Abstract
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder primarily associated with aging. This devastating condition is characterized by significant memory loss, abnormal behavior, personality shifts, and a decline in cognitive function. Despite extensive research, no cure for Alzheimer’s disease currently exists, and available treatment options have shown limited effectiveness. Developing therapeutic interventions to slow down or prevent the onset and progression of Alzheimer’s disease is crucial to address the growing burden of this condition. Ayurvedic medicinal herbs have emerged as a promising avenue for drug research, with numerous compounds derived from these herbs currently undergoing clinical trials. Scientific studies have explored the potential application of various Ayurvedic medicinal plants and their derivatives in the treatment of Alzheimer’s disease. Although the precise mechanisms of action remain largely unknown, extensive phytochemical investigations have identified a wide range of beneficial compounds within these plants. These compounds include lignans, flavonoids, tannins, polyphenols, triterpenes, sterols, and alkaloids, each exhibiting diverse pharmacological activities. These activities encompass anti-inflammatory, anti-amyloidogenic, anticholinesterase, hypolipidemic, and antioxidant effects. This review highlights the phytochemistry and ethnomedicinal applications of various plants, along with their bioactive compounds. It underscores the potential of Ayurveda, one of the world’s oldest holistic healing systems, in identifying effective therapeutic interventions for neurodegenerative disorders like Alzheimer’s disease. The promising pharmacological activities of Ayurvedic medicinal herbs and their constituents suggest their potential as novel treatments for Alzheimer’s disease. These findings offer hope for addressing the challenges posed by this debilitating condition.
Graphical Abstract

Highlights
-
Highlighted the summarized pathophysiology of the Alzheimer’s disease.
-
Importance of plants in treating Alzheimer’s disease.
-
Bioactive compounds involved in the treatment of Alzheimer’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Globally, Alzheimer disease, the predominant form of age-related dementia, affects approximately 24 million people [1]. The increasing aging population worldwide results in the doubling of patients every 20 years. Therapeutic interventions capable of postponing the onset or slowing the progression of Alzheimer’s disease could drastically reduce these numbers in the next five decades [1]. Alzheimer’s disease primarily targets the elderly, evolving into a significant global health concern as data illustrates that dementia affected 47 million people in 2015, a figure projected to escalate to 131 million by 2050 [1]. Dietary habits, a crucial lifestyle variable, greatly influence Alzheimer’s disease risk, and several studies have correlated its preventive potential with bioactive compounds derived from various food sources [2]. Ample research points towards optimal nutrition as one of the key lifestyle factors that can mitigate AD risk. Balanced nutrition can provide neuroprotection, suggesting that bioactive compounds could influence the core pathogenic pathways of Alzheimer’s disease [3,4,5]. Ever since Alois Alzheimer first characterized pre-senile dementia in 1907, the defining clinicopathological markers of AD have been cognitive impairment in conjunction with the evolution of senile plaques (SP) and neurofibrillary tangles (NFT). Alzheimer’s disease manifests as a consistent decline in cognitive capabilities, spurred by senile plaques in the brain’s hippocampal region. The disease, the most prevalent form of dementia among middle-aged and older adults, currently affects over 5 million Americans. Predictions suggest this number will surge to 7.7 million by 2030 [6]. Neuronal loss in the hippocampus, cortex, and subcortical regions primarily contributes to the disease’s pathophysiology [7].
AD typically begins with subtle symptoms, such as short-term memory loss, trouble learning new information, mood swings, language difficulties, and misplacing objects [8]. As the disease progresses, individuals may experience frustration, hostility, and irritability. In severe cases, Alzheimer’s leads to complete incontinence, total memory loss, and a disconnection from time and place, making patients entirely dependent on others and requiring intensive care [9].
Anti-dementia drugs usage can induce additional health conditions in patients, with effects varying based on individual response and the specific medication. Adverse effects have been reported in patients consuming anti-dementia drugs such as acetylcholinesterase inhibitors and N-methyl-d-aspartate (NMDA) receptor antagonists. Acetylcholinesterase inhibitors frequently cause side effects like nausea, diarrhea, and vomiting, alongside psychological effects like agitation, insomnia, and hallucinations. Patients on NMDA receptor antagonists may experience dizziness and lightheadedness [8,9,10,11]. Reported adverse effects include excitement, insomnia, nausea, vomiting, diarrhea, hallucinations, delusions, visual hallucinations, poriomania and violent behavior [12]. Hence, the focus has shifted towards developing herbal-based treatments for Alzheimer’s disease.
Herbal medicine offers several potential strategies for slowing Alzheimer’s diseaseprogression and managing symptoms. The production and sale of medications derived from medicinal plants have gained momentum, and their scientific and financial significance in the healthcare sector is apparently on the rise [6]. In this review article, we aim to bring forth the novelty and significance of our study by addressing specific gaps in existing knowledge. Notably, our work stands out for its inclusion of lesser-known and underexplored medicinal plants in the context of Alzheimer’s disease treatment, expanding the scope beyond commonly studied herbs. Furthermore, we adopt a distinctive ethnopharmacological perspective, delving into traditional knowledge and indigenous practices related to Alzheimer’s treatment, which may have been overlooked in previous reports. By adopting an ethnopharmacological perspective, we delve into the rich tapestry of traditional knowledge and indigenous practices related to Alzheimer’s disease treatment. This approach not only provides a unique angle to our investigation but also underscores the importance of considering cultural and historical context in the study of medicinal plants. By bridging the gap between traditional wisdom and contemporary scientific research, we aim to shed light on the potential therapeutic value of these medicinal plants, offering a holistic perspective on their relevance in the context of Alzheimer’s disease. Our review provides a comprehensive phytochemical analysis, offering an in-depth understanding of the bioactive compounds within these medicinal plants and potentially uncovering new therapeutic leads. Additionally, we focus on elucidating the mechanisms of action of these plants in Alzheimer’s disease treatment, bridging gaps in understanding the precise pathways through which they exert their effects. Furthermore, we emphasize the practical implications of the identified medicinal plants and their active compounds in addressing Alzheimer’s disease, underscoring their potential for clinical applications and drug discovery. This review underscores the importance of holistic healing systems like ethnopharmacology and the wealth of traditional knowledge they offer, adding a novel dimension to the study of Alzheimer’s disease treatment.
2 Methodology
In this review, to collect data, different combinations of keywords Alzheimer’s disease,, medicinal herbs, pharmacological activities, phytochemistry, ethnomedicinal useswere entered into databases consisting of international databases of Web of Science, PubMed, and Scopus. The articles only in English languages published between 1990 and 2023 were only searched. Then, the articles on application of medicinal plants for prevention and treatment of AD were selected, and those demonstrating potent effects of these plants and/or their compounds were reported.
3 Pathophysiology of the disease
The brain is a remarkable organ, housing over 100 billion nerve cells known as neurons. These neurons collaborate by forming intricate networks, allowing them to carry out specific functions. Some of these functions involve vital cognitive processes such as thinking, learning, and remembering, while others contribute to sensory experiences like seeing, hearing, and smelling. In their endeavor to perform these functions, brain cells function akin to tiny factories. They diligently receive necessary supplies, produce energy, construct essential equipment, and efficiently dispose of waste materials. Furthermore, these cells possess the remarkable ability to process and store information while constantly communicating with one another [13].
To maintain this elaborate system, coordination among the neurons is essential, requiring significant amounts of fuel and oxygen to sustain the brain’s incessant activity. The brain’s capacity to carry out these intricate processes is a testament to its complexity and significance in governing our thoughts, actions, and overall well-being (Fig. 1).
Alzheimer’s brain is formed due to many reasons such as Aging, Aβ amyloidosis, phosphorylated tau, calcium dysregulation, neuroinflammation, neurovascular disintegration, and mitochondrial dysfunction. Accumulation of the Aβ due to neuronal damage which leads to activation of microglia releases cytokines such as IL-1β and IL-18 which results in a synaptic loss
The beta-amyloid peptide (BAP) plays a crucial role in the onset of Alzheimer’s disease, consisting of 39–42 amino acid residues. Though a definitive cure for Alzheimer’s is yet to be discovered, current available drugs can offer some management. Studies indicate that natural antioxidants like vitamin E, vitamin C, and beta-carotene may help scavenge free radicals that emerge during the disease’s progression [14].
Memory loss in Alzheimer’s is believed to be linked to reduced levels of the nerve transmitter acetylcholine. By inhibiting the enzyme acetylcholinesterase, responsible for breaking down this transmitter, it becomes possible to elevate acetylcholine levels in the brain. Medications that hinder the breakdown of acetylcholine can slow down the progression of the disease [14]. Research into the causes of Alzheimer’s is multifaceted, involving investigations into genetic and environmental factors. Certain genes have already been associated with Alzheimer’s development, while environmental influences are also considered significant. Factors such as prolonged exposure to silicon or aluminum, chronic exposure to toxins, free-radical damage, and traumatic head injuries have been linked to Alzheimer’s disease.
Despite Alzheimer’s disease being characterized by German physician Aloise Alzheimer over a century ago, the exact underlying processes leading to its development remain unknown. Symptoms of Alzheimer’s include dementia, decline in memory and spatial awareness, mobility impairment, feelings of sadness, delusions, and hallucinations. In the advanced stages, patients may lose their ability to communicate verbally, become dependent on others, and struggle to perform basic daily tasks [15].
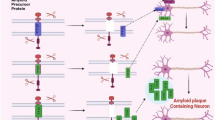
Under normal conditions, the Amyloid Precursor Protein (APP) undergoes metabolism via the secretory pathway, where it is transported from the endoplasmic reticulum (ER) to the Golgi apparatus (GA) and then to the cellular membrane. Here, it undergoes proteolytic modifications catalyzed by the enzyme β-secretase, resulting in the production of a soluble sAPP protein and another fragment that is further cleaved by γ-secretase. This process generates the intracellular domain known as AICD and the p3 domain. However, in disease conditions, the intended route for APP processing is altered, leading it to enter an endosomal-lysosomal APP proteolytic pathway [14, 15].
β-Secretase, located closer to the N-terminus of APP within cellular organelles, influences APP biochemical changes, while γ-secretase acts closer to the C-terminus, immersed in the cytoplasm. The enzymatic activity of β-secretase leads to the formation of the sAPPβ protein, along with the generation of two components, AICD and amyloid beta, an insoluble peptide. In Alzheimer’s disease patients, the cleavage of APP by secretases is significantly higher than in healthy individuals, resulting in an increased concentration of APP in the extracellular matrix (ECM). Here, it binds to various entities such as apolipoprotein E (APOE), microglia, degenerated axons, and astrocytes, which are induced by proinflammatory cytokines [16].
Senile plaques, formed by amyloid beta aggregation, can infiltrate blood vessels, impeding blood flow to the brain, and causing neuronal damage. This process activates various pathways, including microglia activation and the complement system, leading to increased production of free radicals and Ca2+ influx, ultimately resulting in neuronal death. The activation of glycan end-product receptors via APP may lead to the production of proinflammatory substances such as excitotoxins, cytokines, and tumor necrosis factor (TNF-alpha), which can affect the structure of pyramidal neurons [17].
Tau proteins play a critical role in stabilizing microtubules, which are structural components of the cytoskeleton in neurons. Increased tau protein phosphorylation leads to the formation of polymers called tau tangles, a hallmark of AD. This elevated phosphorylation of tau can be triggered by increased Ca2+ ion concentration in nerve cells induced directly by amyloid beta aggregation. Consequently, microtubules depolymerize, causing deformation of the cytoskeleton and disruption of intracellular transport, leading to compromised neuron function. Tau tangles form toxic aggregates that activate microglia and cause inflammation, ultimately leading to neuronal damage and cell death [14,15,16].
Recently, Sharma et al., introduced the significant potential of Licorice (Glycyrrhizaglabra Linn.) in the realm of neuropharmacology, particularly for aging-related disorders. The review is grounded in an exhaustive examination of databases to accumulate and scrutinize information regarding Licorice’s diverse pharmacological effects, its neuroprotective capabilities, safety concerns, and its role in treating various neurological diseases. Marking a novel approach in this field, network pharmacology has been utilized to investigate Licorice’s mechanisms in neurological contexts. Central to our discussion are the plant’s phytoconstituents—liquiritin, glycyrrhizic acid, and others—which have demonstrated promising results in laboratory and animal studies for combating neurological issues such as long-term depression, age-related diseases, Alzheimer’s, and addictions. These effects are mediated through interactions with neural proteins, although these findings are presently confined to animal-based research. This review underlines the necessity of clinical trials to validate these findings in humans, particularly in light of Licorice’s associated risks like mild hypertension and hypokalemia. Their exploration into Licorice’s therapeutic potential aims to contribute to the burgeoning field of neuropharmaceuticals, offering new perspectives and directions for future research.
Despite continuous research efforts, the exact cause and development of this condition, Alzheimer’s disease, have not been fully understood yet. Nevertheless, certain distinct processes have been identified at both the cellular and tissue levels. One prominent feature observed in Alzheimer’s disease is the accumulation of amyloid beta (Aβ). Aβ is a short peptide produced from the amyloid precursor protein (APP), which is naturally present in the body, leading to the formation of senile plaques [16, 18].
In addition to its role in forming plaques, APP plays a crucial role in key regulatory activities, including the regulation of axonal development and its impact on synaptic plasticity under normal physiological conditions [16]. In Alzheimer’s diseasepathophysiology, beta secretase (BACE1) and Keap1 are two key players with significant roles. BACE1 is an enzyme responsible for producing beta-amyloid peptides, which are known to aggregate and form plaques in the brains of Alzheimer’s disease patients. These plaques contribute to neurodegeneration and cognitive decline. On the other hand, Keap1 is a protein involved in regulating the cellular response to oxidative stress. It interacts with a transcription factor called Nrf2, which plays a crucial role in the antioxidant defense system. In Alzheimer’s disease, impaired Keap1-Nrf2 signaling leads to decreased antioxidant capacity, resulting in increased oxidative stress and neuronal damage. Plant-based drugs have shown promise in managing the intricate pathophysiology of Alzheimer’s disease. Some plant compounds, such as flavonoids and polyphenols, possess antioxidant and anti-inflammatory properties [13,14,15,16]. These compounds can scavenge free radicals, reduce oxidative stress, and protect neurons from damage. Additionally, certain plant-derived drugs have been found to modulate BACE1 activity, inhibiting the production of beta-amyloid peptides. By targeting BACE1, these drugs aim to reduce the formation of plaques in the brain, potentially slowing down the progression of Alzheimer’s disease. Overall, plant drugs offer a multifaceted approach to managing Alzheimer’s disease pathophysiology. They can help mitigate oxidative stress, reduce inflammation, and inhibit the production of beta-amyloid peptides, providing potential benefits in the management of this complex neurodegenerative disorder [1, 5, 16].
4 Medicinal plants for the treatment of Alzheimer’s disease
The plant-derived products have undergone stringent standardization, and their effectiveness and safety in specific applications have been established. Some of them were listed below.
This table provides an overview of the subject, but please consult with a healthcare provider or a pharmacist before starting any herbal regimen, especially in the case of serious conditions like Alzheimer’s disease. Additionally, it’s worth noting that while these plants may have potential therapeutic effects, they cannot cure Alzheimer’s disease. The management of Alzheimer’s disease requires a multi-pronged approach, including medication, lifestyle modifications, and supportive care.
4.1 Ginkgo biloba (GB)
Ginkgo biloba (GB) is a well-researched and commonly used herb in the treatment of cognitive impairment and Alzheimer’s disease. Its potential effectiveness is attributed to antioxidant and antiapoptotic properties [19, 20]. The leaves of this plant are utilized for treating cognitive impairment in Alzheimer’s patients, containing various essential components such as flavonoids, steroids (stigmasterol and sitosterol), organic acids (ascorbic, shikimic acid, and vanillic acid), ginkgolides, bilobalide, and terpenoids [21]. Terpenoids, including major sesquiterpene bilobalide and major diterpenes and ginkgolides, are crucial compounds found in Ginkgo biloba [22]. Flavone glycosides constitute approximately 22–27% of the plant extracts, while terpene lactones account for about 5–7% [23]. Among the terpene lactones are A, B, and C ginkgolides, along with bilobalide, while flavone glycosides include quercetin, isorhamnetin, and kaempferol.
Flavonoids present in Ginkgo biloba play a significant role in preventing various forms of oxidative and peroxidative brain damage in AD Alzheimer’s disease [24]. The herb is believed to exert its therapeutic effects through several mechanisms, including antiapoptotic, anti-oxidative, anti-amyloidogenic, and free radical scavenging pathways, all of which aid in the prevention and treatment of AD Alzheimer’s disease [25]. The plant extract inhibits Amyloid β-induced neurotoxicity by controlling glutathione peroxidase and SOD activity in vitro, limiting neuronal apoptosis, ROS build-up, glucose absorption, mitochondrial dysfunction, and activation of the ERK and c-JUN N-terminal kinase (JNK) pathways. GB has been shown to enhance cognitive performance in Alzheimer’s patients by increasing oxygen delivery and aiding the body in removing free radicals, thus improving memory [21].
The presence of flavonoids, terpenoids, and organic acids in Ginkgo biloba contributes to its neuroprotective properties [26]. Scientific investigations have demonstrated the potential of GB as a cognition enhancer, particularly if administered during the early stages of Alzheimer’s disease AD [27] (Table 1).
4.2 Bacopa monnieri (BM)
Bacopa monnieri (BM) is a time-honored nootropic herb with a history of use in treating neurological conditions. A phytochemical study of BM extract revealed the presence of diverse bioactive components, including triterpenoids, alkaloids (such as Nicotine, Brahmine, and Herpestine), saponins, glycosides, d-mannitol, hersaponin, monnierin, and alcohols [28]. This plant also contains various phytocompounds like bacosides A and B, bacosaponins A, B, and C, bacopasides III to V, bacopasaponins D, E, and F, jujubogenin, bisdesmosides, betulic acid, alkaloids, polyphenols, sterols, and sulfhydryl compounds, all of which suggest its antioxidant activity [29].
The antioxidant and neuroprotective properties of BM make it a highly promising herb for treating Alzheimer’s disease [28]. The main substances responsible for BM’s neuroprotective effects are bacoside A and bacoside B [28]. These bacosides facilitate kinase activity, restore synaptic activity, support neuronal synthesis, and ultimately aid in the transmission of nerve impulses to promote the healing of injured neurons [21] (Table 1).
BM’s antioxidant activity also leads to the upregulation of various antioxidant molecules, including SOD and GSH, thereby mitigating H2O2-mediated oxidative stress both in vivo and in vitro. Additionally, this herb reduces lipoxygenase activity, contributing to the recovery from oxidative stress [28]. The diverse range of bioactive compounds in Bacopa monnieri underscores its potential as a valuable natural remedy for promoting brain health and cognitive function.
4.3 Salvia officinalis (SO)
Several species of the Salvia genus are known for their medicinal properties in treating brain-related disorders [30]. Among them, Salvia officinalis, a fragrant herb with well-established pharmacological effects, is widely recognized for its potential in addressing brain disorders, including Alzheimer’s disease [31]. The Salvia plant contains over 160 polyphenols, including various phenolic acids and flavonoids. Some of the phenolic substances found in Salvia species are yunnaneic acid, lithospermic acids, sagernic acids, rosmarinic acid, salvianolic acids, sage-coumarin, and caffeic acid and its derivatives. The flavonoids present include kaempferol, apigenin, luteolin, quercetin, and hispidulin. Moreover, Salvia species are rich in terpenoids such as α and β-thujone, 1,8-cineole, α-humulene, camphor, viridiflorol, and β-caryophyllene, abundant in their essential oils. The plants also contain significant amounts of diterpenes and triterpenes, including tanshinones, carnosic acid, carnosol, and ursolic acid [30].
The phytochemicals found in Salvia plants induce various biological processes associated with cognition and have effects on amyloid-β, oxidative stress, cholinergic activity, inflammation, as well as anxiolytic and antidepressant behaviors [30]. Notably, two antioxidants, rosmarinic acid and carnosic acid, present in Salvia officinalis, play a vital role in protecting the brain from oxidative damage [27]. The herb’s antioxidant properties, particularly rosmarinic acid and other chemical components, have shown effective antioxidant activity in in vitro models [32]. Furthermore, Salvia officinalis exhibits characteristics of CNS acetylcholine receptor activation and muscarinic and nicotinic binding [33]. It interacts with the muscarinic and cholinergic pathways involved in memory retention, thereby improving memory retention [34]. Salvia species, especially Salvia officinalis, hold great promise as natural remedies for promoting brain health and cognitive function.
4.4 Curcuma longa (CL)
Curcumin, the orange-yellow component obtained from turmeric (Curcuma longa), has been recognized for its medicinal properties, particularly in treating Alzheimer’s disease [27]. This natural polyphenol possesses various beneficial effects and has been widely utilized in medications [35]. Its anti-amyloidogenic, anti-inflammatory, and antioxidant properties make it a valuable therapeutic option for AD treatment [26].
Turmeric’s active ingredients include water-soluble curcuminoids and turmerone oil. Among the curcuminoids, there are cyclocurcumin, bisdemethoxycurcumin, demethoxycurcumin, and curcumin. Curcumin, in particular, has been associated with a reduced risk of AD [36]. Curcumin has been used for the treatment of various ailments since ancient times, and its anti-inflammatory properties have been linked to a lower risk of Alzheimer’s [29].
Curcumin’s potent neuroprotective properties stem from its antioxidant and anti-inflammatory effects, making it effective in addressing various neurological conditions. It binds with Amyloid-β proteins, leading to reduced oxidative stress, improved cognition, and decreased inflammation [37]. Interestingly, low doses of curcumin taken over an extended period have shown greater efficacy in treating Alzheimer’s disease compared to high doses. The modest dosage of turmeric has been found to decrease proinflammatory cytokine levels associated with neuroinflammatory pathways involved in neuritic plaque formation [6].
Hypercholesterolemia and hyperlipidemia contribute to amyloid plaque formation by increasing intracellular cholesterol esters. Researchers hypothesize that curcumin may provide therapeutic benefits against Alzheimer’s disease by preventing cholesterol production and lowering serum peroxides [38]. The multifaceted properties of curcumin make it a promising natural compound for potential treatments aimed at managing and possibly preventing Alzheimer’s disease.
4.5 Rosmarinus officinalis (RO)
Rosemary (RO), a perennial plant, has a long history of use in traditional medicine for various purposes. Its active compounds include natural COX-2 inhibitors, such as eugenol, apigenin, oleanolic acid, carvacrol, thymol, and ursolic acid [39]. The essential extract from rosemary is responsible for its antiseptic and medicinal properties [40]. Another important extract, rosmarinic acid, a polyphenol carboxylic acid, exhibits a wide range of pharmacological properties, including antiviral, antioxidant, neuroprotective, anti-inflammatory, antibacterial, and anticancer effects.
Alzheimer’s disease is often associated with the “amyloid hypothesis,” which attributes the progressive death of brain neurons to the build-up and aggregation of β-amyloid. However, rosemary’s rosmarinic acid has shown the ability to reduce the production of NF-κB and TNF-α, suggesting a potential to lower the risk of β-amyloid-induced memory loss [41]. Remarkably, rosemary exhibits significant neuroprotective properties against neurodegenerative conditions like Alzheimer’s and dementia tested in in vitro models.
Natural COX-2 inhibitors found in rosemary may be as effective in preventing Alzheimer’s disease as synthetic COX-2 inhibitors, according to some studies [27]. Moreover, rosemary inhibits acetylcholine-cholinesterase (AChE) and butyryl cholinesterase, the enzymes responsible for breaking down acetylcholine in the brain. Terpenes and rosemarinic acid in rosemary are likely responsible for these anti-AChE and anti-BChE actions. This inhibition of cholinesterase enzymes can potentially reduce memory loss, depression, anxiety, and symptoms of Alzheimer’s disease by increasing total choline levels in the brain.
Additionally, rosemary induces the production of nerve growth factors, which play a vital role in the growth and protection of neurons, offering potential relief for Alzheimer’s disease [40]. With its diverse beneficial effects on brain health and cognitive function, rosemary emerges as a promising natural remedy in the management of neurodegenerative disorders like Alzheimer’s.
4.6 Melissa officinalis (MO)
Melissa officinalis, commonly known as lemon balm, is a perennial herb with bushy, hairy leaves that are heart-shaped and have a coarse surface, growing upright [19]. Extracts from lemon balm contain various beneficial phenolic compounds, such as phenolic acids, cholinergic acid, flavonoids (including apigenin and luteolin), rosmarinic acid, metrilic acid, caffeic acid, as well as triterpenes like ursolic acid and oleanolic acid [42]. This herb exhibits an array of medicinal properties, including anxiolytic, carminative, sedative, antidepressant, and anti-inflammatory effects [33, 38].
Research suggests that lemon balm has the potential to inhibit acetylcholinesterase and display antioxidant activity, making it valuable in the prevention and treatment of Alzheimer’s disease [27]. Furthermore, it possesses anti-cholinesterase action, binds to cholinergic receptors, and exhibits neuroprotective properties [43]. Some of the substances in lemon balm interact with muscarinic acetylcholine and nicotinic receptors, leading to reduced activity of the acetylcholinesterase enzyme [43]. By modulating the cholinergic system, it holds promise in the management of Alzheimer’s disease [44]. Moreover, lemon balm has been observed to reduce agitation and improve cognitive performance in individuals with mild to moderate Alzheimer’s [45, 46].
Another beneficial aspect of lemon balm is its ability to destabilize the production of β-amyloid, a factor that exacerbates Alzheimer’s disease, primarily attributed to the presence of rosmarinic acid [47]. With its multifaceted benefits on brain health, lemon balm shows potential as a natural and therapeutic option for individuals with Alzheimer’s disease.
4.7 Glycyrrhiza glabra (GG)
Glycyrrhiza glabra, commonly known as licorice, is a perennial herbaceous plant with long, cylindrical, flexible roots and runners. The roots and dried runners of the plant are traditionally used for medicinal purposes, as they contain essential phytoconstituents such as triterpenoids, saponins, glycyrrhizin, flavonoids, isoflavonoids, chalcones, coumarins, lignins, amino acids, gums, and volatile oils. The main active constituent is glycyrrhizin, along with glycyrrhitinic acid and their derivatives, belonging to the class of triterpenoids, while glabridin is the most abundant isoflavanone [48].
Licorice has been employed since ancient times to treat various ailments, including viral diseases, peptic ulcers, asthma, malaria, pharyngitis, abdominal pain, insomnia, and psychiatric disorders [48]. These traditional uses align with its pharmacological properties, as it exhibits anti-inflammatory, anti-cancerous, antiviral, anti-diabetic, anti-asthmatic, and anti-malarial effects [49].
Research has also suggested the potential use of licorice in the treatment of neurological disorders such as Alzheimer’s disease, cognitive impairment, and dementia [47]. Studies in mice have demonstrated that the aqueous root extract of licorice enhances memory and learning abilities [50,51,52]. The memory-enhancing effect is attributed to its antioxidant and anti-inflammatory properties, which reduce exposure to oxidative stress and protect brain cells from damage, thereby improving neuronal function. This suggests a possible neuroprotective role of licorice in preventing neurological disorders like Alzheimer’s and Parkinson’s diseases.
Specifically, the active component glycyrrhizin has shown neuroprotective effects against various neurological conditions induced by scopolamine [53]. Additionally, flavonoids extracted from licorice have been reported to have neuroprotective effects against neural cell death caused by seizures [21, 54].
Furthermore, licorice extract has demonstrated potential neuroprotective activity in patients suffering from acute ischemic stroke, alleviating neurological symptoms [55]. It has also shown promising results in reducing pain symptoms in rats induced with neuropathic pain in in vivo models [56]. Various phytoconstituents isolated from licorice, such as flavonones and chalcones, have shown effectiveness against age-related neurodegenerative disorders like Alzheimer’s and Parkinson’s disease [57]. The active components glycyrrhizin, glycrrhetinic acid, and glycyrrhizic acid in licorice root extracts were found to prevent the death of neuronal cells caused by amyloid-β peptide present in plaques, thus alleviating symptoms of Alzheimer’s disease [58]. The positive effects of licorice extract compon’nts on neuronal cell death indicate its potential as a neuroprotective agent, which warrants further exploration. However, in-depth studies on potential side effects and in vivo toxicity are necessary for future developments.
4.8 Galanthus nivalis (GN)
Galanthus nivalis (GN) is a bulbous perennial herbaceous plant belonging to the family Amaryllidaceae and is recognized as one of the 20 most important alkaloid-producing plants [59]. It is commonly known as snowdrops due to its milk-white flowers, deriving its name from the Greek words “gala” (milk) and “nthos” (flower). GN has gained economic significance as an ornamental plant and has a historical use as a folk medicine dating back to ancient Greek times for the treatment of pain and neurological disorders [60].
Phytochemical studies of GN have revealed the presence of various important phytoconstituents, including terpenoids, flavonoids, phenolics, and a significant class of alkaloids. These compounds exhibit diverse biological activities, such as anti-tumor, anti-inflammatory, anti-viral, and acetylcholinesterase inhibitory activity [61, 62]. Of particular importance is galantamine, an alkaloid belonging to the isoquinoline class, which has been found effective against Alzheimer’s disease. Galantamine was first discovered and isolated in the 1950s, and its synthetic form was approved for Alzheimer’s treatment in the United States and the European Union in 2000 [63, 64]. In a research studies by Sweeney et al., a 2 mg/kg dose of galantamine produced optimal results in a swim-maze test and improved passive avoidance behaviors in NMB-lesioned mice [61].
The treatment of Alzheimer’s disease revolves around cholinesterase activity, with a’ocus on Acetylcholinesterase (AchE) inhibition. Galantamine has been reported to possess anticholinesterase activity by inhibiting the action of AchE. Moreover, it activates nicotinic receptors through conformational changes, initiating cholinergic nicotinic transmission, which has protective effects against β-amyloid cytotoxicity on neuronal cells [58]. In vitro studies by Rhee et al. in 2003 demonstrated that the methanolic extract of GN exhibited 96% inhibitory activity against acetylcholinesterase, further supporting its neuroprotective potential [58].
These research findings underscore the importance of GN as an ethnobotanical plant with significant neuroprotective potential and a promising candidate for the treatment of Alzheimer’s disease.
4.9 Huperzia serrata (HS)
Huperzia serrata (Thunb ex. Murray) Trevis, commonly known as toothed firmoss, belongs to the family Lycopodiaceae. This plant is rich in secondary metabolites, including lyopodium alkaloids, triterpenes, flavones, and phenolic acids. Traditionally, it has been used to treat various ailments such as colds, fever, bruises, pain, strains, contusions, and rheumatism. Moreover, it exhibits a wide range of pharmacological properties, including anticonvulsant, anti-inflammatory, anti-nociceptive, anti-Alzheimer, anti-schizophrenia, anti-apoptosis, protection against organophosphate poisoning and myasthenia gravis, antioxidant, and mitochondrial protection [65].
Huperzia A, a crucial alkaloid isolated from H. serrata by Chinese phytochemists, has been reported to possess anti-Alzheimer’s activity [65]. Studies on the effects of H. serrata against Alzheimer’s disease showed positive effects on Acetylcholinesterase (AchE) but not Butyrylcholinesterase (BAChE). Cognitive behavior tests conducted in a scopolamine-induced cognitive impairment model indicated that H. serrata ameliorated cognitive impairment, suggesting its potential in alleviating dementia associated with Alzheimer’s disease. A dose of 30 mg/kg/day in animals might be part of preclinical studies to evaluate the efficacy and safety profile of H. serrata extract, and more specifically, its active compound huperzine A [65, 66].
A standardized green extract of H. serrata, NSP01, was formulated using microwave-assisted technologies. Phytochemical screening of NSP01 extract revealed the presence of three major compounds: Huperzine A, caffeic acid, and ferulic acid. Further, its neuroprotective activity was evaluated in primary neuronal cultures injured with glutamate and another in vivo model where cortical neurons were cultured. The NSP01 extract protected neurons and neurite networks from glutamate-induced damage and improved neuron survival in the subsequent study [66].
Interestingly, the major components, Huperzine A, caffeic acid, and ferulic acid, exhibited a synergistic neuroprotective potential when used in combination, enhancing neuronal survival and neurite network. This finding highlights the potentiality of H. serrata as a neuroprotectant, showing improved neuronal activities when administered in Alzheimer’s-induced in vivo models or cultured neurons [66]. These research outcomes demonstrate the promising neuroprotective properties of H. serrata and its potential in addressing Alzheimer’s disease.
4.10 Lepidium meyenii Walp. (LMW)
Maca L. mayenii Walp., belonging to the Brassicaceae family, is mainly found in specific regions of Peru, thriving at elevations between 3500 and 4500 m above sea level. This plant has a rich history of traditional use as folk medicine and continues to be employed for treating various ailments, including sexual and menstrual disorders, memory loss, cancer, and depression. A thorough phytochemical analysis of its leaves and roots revealed the presence of diverse secondary metabolites.
In a study conducted by Lee and Chang in 2019 [67], LC-Q-TOF analysis of Maca L. mayenii Walp.’s leaves and root extract identified biologically important secondary metabolites such as saponins, phenols, flavonoids, steroids, alkylbenzenes, and amines. Notably, the leaves exhibited higher total saponin, phenol, and flavonoid content compared to the roots. Evaluation of antioxidant properties through DPPH radical scavenging and FRAP assays in vitro models indicated stronger activity in the leaves, suggesting their potential for greater therapeutic benefits than the roots.
Peruvian maca is renowned for its diverse medicinal effects, including neuroprotective, dermatological, antidiabetic, memory-enhancing, fertility, energizing, and antioxidant properties [68]. Studies on the methanolic extract of maca, re-extracted in n-pentane, revealed neuroprotective effects. In vivo experiments on adult rats that had experienced a stroke showed that 3 mg/kg was the most effective dose with positive effects on neuronal health. Similarly, in vitro studies on scampi neuronal cells exposed to hydrogen peroxide (H2O2) to induce oxidative stress demonstrated a significant reduction in oxidative stress after treatment with various concentrations (0.1, 0.3, 1.3, 10, and 30 µg/ml) of the pentane extract, indicating the potential neuroprotective effects of Maca L. mayenii Walp. [69, 70].
Another crucial class of secondary metabolites, macamides, isolated from Maca L. mayenii Walp., exhibited neuroprotective effects in both in vitro and in vivo experiments. Macamides were found to mitigate the neurotoxic effects caused by exposure of U-87 MG glioblastoma cells to MnCl2. Furthermore, an in vivo study investigating the receptors involved in the neuroprotective effects showed that the CB1 receptors of macamides played a role in this neuroprotection [71].
Overall, Maca L. mayenii Walp. emerges as a valuable medicinal plant with significant neuroprotective effects. However, it is crucial to conduct a detailed toxicity study using different solvents for extract preparation to validate its potential as a potent drug for treating various neurodegenerative disorders.
4.11 Centella asiatica (Gotu Kola)
Gotu Kola, scientifically known as Centella asiatica, belongs to the Apiaceae family. One of its prominent constituents is saponins. This herb has gained widespread recognition for its multifaceted health benefits, which encompass blood purification, cognitive enhancement, blood pressure reduction, and potential lifespan extension. Moreover, it plays a pivotal role in promoting mental relaxation and alleviating stress. Within the realm of Ayurveda, Gotu Kola aqueous extracts have long been employed to revitalize and rejuvenate brain cells. These extracts have also demonstrated their effectiveness in combatting insomnia. Furthermore, Gotu Kola exhibits promising potential in inhibiting the formation of β-amyloid cells, offering hope in the treatment of Alzheimer’s disease, particularly in cases associated with β-amyloid poisoning. Research conducted on in vivo models Wistar rats has shown that treatment with fresh Centella asiatica leaf extracts (250 mg/kg body weight) significantly improved their learning and memory retention abilities. Additionally, Gotu Kola has proven to be a versatile remedy for various conditions, including depression, rheumatism, mental weakness, abdominal discomfort, and epilepsy. Notably, it has the capability to mitigate oxidative stress responses and reverse Aβ pathology, making it a valuable asset in the field of health and well-being [72].
4.12 Tinospora cordifolia (Giloy)
Belonging to the Menispermaceae family, Guduchi is a plant renowned for its remarkable memory-enhancing properties, exhibiting efficacy not only in normal subjects but also in individuals facing memory deficits. Its influence on cognitive function is partly attributed to choline supplementation, which not only boosts the immune system but also enhances the production of acetylcholine, a neurotransmitter vital for memory and learning processes. In the ancient tradition of Ayurveda, Guduchi is highly regarded as a potent agent for augmenting learning and memory. This reputation is well-founded in modern research as well. Specifically, the use of an aqueous extract (200 mg/kg body weight) derived from the roots of Guduchi has been shown to yield significant improvements in verbal learning and logical memory [72]. This underscores the plant’s potential as a valuable resource for cognitive enhancement and underscores its historical significance in traditional healing practices [2, 3, 72].
4.13 Convolvulus pluricaulis (Shankhpushpi)
Shankhpushpi, scientifically known as Convolvulus pluricaulis, belongs to the Convolvulaceae family and is recognized for its memory-enhancing properties. Previous studies have shed light on its cognitive benefits, showing that aqueous and ethyl acetate extracts derived from this plant have the potential to significantly improve memory and learning capacities. In India, Shankhpushpi has a long history of use as a nervine stimulant to enhance memory and cerebral function. This usage is reflected in various formulations, highlighting its importance in traditional herbal remedies. Scientific exploration has revealed that a rich array of secondary metabolites, such as steroids, anthocyanins, flavonol glycosides, and triterpenoids, contribute to its memory-enhancing and nootropic effects. These compounds are believed to promote nerve relaxation by regulating the synthesis of stress hormones like cortisol and adrenaline. Studies on rodents have further corroborated the memory-boosting attributes of Shankhpushpi. Ethanol extracts of Shankhpushpi, as well as its aqueous and ethyl acetate fractions (250 mg/kg body weight), have demonstrated a remarkable capacity to enhance memory retention and learning abilities in rats. Similarly, when administered to aged mice for a week, Shankhpushpi displayed the potential to enhance memory. Moreover, Shankhpushpi has been observed to enhance acetylcholinesterase activity in specific regions of the hippocampus, particularly CA1 and CA3, which are closely associated with memory function and learning capacities [72]. The raw extract of the herb C. pluricaulis and its derivatives have demonstrated a broad spectrum of neuropharmacological benefits in studies, including improvements in memory, anxiety relief, sedation, antidepressant, stress mitigation, neuroprotection, anti-inflammatory, antioxidant, pain relief, sedative, anticonvulsant activities, and potential reversal of Alzheimer’s symptoms. Network pharmacology analyses suggest that C. pluricaulis compounds engage with a variety of proteins, synapses, and signaling pathways, particularly influencing serotonergic synapses critical for neurotransmission, Alzheimer’s disease, long-term depression, alcohol addictions, cognitive disorders, psychological conditions, and boosting serotonin levels in the brain [73]. This finding suggests a mechanistic link between Shankhpushpi’s memory-enhancing effects and its impact on the cholinergic system. Thus, Shankhpushpi’s metabolites are endowed with nootropic and memory-enhancing properties, heightening its pharmacological significance. Several studies have consistently shown that the administration of Convolvulus pluricaulis extracts can enhance memory in older mice and improve retention and spatial learning performance in newborn rat pups, further underscoring its potential as a memory-enhancing agent [1, 7, 72].
5 The role of bioactive compounds in the prevention of Alzheimer’s disease
A balanced and nutritious diet is a crucial aspect of maintaining a healthy lifestyle, and it appears to play a significant role in safeguarding against neurological diseases, including Alzheimer’s disease. Consuming a diet rich in bioactive compounds has been linked to a reduced risk of developing dementia [71, 72]. However, the extent to which these beneficial effects of bioactive compounds observed in vitro and animal model studies translate to neuroprotective benefits in humans under real-world conditions is not yet fully understood, as human interventional studies are limited.
Moreover, there is a lack of research on whether the quantities and specific chemical types of nutrients present in meals are effective in making these compounds readily available to the body. Nonetheless, the positive effects of these bioactive substances are supported by experimental studies that elucidate the molecular mechanisms underlying their potential in preventing Alzheimer’s disease. This review highlights the promising benefits of certain bioactive compounds, many of which belong to chemical classes such as phenolic compounds, fat-soluble vitamins, important omega-3 fatty acids, isothiocyanates, or carotenoids. These compounds hold great promise in promoting brain health and may contribute to reducing the risk of Alzheimer’s disease.
5.1 Phenolic compounds
Olive oil, a rich source of phenolic compounds such as oleuropein, hydroxytyrosol, and oleocanthal, holds significant importance in promoting brain health. Oleuropein, a glycosylated seco-iridoid, possesses powerful antioxidant properties and protects brain cells from neurotoxin-induced apoptosis [72, 74]. It also has the ability to reduce A levels and inhibit glutaminyl cyclase, an enzyme involved in A production. When oleuropein (Fig. 2a) is metabolized in the digestive system, it transforms into hydroxytyrosol (Fig. 2b), another potent antioxidant present in olive oil with greater bioavailability [72, 74]. Hydroxytyrosol has been shown to protect neuronal cells from A-induced damage and induce phase II detoxification enzymes [75].
Genistein (Fig. 2c), an isoflavone found in soy products, may also contribute to preventing AD by reducing oxidative stress and protecting mitochondria [77, 78]. Diets rich in soy products containing genistein have been associated with a lower risk of dementia [78].
Additionally, olive oil contains oleocanthal, a phenolic molecule known for its anti-inflammatory effects by blocking the cyclooxygenase (COX) enzyme responsible for producing pro-inflammatory prostaglandins. Oleocanthal (Fig. 2d) has shown promise in inhibiting A aggregation and promoting its clearance from the brain [72]. In vitro studies on tau protein derived from E. coli indicated that oleocanthal could prevent protein accumulation, suggesting its potential in improving the pathological processes involved in Alzheimer’s development [76, 77].
Research studies have also highlighted the neuroprotective effects of olive oil, particularly within the context of the Mediterranean diet (MD). MD, rich in olive oil and other fat sources, has been associated with a lower risk of Alzheimer’s disease [78]. Observational studies have shown that greater adherence to MD principles is linked to a reduced risk of developing Alzheimer’s disease and a slower decline in cognitive functions [79]. Clinical trials have further demonstrated that MD with olive oil can lead to improved working memory, attention, and overall cognitive function [10].
Anthocyanins, another group of neuroprotective phenolic compounds, are responsible for the red, violet, and blue hues in many fruits and vegetables. These compounds exhibit antioxidant properties and have been shown to protect the brain from oxidative stress by reducing the formation of free radicals [80]. Furthermore, anthocyanins have been found to prevent A peptides from aggregating and to reduce excessive production of reactive species in the brain [81]. Berries, a significant source of anthocyanins, have been linked to delayed cognitive decline [82, 83].
Curcumin (Fig. 2e), a natural component of turmeric, is another phenolic molecule with neuroprotective characteristics. Curcumin inhibits protein oxidation, attenuates inflammation, and prevents A from aggregating [84]. Observational studies have suggested that curry, rich in curcumin, may support cognitive processes and slow cognitive decline [85]. Clinical trials have shown that curcumin supplements can improve attention and memory, reduce A and tau protein aggregation, and have a strong neuroprotective effect [86].
Genistein found in hop extracts, with their ability to inhibit γ-secretase activity, offer another intriguing neuroprotective effect, diminishing A synthesis and preventing cognitive deficits [87,88,89].
Overall, the neuroprotective effects of these phenolic compounds found in olive oil, anthocyanins, curcumin, and genistein hold promise in preventing Alzheimer’s disease and promoting brain health. However, further research and clinical studies are needed to fully understand their potential therapeutic benefits in humans.
5.2 Omega-3 fatty acids and fat-soluble
Bioactive molecules present in vitamins and essential fatty acids play a crucial role in the prevention of Alzheimer’s disease. The central nervous system (CNS) is particularly susceptible to oxidative stress due to its high oxygen consumption and polyunsaturated fatty acid content. Using lipophilic antioxidants and vitamins to reduce oxidative stress is considered beneficial in lowering the risk of this disease.
Docosahexaenoic acid (DHA) (Fig. 2f), a polyunsaturated fatty acid primarily found in fish, is a promising compound for addressing CNS issues associated with aging. It is a member of the same long-chain omega-3 family as eicosapentaenoic acid (EPA) (Fig. 2g) and n-3 docosapentaenoic acid (DPA) DHA (Fig. 2h) is the most prevalent n-3 fatty acid in the brain and a significant component of structural membrane phospholipids in brain cells [90, 91]. As individuals age, the activity of the enzyme involved in DHA synthesis decreases, leading to a decline in DHA production. DHA levels in the brain are influenced by both dietary intake and liver conversion from shorter-chain precursors. DHA competes with arachidonic acid, a precursor of prostaglandins, reducing brain inflammation by inhibiting the production of these eicosanoids. It also influences APP metabolism and can protect the nervous system by promoting neuroprotectin D1 and brain-derived neurotrophic factor (BDNF), inhibiting neuronal damage, and promoting neurogenesis [16, 21].
Vitamin D is another neuroprotective bioactive molecule that can be obtained through sunlight exposure and dietary sources like fish. Vitamin D has anti-inflammatory and anti-amyloid actions, enhancing A clearance in the brain and regulating A production and enzymatic degradation. It also influences calcium homeostasis, which is crucial in neurodegenerative diseases [23, 53]. Studies have shown that vitamin D supplementation improves memory and promotes neurogenesis in animal models [25]. Low vitamin D levels are associated with a higher risk of Alzheimer’s disease, highlighting its importance in maintaining mental health in elderly individuals at risk for dementia.
Vitamin E, with its eight constituents, including tocopherols and tocotrienols, found in nuts, seeds, and vegetable oils, may also aid in preventing AD. Vitamin E suppresses the decrease in glutathione and catalase levels, which are hallmarks of Alzheimer’s disease. It serves as an anti-inflammatory agent by inhibiting COX and reducing NF-B activity [27,28,29]. Observational studies suggest that vitamin E’s antioxidant properties protect against dementia.
In summary, bioactive molecules found in vitamins and essential fatty acids, such as DHA, vitamin D, and vitamin E, hold significant potential in preventing and mitigating AD by reducing oxidative stress, promoting neurogenesis, and regulating key processes involved in the development of AD. Further research and clinical studies are needed to fully understand and harness the therapeutic benefits of these bioactive compounds.
5.3 Isothiocyanates
Isothiocyanates represent an essential group of bioactive compounds with potential benefits in preventing Alzheimer’s disease. These compounds are derived from glucosinolates found in cruciferous vegetables. The presence of a sulfur atom in their molecules grants isothiocyanates antioxidant properties, especially those with an aromatic ring adjacent to the thiocyanate group. Additionally, they exhibit COX inhibitory and anti-inflammatory effects. Some isothiocyanates have been shown to suppress acetylcholinesterase activity, leading to increased acetylcholine levels, a neurotransmitter typically reduced in Alzheimer’s patients [21].
Among the extensively studied isothiocyanates is sulforaphane (Fig. 2i), derived from glucoraphanin during the processing of plant tissues. Sulforaphane enhances the antioxidant activity of glutathione peroxidase and glutaredoxin. It boosts sulfiredoxin activity, which helps regenerate other antioxidant enzymes in neurons and glial cells. Furthermore, sulforaphane modulates the activity of proteasomes in nerve cells, aiding in clearance processes [30].
Epidemiological studies provide insights into the neuroprotective potential of isothiocyanates. Nurk et al. conducted a study on 2031 individuals aged 70 to 74, evaluating their dietary intake and cognitive abilities. Those who consumed more cruciferous vegetables like cabbage, cauliflower, broccoli, and Brussels sprouts exhibited better cognitive outcomes compared to those who consumed them less frequently [31]. Further research in this area also yielded positive results [32].
The presence of isothiocyanates in cruciferous vegetables highlights their potential role in maintaining cognitive health and potentially offering protection against AD. However, additional studies are required to fully comprehend their mechanisms of action and therapeutic benefits in the context of neurodegenerative diseases [92]
5.4 Carotenoids
Carotenoids are pigments obtained from plants, contributing vibrant yellow, orange, and red colors to various vegetables and fruits. They can also be found in microalgae, making them an essential part of the marine diet for marine creatures. Carotenoids play a crucial role in photosynthesis and protect against photooxidation. Among the carotenoids, astaxanthin stands out as one of the most beneficial compounds. It acts as a potent free radical scavenger, reducing oxidative stress, lipid peroxidation, and protein peroxidation. Additionally, astaxanthin enhances the activity of antioxidant enzymes like catalase and superoxide dismutase. Its neuroprotective effects include shielding neuronal cells from apoptosis and stimulating neurogenesis by inhibiting caspase-3 activity and modulating mitogen-activated kinases [33].
Clinical research by Katagiri et al. confirmed the neuroprotective benefits of astaxanthin (Fig. 2j) in volunteers aged 45 to 64 who received an astaxanthin extract (6 or 12 mg/day) for 12 weeks. Both intervention groups showed better performance on cognitive and learning tasks compared to the placebo group [34]. Two other carotenoids, lutein (Fig. 2k) and zeaxanthin (Fig. 2l), have also been associated with cognitive benefits. A study of 2796 individuals over the age of 60 found that higher intake of lutein and zeaxanthin was linked to enhanced cognitive abilities [35]. Another study by Power et al. showed that supplementation with lutein (10 mg), zeaxanthin (2 mg), and meso-zeaxanthin (10 mg) for 12 months improved cognitive abilities and episodic memory in participants [26].
Lycopene (Fig. 2m), another neuroprotective carotenoid, is a potent antioxidant that neutralizes singlet oxygen, reduces lipid oxidation, and protects DNA from oxidative damage [36]. In vitro research by Hwang et al. demonstrated that lycopene increased cell survival and reduced apoptosis in human neural cultures exposed to A. It also lowered free radical levels and protected mitochondria from amyloid-induced dysfunction [29]. These findings suggest that lycopene may have a role in preventing neurodegenerative processes. Overall, carotenoids show promise in supporting brain health and protecting against neurodegenerative conditions.
6 Future prospective
The exploration of ethnomedicinal plants as treatments for Alzheimer’s disease (AD) holds considerable promise due to their potential to target the disease’s multifaceted nature. Yet, critical knowledge gaps must be addressed to harness their full potential. Future research should prioritize the identification of active compounds within these plants, supported by robust clinical trials to validate efficacy and safety. Standardization of extracts and dosing, along with detailed toxicology profiles, will be essential for safe integration into treatment regimes. Additionally, a deeper understanding of the mechanisms of action, pharmacokinetics, and drug interactions is needed, alongside considerations of genetic factors that may influence therapeutic outcomes. Importantly, this research must be conducted with an appreciation for the cultural contexts from which these plants are derived, ensuring ethical practices in bioprospecting and a respectful integration of traditional knowledge with scientific innovation. Addressing these areas could lead to breakthroughs in AD treatment and a greater recognition of the value inherent in traditional medicine.
7 Conclusion
The potential of ethnomedicinal plants in the treatment of Alzheimer’s disease (AD) is rooted in their ability to influence a variety of mechanistic pathways implicated in the disease. These plants offer a range of bioactive compounds that may act on key pathological targets, such as amyloid-beta aggregation, tau protein hyperphosphorylation, oxidative stress, metal dysregulation, inflammation, and cholinergic deficit. The anti-amyloidogenic properties of some plant extracts can interfere with the formation or aggregation of amyloid plaques, while others may modulate tau phosphorylation, which is crucial in the development of neurofibrillary tangles. Antioxidant compounds found in ethnomedicines can mitigate oxidative stress, and metal-chelating agents within these plants can address the imbalance of metals that contribute to AD pathology. Anti-inflammatory agents can reduce the neuroinflammation often observed in AD, and compounds that enhance cholinergic transmission can address the neurotransmitter deficits associated with cognitive decline. However, the translation of these mechanistic insights into effective treatments remains a challenge due to the complexity of AD. Future research must continue to dissect these pathways and validate the therapeutic potential of ethnomedicinal plants through clinical trials, ensuring safety and efficacy for patients and embracing a multifaceted approach to managing this debilitating disease. The approach to drug discovery for neurodegenerative diseases has become increasingly expensive, risky, and ineffective, posing significant challenges for the pharmaceutical industry. As a result, there is a growing interest in exploring alternative sources of drugs, particularly from herbal products. Synthetic pharmaceuticals are often associated with undesirable side effects, highlighting the need for drug alternatives with minimal or no adverse effects. Medicinal plants offer a promising avenue for treating Alzheimer’s disease, and several studies have demonstrated their potential efficacy. By incorporating medicinal herbs into treatment, the quality of life for Alzheimer’s and memory-impaired patients can be improved. While these plants show promise in treating the disease, further research is needed to fully understand their mechanisms of action. Future clinical trials with larger sample sizes should investigate the role and underlying mechanisms of various therapeutic herbs in the treatment of Alzheimer’s disease.
Data availability
Not applicable.
References
Perry EK, Pickering AT, Wang WW, Houghton PJ, Perry NSL. Medicinal plants and Alzheimer disease from ethnobotany to phytotherapy. J Pharm Pharmacol. 1999;51(5):527–34.
Howes M-JR, Houghton PJ. Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav. 2003;75(3):513–27.
Islam A, Saif Khandker S, Alam F, Khalil I, Amjad Kamal M, Hua Gan S. Alzheimer disease and natural products: future regimens emerging from nature. Curr Top Med Chem. 2017;17(12):1408–28.
Gu Y, Scarmeas N. Dietary patterns in Alzheimer disease and cognitive aging. Curr Alzheimer Res. 2011;8(5):510–9.
Sharma P, Srivastava P, Seth A, Tripathi PN, Banerjee AG, Shrivastava SK. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer disease and potential therapeutic strategies. Prog Neurobiol. 2019;174:53–89. https://doi.org/10.1016/j.pneurobio.2018.12.006.
Rao RV, Descamps O, John V, Bredesen DE. Ayurvedic medicinal plants for Alzheimer disease: a review. Alzheimers Res Ther. 2012;4(3):1–9. https://doi.org/10.1186/alzrt125.
Bredesen DE. Neurodegeneration in Alzheimer disease: caspases and synaptic element interdependence. Mol Neurodegener. 2009;4(1):1–10.
Mimica N, Presečki P. Side effects of approved antidementives. Psychiatr Danub. 2009;21(1):108–13.
Winslow BT, Onysko MK, Hazlewood KA. Treatment of Alzheimer disease. 2011. www.aafp.org/afpAmericanFamilyPhysician1403.
Winslow BT, Onysko M, Stob CM, Hazlewood KA. Treatment of Alzheimer disease. Am Fam Physician. 2011;83(12):1403–12.
Tricco AC, et al. Comparative effectiveness and safety of cognitive enhancers for treating Alzheimer disease: systematic review and network metaanalysis. J Am Geriatr Soc. 2018;66(1):170–8.
Imai H, et al. Prevalence of and risk factors for adverse events in Alzheimer’s patients receiving anti-dementia drugs in at-home care. PLoS ONE. 2020;15(4): e0231226.
Hodes RJ, Buckholtz N, Cahan V, Morrison-Bogorad M. Eyes on the prize: federal Alzheimer’s research effort aims to facilitate interventions. Alzheimer’s Dement. 2008;4(1):S37–47.
Grodzicki W, Dziendzikowska K. The role of selected bioactive compounds in the prevention of Alzheimer disease. Antioxidants. 2020;9(3):229. https://doi.org/10.3390/antiox9030229.
Yin R-H, Yu J-T, Tan L. The role of SORL1 in Alzheimer disease. Mol Neurobiol. 2015;51(3):909–18.
Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer disease. Alzheimer’s Dement Transl Res Clin Interv. 2018;4:575–90.
Sharma R, Singla RK, Banerjee S, Sharma R. Revisiting Licorice as a functional food in the management of neurological disorders: bench to trend. Neurosci Biobehav Rev. 2023;155: 105452. https://doi.org/10.1016/j.neubiorev.2023.105452. (Epub 2023 Nov 2).
Delacourte A. Tauopathies: recent insights into old diseases. Folia Neuropathol. 2005;43(4):244–57.
Baht R, Ba İY. The essential oil of lemon balm (Melissa officinalis L.), its components and using fields. Anadolu Tarım Bilimleri Dergisi. 2006;21(1):116–21.
Canevelli M, Adali N, Kelaiditi E, Cantet C, Ousset PJ, Cesari M. Effects of Gingko biloba supplementation in Alzheimer disease patients receiving cholinesterase inhibitors: data from the ICTUS study. Phytomedicine. 2014;21(6):888–92. https://doi.org/10.1016/j.phymed.2014.01.003.
Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn Rev. 2012;6(12):81–90. https://doi.org/10.4103/0973-7847.99898.
Nowak A, et al. The use of Ginkgo biloba L. as a neuroprotective agent in the Alzheimer disease. Front Pharmacol. 2021;12: 775034. https://doi.org/10.3389/fphar.2021.775034.
Xie L, Zhu Q, Lu J. Can we use Ginkgo biloba extract to treat Alzheimer disease? Lessons from preclinical and clinical studies. Cells. 2022;11(3):479. https://doi.org/10.3390/cells11030479.
Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55(11):1409–15.
Dash S. Ginkgo biloba in Alzheimer disease. Austin J Clin Neurol. 2015;2(3):1028.
John OO, et al. Phytotherapy: a promising approach for the treatment of Alzheimer disease. Pharmacol Res Mod Chin Med. 2022;2: 100030. https://doi.org/10.1016/j.prmcm.2021.100030.
Singhal A, Bangar O, Naithani V. Medicinal plants with a potential to treat Alzheimer and associated symptoms. Int J Nutr Pharmacol Neurol Dis. 2012;2(2):84. https://doi.org/10.4103/2231-0738.95927.
Dubey T, Chinnathambi S. Brahmi (Bacopa monnieri): an ayurvedic herb against the Alzheimer disease. Arch Biochem Biophys. 2019;676: 108153. https://doi.org/10.1016/j.abb.2019.108153.
Roy A. Role of medicinal plants against Alzheimer disease. Int J Complement Altern Med. 2018;11(4):205–8. https://doi.org/10.15406/ijcam.2018.11.00398.
Lopresti AL. Salvia (Sage): a review of its potential cognitive-enhancing and protective effects. Drugs R&D. 2017;17(1):53–64. https://doi.org/10.1007/s40268-016-0157-5.
Miraj S, Kiani S. A review study of therapeutic effects of Salvia officinalis L. Pharm Lett. 2016;8(6):299–303.
Datta S, Patil S. Evaluation of traditional herb extract Salvia officinalis in treatment of Alzheimer disease. Pharmacogn J. 2020;12(1):131–43. https://doi.org/10.5530/pj.2020.12.20.
Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi AH, Khani M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer disease: a double blind, randomised, placebo controlled trial. J Neurol Neurosurg Psychiatry. 2003;74(7):863–6. https://doi.org/10.1136/jnnp.74.7.863.
Akram M, Nawaz A. Effects of medicinal plants on Alzheimer disease and memory deficits. Neural Regener Res. 2017;12(4):660–70. https://doi.org/10.4103/1673-5374.205108.
Obulesu M, Rao DM. Effect of plant extracts on Alzheimer disease: an insight into therapeutic avenues. J Neurosci Rural Pract. 2011;2(1):56–61. https://doi.org/10.4103/0976-3147.80102.
Gregory J, Vengalasetti YV, Bredesen DE, Rao RV. Neuroprotective herbs for the management of Alzheimer disease. Biomolecules. 2021;11(4):543. https://doi.org/10.3390/biom11040543.
Islam F, et al. Bioactive compounds and their derivatives: an insight into prospective phytotherapeutic approach against Alzheimer disease. Oxid Med Cell Longev. 2022. https://doi.org/10.1155/2022/5100904.
Akram M, Nawaz A. Effects of medicinal plants on Alzheimer disease and memory deficits. Neural Regen Res. 2017;12(4):660–70. https://doi.org/10.4103/1673-5374.205108.
Bui TT, Nguyen TH. Natural product for the treatment of Alzheimer disease. J Basic Clin Physiol Pharmacol. 2017;28(5):413–23. https://doi.org/10.1515/jbcpp-2016-0147.
Hamidpour R. Rosmarinus officinalis (Rosemary): a novel therapeutic agent for antioxidant, antimicrobial, anticancer, antidiabetic, antidepressant, neuroprotective, anti-inflammatory, and anti-obesity treatment. Biomed J Sci Tech Res. 2017;1(4):1098–103. https://doi.org/10.26717/bjstr.2017.01.000371.
Habtemariam S. The therapeutic potential of rosemary (Rosmarinus officinalis) Diterpenes for Alzheimer disease. Evid-based Complement Altern Med. 2016. https://doi.org/10.1155/2016/2680409.
López V, Martín S, Gómez-Serranillos MP, Carretero ME, Jäger AK, Calvo MI. Neuroprotective and neurological properties of Melissa officinalis. Neurochem Res. 2009;34(11):1955–61. https://doi.org/10.1007/s11064-009-9981-0.
Miraj S, Azizi N, Kiani S. A review of chemical components and pharmacological effects of Melissa officinalis L. Pharm Lett. 2016;8(6):229–37.
Beheshti S, Shahmoradi B. Therapeutic effect of Melissa officinalis in an amyloid-β rat model of Alzheimer disease. J Herb Med Pharmacol. 2018;7(3):193–9. https://doi.org/10.15171/jhp.2018.31.
Pratap GK, Ashwini S, Shantaram M. Alzheimer disease: a challenge in managing with certain medicinal plants—a review. Int J Pharm Sci Res. 2017;8(12):4960–72. https://doi.org/10.13040/IJPSR.0975-8232.8(12).4960.
Sharma R, Kuca K, Nepovimova E, Kabra A, Rao MM, Prajapati PK. Traditional ayurvedic and herbal remedies for AD: from bench to bedside. Expert Rev Neurother. 2019;19(5):359–74. https://doi.org/10.1080/14737175.2019.15968033.
Mahboubi M. Melissa officinalis and rosmarinic acid in management of memory functions and Alzheimer disease. Asian Pac J Trop Biomed. 2019;9(2):47–52. https://doi.org/10.4103/2221-1691.250849.
Wahab S, Annadurai S, Abullais SS, Das G, Ahmad W, Ahmad MF, Kandasamy G, Vasudevan R, Ali MS, Amir M. Glycyrrhiza glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants (Basel). 2021 Dec 14;10(12):2751. https://doi.org/10.3390/plants10122751. PMID: 34961221; PMCID: PMC8703329.
Murray MT. Glycyrrhiza glabra (Licorice). In: Textbook of natural medicine. 5th ed. St. Louis: Elsevier; 2020. p. 641- 647.e3. https://doi.org/10.1016/b978-0-323-43044-9.00085-6.
Hasan K, Ara I, Sha M, Mondal A, Kabir Y. Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra. Heliyon. 2021;7(June): e07240. https://doi.org/10.1016/j.heliyon.2021.e07240.
Chakravarthi KK, Avadhani R, Narayan RS. Effect of Glycyrrhiza glabra root extract on learning and memory in wistar albino rats. Int J Pharm Pharm Sci. 2012;4(4):199–202.
Parle M, Dhingra D, Kulkarni SK. Memory-strengthening activity of Glycyrrhiza glabra in exteroceptive and interoceptivebehavioral models. J Med Food. 2004;7(4):462–6. https://doi.org/10.1089/jmf.2004.7.462.60.
Sharma R, Kabra A, Rao MM, Prajapati PK. Herbal and holistic solutions for neurodegenerative and depressive disorders: leads from ayurveda. Curr Pharm Des. 2018;24(22):2597–608. https://doi.org/10.2174/1381612824666180821165741.
Paudel Y, Angelopoulou E, Semple B, Piperi C, Othman I, Shaikh MF. Potential neuroprotective effect of the HMGB1 inhibitor glycyrrhizin in neurological disorders. ACS Chem Neurosci. 2020. https://doi.org/10.1021/acschemneuro.9b00640.
Zeng L, Zhang H, Xu C, Bian Y. Neuroprotective effects of flavonoids extracted from licorice on kainate-induced seizure in mice through their antioxidant properties. J Zhejiang Univ. 2013;14(11):1004–12. https://doi.org/10.1631/jzus.B1300138.
Ravanfar P, Haghighi AB. Plants’ natural products as alternative promising anti-Candida drugs. Pharmacogn Rev. 2018. https://doi.org/10.4103/phrev.phrev.
Joshi TA, Tatke P. Neuroprotective effects of licorice extracts in tibial and sural transection induced neuropathic pain in rats. Indian J Pharm Educ Res. 2020;54(2):S285–94. https://doi.org/10.5530/ijper.54.2s.85.
Ramalingam M, Kim H, Lee Y, Lee YI. Phytochemical and pharmacological role of liquiritigenin and isoliquiritigenin from radix glycyrrhizae in human health and disease models. Front Aging Neurosci. 2018;10(November):1–15. https://doi.org/10.3389/fnagi.2018.00348.
Munawar T, Mashwani ZUR, Bibi Y, Ahmad F. Ethnomedicinal study of plants used for neurodegenerative diseases: a review. Proc Pak Acad Sci Part B. 2021;57(3):13–26.
Berkov S, Codina C, Basti J. The genus Galanthus: a source of bioactive compounds. In: Phytochemicals—a global perspective of their role in nutrition and health. London: IntechOpen; 2012. https://doi.org/10.5772/28798.
Heinrich M, Teoh HL. Galanthamine from snowdrop—the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J Ethnopharmacol. 2004;92(2–3):147–62. https://doi.org/10.1016/j.jep.2004.02.012.
Ding Y, Qu D, Zhang K, Cang X, Kou Z, Xiao W. Phytochemical and biological investigations of Amaryllidaceae alkaloids: a review. J Asian Nat Prod Res. 2016. https://doi.org/10.1080/10286020.2016.1198332.
Kong CK, Low LE, Siew WS, Yap W, Khaw K. Biological activities of snowdrop (Galanthus spp., family Amaryllidaceae). Front Pharmacol. 2021;11(2): 552453. https://doi.org/10.3389/fphar.2020.552453.
Kim JK, Park SU. Letter to the editor: Pharmacological aspects of galantamine for the treatment of Alzheimer’s disease. Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National Department of Crop Science, Chungnam National University; 2017. p. 35–9.
Hussain G, Rasul A, Anwar H, Aziz N, Razzaq A, Wei W. Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int J Biol Sci. 2018;14:341. https://doi.org/10.7150/ijbs.23247.
Ohba T, et al. Japanese Huperzia serrata extract and the constituent, huperzine A, ameliorate the scopolamine-induced cognitive impairment in mice. Biosci Biotechnol Biochem. 2015;79(11):1838–44. https://doi.org/10.1080/09168451.2015.1052773.
Callizot N, et al. Huperzia serrata extract ‘NSP01’ with neuroprotective effects-potential synergies of huperzine A and polyphenols. Front Pharmacol. 2021;12: 681532.
Lee Y-K, Chang YH. Physicochemical and antioxidant properties of methanol extract from Maca (Lepidium meyenii Walp.) leaves and roots. Food Sci Technol. 2019;39:278–86.
Peres NDSL, et al. Medicinal effects of Peruvian maca (Lepidium meyenii): a review. Food Funct. 2020;11(1):83–92.
Figueroa ME, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–67.
Rahman MM, Islam MR, Supti FA, Dhar PS, Shohag S, Ferdous J, Shuvo SK, Akter A, Hossain MS, Sharma R. Exploring the therapeutic effect of neurotrophins and neuropeptides in neurodegenerative diseases: at a glance. Mol Neurobiol. 2023;60(8):4206–31. https://doi.org/10.1007/s12035-023-03328-5. (Epub 2023 Apr 13).
Mukerjee N, Al-Khafaji K, Maitra S, Suhail Wadi J, Sachdeva P, Ghosh A, et al. Recognizing novel drugs against Keap1 in AD using machine learning grounded computational studies. Front Mol Neurosci. 2022;15:1036552.
Francis PT, Plamer AM, Anape M, et al. The cholinergic hypothesis of Alzheimer’s dieases: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66(2):137–47.
Sharma R, Singla RK, Banerjee S, Sinha B, Shen B, Sharma R. Role of Shankhpushpi (Convolvulus pluricaulis) in neurological disorders: an umbrella review covering evidence from ethnopharmacology to clinical studies. Neurosci Biobehav Rev. 2022;140: 104795. https://doi.org/10.1016/j.neubiorev.2022.104795.
Rodríguez-Morató J, Xicota L, Fitó M, Farré M, Dierssen M, de la Torre R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules. 2015;20(3):4655–80.
Daccache A, et al. Oleuropein and derivatives from olives as Tau aggregation inhibitors. Neurochem Int. 2011;58(6):700–7.
Martín-Peláez S, Covas MI, Fitó M, Kušar A, Pravst I. Health effects of olive oil polyphenols: recent advances and possibilities for the use of health claims. Mol Nutr Food Res. 2013;57(5):760–71.
Li W, Sperry JB, Crowe A, Trojanowski JQ, Smith AB III, Lee VM. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. J Neurochem. 2009;110(4):1339–51.
Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer disease. Ann Neurol. 2006;59(6):912–21. https://doi.org/10.1002/ana.20854.
Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63(12):1709–17.
Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. 2013;74(4):580–91.
Li D, Wang P, Luo Y, Zhao M, Chen F. Health benefits of anthocyanins and molecular mechanisms: update from recent decade. Crit Rev Food Sci Nutr. 2017;57(8):1729–41.
Gutierres JM, et al. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci. 2014;96(1):7–17. https://doi.org/10.1016/j.lfs.2013.11.014.
Devore EE, Kang JH, Breteler MMB, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72(1):135–43.
Venigalla M, Gyengesi E, Münch G. Curcumin and apigenin–novel and promising therapeutics against chronic neuroinflammation in Alzheimer disease. Neural Regen Res. 2015;10(8):1181.
Ng T-P, Chiam P-C, Lee T, Chua H-C, Lim L, Kua E-H. Curry consumption and cognitive function in the elderly. Am J Epidemiol. 2006;164(9):898–906.
Small GW, et al. Memory and brain amyloid and tau effects of a bioavailable form of curcumin in non-demented adults: a double-blind, placebo-controlled 18-month trial. Am J Geriatr Psychiatry. 2018;26(3):266–77. https://doi.org/10.1016/j.jagp.2017.10.010.
Devi KP, Shanmuganathan B, Manayi A, Nabavi SF, Nabavi SM. Molecular and therapeutic targets of genistein in Alzheimer disease. Mol Neurobiol. 2017;54(9):7028–41.
Ozawa M, et al. Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama study. Am J Clin Nutr. 2013;97(5):1076–82. https://doi.org/10.3945/ajcn.112.045575.
Sasaoka N, et al. Long-term oral administration of hop flower extracts mitigates Alzheimer phenotypes in mice. PLoS ONE. 2014;9(1): e87185.
Freund Levi Y, et al. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer disease: the OmegAD study. J Intern Med. 2014;275(4):428–36. https://doi.org/10.1111/joim.12166.
Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by SB, KP, MD, RS, JD, VHK, DD, MZA and BAA. The first draft of the manuscript was written by SB, KP, MD, RS, JD, VHK, DD, MZA and BAA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bordoloi, S., Pathak, K., Devi, M. et al. Some promising medicinal plants used in Alzheimer’s disease: an ethnopharmacological perspective. Discov Appl Sci 6, 215 (2024). https://doi.org/10.1007/s42452-024-05811-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05811-7