Abstract
Edible coatings for fruits and vegetables are the subject of intensive agro-based research. These coatings provide value to the product due to their multifunctionality and sustainability. The current study focuses on the development and evaluation of plant-based edible active coatings for Jara lebu (Citrus medica), with the aim of determining the effectiveness of these coatings in extending the shelf-life and preserving the quality attributes. Different blends of corn starch and various plant extracts were formulated and all formulations were applied by immersion onto the fruit surface. The study had five treatment groups: T0, T1, T2, T3, and T4. T0 served as the control group, while T1 consisted of a mixture of 2% corn starch and 0.5% glycerol. T2 included a combination of 2% corn starch, 0.5% glycerol, and 1.5% holy basil. T3 comprises 2% corn starch, 0.5% glycerol, and 1.5% wild turmeric. Lastly, T4 consisted of 2% corn starch, 0.5% glycerol, and 1.5% Indian pennywort. Control and coated samples were kept under the same conditions for 35 days before being evaluated for changes in their physiological, physicochemical, and sensory qualities. Coated sample T2 significantly prolonged the shelf-life of Jara lebu samples, having the least weight reduction (26.25%) and retaining most of the essential nutrients (TSS = 7.09%, pH = 3.0, vitamin C = 22.03 mg/100 g, TPC = 44.57 mg GAE/g DW, TFC = 45.24 mg QE/g DW, antioxidant = 86.09%). This sample received the highest overall acceptability score, a maximum of 8.24. Sensory evaluations revealed no adverse effects on taste, aroma or appearance, suggesting these coatings can be an eco-friendly and efficient method for preserving the freshness and quality of Jara lebu and potentially other citrus fruits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Citrus fruits are globally esteemed for their palatable taste, abundant nutritional composition, and convenient accessibility, hence prompting their cultivation in tropical and subtropical regions [1]. Jara lebu (Citrus medica), commonly known as Citron, is a fruit indigenous to Southeast Asia [2]. The edaphic and climatic conditions in Bangladesh, particularly in the Sylhet region, are favorable to the growing of citrus fruits. As a result, an estimated annual production of around 1293 MT of lime and lemon is cultivated in Bangladesh [3]. Among these, Jara lebu is famous in the Sylhet region, and the fruit skin is eaten raw with rice [4]. Based on the data provided by the Department of Agricultural Extension (DAE), it can be observed that Bangladesh’s yearly export of Jara lebu amounts to approximately 100 MMT [5]. However, a significant proportion of lemon, up to 30%, is annually lost due to inadequate post-harvest management [6]. There is a pressing need to mitigate post-harvest spoiling by implementing novel technologies that can enhance the shelf-life of Jara lebu. One effective method for extending the duration of fruit shelf-life has entailed the utilization of suitable coating materials applied to the outer layer of the fruit peel [7]. Applying an edible coating to the outer surface of fruits is a highly effective method for improving the shelf-life of the fruit, in addition to the material surface coating. Coating materials applied on natural fruit do not alter its texture or flavor. Instead, they create a barrier that hinders the fruit’s ripening mechanism, therefore delaying the ripening process by limiting gas exchange [8].
In contemporary times, the majority of citrus fruits that are harvested for commercial purposes are subjected to a process known as waxing, wherein a synthetic fungicide coating is applied to the fruits within packing facilities. This practice has been found to have adverse effects on both human health and the environment. Food businesses face significant challenges in managing the postharvest phase of citrus fruits to minimize losses during the supply chains. In order to mitigate postharvest losses, many strategies have been employed to extend the shelf-life of fresh produces. When considering the health of consumers, one of the most effective options to enhance the shelf life of fruits after they are harvested is the use of edible coatings, which are ecofriendly solutions [9]. Edible coatings have demonstrated considerable potential in prolonging the shelf-life of diverse fruit items, exhibiting comparable efficacy to traditional coating methods. Edible coatings are formulated using biodegradable and edible ingredients derived from natural sources, thereby addressing environmental considerations and meeting consumer preferences. Edible coatings have proven to be an effective kind of primary packaging that delays the ripening process and maintains the nutritious properties of the product [10].
There are multiple compelling reasons that have led to a growing interest in plant-based edible coatings. To begin with, these coatings frequently originate from renewable resources, so establishing their status as sustainable solutions. The incorporation of various bioactive compounds into plant-based products improves the overall quality and lifespan of perishable foods as a result of their natural adaptability [11]. The utilization of bio-active plant extracts as a substitute for synthetic post-harvest treatments has been shown to greatly enhance antioxidant and antibacterial efficacy. The utilization of an active edible coating provides several advantages. These coatings are utilized on the external layer of a fruit in order to inhibit the process of dehydration and the occurrence of contamination. Edible coatings are used to safeguard food products from environmental damage, including oxidation, moisture loss, and microbial growth [12].
The use of edible plant-based coatings is not only a pragmatic approach, but also reflects the holistic character of traditional practices in regions where the fruit is indigenous. The presence of fungal and mold growth in citrus fruits poses a significant risk to human health due to the abundant generation of mycotoxins. P. digitatum, P. italicum, and G. candidum are the primary fungal infections that cause spoilage and postharvest disease in citrus fruits, resulting in economic losses [11]. Researchers have discovered that plant extracts can be used effectively in food preservation to prevent the deterioration of food by providing them with antimicrobial protection [13]. The modernization of these practices could therefore pave the way for more sustainable and health-conscious preservation techniques. Prior research has shown that using sustainable coating materials on freshly harvested citrus fruits is an efficient postharvest technique that improves quality and prolongs the time they can be stored. This is accomplished by enhancing the fruit's visual aesthetics and reducing postharvest losses [14]. Nevertheless, the development of a proficient edible coating necessitates thorough and meticulous research. Several factors have been identified as significant determinants of the effectiveness of a coating. These factors include the selection of the base material, the inclusion of active components, the application method employed, and the interaction between the coating and the surface of the fruit [15]. It is also important to comprehend the physicochemical characteristics, biodegradability, and potential sensory impacts of the fruit coating in order to ensure customer acceptance. A primary objective of this research was to assess how effective these coatings were at extending the shelf-life and maintaining the quality of Jara lebu. The study measured various physiological, physicochemical, and sensory qualities to determine this. In this context, the objective of this study was to develop and evaluate an active edible coating made of corn starch and various plant extracts (Holy basil, Wild turmeric, and Indian pennywort) to increase the shelf-life of Jara lebu in a sustainable way, thereby reducing post-harvest losses and quality attributes of this valuable fruit. The major contributions of this study are:
-
a.
The research presents innovative edible coatings made from plant-based materials. The combination of natural ingredients for edible coatings is relatively unexplored, especially for citrus fruits like Jara lebu.
-
b.
The study underscores the sustainability of using plant-based materials for food preservation, highlighting the eco-friendly nature of the coatings and their efficiency in maintaining fresh quality.
-
c.
The research demonstrates the effectiveness of these coatings in significantly extending the shelf-life of Jara lebu by reducing weight loss and maintaining essential nutrients. This contribution is crucial for agricultural economics and food sustainability.
Although the study focuses on Jara lebu, the successful application of these coatings suggests potential adaptability to other citrus fruits, which could broaden the impact of the research.
2 Materials and methods
2.1 Experimental site and plant materials
The research was carried out at the laboratories of the Food Engineering and Technology Department and the Horticulture Department at Sylhet Agricultural University, Sylhet, Bangladesh. Jara lebu (Citrus medica) samples were collected from Jaintapur, Sylhet. In order to get bioactive extract concentrations, plant materials including holy basil (Ocimum tenuiflorum), wild turmeric (Curcuma aromatica), and Indian pennywort (Centella asiatica) were gathered from various locations within the Sylhet and Mymensingh regions of Bangladesh. The corn starch (Merck, Germany) used for the preparation of the basic coating was obtained from a nearby scientific store located in Sylhet city. All other chemicals used in this study were from Merck, Germany.
2.2 Preparation of plant extract
The mature leaves of holy basil, wild turmeric, and Indian pennywort were gently washed with 2.5% chlorine solution. The wild turmeric was peeled and subsequently pulverized using a blender. Holy basil and Indian pennywort were individually ground using a blender. The resultant mixture underwent filtration in order to eliminate the presence of fibers. The pasteurization process involved subjecting each plant extract to a temperature of 70 °C for a duration of 45 min. After immediately cooling the plant extract to room temperature, 1.9–2.0 g L−1 ascorbic acid was added to stabilize it. To regulate the pH at a level of 4, citric acid was introduced at a concentration of 4.5–4.6 g per L [16].
2.3 Preparation of active edible coating
The use of 2% corn starch [9] as a commercial gelling agent prior to usage as a coating agent resulted in enhancements in both the viscosity of the stabilized substance and its coating efficiency. Subsequently, the gel was placed in a brown amber bottle in order to mitigate the effects of oxidation [17]. The mixture of 2 g corn starch and 100 mL distilled water was heated at 100 °C for continuous heating on a magnetic stirrer until total dissolution was achieved, which took approximately 30 min. The combination was subsequently cooled to ambient temperature, which typically ranges between 28 and 35 °C. The solution was mixed with 1.5% (w/v) of each plant extract, which was added one at a time and stirred for two to three minutes to make the mixture smooth [18].
2.4 Coating of fruits
Fresh limes of comparable size, color, and firmness were sorted and subjected to a cleaning process involving immersion in a 0.5% solution of commercial bleach for a duration of three minutes [19]. Subsequently, the limes were thoroughly washed and let dry naturally. Five lemons were coated for each batch of coating formulations. The study had five treatment groups and the details are shown in Table 1. Lemons were submerged in the coating solution for 30 s, then withdrawn for 10 s. The operation was conducted three times, involving one instance of suspension and one instance of coating. The lemons were subjected to a coating process and afterwards underwent a two hr period of allowing excess coating solution to drop off at ambient temperature and with airflow. In order to ensure the application of the coating, the samples were thereafter subjected to air drying at ambient temperature for a minimum duration of 30 min. Samples were stored at room temperature and 80–85% relative humidity for 35 d.
2.5 Determination of physiological and physicochemical characteristics
2.5.1 Firmness
The determination of the firmness of the sample was conducted in accordance with the procedure outlined by Formiga et al. [20]. The evaluation of the firmness of the entire sample, including both the skin and flesh, was conducted utilizing a texture analyzer (Agrosta®100, Agrosta SARL, Serqueux, France). The texture analyzer employed a load cell with a maximum capacity of 10 kg and a maximum penetration speed of 27 mm/s. A probe with a diameter of 10 mm was utilized to assess the firmness of the material. The measurements were conducted on the entire fruit. The probe was inserted into the fruit at a velocity of 27 mm/s, with a penetration depth of 1 mm, and afterwards retracted to its initial position. In three locations, 1 cm incisions were made to test the samples' skin and flesh hardness.
2.5.2 Weight loss
The measurement of weight loss in the sample was conducted according to the methodology outlined by Ahmed et al. [21]. The net weight was determined using a laboratory-grade electronic weighing balance (Model: ATY 224, Shimadzu, Kyoto, Japan) with a precision of 0.0001 g. The data was collected at regular intervals of 5 d during a period of 35 d. By comparing the initial and final weights at various times, weight loss of each samples were measured. This value was then reported as a percentage, relative to the initial weight.
2.5.3 Color
The color of the plant extract samples was assessed utilizing a colorimeter (PCE-CSM4 model, PCE Instruments, UK), following the procedure given by Zinia et al. [22] with certain modifications. The measurements encompassed brightness (L*), red/green (a*), yellow/blue (b*), chromaticity (C), and hue angle (h°). The color of each sample was assessed at three points, and the average of three observations was used to calculate L*, a*, and b*. The calculation of color differences (ΔE) was performed by means of the following equation, in relation to the control [23].
2.5.4 Total soluble solids (TSS), titratable acidity (TA), pH, and vitamin C
The measurement of total soluble solids (TSS) was conducted using a refractometer (Bellingham + Stanley, UK) at a temperature of 30 °C, with the findings being reported in °Brix. The titratable acidity was assessed using a method outlined by the Association of Official Agricultural Chemists (AOAC) [24], with some adjustments made. Juice was diluted by mixing 4 mL with 25 mL of distilled water. The solution underwent homogenization and was thereafter titrated with a 0.1 N solution of sodium hydroxide (NaOH). The titration process involved the addition of 2 drops of phenolphthalein as an indicator. The determination of vitamin C in the sample was evaluated utilizing a modified methodology derived from two contemporary studies [25, 26]. The absorbance measurement was performed using a UV–Vis spectrophotometer (Model: UV-1800, Shimadzu, Kyoto, Japan) at 266 nm wavelength. The vitamin C concentration in the sample was measured in mg/mL, utilizing the ascorbic acid standard curve with a coefficient of determination (R2) value of 0.9955. The pH value was measured using a digital pH meter (Model: HI-2211, Hanna Instrument, USA). In the course of the analysis, a 5 mL volume of fruit juice was subjected to homogenization with 45 mL of distilled water. Subsequently, the electrode was submerged into the homogenized solution, and the corresponding reading was properly recorded.
2.5.5 Total phenol content (TPC), flavonoid content, and antioxidant activity
Folin-Ciocalteu reagent was used to measure total phenol content (TPC) using gallic acid as the reference standard [27]. The determination of antioxidant activity was conducted using two methods: the 2, 2-diphenyl-2-picrylhydrazyl (DPPH) method [28] and the ferric reducing antioxidant power assay (FRAP) [29]. Methods developed by Hajlaoui et al. [30] were used to calculate total flavonoid content, with certain adjustments as outlined by Naeem et al. [31].
2.6 Sensory analysis
An untrained group of 50 people (24 men and 26 women) with a median age of 25, who were familiar with the fruit, were recruited to do sensory evaluations. The main eligibility requirement for panel testers was their proficiency in discerning the taste, aroma, and color of the juice. In the periods between sample assessments, the panelists were instructed to do oral rinsing using drinking water. The sensory attributes of the juice samples, including taste, color, aroma, and overall acceptability, were evaluated using a nine-point hedonic scale. A rating of 9 indicated extreme liking, while a rating of 1 indicated extreme disliking.
2.7 Shelf-life study
The study on shelf-life was carried out through the observation and evaluation of several physicochemical and microbiological parameters of both the sample and the juice derived from it.
2.8 Statistical analysis
The results are presented as means ± standard deviations. A one-way analysis of variance (ANOVA) was used to compare the various treatments. A comparison test with a significance level of p < 0.05 was used to evaluate whether there were significant differences between mean values. SPSS 16.0 software (SPSS Inc., Chicago, IL) was used for the statistical analysis.
3 Results and discussion
3.1 Fruit firmness and weight loss
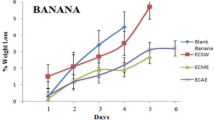
The firmness of fruit is a significant attribute in post-harvest quality assessment due to consumer preference for firmer fruits over softer ones [32]. Figure 1 depicts the firmness of the fruit samples throughout storage. Regardless of edible coating treatments, the fruit firmness of Jara lebu deteriorated with increasing storage length till 20 d of storage. For instance, on the very first day of storage, there was hardly any difference in fruit firmness across the treatments, but it fell to 10.22, 12.22, 12.45, 12.12, and 12.12 kg/cm2 for T0, T1, T2, T3, and T4, respectively, after 20 d of storage. Control treatments were shown to have a faster loss in fruit firmness than other treatments. Fruit firmness and shriveling are caused by a loss of pectic compounds in the cell wall’s middle lamella, which leads to a loss of cell wall integrity and softening and shriveling [33]. Loss of firmness during storage may be attributed to fruit senescence, which occurs when fruit is exposed to conventional storage conditions for an extended period of time and give consumers an unattractive visual appearance [34]. Within 20 d of storage, fruit firmness increased steadily in all treated and untreated fruits, reaching levels comparable to the first day of storage after 35 d. Long-term storage in typical room settings may cause skin hardening due to water loss and wrinkles, resulting in a high firmness value [35]. In contrast, both the control and coated samples lost weight steadily over time while being stored (Fig. 1). The control sample (T0) differed significantly from other samples. After 20 d, the proportion of weight loss in the control group was much higher. T1, T2, and T3 samples were similar (about 30%), with T2 exhibiting the least weight reduction (26.25%). At the 35th d, the control therapy had the greatest weight reduction (53.31%), while the T2 treatment had the least (36.24%). The primary cause of water loss from the surface of the fruit is respiration and transpiration and edible films and coatings offer a potentially efficient method for preserving fresh fruits by minimizing moisture loss [36]. Because of the hydrophilic nature of the edible coating, more water is retained, resulting in a decrease in transpiration and respiration rate. Plant extracts can change the physicochemical properties of coated fruits and boost their water-binding capability. Although starch coatings have a high water vapor permeability, the inclusion of plant extracts can improve their moisture barrier properties. These findings are consistent with those of Adetunji et al. [16] and Shanta et al. [25], who indicated that coatings or films preserved water content significantly.
3.2 Color
The findings indicated that the color indices L* (lightness/darkness), a* (redness/greenness), and b* (yellowness/blueness) underwent significant shifts over the storage period (Fig. 2). The lightness of both the control and coated samples exhibited a gradual increase throughout the storage period. The control samples exhibit a higher rate of increase in the L value compared to the coated samples. Since L is a measure of the lightness–darkness axis, this increase in value is evidenced by the disappearance of the darker green color and resulting appearance of the lighter yellow color [37]. The findings indicated that the a* values of the coated samples were significantly more negative, and thus, the color of the coated ones seemed greener compared to the control samples (p < 0.01). The transition of the a* value from negative to positive often signifies a decrease in the green color intensity of the samples. The b* value also exhibited a comparable trend, indicating significant (p < 0.01) increases over time for both the control and coated samples. This can be attributed to the presence and increase of carotenoid pigments as chlorophyll degrades. Similar findings can be observed from the findings of Maftoonazad & Ramaswamy [37].
3.3 TSS, TA, and pH
Figure 3 presents the TSS, TA, and pH content of Jara lebu samples during the storage periods. Irrespective of the post-harvest treatments applied, the TSS exhibited an increase during the storage period (Fig. 3a). The control treatment (T0) exhibited a higher observed change in TSS, while the T2 treatment showed a lower change in TSS at the end of the storage period. TSS may have increased due to the breakdown of polysaccharides (starch, pectin, and other insoluble carbohydrates) to simpler sugars such as glucose and fructose [32]. Edible coating treatments may delay TSS increase due to delayed ripening, lower metabolic activity due to intact fruit peel, and slower senescence [38]. A gradual decrease in TA is also observed in the control and coated samples throughout the storage period (Fig. 3b). Upon reaching the end point of the storage period, it becomes noticeable that the T2 sample exhibited the least amount of reduction, but the control sample experienced a greater decrease, followed by T1, T4, and T3, in descending order. Same type of trend in acidity can be observed in the study by [32] and [39]. The pH fluctuations of Jara Lebu resulting from various coating treatments are depicted in Fig. 3c. Until the fifth day, the pH levels of all the samples remained quite constant. After that a significant increase is seen in all samples. Following the storage period, the T2 sample had a comparatively lower rise in pH levels, whereas the control group demonstrated a large increase in pH values. The observed gradual alteration in pH aligns with the findings presented by [39]. This phenomenon exhibits potential promise as it is attributed to the oxidation of acid during storage, leading to an increase in pH levels. This observation aligns with the conclusions drawn by [40].
3.4 Vitamin C
The application of the coating influenced the concentration of vitamin C in the citrus fruits. Over the course of the storage period, there was a gradual decline in the overall vitamin C concentration on a daily basis. Figure 4 shows that control samples had considerably lower ascorbic acid content after 5 d of storage than other coated samples. After 15 d, there was a significant difference between T0 (29.12 mg/100 g) and T3 (33.67 mg/100 g). The vitamin C concentration of coated samples was considerably different from control samples after 30 d of storage where T3 and T4 retained substantially more vitamin C than other treatments. On the final day of the storage period, the maximum level of vitamin C retention was observed in T4 (26.87 mg/100 g) treatments and, there was a similarity among T2 (22.03 mg/100 g), T3 (22.98 mg/100 g). Evidence from edible coatings corroborated findings of [41] that vitamin C degraded more slowly. The observed decline in ascorbic acid concentration may be attributable to alterations in metabolic processes and an elevation in acidity levels associated with certain coating materials. During storage, coating made it easier for ascorbic acid to dissipate into de-hydro ascorbic acid by reducing the rate of respiration and maturation [31]. These findings demonstrated that edible coatings have a significant impact on biological reactions such as the creation of organic acids (ascorbic acid) that occur inside lemon fruits during the post-harvest life [42].
3.5 Total phenol, flavonoid, and antioxidant activity (DPPH)
The total phenolic content (TPC) varied significantly across treatments. TPC content decreased after 5 d of storage and then steadily increased throughout the storage duration (Fig. 5a). TPC was significantly lower in the control treatment than in all edible coated samples from 10 to 35 d of storage. Control samples had the lowest amount of TPC (32.03 mg GAE/g DW) after 35 d of storage, whereas T3 treated samples had the maximum amount of TPC (47.57 mg GAE/g DW), followed by T4 (44.89 mg GAE/g DW), T2 (44.57 mg GAE/g DW), and T1 (41.33 mg GAE/g DW). TPC levels were highest in coated fruit across all storage conditions, showing that active edible coating preserved more antioxidants in fruit. The gas barrier of the coatings can be used to describe the function of edible coatings and active edible coatings on preserving TPC in Jara Lebu fruit during storage time [43]. As a result, the defensive system was strengthened, and the fruit quality was preserved during storage following harvest. These findings agreed with those of Saberi et al. [44]. Flavonoids are abundant in lemons, with narirutin, didymin, and hesperidin being the most abundant in lemon juice. Flavonoids, such as hesperidin, play a significant part in plant defense mechanisms and are produced as phenolic substances [45, 46]. Total flavonoid content in samples taken from coated and controls decreased after 5 d of storage and then continuously increased up to 35 d of storage (Fig. 5b). To deal with the abrupt shift in the environment, flavonoid concentration would initially increase during postharvest storage and then fall due to enormous flavonoid compound consumption [47]. Total flavonoid content levels in control fruit samples dropped after 20 d of storage, whereas total flavonoid content levels in coated fruits were significantly higher. Control fruit samples had the lowest amount of total flavonoid content (31.49 mg QE/g DW) of Jara Lebu fruit extract after 25 d of storage, whereas T2 treated samples had the maximum amount of total flavonoid content (45.24 mg QE/g DW), followed by T1 (43.85 mg QE/g DW), T3 (40.62 mg QE/g DW), and T4 (39.44 mg QE/g DW). T2 treated fruit samples had the greatest total flavonoid content from 5 d of storage than all other treatments. Figure 5c depicts changes in total antioxidant capacity in Jara Lebu throughout storage in terms of DPPH scavenging activity. All samples, regardless of treatment, had increasing antioxidant activity as storage time increased up to 20 d. After 20 d of storage, antioxidant capacity in the control treatment rapidly dropped but antioxidant capacity in coating treated samples increased until 25 d of storage and then began to decline. At the end of the storage, antioxidant activities of all the treated samples were reached with the values of 76.59%, 82.23%, 86.09%, 84.12%, and 81.95% for T0, T1, T2, T3, and T4 treatments, respectively. According to the results, all of the coated samples had significantly higher antioxidant activity than the control treatment, with T2 having the maximum antioxidant capacity during the later stages of storage. The use of plant-based edible coverings resulted in the preservation of increased levels of antioxidant capacity in the samples across time. The phenomena could be ascribed to the oxygen barrier qualities of plant-based edible coatings, which delay the biochemical and physiological changes that occur during storage, hence slowing enzyme activity and oxidative degradation of antioxidant molecules [48]. Citrus fruit antioxidant activity has been linked to phenolic chemicals, flavonoid compounds, and ascorbic acid [49]. Our findings are consistent with [50] and [31].
3.6 Sensory analysis
Sensory acceptance of the coated Jara lebu samples was mostly favorable, with average ratings of more than 7 (7.8 > liked moderately) (Fig. 6). This shows that all of the coated samples were well received in terms of sensory perception. The T2 sample had the greatest overall acceptability score, with a maximum of 8.24. In terms of general acceptability, the control sample received the lowest score. When it came to taste, aroma, and appearance, all of the coated samples obtained the same level of scoring compared to the control sample.
4 Conclusion and future works
In order to evaluate the effectiveness of an edible coating applied to fruits and vegetables, quality parameters of the coated products are typically examined as indicators. The findings of this study provide confirmation that the application of coating containing Jara lebu samples, corn starch, glycerol, and holy basil contributes to the preservation of physiological, physicochemical, and sensory quality criteria. Finally, the treated samples outperformed the control sample in every physicochemical and sensory feature. T2 was discovered to be the best of the coated samples. T2 significantly prolonged the shelf-life of Jara lebu samples, having the least weight reduction (26.25%) and retaining most of the essential nutrients. T2 had a lower change in TSS (7.09%) at the end of the storage period, a lower rise in pH levels (3.00), good retention of vitamin C (22.03 mg/100 g) and TPC T2 (44.57 mg GAE/g DW), the highest total flavonoid content (45.24 mg QE/g DW), and antioxidant capacity (86.09%). This sample received the highest overall acceptability score, a maximum of 8.24. In conclusion, edible coatings have emerged as a viable technique to address the issue of postharvest losses by offering supplementary protection to food products. These coatings operate as selective barriers to gases, thereby extending the shelf-life of the food. It is important to highlight that there is no universally applicable edible coating, as each composition is specific to the individual characteristics of the food. Future research could include testing the efficacy of these coatings on a broader range of fruits and vegetables to generalize the findings and potentially revolutionize post-harvest treatments in agro-based industries, further optimizing and characterizing the coatings to enhance their protective qualities and sustainability, possibly by experimenting with different concentrations and combinations of plant extracts, and conducting a comprehensive economic analysis. Another potential future scope for the current work is the use of ultrasonication to the coating solution, which could result in the production of nanoparticles. The morphology, size distribution, and chemical content of the nanoparticles can be studied further using procedures such as scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray diffraction (XRD). Furthermore, investigating the rate of ethylene production and respiration at various storage durations might be a viable area for future research.
Data availability
All data are linked to this manuscript. There is no additional data available to be shared.
References
Liu Y, Heying E, Tanumihardjo SA. History, global distribution, and nutritional importance of citrus fruits. Comp Rev Food Sci Food Safety. 2012;11(6):530–45. https://doi.org/10.1111/j.1541-4337.2012.00201.x.
Luro F, Venturini N, Costantino G, Paolini J, Ollitrault P, Costa J. Genetic and chemical diversity of citron (Citrus medica L.) based on nuclear and cytoplasmic markers and leaf essential oil composition. Phytochemistry. 2012;77:186–96. https://doi.org/10.1016/j.phytochem.2011.12.013.
Statistics BB. Statistical yearbook of Bangladesh. Statistics Division, Ministry of Planning, Dhaka, Government of the People’s Republic of Bangladesh. 2011.
Kaysar MI, Hoq MS, Mia MS, Islam MS, Islam MM. An economic analysis of Jara and Colombo lemon production in Bangladesh. J Bangladesh Agric Univ. 2017;15(2):289–96.
Saha JK, Koiry S, Datta T. Technical, Al locative and Economic Efficiency of Citron (Jara Lebu) Cultivation in Some Selected Areas of Sylhet District in Bangladesh.
Hasan M, Farid MS, Marium B, Begum M. Post-harvest loss assessment and marketing practices of fruits: an empirical study of Maulvibazar District in Bangladesh. J Econ Manag Trade. 2022. https://doi.org/10.9734/jemt/2022/v28i130385.
Hagenmaier RD. The flavor of mandarin hybrids with different coatings. Postharvest Biol Technol. 2002;24(1):79–87. https://doi.org/10.1016/S0925-5214(01)00121-1.
Singh AK. Recent advancements in polysaccharides, proteins and lipids based edible coatings to enhance guava fruit shelf-life: a review. Int J Biol Macromol. 2024;262: 129826. https://doi.org/10.1016/j.ijbiomac.2024.129826.
Kumar N, Upadhyay A, Shukla S. Effects of essential oils and ultrasonic treatments on properties of edible coatings and their application on citrus fruits. Starch-Stärke. 2023. https://doi.org/10.1002/star.202300104.
Kumar Pandey V, Shams R, Singh R, Dar AH, Pandiselvam R, Rusu AV, Trif M. A comprehensive review on clove (Caryophyllus aromaticus L.) essential oil and its significance in the formulation of edible coatings for potential food applications. Front Nutr. 2022;9:987674. https://doi.org/10.3389/fnut.2022.987674.
Kumar N, Upadhyay A, Shukla S, Bajpai VK, Kieliszek M, Yadav A, Kumaravel V. Next generation edible nanoformulations for improving post-harvest shelf-life of citrus fruits. J Food Meas Charact. 2024;18(3):1825–56. https://doi.org/10.1007/s11694-023-02287-8.
Pandey VK, Srivastava S, Singh R, Dar AH, Dash KK. Effects of clove essential oil (Caryophyllus aromaticus L.) nanoemulsion incorporated edible coating on shelf-life of fresh cut apple pieces. J Agric Food Res. 2023;14:100791. https://doi.org/10.1016/j.jafr.2023.100791.
Pandey VK, Islam RU, Shams R, Dar AH. A comprehensive review on the application of essential oils as bioactive compounds in Nano-emulsion based edible coatings of fruits and vegetables. Appl Food Res. 2022;2(1): 100042. https://doi.org/10.1016/j.afres.2022.100042.
Mohammadi M, Rastegar S, Rohani A. Enhancing shelf-life and quality of Mexican lime (Citrus aurantifolia cv.) fruit: utilizing edible coating from wild sage seeds enriched with pomegranate seed oils. J Food Meas Charact. 2024;18(1):331–44. https://doi.org/10.1007/s11694-023-02176-0.
Huber KC, Embuscado ME, editors. Edible films and coatings for food applications. New York: Springer, New York; 2009. https://doi.org/10.1007/978-0-387-92824-1.
Adetunji CO, Fawole OB, Oloke JK, Adetunji JB, Makanjuola OR. Effect of edible coatings from aloe vera gel on citrus sinensis during ambient storage. 2012; 11(1):77–84.
He Q, Changhong L, Kojo E, Tian Z. Quality and safety assurance in the processing of Aloe vera gel juice. Food Control. 2005;16(2):95–104. https://doi.org/10.1016/j.foodcont.2003.12.001.
Nouri L, Nafchi AM. Antibacterial, mechanical, and barrier properties of sago starch film incorporated with betel leaves extract. Int J Biol Macromol. 2014;66:254–9. https://doi.org/10.1016/j.ijbiomac.2014.02.044.
Maftoonazad N, Ramaswamy HS. Postharvest shelf-life extension of avocados using methyl cellulose-based coating. LWT-Food Sci Technol. 2005;38(6):617–24. https://doi.org/10.1016/j.lwt.2004.08.007.
Formiga AS, Junior JS, Pereira EM, Cordeiro IN, Mattiuz BH. Use of edible coatings based on hydroxypropyl methylcellulose and beeswax in the conservation of red guava ‘Pedro Sato.’ Food Chem. 2019;290:144–51. https://doi.org/10.1016/j.foodchem.2019.03.142.
Ahmed A, Ali SW, Imran A, Afzaal M, Arshad MS, Nadeem M, Mubeen Z, Ikram A. Formulation of date pit oil-based edible wax coating for extending the storage stability of guava fruit. J Food Process Preserv. 2020;44(2): e14336. https://doi.org/10.1111/jfpp.14336.
Zinia SA, Nupur AH, Karmoker P, Hossain A, Jubayer MF, Akhter D, Mazumder MA. Effects of sprouting of soybean on the anti-nutritional, nutritional, textural and sensory quality of tofu. Heliyon. 2022. https://doi.org/10.1016/j.heliyon.2022.e10878.
Pathare PB, Opara UL, Al-Said FA. Colour measurement and analysis in fresh and processed foods: a review. Food Bioprocess Technol. 2013;6:36–60. https://doi.org/10.1007/s11947-012-0867-9.
AOAC. Association of Official Analytical Chemists, official methods of analysis, AOAC international. 20th edition. Revision. Washington DC. 3172, 2016.
Shanta SS, Ahmed T, Jubayer MF, Sharma M, Sridhar K, Hoque MM, Rana MR, Inbaraj BS. Effect of taro corm mucilage and black seed oil as edible coatings on the shelf-life and quality of fresh guava. Agronomy. 2023;13(2):538. https://doi.org/10.3390/agronomy13020538.
Guntarti A, Ahda M, Nabilla H, Susanti H. The storage effect against Vitamin C content in crystal guava (Psidium guajava L.) Juice. J Sci Islamic Republic of Iran. 2021;32(1):39–42. https://doi.org/10.22059/jsciences.2020.312239.1007585.
Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144–58. https://doi.org/10.5344/ajev.1965.16.3.144.
Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25–30. https://doi.org/10.1016/S0023-6438(95)80008-5.
Benzie IF, Strain JJ. Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in enzymology 1999 (Vol. 299, pp. 15–27). Academic press. https://doi.org/10.1016/S0076-6879(99)99005-5.
Hajlaoui H, Trabelsi N, Noumi E, Snoussi M, Fallah H, Ksouri R, Bakhrouf A. Biological activities of the essential oils and methanol extract of tow cultivated mint species (Mentha longifolia and Mentha pulegium) used in the Tunisian folkloric medicine. World J Microbiol Biotechnol. 2009;25:2227–38. https://doi.org/10.1007/s11274-009-0130-3.
Naeem A, Abbas T, Ali TM, Hasnain A. Application of guar gum-based edible coatings supplemented with spice extracts to extend post-harvest shelf life of lemon (Citrus limon). Qual Assur Safety Crops Foods. 2019;11(3):241–50. https://doi.org/10.3920/QAS2018.1310.
Khatri S, Shrestha S, Pokharel KP. Evaluation of manual fruit harvesters and storability characteristics of harvested sweet orange under ordinary room storage condition. Sustain Food Agric (SFNA). 2021;2(2):84–91. https://doi.org/10.26480/sfna.02.2021.84.91.
Mahajan BV, Singh R, Kumar M. Quality assurance and shelf-life extension of Kinnow mandarin fruit under supermarket conditions. Int J Fruit Sci. 2016;16(1):94–102. https://doi.org/10.1080/15538362.2015.1061959.
Alhassan AF, Adjei PY, Mohammed S. Effect of maturity stage and storage duration on physico-chemical properties of citrus (Citrus sinesis var. Late Valencia). Eur Sci J. 2014;10(36).
Faasema J, Abu JO, Alakali JS. Effect of packaging and storage condition on the quality of sweet orange (Citrus cinesis). J Agric Technol. 2011;7(3):797–804.
Majeed T, Dar AH, Pandey VK, Dash KK, Srivastava S, Shams R, Jeevarathinam G, Singh P, Echegaray N, Pandiselvam R. Role of additives in starch-based edible films and coating: a review with current knowledge. Prog Org Coat. 2023;181: 107597. https://doi.org/10.1016/j.porgcoat.2023.107597.
Maftoonazad N, Ramaswamy HS. Application and evaluation of a pectin-based edible coating process for quality change kinetics and shelf-life extension of lime fruit (Citrus aurantifolium). Coatings. 2019;9(5):285. https://doi.org/10.3390/coatings9050285.
Huang R, Xia R, Lu Y, Hu L, Xu Y. Effect of pre-harvest salicylic acid spray treatment on post-harvest antioxidant in the pulp and peel of ‘Cara cara’ navel orange (Citrus sinenisis L. Osbeck). J Sci Food Agric. 2008;88(2):229–36. https://doi.org/10.1002/jsfa.3076.
Sen P, Rafi KN, Uddin Z, Aziz MG. Evaluation of postharvest shelf life and quality of lemon treated with different coatings during storage. Food Sci Eng. 2020;1(2):74–84.
Ahmed MS, Sanjay S. Studies on extension of storage life of Amrapali mango. Orissa J Horticult. 2000;28(2):73–6.
Bisen A, Pandey SK, Patel N. Effect of skin coatings on prolonging shelf life of kagzi lime fruits (Citrus aurantifolia Swingle). J Food Sci Technol. 2012;49(6):753–9. https://doi.org/10.1007/s13197-010-0214-y.
Nasirifar SZ, Maghsoudlou Y, Oliyaei N. Effect of active lipid-based coating incorporated with nanoclay and orange peel essential oil on physicochemical properties of Citrus sinensis. Food Sci Nutr. 2018;6(6):1508–18. https://doi.org/10.1002/fsn3.681.
Contreras-Oliva A, Rojas-Argudo C, Pérez-Gago MB. Effect of solid content and composition of hydroxypropyl methylcellulose–lipid edible coatings on physico-chemical and nutritional quality of ‘Oronules’ mandarins. J Sci Food Agric. 2012;92(4):794–802. https://doi.org/10.1002/jsfa.4649.
Saberi B, Golding JB, Chockchaisawasdee S, Scarlett CJ, Stathopoulos CE. Effect of biocomposite edible coatings based on pea starch and guar gum on nutritional quality of “Valencia” orange during storage. Starch-Stärke. 2018;70(5–6):1700299. https://doi.org/10.1002/star.201700299.
Liu S, Lou Y, Li Y, Zhang J, Li P, Yang B, Gu Q. Review of phytochemical and nutritional characteristics and food applications of Citrus L. fruits. Front Nutr. 2022;9:968604. https://doi.org/10.3389/fnut.2022.968604.
Wedamulla NE, Fan M, Choi YJ, Kim EK. Citrus peel as a renewable bioresource: transforming waste to food additives. J Funct Foods. 2022;95: 105163. https://doi.org/10.1016/j.foodres.2020.109114.
Jurić S, Bureš MS, Vlahoviček-Kahlina K, Stracenski KS, Fruk G, Jalšenjak N, Bandić LM. Chitosan-based layer-by-layer edible coatings application for the preservation of mandarin fruit bioactive compounds and organic acids. Food Chem: X. 2023;17: 100575. https://doi.org/10.1016/j.fochx.2023.100575.
Bonilla J, Atarés L, Vargas M, Chiralt A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocolloids. 2012;26(1):9–16. https://doi.org/10.1016/j.foodhyd.2011.03.015.
Gil-Izquierdo A, Gil MI, Ferreres F. Effect of processing techniques at industrial scale on orange juice antioxidant and beneficial health compounds. J Agric Food Chem. 2002;50(18):5107–14. https://doi.org/10.1021/jf020162+.
Haider ST, Ahmad S, Anjum MA, Naz S, Liaqat M, Saddiq B. Effects of different postharvest techniques on quality management and shelf life of ‘Kinnow’ mandarin fruit. J Food Meas Charact. 2021;15:2549–61. https://doi.org/10.1007/s11694-021-00820-1.
Acknowledgements
Nothing to declare.
Funding
We express our gratitude to the UGC-funded SAURES (Sylhet Agricultural University Research System) for providing financial support for this research endeavor. The funding body provided funds for the execution of experiments, reagents, travel, and data collection. The authors bear the entire cost of publishing the research.
Author information
Authors and Affiliations
Contributions
Md. Mahfuzur Rob and Md. Fahad Jubayer: Conceptualization, supervision, writing original draft, reviewing and editing; Md. Mahfujul Haque Pappu, Tahsin Nusrat Era, and Masuma Zahan Akhi: Data collection, formal analysis, writing, reviewing, and editing; Md. Shoaib Arifin: Formal analysis, writing original draft, reviewing and editing; Debu Kumar Bhattacharjya and Md. Shahidullah Kayshar: writing, reviewing, and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Sylhet Agricultural University Research System (SAURES), Sylhet, Bangladesh. The experiments pertaining to sensory evaluation were carried out in adherence to established ethical guidelines, wherein each panelist was required to provide informed written consent prior to participating in the study. It is important to note that there are no strict ethical approval requirements for sensory tests in Bangladesh.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rob, M.M., Pappu, M.M.H., Arifin, M.S. et al. Application and evaluation of plant-based edible active coatings to enhance the shelf-life and quality attributes of Jara lebu (Citrus medica). Discov Food 4, 26 (2024). https://doi.org/10.1007/s44187-024-00094-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44187-024-00094-8