water chestnut powder were also validated by Ahmed et al. [36], as they reported starches to be the main components having functional roles in water chestnut. Research has demonstrated that the functional, pasting, rheological, thermal, and structural properties of water chestnut flour are significantly influenced by the particle size. The process of size reduction increases the surface area of particles, which in turn affects their ability to hold on water and oil [36]. For this reason, while creating edible coatings, heated water chestnut powder was used rather than unheated.
3.3 Functional properties of water chestnut powder
3.3.1 Water and oil holding capacities
WHC indicates the quantity of water, the powder can absorb. Similarly, OHC is that how much amount of oil a powder can hold. WHC depends on the pore size of the gum and charges on protein molecule whereas OHC of gums largely depends on the structure of gums including both chemical and conformational structure [23]. The absorption of moisture in polysaccharides also depends on the quantity of residual proteins and hydroxyl groups. If any gum or powder has WHC, it means this powder is able to form emulsion or gel. While, modification of starch with heating increases the WHC and OHC of the flours [37]. WHC of water chestnut powder was improved by heating, as have been shown in Table 3, WHC and OHC of water chestnut sample (W1) was found to be 2.26% and 0.98% without heating, and 4.41% and 1.05% with heating (W2). It indicates that water chestnut powder could form better emulsion and has high water and oil holding capacity when heated. In a similar study, it was observed that the WHC of water chestnut starch increases on heating it [14]. According to Ahmed et al. [36], size of particles of water chestnut powder plays important role in changing the WHC and OHC, whereas in current work heated water chestnut powder with fine particle size was used to develop coatings, which presented high WHC and OHC as compared to the unheated powder. Increased surface area of particles due to the reduced size as a result of fine grinding also helps in increasing the WHC and OHC of the water chestnut powders. Water chestnut flour with a particle size of 88 µm showed higher WHC and OHC as compared to the flour with a particle size 74 µm [36], as in current experiments fine powder with 80 µm particle size was used to develop coatings. Similarly, Yadav et al. [16] have also confirmed that heating positively modifies the starch of water chestnut, enhancing its functional properties to develop edible films.
3.3.2 Emulsifying properties
Emulsion capacity is the ability of the gum to form emulsion, while the emulsion stability is their ability to keep the emulsion stable without any separation. Generally, emulsions made from more quantity of gums are considered more stable [38]. Table 4 indicates that both the emulsions (T1 and T2) have significantly different emulsion capacities (98.20%, 98.36%), similarly, the emulsion made from 2.5 g of water chestnut powder (T2) has more emulsion stability (97.05%) than the emulsion made from 2 g (T1) of water chestnut powder (95.87%). The reason behind this increase in emulsion capacity and emulsion stability of T2 was high amount of water chestnut powder, providing more starch to develop and stabilize emulsions. These results are supported by the outcomes recorded by Ainee et al. [9], where it was noted that emulsifying capacity of emulsions was enhanced by the increase in concentration of gum (galactomannan). Different concentrations of galactomannan (0.5%, 0.75% and 1.0%) were used to make the emulsion and it was noted that by increasing the galactomannan quantity up to 1%, an increase in the emulsifying property of the coating was found. Similar outcomes were revealed by Rashid et al. [39], when the emulsion stability of edible coating made by flax seeds and fenugreek seeds was found about 90%. Supportive results for high emulsifying properties of fine sized water chestnut powders have also been reported by Ahmed and Al-Attar [40] and Ahmed et al. [36], validating the current results, as the moderately heated water chestnut powders with fine particle size have ability to absorb and retain more water, thus forming good emulsions.
3.3.3 Foaming capacity
Foaming capacity is the ability of gum molecules to reduce surface tension of air and water interface to form foam. Formation of foams from gums results from the whipping of gums on high speed, so it develops a stable network, which carries and holds tiny particles of gum to form foam [38]. As the Table 4 indicates, T2 have higher foaming capacity (20.88%) than T1 (20.54%). The concentration of water chestnut powder was higher in T2, which resulted in higher foaming capacity of T2 than T1. Similar outcomes were also discovered by Hussain et al. [35], when in their study results the foaming capacity of water chestnut powder was estimated in the range of 21–23.15%. It was also revealed that the foaming capacity of commercially processed flour was less because of long dry heating period during the flour formation, as because most proteins are denatured due to high heating [35]. Different drying conditions alter both the functional and rheological behaviors of water chestnut powder, but they have no effect on its physicochemical qualities, according to Ahmed et al. [36]. On the other hand, powder with small particles and fine heating can be a preferable choice for use in various coatings. These results were highly relevant to the current ones. Foaming capacity is an important property when films are in contact with water during processing and storage. The degree of cross-linking and the hygroscopicity of the starch components used in the film-forming process have a major influence on the foaming capacity. The emulsions' ability to foam may also increase as a result of increasing the amount of starch in the films [15].
3.3.4 Viscosity
Viscosity of any emulsion is indicated as the thickness of emulsion. It is dependent on the temperature and shear rate, as the temperature and share rate increase the viscosity of emulsion decreases [38]. Table 4 shows that T2 (12.20 mPa.s) has more viscosity than T1 (11.24 mPa.s). As the concentration of water chestnut powder was higher in T2 (2.5%), the viscosity was also higher and vice versa. These results were in strong relevance with those provided by Mir et al. [41], as they developed water chestnut powder to use in gluten free cakes. According to Ansari et al. [42], starches present in water chestnut powder are responsible for viscosity of the emulsions, due to their various functional and rheological features. Similarly, Mei et al. [15] has reported that viscosity of water chestnut based edible coatings is highly affected by the quantity, nature and processing of starch, as in the present work heated powder of water chestnut has been tested at two different concentrations, and emulsions having high amount (2.5%) of water chestnut powder presented higher value of viscosity as compared to that having low amount (2%) of water chestnut powder. Present findings were also confirmed by Gul et al. [37], when heating and increasing the amount of water chestnut starch resulted in high viscosity of emulsions.
3.4 Physiochemical properties of the coated apples
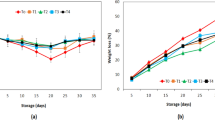
3.4.1 Weight loss
Respiration and transpiration are the main reason that causes the weight loss in fruits and vegetables. Transpiration involves the loss of moisture or water vapors from the fruit’s surface. As the time passes, weight loss of fruits gradually increases by loss of moisture from the fruit. Many undesirable changes take place due to weight loss during the storage life, and quality of the fruit changes. Weight loss increases the shrinkage of fruit and develop impaired flavor along with unfavorable textural changes in the fruit [28, 29]. The main function of edible coating is that it reduces the rate of transpiration and respiration by acting as a barrier between the fruit and surrounding, so helps to reduce the weight loss of fruits. As shown in Tables 5 and 6, treatment and storage both have highly significant effect on the weight loss of apples. Weight loss range of apples was (27.9–37.52%). The least weight loss was observed in T2 (27.9%) while, highest weight loss was noted in T0- (37.92%). Similar results were observed by Nawab et al. [43], as their findings showed that during the storage, the weight of tomatoes was decreased for all samples, except for treated ones. Polysaccharide based edible coating made from wheat straw and oats bran for apples by Ali et al. [44] also provided similar results, as it reduced the respiration rate and weight loss of apples. Present findings were also strongly validated from a recent work by Bashir et al. [8], when influence of water-chestnut starch-based coatings on weight loss and decay of apples was studied, with very much positive indications.
3.4.2 Decay percentage
The spoilage and decay of fruits and vegetables often occurs by light, temperature, microbial attack and moisture. Blemishes, patches of mold and slime, loss of color, bruises, undesirable odor and taste are mostly result from decay and spoilage of the fruits. Amidst all, loss of moisture is the main cause of decay of the fruits [29]. As shown in Tables 5 and 6, treatment and storage both have highly significant effect on the decay percentage of the apples. It was exhibited that decay percentage of apples was (5.98% to 10.03%). The least decay was observed in T2 (5.98%). On the other hand, highest spoilage was noted in T0- (10.03%). Current results were also witnessed by Bashir et al. [8], when water chestnut based edible coatings were applied on apples to reduce the decaying of the fruits during the storage. Our results were also supported by the outcomes recorded by Mehyar et al. [45], as they discovered that polysaccharide based edible coatings reduces the spoilage in fruits. Potassium sorbate was added in various proportions in the polysaccharide-based guar gum coatings, which were used to coat apples and tomatoes. The results showed that the coating reduced the spoilage of apples and tomatoes, thus prevented them from decaying. These edible coatings showed good results for apples than that of tomatoes [45]. In another study on papaya fruits, it was observed that aloe vera gel based edible coating was effective against spoilage of fruits. The papaya fruits coated with aloe vera gel coating had minimum disease incidence than uncoated [46].
3.4.3 Firmness
Firmness of fruit is considered as initial measure of texture of the fruit. Firmness also determines the eating quality of the fruit. Firmer fruits are preferred over soft ones because they are considered better in quality. During the storage, firmness of the food decreases as the freshness decreases because of moisture loss from the fruit. Table 6 displayed that the range of force required to check the firmness of apples was 5.82–6.05 kg/cm2. The least level of force to determine the firmness was recorded in T0- On the other hand, the higher values of forces to determine the firmness level were recorded T0+. While both T1 and T2 showed good results to retain the firmness of the fruits during the storage duration, as the values of forces on these apples were very comparable to positive control, and comparatively higher to that of shown by negative control. According to Thakur et al. [29] the firmness of fresh and stored fruits, and coated and uncoated fruits, varies significantly, as the during the storage duration the firmness decreases, while the edible coatings maintain the firmness of the fruits during the storage. Tragacanth gum used as edible coating showed similar outcomes. The firmness of apples also remained preserved, and softening of apples was also delayed by the use of tragacanth based edible coating by Jahanshahi et al. [47]. Dietary fiber was incorporated in the edible coating to produce polysaccharide-based coating on apples by Moreira et al. [48], while their results indicated the preservation of firmness and color, after using the edible coating. Retention of firmness of apples coated with water chestnut based edible coatings were also reported by Bashir et al. [8], validating the present findings.
3.4.4 Total soluble solids
Total soluble solids are the quantity of total solids that are present in a solution. TSS is measured by index of refraction by using refractometer and is calculated as brix [28]. Brix is referred as level of sucrose sugar present in the solution. Measurement of TSS aids in the estimation of sugars and ripening stage of the fruits. As shown in Tables 5 and 6, the treatments along with storage period have a significant effect on the TSS concentration of the apples. The range of mean values of TSS in the apples was 10.45–10.99° brix (Table 6). The minimum value for TSS was observed in T2 (10.45° brix) followed by T1 (10.53° brix). While, the maximum value of TSS was observed in T0− (10.99° brix). According to Thakur et al. [29] the loss of moisture contents due to respiration, during the storage periods results in increment in the TSS of the fruits. Whereas, the edible coatings help in controlling this moisture loss thus maintain the TSS of the fruits. Similar results were observed in a study by Hazrati et al. [49], as during the storage period of peach fruit, its soluble solid concentration was maintained. They were coated with aloe vera gel coating and kept for storage for 30 days. After 30 days, the value of TSS was observed, and it was lower than uncoated peach fruits. In a similar study on mangoes, it was discovered that chitosan based edible coatings maintained the TSS content. Ripening of fruit during storage period causes increase in the TSS content; it was found that uncoated fruits had higher TSS content than coated treatments [50].
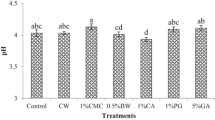
3.4.5 pH
During the ripening stage, different organic acids like citric and malic acids are produced in the fruits, and these produced acids aids in providing various features to the fruit including flavor and taste. Moreover, juice quality and color of fruit is also enhanced by these produced acids [51]. pH, which mainly depends upon these acids, also contributes to the freshness and shelf life of the fruits, as a low pH results in less microbial infestation of fruits and their juices. As have been shown in Tables 5 and 6, the least level of pH was recorded in T2 (3.91), on the other hand, the highest pH level was noted in T0− (4.08). The reason behind this was that coatings with high amount of water chestnut powder resulted in maintaining the low pH of the fruits through controlling and moderating the ripening and decaying process, while in uncoated apples this pH was increased. Similar results were also discovered by Naeini et al. [52], when tragacanth based edible coating was tested on pomegranate fruit. The outcomes showed that tragacanth-based coating maintained the acidity and pH of pomegranate fruits. A study on strawberry by Gol et al. [53] also showed similar outcomes, when it was observed that edible coating made by CMC, HPMC and chitosan displayed a delay in the change of pH in the strawberries during the storage. Chitosan based edible coatings on strawberries displayed positive effect on pH [53]. During the storage of fruits and vegetables without any appropriate conditions or treatments decaying leads toward production of certain acids, which cause a reduction in pH of the commodity [28], while coatings used in the current work might have moderated this production of acids by slowing down the decaying as discussed earlier.
3.4.6 Titratable acidity
Titratable acidity is actually the quantity of acids in the food. For the estimation of total acid concentration in the food, titration of an acid with a standard base is done. This method is more accurate than pH to measure the effect of acids on the fruit flavor. In general, the concentration of acidity is usually higher during the harvesting, and the value decreases during the ripening process, because of the reduction in the value of organic acids due to their utilization as substrate [28, 51]. Table 5 displays that range of titratable acidity of apples was 0.18–0.25. The minimum value was noted in T0− (0.18%). On the other hand, highest value was recorded in T2 (0.25%), followed by T1 (0.24%) and T0+ (0.23%), respectively. According to Thakur et al. [29], the reason behind the cessation of acid breakdown in coated apple fruits during storage could be the result of their higher titratable acidity, which is a result of the decreased utilization of acids in the metabolic process. Similar results were discovered by Naeini et al. [52], when tragacanth based edible coating had substantial influence on the titratable acidity content of pomegranate fruit. The outcomes showed that tragacanth-based coating maintained the acidity of pomegranate. A study by Misir et al. [46] also showed same outcomes, as aloe vera led to delay in increase of acidity in the coated berries. It was also discovered that aloe vera gel-based coating coated oranges had more titratable acidity content than uncoated fruits. A decrease in pH results in an increase in the titratable acidity of the fruits, while coated fruits maintain their pH and acidity for longer periods of times due to the controlled biochemical reactions under controlled atmospheric conditions [28].
3.4.7 Antioxidant capacity
Antioxidants are compounds that aids in delaying and preventing some types of cell damage. Antioxidants are in abundant quantity in the fruits and vegetables [18, 31] Antioxidants lower the danger of diseases by scavenging the free radicals, as the oxidative stress is caused by these free radicals. Total antioxidant capacity can also be defined by measuring the quantity of free radicals that are scavenged by the solution [19]. Tables 5 and 6 shows that treatment as well as storage has a highly significant effect on the antioxidant capacity of the apples. The minimum value was recorded in T0- (217.26 µg trolox equivalent/g), while the highest value was recorded in T2 (305.48 µg trolox equivalent/g). Similar results were also discovered by Abid et al. [54]. In that study, ultrasound technique was used to prevent the quality loss of the apples. It was investigated that the antioxidant content of apples was found in the range of 299.64–363.05 µg trolox equivalent/g. With the passage of time during the storage, the antioxidant capacity of the apples was found to be decreased, which was comparable with the outcomes discovered by Jafari et al. [55], as their findings showed that antioxidant content of apples reduces during the storage. The reduction in ascorbic acid causes retardation of antioxidant activity of the apples. Also, the phenolic compounds and flavonoids are the major reasons of antioxidant capacity of fruits, which are lost from the fruits during the storage, under uncontrolled conditions [23]. The decline of antioxidant activity in fruits during storage could be related to the decrease in the bioactive compounds during the storage [56]. Present findings were strongly corelated with a recent work of Bashir et al. [8], aiming to preserve the antioxidant capacity of apples coated with water chestnut starch based edible coatings, as the coated apples presented significantly high values of antioxidant activities as compared to the uncoated ones, even during the storage duration. Current results were also in line with the findings of Thakur et al. [29], when the coated apples exhibited higher values of antioxidant activities as compared to the uncoated apples.
3.4.8 Total phenolic content
Essential plant components that supervise the antioxidant activity are termed as total polyphenols. In foods, phenolic content is present as polymeric form of secondary metabolites. They facilitate the scavenging of free radicals in plants [31]. Table 6 displayed that the range of total phenolic content of in four different treatments of apples was from 427.81 to 546.13 µg GAE/g. The least value of total phenolic content was noted in T0- (427.81 µg GAE/g). While the highest value was recorded in T2 (546.13 µg GAE/g). Whereas, both T1 and T0+ also presented significantly high amounts of total phenolic content as compared to the negative control (T0-), which showed significantly low values of total phenolic content. Similar outcomes were also recorded by Abid et al. [54], when ultrasound treatment was used on the apples to determine its effect on the total phenolic contents of the apples. The range of phenolic content in apple was observed from 757.49 to 829.32 µg, as the ultrasound treatment raised total phenolic content. Similarly, the initial range of phenols in our study was estimated as 756.56–758.66 µg GAE/g (Table 5). The reduction of total phenols during storage is associated with the oxidation of different bioactive components through polyphenol oxidase and peroxidase, while edible coatings prevent such oxidation reactions [52]. In a similar study, with in line findings, Bashir et al. [8] also reported the preservation of phenolic contents and other bioactives (flavonoids, ascorbic acid and anthocyanins) of apples coated water chestnut-based materials, during the storage. Present findings were also supported from the results of Thakur et al. [29], when total phenolic contents of coated apples were maximally retained during the storage as compared to the uncoated apples.
3.5 Sensory analysis of apples
3.5.1 Color
Color is the most important sensory property in any food element. Color provides primary impression of the freshness, quality and the flavor of the food. Color determines the acceptability, provides perception about taste and makes the food to look more appealing [12]. As displayed in the Table 8, the score range of color in apples was 6.73–7.73. The maximum value was observed in T2 (7.73) and the minimum score for color was observed in T0- (6.73). Whereas the results presented in Table 7 showed that color scores of uncoated apples were highly decreased during the storage, as compared to the coated ones, while the T2 got comparatively high color scores even during the storage interval inspections. A study of starch based edible coating on apples by Thakur et al. [29] also showed the similar results, as the starch-based coating caused delay in color change and a notable reduction in the fruit greasiness in the coated fruits. Similar outcomes were also reported by Farina et al. [57], where Fuji apples were coated with aloe vera gel along with some additives. Treatments made of different concentrations of aloe vera gel slowed down the browning processes, maintaining color during the storage, while also reduced the postharvest losses. In another similar study on apples, having application of polysaccharide-based coating made from seeds (fenugreek and flax seeds), the outcomes showed that coatings improved color of apples and extended their shelf life [3]. Similar findings were also reported when dietary fiber was incorporated in edible coating to produce polysaccharide-based coating on apples by Moreira et al. [48], and results indicated the retention of firmness and color during the storage, after using this edible coating.
3.5.2 Texture
Texture relates to the characteristics and surface appearance of the food. Texture is the fundamental feature that includes the sensory quality of the food. Texture is used as a quality parameter to determine the physical properties of the food. Texture is generally described as smooth or rough, coarse or fine, and soft or hard [58]. As the overall means of texture scores have been shown in Table 8, the maximum score of texture of apples was observed for T2 (7.66). On the opposite side, the minimum score for texture was observed in T0- (6.62). Similarly, the results presented in Table 7, showed that treatments and storage days have significant effect on the texture scores of the coated and uncoated apples. As the storage duration was increased the texture scores were decreased more prominently in uncoated samples (T0-), while T2 still showed good texture scores. Ghavidel et al. [59] used a protein based edible coating made from whey protein concentrate and soy protein isolate for coating of fruits. Outcomes showed that protein-based formulation improves the shelf life of apples along with maintaining the quality of apples by retention of color and texture. Present findings were also supported from the recent experiments of Naqash et al. [60], as apples coated with pectin maintained their texture stability for longer as compared to the uncoated ones. Coatings usually preserve the moisture contents of the fruits and also slow down the decay process, thus keeping fruits fresh with a firm texture, for longer period of time.
3.5.3 Taste
Taste is sensed when the taste receptors receive the taste stimuli. Taste is stimulated by taste buds present on tongue and in oral cavity. Basic tastes include sweet, sour, salty, bitter and umami. Table 7 showed that there is a highly significant effect of storage and treatment on the taste of apples. Taste score of apples was in the range from 6.56 to 7.69. The minimum value was noted in T0- (6.56), while the highest value was recorded in T2 (7.69) (Table 8) According to Thakur et al. [29], taste of apples is due to the sugars and organic acids, while as the storage period is increased due to the degradation of these sugars and organic acids the taste scores of the apples are decreased. A similar previous study has shown that chitosan based edible coating on strawberry exhibited the similar outcomes. It was observed that the edible coatings preserved the characteristic taste and flavor of strawberry fruits during the storage [61]. In a similar study, cucumbers were coated with herbal coating and they displayed the greater retention of taste and color than uncoated cucumbers [62]. Providing in line results with the present findings, Naqash et al. [60] found that taste of apples could be preserved during storage, through emulsions based edible coatings.
3.5.4 Flavor
Flavor is the sensory perception that includes both the chemical senses of taste and smell. Flavor aids in selection, acceptance and ingestion of food material. By keeping the taste good, but changing the smell of the food, the flavor of food can be changed. Table 8 displayed that the flavor scores of apples were in the range of 6.54–7.69. The minimum value was noted in T0− (6.54), while on the other hand, highest value was recorded in T2 (7.69). Whereas, data presented in Table 7, showed that storage days and treatments have significant effect on the flavor scores of the apples, which were found to be decreased during the storage, while T2 provided good flavor scores even during storage duration. Naqash et al. [60] also used emulsions based edible coatings on the apples and found positive indications for sensory parameters, as the consumers preferred the flavor of coated apples over uncoated ones, during the storage durations. Chitosan based edible coating on strawberry exhibited the similar outcomes. It was observed that the edible coated preserved the characteristic taste and flavor of strawberry fruits [61]. High flavor scores of water chestnut powder coated apples might be due to the preservation of volatile compounds of the apples by the barrier properties of the edible coatings.
3.5.5 Overall acceptability
Overall acceptability means to estimate the degree of liking or disliking of food on the basis of all other sensory characteristics. Table 7 showed that there is a significant effect of storage days and treatments on the overall acceptability of the apples, as the scores were found to be decreased with the increase in storage duration, for all treatments. Meanwhile the T2 showed comparatively high scores as compared to the other treatments. Table 8 displayed that the overall acceptability score of apples were found to be ranging 6.58–7.76. The minimum value was noted in T0- (6.58), on the other hand, the highest value was recorded in T2 (7.76), followed by T1 (7.5) and T0+ (7.29), respectively. These results showed that coatings having 2.5% water chestnut powder showed good results for the overall acceptability of the apples. In a similar study, Bashir et al. [8] reported that apples coated with water chestnut based edible coatings were acceptable even after long term cold storage, possibly due to the reduced chilling injury. According to the findings of Cruz et al. [63], pears with an edible coating could have their shelf life increased and their quality preserved. It was investigated whether or not consumers thought coated pears were a decent product. According to their findings, applying an edible coating is a good substitute for preserving the freshness of fruits for extended periods of time.
4 Conclusion
This study assessed the possibility of water chestnut powder based edible coatings to maintain the quality and shelf life of apples during 80 days of storage. Before developing coatings, heated water chestnut powder (W2) with fine particle size was compared with unheated one (W1), where W2 showed significantly high WHC and OHC, as compared to W1. Then this heated water chestnut powder was used at 2% (T1) and 2.5% (T2) to develop coatings for the ripened apples, which were compared to the apples coated with 2% guar gum solution (T0+) and uncoated apples (T0-), during 80 days of storage. Analysis of emulsions developed for coatings showed that emulsion having 2.5% water chestnut powder (T2) showed significantly high emulsion capacity, emulsion stability, foaming capacity and viscosity as compared to T1. Apples in T2 were found to be good in term of different physicochemical criteria as total phenolic contents and antioxidant activity results of T2 were significantly high. T2 also retained the significantly high firmness of the apples during 80 days of storage. It also maintained the titratable acidity through preventing decrease of pH, and prevented the weight loss and decay of the fruits. According to the results, it was observed that T2 enhanced the sensorial features of the apples by preventing the loss of excess moisture from the fruits, thus maintained the quality during the storage duration as scores for color, flavor, taste, texture and overall acceptability were significantly high for T2, as compared to the other treatments. It was concluded that water chestnut based edible coatings could be used on the fruits, to maintain their quality characteristics, to preserve their antioxidant potential, and to prolong their shelf life at ambient conditions.
Data availability
Data relevant to this study can be provided upon request.
References
Sani IK, Masoudpour-Behabadi M, Sani MA, Motalebinejad H, Juma AS, Asdagh A, Mohammadi FJFC. Value-added utilization of fruit and vegetable processing by-products for the manufacture of biodegradable food packaging films. Food Chem. 2022;405:134964. https://doi.org/10.1016/j.foodchem.2022.134964.
Esmaeili F, Mehrabi M, Babapour H, Hassani B, Abedinia A. Active coating based on carboxymethyl cellulose and flaxseed mucilage, containing burdock extract, for fresh-cut and fried potatoes. LWT. 2024;192:115726. https://doi.org/10.1016/j.lwt.2024.115726.
Rashid F, Ahmed Z, Hussain S, Kausar T, Nadeem M, Ainee A, Mehmood T. Optimization of fenugreek and flax polysaccharides-based edible coating formulation to preserve the quality and storability of apple after harvesting. J Food Process Preserv. 2020;44(10):e14812. https://doi.org/10.1111/jfpp.14812.
Zhou K, Hu L, Liu B, Li Y, Gong X, Ma F. Identification of apple fruits rich in health-promoting dihydrochalcones by comparative assessment of cultivated and wild accessions. Sci Hort. 2018;233:38–46. https://doi.org/10.1016/j.scienta.2018.01.042.
Kauser S, Murtaza MA, Hussain A, Imran M, Kabir K, Najam A, Yaqub S. Apple pomace, a bioresource of functional and nutritional components with potential of utilization in different food formulations; a review. Food Chem Adv. 2023. https://doi.org/10.1016/j.focha.2023.100598.
Galus S, Arik Kibar EA, Gniewosz M, Kraśniewska K. Novel materials in the preparation of edible films and coatings—a review. Coatings. 2020;10(7):674. https://doi.org/10.3390/coatings10070674.
Sharma P, Shehin VP, Kaur N, Vyas P. Application of edible coatings on fresh and minimally processed vegetables: a review. Int J Veg Sci. 2019;25(3):295–314. https://doi.org/10.1080/19315260.2018.1510863.
Bashir O, Amin T, Hussain SZ, Naik HR, Goksen G, Wani AW, Proestos C. Development, characterization and use of rosemary essential oil loaded water-chestnut starch based nano emulsion coatings for enhancing post-harvest quality of apples var. Gold Delic Curr Res Food Sci. 2023;7:100570. https://doi.org/10.1016/j.crfs.2023.100570.
Ainee A, Hussain S, Nadeem M, Al-Hilphy AR, Siddeeg A. Extraction, purification, optimization, and application of galactomannan-based edible coating formulations for guava using response surface methodology. J Food Qual. 2022. https://doi.org/10.1155/2022/5613046.
Din GMU, Hussain A, Ashraf H, Kausar T, Fatima H, Akram S, Korma SA. Physicochemical, nutritional and organoleptic characteristics of cookies based on water chestnut (Trapa natans) and wheat. Food Chem Adv. 2024. https://doi.org/10.1016/j.focha.2024.100691.
Vishwakarma RK, Arora S, Bala M. Formulation and characterization of novel cereal gluten-free pasta from semi-popped Makhana, Water Chestnut, and Potato. Food Bioprocess Technol. 2024. https://doi.org/10.1007/s11947-024-03349-5.
Frangipane MT, Massantini R, Corona P. Better selection of chestnut cultivars: experimenting the sensory characterization of the Marrone chestnuts compared to the “Chataigne” chestnuts. Euro Food Res Technol. 2024. https://doi.org/10.1007/s00217-023-04438-z.
Zeng F, Chen W, He P, Zhan Q, Wang Q, Wu H, Zhang M. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels. Carb Polym. 2020;246:116551. https://doi.org/10.1016/j.carbpol.2020.116551.
Kaur K, Kaur G, Singh A. Water chestnut starch: extraction, chemical composition, properties, modifications, and application concerns. Sust Food Technol. 2023;1(2):228–62. https://doi.org/10.1039/D2FB00041E.
Mei J, Yuan Y, Guo Q, Wu Y, Li Y, Yu H. Characterization and antimicrobial properties of water chestnut starch-chitosan edible films. Int J Biol Macromol. 2013;61:169–74. https://doi.org/10.1016/j.ijbiomac.2013.06.026.
Yadav BS, Guleria P, Yadav RB. Hydrothermal modification of Indian water chestnut starch: influence of heat-moisture treatment and annealing on the physicochemical, gelatinization and pasting characteristics. LWT-Food Sci Technol. 2013;53(1):211–7. https://doi.org/10.1016/j.lwt.2013.02.007.
Adkar P, Dongare A, Ambavade S, Bhaskar VH. Trapa bispinosa Roxb.: a review on nutritional and pharmacological aspects. Adv Pharmacol Pharm Sci. 2014. https://doi.org/10.1155/2014/959830.
Garg S, Anvar Hussain NA, Syed I, Asaithambi N, Mundhada S. Water Chestnut (Trapa natans). In: Antioxidants in vegetables and nuts-properties and health benefits. 2020; 453–465. https://doi.org/10.1007/978-981-15-7470-2_22
Uchikura T, Miura Y, Yoshimura M, Ito H, Amakura Y. Identification of antioxidative hydrolyzable tannins in water chestnut. Molecules. 2023;28(18):6563. https://doi.org/10.3390/molecules28186563.
Singh GD, Sharma R, Bawa AS, Saxena DC. Drying and rehydration characteristics of water chestnut (Trapa natans) as a function of drying air temperature. J Food Eng. 2008;87(2):213–21. https://doi.org/10.1016/j.jfoodeng.2007.11.027.
AOAC. Association of Official Agricultural Chemists) In: Horwitz W, Latimer G , editors. The official methods of analysis of AOAC International (20th ed.). Gaithersburg: AOAC International; 2016.
Gorsi FI, Hussain A, Kausar T, Nisar R, Siddique T, Yaqub S, Akram S. Structural and thermal interaction studies of aloe vera (aloe barbadensis miller) gel powder and developed food bars. J Therm Anal Calorim. 2024. https://doi.org/10.1007/s10973-024-13007-9.
Hussain A, Kauser T, Aslam J, Quddoos MY, Ali A, Kauser S, Gorsi FI. Comparison of different techno-functional properties of raw lemon pomace and lemon pomace powder, and development of nutritional biscuits by incorporation of lemon pomace powder. Caraka Tani J Sust Agric. 2023;38(1):176–92.
Huang X, Kakuda Y, Cui W. Hydrocolloids in emulsions: particle size distribution and interfacial activity. Food Hydrocoll. 2001;15(4–6):533–42. https://doi.org/10.1016/S0268-005X(01)00091-1.
Akhter R, Masoodi F, Wani TA, Rather SA. Functional characterization of biopolymer based composite film: incorporation of natural essential oils and antimicrobial agents. Int J Bio Macromol. 2019;137:1245–55. https://doi.org/10.1016/j.ijbiomac.2019.06.214.
Siddique F, Hussain A, Mahdi AA, Hassan M, Noreen S, Siddique T, Korma SA. Comparison of chemically treated, pasteurized, and microwave-treated (at different time durations) chia seeds added To Mango-Whey Beverage, during different storage periods, for physicochemical and sensory parameters. J Food Qual. 2024. https://doi.org/10.1155/2024/6688945.
Thakur R, Pristijono P, Golding JB, Stathopoulos CE, Scarlett CJ, Bowyer M, Vuong QV. Development and application of rice starch based edible coating to improve the postharvest storage potential and quality of plum fruit (Prunus salicina). Sci Hort. 2018;237:59–66. https://doi.org/10.1016/j.scienta.2018.04.005.
Zakriya M, Hussain A, Mahdi AA, Yasmeen F, Kausar T, Rehman A, Korma SA. Effect of different types of ethylene scavengers used in different combinations, on the post-harvest quality and phytochemicals retention of tomatoes (Solanum lycopersicum L.). Chem Biol Technol Agric. 2023;10(1):90. https://doi.org/10.1186/s40538-023-00465-w.
Thakur R, Pristijono P, Scarlett CJ, Bowyer M, Singh SP, Vuong QV. Starch-based edible coating formulation: Optimization and its application to improve the postharvest quality of “Cripps pink” apple under different temperature regimes. Food Pack Shelf Life. 2019;22:100409. https://doi.org/10.1016/j.fpsl.2019.100409.
Cheng Z, Moore J, Yu L. High-throughput relative DPPH radical scavenging capacity assay. J Agric Food Chem. 2006;54(20):7429–36. https://doi.org/10.1021/jf0611668.
Hussain A, Kausar T, Din A, Murtaza MA, Jamil MA, Noreen S, Ramzan MA. Determination of total phenolic, flavonoid, carotenoid, and mineral contents in peel, flesh, and seeds of pumpkin (Cucurbita maxima). J Food Process Preserv. 2021;45(6):e15542. https://doi.org/10.1111/jfpp.15542.
Rafique HS, Hussain A, Nadeem M, Rehman A, Siddique T, Najam A, Laaraj S. Impact of different proportions of sweet potato (Ipomoea batatas L.) flour on physical, chemical and sensory parameters of straight grade flour-based cake rusk. Food Hum. 2023;1:1282–96. https://doi.org/10.1016/j.foohum.2023.09.024.
Steel R, Torrie J, Dickey D. Principles and procedures of statistics A biometrical approach 3rd ed. New York: McGraw Hill Book Company Inc.; 1997, pp. 334–381.
Beigh MA, Hussain SZ, Naseer B, Rouf A, Ahmad RT. Storage studies of water chestnut flour. J Food Process Preserv. 2020;44(2):e14321. https://doi.org/10.1111/jfpp.14321.
Hussain SZ, Beigh MA, Naseer B, Naik HR. Visco-thermal and structural characterization of water chestnut flour. J Food Sci Technol. 2020;57(8):2949–59. https://doi.org/10.1007/s13197-020-04327-3.
Ahmed J, Al-Attar H, Arfat YA. Effect of particle size on compositional, functional, pasting and rheological properties of commercial water chestnut flour. Food Hydrocoll. 2016;52:888–95. https://doi.org/10.1016/j.foodhyd.2015.08.028.
Gul K, Riar CS, Bala A, Sibian MS. Effect of ionic gums and dry heating on physicochemical, morphological, thermal and pasting properties of water chestnut starch. LWT-Food Sci Technol. 2014;59(1):348–55. https://doi.org/10.1016/j.lwt.2014.04.060.
Ahsan S, Tariq A, Liaqat A, Khaliq A, Farooq MA, Hussain A, Hussain A. Development of active packaging film, based on Phyllanthus wightianus and their application to meat product preservation. J Food Measur Charact. 2024. https://doi.org/10.1007/s11694-024-02545-3.
Rashid F, Ahmed Z, Ferheen I, Mehmood T, Liaqat S, Ghoneim MM, Rahman A. Effect of fenugreek and flaxseed polysaccharide-based edible coatings on the quality attributes and shelf life of apple fruit during storage. Food Sci Nutr. 2024;12(3):2093–103. https://doi.org/10.1002/fsn3.3909.
Ahmed J, Al-Attar H. Effect of drying method on rheological, thermal, and structural properties of chestnut flour doughs. Food Hydrocoll. 2015;51:76–87. https://doi.org/10.1016/j.foodhyd.2015.04.030.
Mir NA, Gul K, Riar CS. Technofunctional and nutritional characterization of gluten-free cakes prepared from water chestnut flours and hydrocolloids. J Food Process Preserv. 2015;39(6):978–84. https://doi.org/10.1111/jfpp.12311.
Ansari L, Ali TM, Hasnain A. Effect of chemical modifications on morphological and functional characteristics of water-chestnut starches and their utilization as a fat-replacer in low-fat mayonnaise. Starch-Stärke. 2017;69(1–2):1600041. https://doi.org/10.1002/star.201600041.
Nawab A, Alam F, Hasnain A. Mango kernel starch as a novel edible coating for enhancing shelf-life of tomato (Solanum lycopersicum) fruit. Int J Biol Macromol. 2017;103:581–6. https://doi.org/10.1016/j.ijbiomac.2017.05.057.
Ali U, Kanwar S, Yadav K, Basu S, Mazumder K. Effect of arabinoxylan and β-glucan stearic acid ester coatings on post-harvest quality of apple (Royal Delicious). Carb Polym. 2019;209:338–49. https://doi.org/10.1016/j.carbpol.2019.01.002.
Mehyar GF, Al-Qadiri HM, Abu-Blan HA, Swanson BG. Antifungal effectiveness of potassium sorbate incorporated in edible coatings against spoilage molds of apples, cucumbers, and tomatoes during refrigerated storage. J Food Sci. 2011;76(3):M210–7. https://doi.org/10.1111/j.1750-3841.2011.02059.x.
Misir J, Brishti FH, Hoque MM. Aloe vera gel as a novel edible coating for fresh fruits: A review. Am J Food Sci Technol. 2014;2(3):93–7.
Jahanshahi B, Jafari A, Vazifeshenas MR, Gholamnejad J. A novel edible coating for apple fruits. J Horti Postharv Res. 2018;1(1):63–72. https://doi.org/10.22077/jhpr.2018.1186.1009.
Moreira MR, Cassani L, Martín-Belloso O, Soliva-Fortuny R. Effects of polysaccharide-based edible coatings enriched with dietary fiber on quality attributes of fresh-cut apples. J Food Sci Technol. 2015;52(12):7795–805. https://doi.org/10.1007/s13197-015-1907-z.
Hazrati S, Beyraghdar Kashkooli A, Habibzadeh F, Tahmasebi-Sarvestani Z, Sadeghi AR. Evaluation of Aloe vera gel as an alternative edible coating for peach fruits during cold storage period. Gesunde Pflanzen. 2017;69(3):131–7. https://doi.org/10.1007/s10343-017-0397-5.
Zahedi SM, Hosseini MS, Karimi M, Ebrahimzadeh A. Effects of postharvest polyamine application and edible coating on maintaining quality of mango (Mangifera indica L.) cv. Langra during cold storage. Food Sci Nutr. 2019;7(2):433–41. https://doi.org/10.1002/fsn3.802.
Hussain A, Kausar T, Siddique T, Kabir K, An QU, Rukhsar F, Mahdi AA. Physiological and biochemical variations of naturally ripened mango (Mangifera Indica L.) with synthetic calcium carbide and ethylene. Sci Rep. 2024;14(1):2121.
Naeini M, Jafari A, Gholamnejad J, Vazifeshenas M. Optimizing shelf life of pomegranate fruits during traditional storage by Tragacanth gum coating. J Hort Postharv Res. 2020;3(1):49–60. https://doi.org/10.22077/jhpr.2019.2690.1081.
Gol NB, Patel PR, Rao TR. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharv Biol Technol. 2013;85:185–95. https://doi.org/10.1016/j.postharvbio.2013.06.008.
Abid M, Jabbar S, Wu T, Hashim MM, Hu B, Lei S, Zeng X. Effect of ultrasound on different quality parameters of apple juice. Ultrason Sonochem. 2013;20(5):1182–7. https://doi.org/10.1016/j.ultsonch.2013.02.010.
Jafari S, Hojjati M, Noshad M. Influence of soluble soybean polysaccharide and tragacanth gum based edible coating to improve the quality of fresh-cut apple slices. J Food Process Preserv. 2018;42(6):e13638. https://doi.org/10.1111/jfpp.13638.
Anjum MA, Akram H, Zaidi M, Ali S. Effect of gum arabic and Aloe vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘Gola’guava fruits. Sci Hort. 2020;271:109506. https://doi.org/10.1016/j.scienta.2020.109506.
Farina V, Passafiume R, Tinebra I, Palazzolo E, Sortino G. Use of aloe vera gel-based edible coating with natural anti-browning and anti-oxidant additives to improve post-harvest quality of fresh-cut ‘fuji’apple. Agronomy. 2020;10(4):515. https://doi.org/10.3390/agronomy10040515.
Lu Y, Zhao S, Jia C, Xu Y, Zhang B, Niu M. Textural properties of Chinese water chestnut (Eleocharis dulcis) during steam heating treatment. Foods. 2022;11(9):1175. https://doi.org/10.3390/foods11091175.
Ghavidel RA, Davoodi MG, Asl AFA, Tanoori T, Sheykholeslami Z. Effect of selected edible coatings to extend shelf-life of fresh-cut apples. Int J Agric Crop Sci. 2013;6(16):1171.
Naqash F, Masoodi FA, Ayob O, Parvez S. Effect of active pectin edible coatings on the safety and quality of fresh-cut apple. Int J Food Sci Technol. 2022;57(1):57–66. https://doi.org/10.1111/ijfs.15059.
Riaz A, Aadil RM, Amoussa AMO, Bashari M, Abid M, Hashim MM. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J Food Process Preserv. 2021;45(1):e15018. https://doi.org/10.1111/jfpp.15018.
Raghav PK, Agarwal N, Saini M. Edible coating of fruits and vegetables: a review. Education. 2016;1:2455–5630.
Cruz V, Rojas R, Saucedo-Pompa S, Martínez DG, Aguilera-Carbó AF, Alvarez OB, Aguilar CN. Improvement of shelf life and sensory quality of pears using a specialized edible coating. J Chem. 2015. https://doi.org/10.1155/2015/138707.
Funding
No funds in any form, from any source were availed for this research work.
Author information
Authors and Affiliations
Contributions
Conceptualization: AA, Data curation: TK, Formal analysis: M, and AA, Funding acquisition and Investigation: BB and AA, Methodology: SAK and RN, Project administration: SY and NF, Resources: TK, Software: KK: Visualization and Validation: RN and KK, Supervision: AH and AAM, Roles/Writing—original draft: AH, SAK and AA, Writing—review and editing: AH, AAM and M.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
No animal or human study was involved in this work, however for sensory evaluation study, informed consent was obtained from all individual participants included in the study (all above 18 years age). This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University of Sargodha, Sargodha, Pakistan, wide order no. UOS/SGD/IFSN/21/2022.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahnoor, Ainee, A., Hussain, A. et al. Effect of water chestnut based edible coating on the physicochemical quality and shelf life of apples. Discov Appl Sci 6, 442 (2024). https://doi.org/10.1007/s42452-024-06114-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06114-7