Abstract
Silver nanoparticles (AgNPs) have shown a wide range of antimicrobial activities over the last 2 decades, but little is known about their antimalarial activity. Therefore, in the present study, AgNPs were surface functionalized by chalcones to create an efficient bioactive molecular surface that can enhance the antimalarial competency of both chalcones as well as chemically synthesized AgNPs. The AgNPs-conjugated chalcones have been synthesized using a chemical method employing the EDC-NHS coupling method. The characterization of AgNPs and AgNPs-conjugated chalcones was done through various analytical techniques. The SYBR Green I assay was performed for in vitro antimalarial activity, and cell cytotoxicity was done on HeLa cell line with MTT assay to calculate the IC50 and CC50, respectively. Haemolytic effect on fresh RBCs of these nanoconjugates were observed for 3 h and 24 h. AgNPs and AgNPs-conjugated chalcones have spectra at 420 nm and between 350 and 375 nm, respectively. The IC50 values of all the three conjugates for antimalarial activity ranged from 0.30 to 0.80 μg/mL. The present study provides a new method of synthesizing AgNPs-conjugated chalcones. Also, these synthesized conjugates show better antimalarial potential and reduced cellular toxicity compared to bared chalcones under an in vitro culture system. However, a further pre-clinical study on the murine model of malaria along with toxicity parameters is needed to provide more clarity.
Graphical abstract

Article Highlights
-
Chalcones have versatile pharmacological properties and can be conjugated with silver nanoparticles to enhance their antimalarial activity.
-
The activity of these synthesized nanoconjugates was highly dependent on exposure time and concentration.
-
The synthetic procedure can be performed with ease, is reproducible with similar chalcone scaffolds, and is inexpensive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Malaria is an ancient human disease that is caused by five species of Plasmodium, namely P. falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi. Among these five, P. falciparum is more prevalent and causes the greatest morbidity and mortality. The disease is mostly endemic in tropical countries, but due to climate change, travellers and immigrants from malaria endemic regions, it could spread to other regions [1]. Nevertheless, the occurrence and spread of drug resistance towards various antimalarial drugs pose a serious danger to initiatives aimed at eliminating malaria [2]. To prevent this, new antimalarial drugs or tools that have broad therapeutic potential, have fewer side effects, have maximum accessibility, and have novel mechanisms of action are urgently needed. The development of antimalarial drugs can take a number of different paths, from tweaking already-existing agents to creating novel drugs that can act on distinct targets [3]. In this context, nanotechnology based formulations manifest to surpass the restrictions of existing medications related to ideal therapeutic benefits, cost effectiveness, and safety and can also help in patients’ compliance with treatment [4]. Among various nanotechnological based drugs and delivery systems, metal nanoparticles are one of them and were chiefly evaluated for their biocidal action against bacteria [5,6,7], fungi [8, 9], and viruses [10, 11]. However, very little is known about the antimalarial potential of metal nanoparticles [12]. Metallic nanoparticles like silver have been found to be an ideal material for a variety of biomedical applications and have been extensively explored in the last few years [13, 14]. The action of silver-based drugs seems to be extremely desirable, as silver nanoparticles (AgNPs) could provide relief from bioavailability problems as they act as a reservoir of Ag+ ions inside the cell, released close to the molecular targets [15]. According to the previous studies, AgNPs are hazardous for prokaryotic organisms [16], but have been found to be comparatively safe for the various eukaryotic species, which include humans as well. Their cytotoxicity is linked to a number of properties, such as reactivity in solution, size distribution, shape, coating or capping, etc., all of which depend on the synthetic process employed to make them [17]. The AgNPs have shown strong action against the vector (female Anopheles mosquito) and malarial pathogen (P. falciparum). Moreover, AgNPs are anticipated to provide a malaria control strategy [17]. Alternatively, metal nanoparticles are also very promising armamentariums in the development of novel antimicrobial agents, due to their nontoxicity, high ability for functionalization, and polyvalent effects [18]. Their cellular uptake is influenced by their form, surface charge, and size. Moreover, the functionalities on the surface of a few metal nanoparticles amplify their cellular interactivity [19].
Chalcones provide a huge collection of bioactive substances with numerous different molecular targets. Chalcones are basically structural derivatives of 1,3-diphenylprop-2-en-1-one. They are members of the flavonoids family and are widely present in various natural plant products [20]. In previous studies, chalcones have exhibited potent antimalarial activity, and these studies revealed the importance of minor structural changes in the chalcone scaffold that can lead to targeting numerous cellular processes [21,22,23]. Therefore, in the present study, the AgNPs were conjugated to chalcone derivatives to surface functionalize for an efficient biomolecular surface and thereby enhance the antimalarial competency of both AgNPs and chalcones in comparison to being used in their bared form.
2 Materials and methods
2.1 2.1. Chemicals and reagents
The chalcone derivatives used in this study are named, (E)-1-(2,5-Dimethoxyphenyl)-3-(4- methoxyphenyl)prop-2-en-1-one, (1); (E)-(3,4,5-Trimethoxyphenyl)-3-(4- methoxyphenyl)prop-2-en-1-one, (2); and, (E)-1-(3,4,5-Trimethoxyphenyl)-3-(3,4- dimethoxyphenyl)prop-2-en-1-one, (3). These three chemically synthesized chalcones were (E)-3-(4-methoxyphenyl)prop-2-en-1-one derivatives with varying numbers of methoxy substituents and published by our group, as shown in Fig. 1 [24, 25].
The other chemicals, drugs, and reagents such as silver nitrate (AgNO3), chloroquine diphosphate, (N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), RPMI 1640, sodium bicarbonate, Gentamycin, dimethyl sulfoxide (DMSO), Dulbecco’s Modified Eagle’s Medium (DMEM), and Trypsin EDTA solution (0.25%) were purchased from Sigma Aldrich, USA. SYBER Green I Nucleic Stain was purchased from Invitrogen by Thermo Fisher Scientific, USA, and Fetal bovine sera, research grade, sterile was purchased from HiMEDIA. In addition, other chemicals that were required in this study were of analytical grade.
2.2 Synthesis of nanomaterials
2.2.1 Synthesis of AgNPs
AgNPs were synthesized at a small laboratory scale by the previously described chemical reduction method [26]. Briefly, in a standard experiment, 50 ml of 0.001 M AgNO3 were heated until boiling. Afterwards, 5 mL of 1% trisodium citrate were added to this solution. During the entire process, solutions were vigorously mixed and heated until a visible change of color was seen (from a pale yellow to a greenish-yellow solution). The solution was then removed from the heating apparatus and stirred until it cooled to normal room temperature [26]. The observed change in color signifies the formation of AgNPs, and this was later validated through different characterization techniques.
2.2.2 Synthesis of AgNPs conjugated-chalcones derivative 1, 2 and 3
For conjugation of chalcone derivatives 1, 2, and 3, EDC-NHS coupling chemistry has been adopted. Briefly, 1 mg of crystalline chalcones dissolved in acetone was added to the EDC and Sulfo-NHS. The weight ratio of EDC-Sulfo NHS was kept at 1:2 in this reaction. After that, the solution was mixed properly and allowed to react by putting it on a rotary shaker at 25 °C for 2–3 h, and further AgNPs were finally added and kept at a rotary shaker at 180 rpm for overnight at 25–28 °C. These conjugates were then stored at 4 °C. Before experimentation, the AgNPs-chalcones solution was then centrifuged at 10,000 rpm to remove unattached chalcones. This was done two times to ensure that no free chalcone molecules are left in the final conjugates. The final solution was used for characterization.
2.3 Characterization of nanomaterials
The characterization of synthesized AgNPs and AgNPs-conjugated chalcone derivatives 1, 2, and 3 was done using various techniques such as Ultraviolet–visible (UV–Vis) spectroscopy, Zeta potential analyser, Fourier-transform infrared spectroscopy (FTIR), and Transmission Electron Microscopy (TEM).
2.3.1 UV–vis spectroscopy
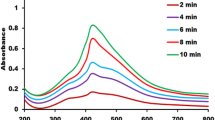
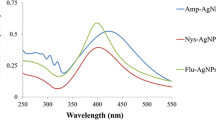
The UV–Vis spectrophotometer (Labindia Analytical UV–VIS 2000 Spectrophotometer) was used to record the UV–visible spectrum of synthesized AgNPs and AgNPs-conjugated chalcone derivatives 1, 2, and 3 at 1 nm resolution. The scanning range was used from 200 to 800 nm with a 1 cm optical path, and each reading was taken at room temperature using a quartz cuvette. By noticing a peak between a region of 400–435 nm and 340–370 nm, this spectrometry was utilised to confirm the synthesis of AgNPs and AgNPs-conjugated chalcones.
2.3.2 Zeta potential analysis
Zeta potential instruments are mainly used for evaluating the surface charge of nanoparticles, either in solid or solution form. In the present study, the surface charges of synthesized nanoparticles and conjugates were measured using Zetasizer Nano ZS, Malvern Instruments Ltd., UK. For this, 12 zeta runs were carried out at 25 °C using 1.5 mL of the synthesized AgNPs and AgNPs-conjugated chalcones solution in a folded capillary zeta cell [27]. A temperature equilibration time of 1 min at 25 °C was used for all measurements, which were performed in triplicate.
2.3.3 Fourier transform infrared spectroscopy analysis
FTIR analysis of the synthesized AgNPs and AgNPs-conjugated chalcones were done by scanning these nanomaterials in the range of 400 to 4,000 cm−1. These analyses were performed on a Perkin-Elmer spectrum at a resolution of 4 cm−1 in the diffuse reflectance mode.
2.3.4 Transmission electron microscopy
The synthesized AgNPs and AgNPs-conjugated chalcones were characterized for their morphological properties, such as size and shape, through TEM using a JEOL JEM 1400 Plus electron microscope operating at a 110 kV accelerating voltage. For this, sample preparation involves the placement of 10 μL of each sample suspension on formvar-coated, 400-mesh copper grids, which were then allowed to air-dry for 1 h.
2.4 Antimalarial activity
2.4.1 Parasites and culture
P. falciparum strain, 3D7 (sensitive to chloroquine) was obtained from the National Institute of Malaria Research (NIMR), New Delhi, India, and cultured and maintained in the Department of Medical Parasitology, PGIMER, Chandigarh, for this study. The strain was maintained in vitro in continuous culture using a modified version of Trager and Jensen's (1976), technique [28]. Briefly, the P. falciparum 3D7 strain was cultured in O + erythrocytes in RPMI-1640 medium (included with glutamine and no sodium bicarbonate). This medium was further constituted with 5.94 g of HEPES buffer, 1 g of dextrose, and 40 μg/L of gentamycin. Sodium bicarbonate (5%) and inactivated human AB + serum (10% v/v) were additionally supplemented in the medium before subculturing. After that, culture plates were incubated in an optimum gas mixture of 5% O2, 5% CO2, and 90% N2 at 37 °C. Parasitized erythrocytes were suspended in the aforementioned culture medium at an initial 5% haematocrit level, and parasitaemia levels were monitored often to be kept between 2 and 4%, with further sub-culturing for parasitaemia above 5% microscopy. Giemsa-stained slides of culture were done daily to track the parasite's growth and reproduction.
2.4.2 SYBR green I drug sensitivity assay
In vitro antimalarial activity was determined through a SYBR Green I fluorescence-based protocol [29, 30]. Briefly, drugs and synthesized nanomaterials were added in twofold serial dilutions, diluted in RPMI-1640 medium, to 96-well plates in a concentration range of 12.5 to 0.19 μg/mL. Asynchronous P. falciparum parasites were diluted in uninfected erythrocytes at 2% haematocrit and 0.2% parasitemia and added to each well to make a final volume of 200 μL. Plates were then incubated at 37 °C for 72 h in an atmosphere containing 5% O2, 5% CO2, and 90% N2. After that, parasites were lysed using SYBR Green I lysis buffer (0.2 μL SYBR Green I/mL MSF) and incubated for 60 min in the dark. Utilising a Tecan Genios Plus plate reader with excitation and emission bands centred at 485 and 530 nm, respectively, with a gain setting of 50, SYBR Green I signal was measured. Fifty percent (50%) inhibitory concentrations (IC50) were analysed using HN-NonLin regression analysis software [31].
2.5 Haemolytic activity
Haemolytic effect of all synthesized nanoparticles and conjugates, and standard antimalarial drugs was determined by incubating the fresh normal erythrocytes with the synthesized nanoparticles and conjugates in phosphate-buffered saline (PBS), respectively. Briefly, fresh erythrocytes suspended in PBS were centrifuged for only 5 min at 1600 rpm three times, and then the residual pellet was re-suspended in PBS at 2% haematocrit. Controls included 0.4% Triton X-100 in PBS (for 100% haemolysis) and PBS alone (for baseline values). After incubation at 37 °C for 3 h and 24 h, the samples were then centrifuged, and the supernatant was used to calculate the haemolytic activity, which has been quantified in terms of haemoglobin release. This was monitored by measuring the absorbance at 415 nm. The experiment was performed in triplicate, and the data was represented as the mean ± SD [32, 33].
2.6 Cytotoxicity
Cytotoxicity of the synthesized nanoparticles and conjugates on mammalian cells was accomplished by employing the HeLa cell line (NCCS, Pune), which was cultured in DMEM supplemented with fetal bovine serum (10% v/v) by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) micro-enzymatic method with a few modifications [34]. Briefly, the cells were incubated with different dilutions of synthesized nanomaterials for 24 h, 48 h, & 72 h, and MTT was used as a reagent for the detection of cytotoxicity. MTT as a stock solution (5 mg/mL in 1X PBS) was freshly prepared, and 20 μL was added into each well, mixed properly, and incubated at 37 °C for at least 3–4 h. After incubation, a short centrifugation for 5 min at 1500 rpm was done, and the supernatant was carefully disposed of. After that, 100 μL of DMSO were pipetted to each well in order to lyse the cell. This action results in the dissolution of the insoluble purple formazan product into a coloured solution. Absorbance was taken at 570 nm to evaluate formazan formation, as an assessment of cell viability. Experiments were done in triplicate. Utilising MS Excel for nonlinear regression analysis of dose response curves, the 50% cytotoxic concentration (CC50) was evaluated.
2.7 Data analysis
All parameters were assessed in triplicate (n = 3) for each sample, and the results were presented as mean ± SD.
3 Results and discussion
3.1 Visual observation
The synthesized nanoparticles were first characterized by visible observation as a change in color from pale light yellow to a greenish yellow solution. This color change of the solution is the first indicator of the formation of AgNPs and was mainly because of the excitation of surface plasmon resonance, which specifies the AgNPs synthesis by reduction of Ag + to Ag0 [35], shown in Fig. 2. Further confirmation of synthesized AgNPs and their stability was done by using other techniques such as UV–vis spectroscopy, Zeta Potential, FTIR-spectroscopy, and TEM analysis.
3.2 UV–vis spectroscopy
The UV–vis spectroscopy measures characteristic surface plasmon resonance absorption peaks to show the synthesis of both bared and AgNPs-conjugated chalcones. This method is among the most effective, straightforward, sensitive, and popular ways to verify the initial synthesis of AgNPs [36]. For each sample, deionized water was taken as a control. In the present study, AgNPs samples showed a peak at 420 nm, as shown in Fig. 3a. According to studies by Ider et al. [37], and Saxena et al. [38] AgNPs exhibits a plasmon resonance absorption peak mainly under the visible range of 380–500 nm owing to the excitation of surface plasmon vibration. The observed band in this range has been associated with AgNPs [39], confirming the synthesis of spherical-shaped AgNPs, which has also been confirmed through TEM in the present study. The UV–vis of three AgNPs-conjugated chalcone derivatives shows two spectrum peaks, i.e., at 360 nm (1), 360–365 nm (2), and 355–365 nm (3), that correspond to 3 chalcones derivatives, and other at 420 nm for AgNPs, as shown in Fig. 3b, c, & d.
3.3 Zeta potential
The electrostatic charge and stability of the functionalized nanomaterials (AgNPs) with 3 chalcone derivatives 1, 2, & 3, were determined using a Zetasizer Nano S (Malvern Instruments Ltd.), as shown in Table 1.
The zeta potential relies on the surface charge and is crucial to determining the stability of nanoparticles in suspension. Moreover, zeta potential is also a deciding factor during the initial adsorption of nanoparticles onto the cell membrane [27]. It can also affect the pharmacokinetic characteristics of nanosystems within the body or the phagocytosis of the nanoparticles in the blood [27]. The zeta potential in this study was found to be -12.30 ± 0.32 for bared AgNPs and − 12.60 ± 0.50, − 7.44 ± 1.83, and − 14.06 ± 0.73 mV, respectively, for AgNPs-conjugated chalcones. Nanoparticles with zeta potentials of more than + 30 mV or less than − 30 mV are thought to be strongly cationic, while those with a zeta potential between − 10 and + 10 mV are thought to be roughly neutral [40]. AgNPs-Chalcone Deriv.2 has the lowest zeta potential (− 7.44 ± 1.83) due to existence of more electronegative atoms in the structure, that reflect its neutral nature.
3.4 Fourier transform infrared spectroscopy analysis
FTIR analysis measures the interaction of infrared radiation with a test substance to ascertain their molecular structure and composition. The results of the FTIR analysis in this study show distinct stretches of the bond at altered peaks. The bared AgNPs show the presence of O–H stretching (around 3213 cm−1), O=C=O stretching at 2354 cm−1; N–O stretching at 1558 cm−1; C–C and C–N stretching at 1,385 cm−1; O–H stretching at 1075 cm−1, as shown in Fig. 4a. However, there was a shift and appearance of new peaks in AgNPs-conjugated chalcones, that were observed at 1659 cm−1 (C=C stretching; alkene); 1337 cm−1 (O–H bending); 1267 cm−1 (C–O stretching); 1170–1023 cm−1 (C–O stretching); 821 cm−1 (C=C bending), shown in Fig. 4b, c, & d. Also, the peaks that appear at 860–680 cm−1 in the three AgNPs-conjugated chalcones are helpful in determining the ortho-meta-para substitution patterns for aromatics.
3.5 Transmission electron microscopy
TEM was used to visualize the morphology (shape and size) of nanoparticles. The TEM images of the prepared AgNPs are shown in Fig. 5a. Almost all the AgNPs particles appear spherical in shape, with variations in sizes ranging from 200 to 50 nm. Moreover, the three AgNPs-conjugated chalcones are also spherical in shape and measures between 100 and 50 nm, as shown in Fig. 5b, c, & d. The endocytic uptake rate after adsorption is influenced by particle size. Thus, nanoparticle toxicity is influenced by the zeta potential and size [41], which allows for specifying the cellular targets [42].
3.6 Antimalarial activity
The antimalarial activity was evaluated by the SYBR Green I fluorescence-based method on P. falciparum 3D7 strains. This method determines the presence of Plasmodium parasite DNA in infected erythrocytes, which is directly related to parasite growth and development and parasite growth inhibition by various antimalarial drugs [43]. The concentration of drug that is required to inhibit the parasite growth by 50% and 90%, means IC50 and IC90 values gained after incubation of 72 h were calculated using HN-NonLin V1.1 as depicted in Table 2. The AgNPs in this study show an IC50 at 0.66 μg/mL after 72 h of incubation, which is somewhat similar to the previous observations illustrated by Vossen et al. [44], showing a substantial decrease in parasitemia in 48 h.
3.7 Haemolysis
Erythrocytes are frequently used as models for mammalian cell membranes, and a variety of mammalian species have had their inner and outer leaflet compositions thoroughly examined [45]. Also, the simple method to isolate erythrocytes makes haemolytic assays one of the most versatile assays for quick assessment of initial toxicity [46], and therefore they are usually employed to measure membrane active antimicrobial agents and other novel compounds [47]. In the present study, fresh erythrocytes were treated with AgNPs and AgNPs-conjugated chalcone derivatives 1, 2, and 3 and incubated for different time intervals (3 h and 24 h) at different concentrations in a serial dilution range of 12.5–0.40 μg/mL. Table 3 illustrates minimum percentages haemolysis below 1%, of the nanomaterials at concentrations of 6.25 μg/mL and 12.5 μg/mL in contrast to the standard control triton X-100 (100% haemolysis). The tested haemolytic values were around 12 and 25 times higher than the IC50 obtained in the antimalarial test of these compounds, which illustrates the non-toxic nature of these conjugates on fresh erythrocytes. Moreover, haemolytic effect of each nanomaterial has increased as the time of incubation has increased.
3.8 Cytotoxicity
During the assessment of nanomaterials, cell viability studies are crucial for providing data on cell survival, metabolic condition, and cell death mode of treated cells [48]. The cytotoxicity of the synthesized nanomaterials in the present study on the HeLa cell line revealed 50% inhibitory cellular cytotoxicity (CC50) at concentrations between 0.80 and 25.00 μg/mL. Table 4 provides a summary of the findings. Time is a critical component of drug activity, as the duration of inhibition of the target or residence time of the drug molecule on the target often determines drug timing [49]. Therefore the cytotoxicity of each synthesized nanoconjugates has been evaluated at different time points, i.e., 24 h, 48 h, and 72 h. The obtained CC50 of each synthesized nanoconjugates shows an increase in toxicity at the cellular level with the increase in exposure over time. Additionally, percentage cell viability was observed to be higher, when HeLa cells were exposed to lower concentrations (12.5 μg/mL) as compared to higher concentrations (25 μg/mL) of each drug and nanoconjugate at three different time points, as shown in Fig. 6. The results also demonstrated that the proliferation of cells was inhibited in a dose- and time dependent manner when drugs or nanoconjugates were exposed for 24 h or 48 h, but at longer exposure, i.e., 72 h, there was a varied response of drugs and nanoconjugates to viable cells. This illustrates that the activity of these synthesized nanoconjugates was highly dependent on exposure time and concentration.
4 Conclusion
Functionalization of nanoparticles involves the conjugation of molecules on the surface of the particles. In the fight against several bacteria, AgNPs have proven to be a viable alternative [50]. In the medical field, functionalizing AgNPs has proven effective for improving medical devices or delivering drugs, and it can also boost the antibacterial activity of these nanoparticles [51]. Moreover, chalcones are well known for their antimalarial activity, but due to their toxicity and low bioavailability, they mostly fail to provide optimum results. The present study illustrates the very first time the new chemical method for the synthesis of AgNPs-conjugated chalcone. The main advantages of this method of synthesis are its reproducibility with similar chalcone scaffolds and can be synthesized easily and inexpensively with minimal chemical and equipment needs. Functionalization of AgNPs with chalcones with known antimalarial activity enhances the antimalarial potential of both AgNPs as well as chalcones in their conjugated form as compared to their bared form. Additionally, these synthesized conjugates have lesser cytotoxicity and prevent haemolysis of normal RBCs even if treated for a longer duration of time. Therefore, the study underline that the surface functionalization of AgNPs could enhance the antimalarial potential and reduce cellular toxicity, and it can be explored further at the preclinical level to check their both toxic and antimalarial activity in an in vivo system, which would provide a more elaborative conclusion. Moreover, a positive outcome of this study may help combat antimalarial drug resistance in the future.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Mischlinger J, Rönnberg C, Álvarez-Martínez MJ, Bühler S, Paul M, Schlagenhauf P, Petersen E, Ramharter M. Imported malaria in countries where malaria is not endemic: a comparison of semi-immune and nonimmune travelers. Clin Microbiol Rev. 2020;33(2):e00104-e119.
Shibeshi MA, Kifle ZD, Atnafie SA. Antimalarial drug resistance and novel targets for antimalarial drug discovery. Infect Drug Resist. 2020;10(13):4047–60.
Chowdhury A, Kunjiappan S, Panneerselvam T, Somasundaram B, Bhattacharjee C. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int Nano Lett. 2017;7:91–122.
Gujjari L, Kalani H, Pindiprolu SK, Arakareddy BP, Yadagiri G. Current challenges and nanotechnology-based pharmaceutical strategies for the treatment and control of malaria. Parasite Epidemiol Control. 2022;17: e00244.
Maiti S, Krishnan D, Barman G, Ghosh SK, Laha JK. Antimicrobial activities of silver nanoparticles synthesized from Lycopersicon esculentum extract. J Anal Sci Technol. 2014;5:1–7.
Patra JH, Baek KH. Biosynthesis of silver nanoparticles using aqueous extract of silky hairs of corn and investigation of its antibacterial and anticandidal synergistic activity and antioxidant potential. IET Nanobiotechnol. 2016;10:326–33.
Patra JK, Baek KH. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front Microbiol. 2017;8:167.
Mallmann EJ, Cunha FA, Castro BN, Maciel AM, Menezes EA, Fechine PB. Antifungal activity of silver nanoparticles obtained by green synthesis. Rev Inst Med Trop Sao Paulo. 2015;57(2):165–7.
Arciniegas-Grijalba PA, Patiño-Portela MC, Mosquera-Sánchez LP, Guerrero-Vargas JA, Rodríguez-Páez JE. ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl Nanosci. 2017;7:225–41.
Narasimha G. Virucidal properties of silver nanoparticles synthesized from white button mushrooms (Agaricus bisporus). Int J Nano Dimens. 2016;3:181–4.
Broglie JJ, Alston B, Yang C, Ma L, Adcock AF, Chen W, Yang L. Antiviral activity of gold/copper sulfide core/shell nanoparticles against human norovirus virus-like particles. PLoS ONE. 2015;10(10): e0141050.
Dauda K, Busari Z, Morenikeji O, Afolayan F. Poly (d-l-lactic-co-glycolic acid) based artesunate nanoparticles: formulation, antimalarial and toxicity assessments. J Zhejiang Univ Sci B. 2017;18:977–85.
Patra CR, Mukherjee S, Kotcherlakota R. Biosynthesized silver nanoparticles: a step forward for cancer theranostics? Nanomedicine. 2014;9:1445–8.
Balachandran YL, Girija S, Selvakumar R, Tongpim S, Gutleb AC, Suriyanarayanan S. Differently environment stable bio-silver nanoparticles: study on their optical enhancing and antibacterial properties. PLoS ONE. 2013;8(10):e77043.
Medici S, Peana M, Nurchi VM, Zoroddu MA. Medical uses of silver: history, myths, and scientific evidence. J Med Chem. 2019;62(13):5923–43.
Li WR, Xie XB, Shi QS, Zeng HY, Ou-Yang YS, Chen YB. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol. 2010;85(4):1115–22.
Rai M, Ingle AP, Paralikar P, Gupta I, Medici S, Santos CA. Recent advances in use of silver nanoparticles as antimalarial agents. Int J Pharm. 2017;526(1–2):254–70.
Sánchez-López E, Gomes D, Esteruelas G, Bonilla L, Lopez-Machado AL, Galindo R, Cano A, Espina M, Ettcheto M, Camins A, Silva AM, Durazzo A, Santini A, Garcia ML, Souto EB. Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials. 2020;10(2):292.
Mout R, Moyano DF, Rana S, Rotello VM. Surface functionalization of nanoparticles for nanomedicine. Chem Soc Rev. 2012;41(7):2539–44.
Sinha S, Medhi B, Sehgal R. Chalcones as an emerging lead molecule for antimalarial therapy: a review. J Mod Med Chem. 2013;1:64–77.
Liu M, Wilairat P, Go ML. Antimalarial alkoxylated and hydroxylated chalones: structure-activity relationship analysis. J Med Chem. 2001;44:4443–52.
Cheng P, Yang L, Huang X, Wang X, Gong M. Chalcone hybrids and their antimalarial activity. Arch Pharm. 2020;353(4): e1900350.
Sinha S, Medhi B, Radotra BD, Batovska DI, Markova N, Bhalla A, Sehgal R. Antimalarial and immunomodulatory potential of chalcone derivatives in experimental model of malaria. BMC Complement Med Ther. 2022;22(1):330.
Sinha S, Batovska DI, Medhi B, Radotra BD, Bhalla A, Markova N, Sehgal R. In vitro anti-malarial efficacy of chalcones: cytotoxicity profile, mechanism of action and their effect on erythrocytes. Malar J. 2019;18(1):421.
Sinha S, Radotra BD, Medhi B, Batovska DI, Markova N, Sehgal R. Ultrastructural alterations in Plasmodium falciparum induced by chalcone derivatives. BMC Res Notes. 2020;13(1):290.
Fang J, Zhong C, Mu R. The study of deposited silver particulate films by simple method for efficient sers. Chem Phys Lett. 2005;401:271–5.
Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop J Pharm Res. 2013;12:225–64.
Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–5.
Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–6.
Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother. 2007;51(6):1926–33.
Noedl H. Non linear evaluation of malaria drug sensitivity data (HN-NonLin V1.1) Bangkok, Thailand: armed forces research institute for medical sciences; (2002) http://www.meduniwien.ac.at/user/harald.noedl/malaria/download.html.].
Sharma P, Sharma JD. In vitro hemolysis of human erythrocytes—by plant extracts with antiplasmodial activity. J Ethnopharmacol. 2001;74:239–43.
Kaushik NK, Sharma J, Sahal D. Anti-plasmodial action of de novo-designed, cationic, lysine-branched, amphipathic, helical peptides. Malar J. 2012;11:256.
Moradhaseli S, Zare Mirakabadi A, Sarzaeem A, Kamalzadeh M, Haji HR. Cytotoxicity of ICD-85 NPs on human cervical carcinoma HeLa cells through Caspase-8 mediated pathway. Iran J Pharm Res. 2013;12(1):155–63.
HI Badiah et al. Synthesis of silver nanoparticles and the development in analysis method 2019 IOP Conf. Ser.: Earth Environ. Sci. 217 012005.
Kumar D, Kumar G, Das R, Agrawal V. Strong larvicidal potential of silver nanoparticles (AgNPs) synthesized using Holarrhena antidysenterica (L.) Wall. bark extract against malarial vector, Anopheles stephensi Liston. Process Saf Environ Protect. 2018;116:137–48.
Ider M, Abderrafi K, Eddahbi A, Ouaskit AS, Kassiba A. Silver metallic nanoparticles with surface plasmon resonance: synthesis and characterizations. J Clust Sci. 2017;28:1051–69.
Saxena A, Tripathi RM, Zafar F, Singh P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater Lett. 2012;67:91–4.
Kumar P, Selvi S, Praba L, Kumar PK, Ganeshkumar RS, Govindaraju M. Synthesis of silver nanoparticles from Sargassum tenerrimum and screening phyto-chemcials for its anti-bacterial activity. Nano Biomed Eng. 2012;4:12–6.
Clogston JD, Patri AK. Zeta potential measurement. Method Mol Biol. 2011;697:63–70.
Schwegmann H, Feitz AJ, Frimmel FH. Influence of the zeta potential on the sorption and toxicity of iron oxide nanoparticles on S. cerevisiae and E. coli. J Colloid Interface Sci. 2010;347(1):43–8.
Rasmussen MK, Pedersen JN, Marie R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat Commun. 2020;11(1):2337.
Avitabile E, Senes N, D’Avino C, Tsamesidis I, Pinna A, Medici S, Pantaleo A. The potential antimalarial efficacy of hemocompatible silver nanoparticles from Artemisia species against P. falciparum parasite. PLoS ONE. 2020;15(9):e0238532.
Vossen MG, Pferschy S, Chiba P, Noedl H. The SYBR green I malaria drug sensitivity assay: performance in low parasitemia samples. Am J Trop Med Hyg. 2010;82(3):398–401.
Helmerhorst EJ, Reijnders IM, Vant Hof W, Veerman EC, Nieuw Amerongen AV. A critical comparison of the hemolytic and fungicidal activities of cationic antimicrobial peptides. FEBS Lett. 1999;449(2–3):105–10.
Farag MR, Alagawany M. Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem Biol Interact. 2018;5(279):73–83.
Pagano M, Faggio C. The use of erythrocyte fragility to assess xenobiotic cytotoxicity. Cell Biochem Funct. 2015;33(6):351–5.
Xiong P, Huang X, Ye N, Lu Q, Zhang G, Peng S, Wang H, Liu Y. Cytotoxicity of metal-based nanoparticles: from mechanisms and methods of evaluation to pathological manifestations. Adv Sci. 2022;9(16): e2106049.
Evans DM, Fang J, Silvers T, Delosh R, Laudeman J, Ogle C, Reinhart R, Selby M, Bowles L, Connelly J, Harris E, Krushkal J, Rubinstein L, Doroshow JH, Teicher BA. Exposure time versus cytotoxicity for anticancer agents. Cancer Chemother Pharmacol. 2019;84(2):359–71.
Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver nanoparticles and their antibacterial applications. Int J Mol Sci. 2021;22(13):7202.
Salleh A, Naomi R, Utami ND, Mohammad AW, Mahmoudi E, Mustafa N, Fauzi MB. The potential of silver nanoparticles for antiviral and antibacterial applications: a mechanism of action. Nanomaterials. 2020;10(8):1566.
Acknowledgements
We are thankful to ICMR, New Delhi for providing financial support in form of Research associate fellowship to Dr. Shweta Sinha, ICMR Ref No. NAN-BMS-45/25/2020. Special thanks are given to Dr. Suman Singh (Principal Scientist, Materials Science and Sensor Applications, CSIO, Chandigarh), for providing a glance on nanomaterials synthesis and Dr Daniela Batovska and Nadezhda Markova, Bulgarian Academy of Sciences, Bulgaria for providing the three synthesized chalcone derivatives.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
S. S.: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing—original draft, Writing—review & editing, Visualization, Project administration, Funding acquisition. A. K.: Methodology, Data curation. R. S.: Resources, Writing—review & editing, Project administration, Funding acquisition, Supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable as no human participant and animal experiments were performed in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sinha, S., Kaur, A. & Sehgal, R. Synthesis and characterization of silver nanoparticle conjugated-chalcones and their evaluation for antimalarial, cytotoxicity and haemolytic potential at in vitro level. Discov Appl Sci 6, 273 (2024). https://doi.org/10.1007/s42452-024-05928-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05928-9