Abstract
Despite its beneficial properties and the efficiency of essential oil in controlling mosquitoes and other hematopoietic insects, this biocontrol agent has several significant limitations, ranging from its chemical instability to its short protection time and sensitivity to oxidation. This research aimed to address these limitations by altering the surface functionality using encapsulated Vitex negundo essential oil (VnEO). The VnEO was extracted by hydrodistilation and analysed by GC–MS. The oil was β-cyclodextrin (β-CD) encapsulated and monochlorotriazine (MCT) modified to improve its fabric interaction and stability, while histological and immunohistochemical examinations were conducted to determine its safety. The fabrics were subjected to FT-IR, SEM, XRD, TGA, fiberometric, and Zeta potential analysis, while the repellency study was conducted in an olfactometer. Insecticidal monoterpenes and sesquiterpenes were confirmed by GC–MS, and an entrapment efficiency of 94.3% was achieved. The repellent interaction with the fabric was confirmed by the formation of two intense bands at 3277 (O–H) and 1710 cm−1 (C=O), broad diffraction peaks at 17.30° and 38.30–57.10° about a 4.70% increase in average fibre size and a 18.8% decrease in pore size, and lower fibre thermal stability. The surface of the fabric is negatively charged, causing an enhancement in the adsorption and affinity of VnEO by grafting of β-CD/MCT. The dermatological investigation suggests that the oil has no significant toxic effects. In conclusion, the encapsulation and fabric grafting employed are effective and safe for the preparation of a long-lasting repellent fabric.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the words of Brown [1] and WHO report of 2015, malaria is the world’s third deadliest disease and occupies a unique place in the annals of history; considering its role in the modern environmental movement and contribution towards the fall of the Roman empire. The human malaria can be caused by five Plasmodium species; transmitted primarily by Anopheles gambiae [2].
In recent years, increased partnership and funding has accelerated malaria control efforts. Consequently, the introduction of various WHO-recommended malaria prevention tools, which include the (i) vector control measure, which involves the use of indoor residual spraying and insecticide-treated nets highly effective in preventing infection and reducing transmission. (ii) preventive chemotherapy, and (iii) RTS, S, or AS01 malaria vaccine (Mosquirix™). Hemingway et al. [3] and Hwang et al. [4] in their studies reported a significantly reduction in deadly severe malaria cases, among young children on Mosquirix™.
There are major setbacks to these preventive tools which include the mosquito resistance to at least one of carbamates, organophosphates, and pyrethroids insecticide classes were reported in 78 countries between 2010 and 2019. Another report showed that about 29 countries showed mosquito resistance to all three classes [5]. Furthermore, insecticide-treated nets have limitations due to their ineffectiveness against exophagic malaria vectors, lowered susceptibility, distribution issues, sustainability issues, and poor coverage rates. Furthermore, there has been a growing concern of plasmodium resistance to the use of preventive chemotherapeutic drugs (quinoline-based compounds, antifolates, and artemisinin derivatives) over the last decade [6].
The several highlighted challenges of the afore-mentioned malaria preventative tools have led to increases in malaria cases and deaths in many developing countries, where children under the age of five, women in their first and second pregnancy, migrants from areas where malaria has not spread or is not transmitted and people who do not have immunity to the disease are the most vulnerable [7]. It was reported in 2019, that the global malaria burden and estimated malaria cases stood at 224 and 227 million, respectively, with an estimated 241 million malaria cases recorded in 2020 [7]. In 2020, the WHO African region is responsible for 95% of all malaria cases and 96% of malaria deaths, and 80% of malaria deaths in the WHO African region were in children under 5 years of age. Nigeria, from an African perspective, accounts for 26.8% of malaria cases and 31.9% of deaths in 2020 [8]. According to the CDC, [9] report, nearly half of the world’s population lives in areas at risk of malaria transmission, and the WHO African region is responsible for the greatest burden of malaria.
Given the unfavourable African malaria statistics and limitations of preventative tools, finding a viable and sustainable alternative has become a topical and critical issue of focus if we are to achieve the global technical strategy for malaria target by 2030 [10]. These have led to the desire to explore the possibility of developing a sustainable and readily affordable mosquito repellent textile. Studies by Jaswal [11] and Monllor et al. [12] have shown that fabric surface alteration can change their qualities and give new functionality. Adding essential oil to fabric for essence release looks to be the most promising of the several strategies that have been developed. The development of an oil/cyclodextrin molecular inclusion complex presently holds the most potential for managing essential oil release for long-term mosquito control [13, 14].
Cyclodextrine (CD) is a group of three industrially prepared compounds (α-, β-, and γ-CDs) with a molecular weight of 200 to 800 g/mol, are made up of a cyclic oligosaccharide consisting of 7 glucopyranose units linked at the α-(1,4) position [15,16,17]. It has a unique structure with a water-resistant cavity and surface, and it may host various organic compounds after grafting, enabling aroma fixation and slow-release function [18]. Sricharussin et al. [19] and Andrade et al. [20] discovered that when β-CD is attached to cellulose fibres, its ability to form complexes is preserved with other molecules while having no effect on the hydrophilic properties of cellulose and does not produce skin irritation or sensitivity.
Vitex negundo essential oil (VnEO) has piqued interest in the area of malaria vector control due to its strong hydrophobic and H-bond interactions and olfactory receptor blocking, subsequently resulting in vector disorientation [21,22,23,24]. According to previous researches, the essential oil of V. negundo has a significant repellent effect on A. gambiae, with an ED50 of 0.14–0.08% v/v [21, 23]. However, its chemical instability, quality control concerns, insolubility in aqueous systems, short-term protection against mosquito bite and susceptible to oxidation have limited the applications [25, 26]. According to Sangsuriya [27], because of the stability of covalent connections with electron-rich groups on textiles or papers, the monochlorotriazinyl group is a reactive anchor for the dye-cyclodextrin molecular inclusion complex on cellulose. On cotton textiles, the presence of CD aids in the greater suppression of essential oil release from finished fabrics; however, the fabrics and encapsulate only have weak Vander-Waal and hydrogen interactions. Hence, their adherence to any of the treated fabrics is short-lived resulting in a temporary fabric finish that can easily be washed off [28]. Their lack of durability on fabrics severely limits their effectiveness as finishing agents. Instead, it is hypothesized that the modified monomolecular complexes can form van der Waal bonds, ionic bonds, or even covalent bonds with the fabric surfaces. This improved monochlorotriazinyl-β-cyclodextrin (MCT-β-CD) derivative with improved stability is currently in high demand [29]. The MCT-β-CD complex can be used to permanently bind β-CD to cotton using the standard reactive dyeing method proposed by Bhaskara-Amrit et al. [30]. Covalent bonds can be formed when the reactive chlorine atom of the triazinyl group of MCT- β-CD reacts with nucleophilic residues on the cotton, like OH [31]. According to Cabrales et al. [32] and Ali et al. [33], it has the potential to serve as a universal anchor for a wide range of textile materials. The purpose of this research is to design a long-lasting repellent textile by altering the surface functionality of fabric with monochlorotriazine modified β-CD encapsulated V. negundo essential oil.
2 Materials and methods
2.1 Collection and extraction of V. negundo leaves
The leaves were collected from a specific location in Nasarawa State (8.4998° N, 8.1997° E) in Nigeria. The authentication of the plant was carried out in the Department of Research on Medical Plants and Traditional Medicine, National Institute of Pharmaceutical Research and Development, Idu, Abuja, and assigned specimen number NIPRD/Hebarium/1101.
Freshly collected leaves were cleaned with distilled water and steam distilled for 45 min, followed by the separation of the distillate into essential oil and hydrosol in batches. Finally, the oil was dried over anhydrous Na2SO4 and stored in an amber vial for further analysis.
2.2 Rearing, and identification of mosquitoes
Mosquito larvae were collected between September and October, 2022, according to the previously reported protocol by Okoli et al. [24]. At the nesting locations, larvae were identified using Coetzee [34] identification key, morphologically classified, and collected from a deep temporary pool with grass growth using a 30 cm long dipper. The stages of immature larvae were carefully transported to the insect in a vial., where they are placed in an open container with 1 L of ground water for 2 h before being given a finely ground low-fat flour-baked food product.
2.3 Experimental animals
Male Wistar rats weighing between 55 and 80 g were purchased from the Animal Holding Facility at Bingham University, Physiology Department. The animals were examined and adapted to the new environmental conditions for a week before the formal experiment. During the day, they were fed chaw and given free access to tap water. They were kept on a 12-h light/dark cycle. All animal studies were performed according to the US National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.4 Experimental design
The rats were weighed and grouped into two groups of five rats each. Before the administration, the dorsal fur of the rats was shaved, and then oil samples were applied topically to the skin of the rats according to the following groupings: group 1 was administered neat V. negundo oil topically, while group 2 was without treatment (normal control). The administration lasted for 14 days before the sacrifice.
2.5 Histopathology and immunohistochemical examination of the skin
Skin tissue samples were taken for histopathological examinations. The tissues were promptly fixed in 10% formalin before being dried in a succession of ethanol treatments and embedded in paraffin. Thin slices 4–5-μm thick were cut using a rotary microtome and stained with hematoxylin and eosin for photomicroscopic examination. The microscopic characteristics of the treated group was then compared to those of the control group.
The skin tissues were exposed to the avidin–biotin peroxidase complex employing immunoperoxidase methods for the immunochemical investigation. The samples were fixed in 10% buffered formalin, embedded in paraffin, and sectioned into 5 μm thick sections. The slices were subsequently subjected to pressure antigen retrieval (20 psi/125 °C). The primary antibody pan-cytokeratin was used for immunohistochemistry. The immunohistochemistry image analysis toolbox (ImageJ computer application) was used to automatically calculate the percentage area of expression, the percentage of positively stained nuclear area (labelling index), and a coloured deconvolution algorithm to separate the counter stains [35].
2.6 Characterisation of V. negundo leaves essential oils
2.6.1 Compositional analysis
The 0.2µL of essential oil (diluted 100: 1 in hexane) were injected into the port at 220 °C and eluted with nitrogen at a rate of 1.2 mL/min through the HP-5 capillary at 244 °C in a Varian CP-3800 gas chromatographer equipped with a Varian Saturn 2000 mass detector. The oven temperature was set at 280 °C and 3 °C per minute. Individual components were identified using retention indices, which were then compared to compounds previously described in the literature. The individual components were identified with retention indexes and then compared to the compounds previously described in literature [36, 37]. In addition, data from computer libraries linked to the GC–MS, Adams Library, NIST’s website using RI values from comparable polarity columns and/or Mondello Library.
2.6.2 Preparation of inclusion complex
The inclusion complex was constructed using a needle technique described in previous studies. By Menezes et al. [38] and Tao et al. [39, 40] with slight modification. A solid mixture was prepared by introducing the oil into a mortar already containing powdered β-CD on various molar guest: host ratio based on the encapsulation performance. The mix was then dried in a desiccator at 28 °C until an inclusion complex with a constant weight was obtained, labelled as VnEOβ-CD and returned to the desiccator for future analysis and use.
2.6.3 Encapsulation efficiency
A freshly produced 50 mL phosphate buffer with a pH of 7.4 was added to the VnEOβ-CD and gently stirred for 24 h before filtering. After that, the filtrate was submitted to GC–MS analysis. The encapsulation efficiency was determined using Eq. (1).
2.6.4 Modification of the inclusion complex
The monochlorotriazine modified inclusion complex was synthesis according to Wacker–Chemie [41]. A clear solution of sodium salt dichlorotriazine was obtained by dissolving cyanuric chloride and NaOH into water at 5 °C. The second step involves the reaction of dichlorotriazine molecule with VnEOβ-CD at 15 °C in a neutral environment. The obtained solution is freeze-drying into a white powder labelled as VnEOβ-CD/MCT.
2.6.5 Fabric treatment
Figure 1 shows the schematic for the preparation of mosquito repellent fabrics. A 30 cm by 45 cm sateen fabrics was first treated with 2% Na2CO3 for 2 min, dried, and then impregnated with VnEO-CD/MCT in a treatment bath at 150 °C. The impregnated fabrics were subsequently padded using mechanical squeeze rollers at 15 kg/cm, yielding a wet pick-up of 70–85%. The fabrics were then dried for 2 min at 100 °C and cured for 3 min at 150 °C in a Werner Mathis AG oven. Afterwards, the treated fabrics were rinsed with distilled water to remove any unreacted chemicals and spray-dried in air with 8% ethanol solution of VnEO. The fabrics were then stored in the laboratory at ambient conditions for up to four weeks—labelled as VnEOβ-CD/MCT-F.
2.6.6 Fabric analysis
The untreated fabric, activated fabric, and treated fabric were characterised using FT-IR (PerkinElmer Spectrum 400, Waltham, MA) measured over the range of 4000–400 cm−1. The surface morphology and fibermetric analyses were investigated using a Philips XL30 scanning electron microscope (Hillsboro, OR). The fabric was mounted on a tub of clean glass and coated with a gold palladium film of 2 nm thickness. for 120 s at 10 mA, under low-pressure argon and explored at 4–25 kV. The X-ray diffraction data (XRD) were obtained using a Rigaku D2000 Bragg–Brentano diffraction meter equipped with a rotating copper anode, a diffraction beam monochrome calibrated to CuK radiation and a scattering detector with geometry in reflection mode. Finally, the thermal responses were measured using a Perkin Elmer thermogravimetric analyzer (Waltham, MA) set at 303–1173 K and a heating rate of 283 K/min in a nitrogen environment. The fabric analysis was conducted on Phenom XL Compact Desktop SEM (Thermo Scientific, UK). A Möbius® Zeta Potential Analyzer (Wyatt Technology Corporation) was used to measure the zeta potential of the 1 mm length treated and untreated fabrics. The 0.1% solids were prepared in 0.001 M KCl at various pH levels, agitated for 600 s with a magnetic stirrer, and the results were recorded.
2.6.7 Laundering durability
In order to obtain sufficient friction and reflect the true durability of washing under normal conditions, the treated fabric has been washed. Water containing 2 g/L sodium carbonate and 5 g/L standard laundry soap, at 27 °C for 15 min was used for the treated fabrics obtained from the 6 wash cycles obtained at an interval of 15 days. The fabric was rinsed in cold distilled water and dried at 30 °C. An aliquot of the wash solution was extracted quantitatively with analytical grade hexane and subjected to GCMS profiling to determine the quantity of essential oil compound in the wash solution. Mosquito repellency study was carried out after each wash cycle on the treated fabric, and the ability of treated fabrics to keep repellent fragrance after washing is expressed as percentage retention according to Nielsen [42] following Eq. (2).
The amount of time required to observe behavioural changes in the flight patterns of 50% female Anopheles gambiae mosquitos was recorded when exposed to 5 g of the treated fabrics (VnEOβ-CD/MCT-F), neat essential oil (positive control), and cloth treated with monochlorotriazine-modified β-CD without V. negundo essential oil (negative control). After introduction of fabric, pure essential oil, and negative control, a period of 10 min was allowed to ensure equilibration of the air-essential oil mixture. This was observed for 90 days, with the introduction of fresh 100 female A. gambiae at pre-determined intervals of 1, 14, 30, 60, and 90 days into the olfactometer.
Following the procedure of Costantini et al. [43] with a slight modification,using an olfactometer over a period of 1800s, the mosquito repellency after six wash cycle was calculated using Eq. 3:
2.7 Data analysis
The results are expressed as the mean value and standard deviation (mean SD) of each repeated sample (n = 3).
3 Result and discussion
3.1 Yield and terpene composition of V. negundo essential oils
The essential oils yield from 50 kg fresh V. negundo plant was 0.40 ± 0.02%w/w and an aliquote of the oil was subjected to GC–MS analysis with the terpene composition and chromatograms reported in Supplementary Table S1 and Figure S1. The analysis of the oil revealed the presence of 28 known compounds of which 80.16%, 7.63%, and 10.88% are monoterpene, sesquiterpene, and unknown content; respectively. The study of Thi My Dung et al. [44] on the chemical composition of essential oil extracted from the leaves of V. negundo Linn From Binh Thuan province by hydrodistillation and microwave hydrodistillation also confirms the presence of greater concentration of monoterpenoids (45.3%) than sesquiterpenoids (41.0%).
The identified chemicals are consistent with Khokra et al. [36] findings on the GC-FID and GC/MS analysis of the chemical contents of V. negundo EOs leaves, flowers, and dried fruits. Some monoterpenes and sesquiterpenes with reported insecticidal properties such as linalool, myrcene, α-terpinolene, α-pinene, and citronellal were present in the oil in varying percentage compositions [45] while α-pinene and myrcene were reported to be present in quantity > 15%.
3.2 Histopathology and immunohistochemical examination of the skin
The assay showed the presence of a focal mild epidermal sclerosis (Supplementary fig. S2) and a low expression of the primary antibody pan-cytokeratin with a nuclear area of groups 1 and 2 is 53% and 81%; respectively (Supplementary fig. S3). This is not in consonance with the study of Kadir et al. [46], which shows the V. negundo may prevent ongoing TAA-induced nephrotoxicity in rats, both biochemically and morphologically. The observed focal mild epidermal sclerosis with low expression of the primary antibody pan-cytokeratin might be the result of the chemotaxonomical variations in the essential oil composition.
3.3 Characterization of the inclusion complex
3.3.1 Entrapment efficiency (%EE)
The optimal entrapment efficiency (EE) of VnEOβ-CD prepared is 94.3 ± 1.5% at a molar guest: host ratio of 1:3 while the lowest is recorded at 1:1 molar ratio (Table 1). By implication the higher the host load the better the entrapment efficiency. This optimal entrapment efficiency is consistent with other previous studies of Kamimura et al. [47] and Kfoury et al. [48], on the complexation of estragole with cyclodextrins and microencapsulation of carvacrol in hydroxypropyl-β-CD with value \(\le\) 99% reported.
According to Dos Passos Menezes et al. [49] lower value of complexation efficiency is may be a result of surface interaction of the essential oil or changes in the equilibrium states of molecules in a solution [40]. On the other hand, the method used may have a significant impact on the entrapment efficiency, as reported in the studies of Wang et al. [50] and Santos et al. [51] where they discovered 90.3% and 91.3% EE for garlic and carvacrol using the co-precipitation technique and freeze-drying at low temperatures,respectively.
3.3.2 Morphological examinations
Figure 2 depicts the surface morphologies of the β-CD and the VnEOβ-CD. In this study, the best complexation result was obtained at molar guest: host 1:3. The morphology of the pure β-CD shows a compact particle with irregular surface. Wen et al. [29] analyzed the morphology of β-CD inclusion complexes of cinnamon essential oil and reported an irregular particle size, whereas the inclusion complex was present in a multilayered crystal form. Due to the absence of a significant net load on the particles included in the complex, β-CD has a strong tendency to accumulate due to itsself-assembly property in water, which has no repulsive force to prevent particle accumulation [52, 53].
When comparing VnEOβ-CD to pure β-CD, we discovered differences in shape and size; these findings could point to the formation of VnEOβ-CD, which is consistent with the studies of Cid-Samamed et al. [54] and Saikosin et al. [55]. One of the primary benefits of the capsules is their ability to provide long-lasting fragrance by slowly releasing the essential oil over time, providing a consistent aroma that can help keep fabrics smelling fresh and clean.
Figure 3 indicates that the surface appearance of the untreated fabric, activated fabric, and treated fabric (VnEOβ-CD/MCT-F). The activated fabric surface is agitated, inflated smooth, and flat with small twists [56]. The surface of the VnEOβ-CD/MCT-F was cylindrical with small particles scattered over it (Fig. 3c). However, the surface of the fabric had a significant number of particles accumulated and aggregated on the surface of the samples. The inclusion complexes deposited to the fabric covered its surface, allowing the finished layer to form. This revealed that this method was effective for applying inclusion complexes to fabrics.
This is in line with Lis et al. [57] application of the citronella oil-cyclodextrin complex for controlled release in biofunctional textiles, which results in entire covering of the textile surface for the development of the final layer (Fig. 4).
The production of aggregates is enabled by the base-catalyzed interaction between the chloride group of the VnEOβ-CD/MCT and the fabric [58, 59] .
3.3.3 Fibermetric analysis
The image of the fiber and pore histograms of the untreated fabric, activated fabric, and repellent treated fabric were reported in Supplementary Fig. S4 and the results are presented in Table 2.
The average fiber and pore sizes of the untreated fabric and activated fabric decrease in by approximately 5% and 23.6%; respectively because the material is damaged by the loss of amorphous compounds when treated with a saturated carbonate solution. This is in consonance with a study on the effect of sodium carbonate on the mechanical and water absorption characteristics of natural fabric by Abdul Karim et al. [60]. However, on treatment of the fabrics with the inclusion complex modified with monochlorotriazine to produce the repellent treated fabric (VnEOβ-CD/MCT-F) there was an increase in the average fiber size and decrease in the pore size by 4.7% and 18.8%; respectively. This is consistent with a study that activated fabric exhibits a high degree of interfacial interaction with substances such as reactive cyclodextrin [18], which might explain the considerable change in surface texture seen.
3.3.4 Fabric functionalization
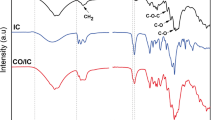
The FTIR spectra of the untreated fabric, activated fabric, essential oil, pure β–CD, VnEOβ-CD, cyanuric chloride, VnEOβ-CD/MCT, and VnEOβ-CD/MCT-F are presented in Fig. 5. The FTIR spectrum of the cyanuric chloride showed some bands in the 3191–2822 cm−1 range and 1669 cm−1 that corresponding to C–H and olefinic groups stretching vibrations [61]. The pure β-CD FTIR spectrum showed bands centered on 3309, 2920, 1637 and 1151–1026 cm−1 corresponding to the strain vibrations of O–H, C–H (CH2 and CH2 groups), C=O, and C–O. Rocking vibrations of the C–H bonds and the C–C skeletal vibrations in the glucopyranose ring correspond to bands in the region 940–700 cm−1 were equally observed. The EO spectrum showed some bands in the 2925–2853, 1779, and 1457 cm−1 that correspond to vibrations of the C–H bonds, C=O group, and C=C group of unsaturated components of the essential oils. These reveals the presence of carbonyl and unsaturated type of monoterpenes and sesquiterpenes in the essential oil.
The VnEOβ-CD spectrum revealed O–H stretching vibration bands at 3308 cm−1 and a significant decrease band intensity at around 2920 cm−1 and 1733 cm−1; the reduction of characteristic peaks confirms the possible interaction of the EO between β-CD which might imply the formation of VnEOβ-CD inclusion complexes. Kringel et al. [62] discovered a link between fluctuation in characteristic peak intensity and complex formation,the inclusion of guest molecules in the β-CD cavity limits the mobility of encapsulated molecules. Similar studies that investigated the inclusion complexes of orange oil, thyme oil, eucalyptus, and limonene discovered a decrease in the strength of various essential oil characteristic bands [49, 62, 63].
Fixation of VnEOβ-CD/MCT on fabric was also confirmed by FTIR results in Fig. 5. VnEOβ-CD/MCT presented the FTIR bands at 2914 (CH), 1603 (C═N) and 1021 cm−1 (Cl–C═N), while a strong band at 3345 cm−1 (OH) is observed for cotton. The fabric, which was treated with modified inclusion complex (VnEOβ-CD/MCT) at curing temperature of 150 °C for 3 min and underwent a washing-off process, exhibited the same characteristic bands of VnEOβ-CD/MCT and fabric, confirming the existence of VnEOβ-CD/MCT grafted on the fabrics. This resulted in the formation of two intense bands on the fabric at 3277 cm−1 and 1710 cm−1 corresponding to the stretching vibrations of O–H and C=O bonds; a consequence of a chemical modification of the fabric [64, 65]. The spectra of the untreated and activated fabrics did not reveal any differences in peak position or intensity; consequently, the activation step is a physical process.
3.3.5 Fabric thermal response
Thermal stability of untreated fabric and treated fabric (VnEOβ-CD/MCT-F) is shown in Fig. 6. The thermal stability of a repellent fabric can have a significant effect on its performance as a repellent [66]. At temperature below 200 °C the vaporization of the physically adsorbed water was negligible for both fabrics. The thermogram of untreated fabric reveals three phases of weight loss. The weight loss in the first phase, between 200 and 350 °C, is ascribed to the dehydroxylation of the chemically bonded water. In the second phase, from 427 to 440 °C, the untreated fabric displays a slight weight loss, which is attributed to the degradation of cellulose, as expected from the literature [67].
In the third stage, in the temperature range of 547–554 °C, the fabric continues to decompose slowly with a weight loss of 1.6%. In contrast, the weight loss of the repellent-treated fabric increased by 5%; therefore, the repellent treatment weakens the fabric and makes it prone to thermal degradation, possibly acting as a conduit to increase the ingress of air for resultant oxidative degradation. A similar weight loss around 426–550 °C was observed for repellent treated fabric. However, there is a distinct deterioration at 728–764 °C, which may be attributable to the contrasting behaviour of internal crystallites and the chemical structure of untreated and repellent-treated fabric as a result of the fabric’s chemical alteration. The decomposition of the modified inclusion complex grafted on fabric lowers its thermal stability compared to that of the untreated fabric, which may compromise its overall performance as a repellent [68].
3.3.6 X-ray diffraction analysis
Diffractogram for the inclusion complex, modified inclusion complex, untreated fabric, activated fabric, and repellent treated fabrics are shown in Figs. 7 and 8.
The morphology of a material, whether it is amorphous or crystalline, can have an impact on the properties of essential oils that are in contact with the fabric [69]. The encapsulated essential oil (VnEOβ-CD) exhibited significant diffraction peaks at 2θ = 33.90°, 39.10°, 46.20°, 55.10°, and 65.50°, which is a crystalline material feature. On modification of the inclusion complex with monochlorotriazine, new peaks appeared at 2θ = 28.40° and 59.10°, with peaks at 46.20°, 55.10°, and 65.50° were slightly altered to 47.50°, 56.30°, and 69.40°, respectively By comparing X-ray diffraction (XRD) patterns, the characteristic peak intensity of the modified inclusion complex substrate increases due to structural changes on inclusion complex during monochlorotriazine grafting, which is consistent with the findings of Khanna and Chakraborty [70] in their study on the optimization of monochlorotriazine- β-CD grafting on cotton.
The grafting of the VnEOβ-CD/MCT on fabric increased the amorphous characteristics of the fabric. The treated fabric showed a medium broad diffraction peak at 2θ = 17.30° compared to the characteristic peaks of the of the untreated and activated fabrics with sharp strong peaks at 17.70, 26.00 and a broad peak at around 38.30°–57.10° (Fig. 8). According to Ambaye et al. [71], amorphous materials lack long-range order in their structure, which can make them more porous and provide a greater surface area for adsorption. This increased surface area can lead to higher adsorption capacities for essential oils, potentially making the amorphous materials more effective at delivering essential oil fragrances or flavours.
3.4 Zeta potential of the fabrics
Figure 9 shows the zeta potential of untreated and treated fabrics in a wide pH. Over the pH range, the findings indicated that untreated fabrics had a range of negative charges on their surfaces.
The presence of abundant terminal sodium oxide, hydroxyl, and chloride groups in the molecular structure of the β-CD/MCT grafted onto the fabric surface was probably responsible for this behavior, as those groups gained H+ ions in the liquid phase and then transformed into cationic groups at pHs lower than 7.5. The zeta potential values of the treated fabrics increased across the entire pH range, rendering the surfaces positively charged at a low pH. When β-CD/MCT interact with positively charged surfaces of the fabrics, they can become adsorbed onto the surface through electrostatic interactions enhancing the affinity and adsorption of VnEO onto the fabric [72]. This enhanced interaction may increase the stability and shelf life of the oil by reducing its volatility and preventing oxidation. It may also enhance the oil's ability to adhere to certain surfaces, which can be useful in applications such as flavouring or fragrance delivery[73].
3.4.1 Repellency potential of the treated fabric and laundering durability
Table 3 shows the time needed to detect behavioural changes in the flying patterns of 50% of the female A. gambiae after being subjected to the treated fabric, pure essential oil, and negative control over a 90-day period. The period for behavioural changes in flying patterns differed considerably after exposure of the female A. gambiae to the fabric and essential oil. The negative control did not exhibit any form of repellency effect on the exposure to the A. gambiae. Further, the efficacy of the V. negundo oil has also been reported in previously published articles by Hebbalkar et al. [74] and Okoli et al. [24].
The regulated release pattern of the essential from the monochlorotriazine modified β-CD functionalized surface of the fabric caused an increase in the distortion in the flying patterns of A. gambiae as the number of days increased. However, from day 1 to day 14, a similar response was noted for the pure essential; however, repellency activity declined from day 12 to a point of no activity. This is most likely due to the chemical volatility, vulnerability to oxidation, and limited protection against mosquito bites. According to the findings of Maes et al. [75] the observed attitudinal shift is highly related to the stabilisation of the essential oil by the encapsulate.
The treated fabric, subjected to six wash cycles and subjected to mosquito repellency study over period of 1800s after each wash cycle, showed a gradual decrease in repellency activity from 100 ± 2.00 to 50 ± 4.10%. Generally, the observed repellency activity is associated with the decrease in the percentage retention of the treated fabric after each wash cycle (Fig. 10).
After the six wash cycles varying amount of α-pinene, sulcatone, sabinene, myrcene, α·3-carene, linalool, cis-sabinene hydrate, camphor, borneol, α-terpineol, (E)-β-ocimenen, decanal, geraniol, linalyl acetate, and bornyl acetate were released from the treated fabric into the wash solution (Table 4). Linalool, cis-sabinene hydrate, α-pinene, sulcatone, sabinene, myrcene, camphor accounted for the largest amount of compound found in the wash solution across the 6 wash cycles. After the first wash cycle there was a loss of 13% repellency possibly due to a loss of α-pinene, sabinene, and myrcene which are lead phytocompounds natural anti-mosquito in V. negundo EO according to the findings of Okoli et al. [23] on the in-silico and excito-repellent studies. After the first wash cycle β-selinene and ledene were the only sesquiterpenes lost to the wash solution.
After the second was cycle the repellent activity further decreased by a significant amount with of α-pinene, sulcatone, sabinene, (E)-β-ocimene, β-selinene, and ledene found in the wash solution. There was a relative stability in the retention characteristic of the treated fabrics after the third, fourth and fifth wash cycle. The sixth wash cycle provided the highest decrease in repellency activity of about 55% due to the consistent loss of sulcatone one of the major anti-repellent compound in V. negundo due to its strong hydrogen bond interaction with key odourant proteins in A. gambiae mosquitos [24]. However, the only sesquiterpenes found in the wash solution were β-selinene and ledene which occurred after the second cycle and their removal is associated with the molecular weights of the sesquiterpenes in relation to the size of the host hydrophobic cavity.
4 Conclusion
The results of this investigation suggest that finishing fabric with MCT-modified encapsulated VnEO is successful, providing important information for the development of biofunctional materials designed to repel mosquitos. The characterization data of treated and untreated fabrics clearly demonstrated the efficacy of MCT on fabric in terms of significant increases in chemical stability, protection time, and oxidation resistance. The treated textiles did, in fact, exhibit good repellency qualities, satisfactory wash cycles, and distinctive features that might fulfil practical usage requirements. The environmental effect of chemical-based aerosol repellents will be eliminated with this innovative alteration strategy, as well as the impact of malaria in endemic areas. Based on the findings, it is possible to infer that finishing fabric with MCT-modified β-CD encapsulated V. negundo essential oil is a potential option for convectional malaria vector control that may satisfy desirable end-use qualities.
Data availability
The data used to support this study is included in the publication.
References
Brown PJ (2004) Malaria and Rome: a history of malaria in ancient Italy (review). Bull Hist Med 78(1):208–210. https://doi.org/10.1353/bhm.2004.0008
Fayehun OA, Macro ICF (2010) Household environmental health hazards and child survival in Sub-Saharan Africa [WP74]. Dhs Working Papers (No. 74; Issue 74)
Hemingway J, Shretta R, Wells TNC, Bell D, Djimdé AA, Achee N, Qi G (2016) Tools and strategies for malaria control and elimination: what do we need to achieve a grand convergence in malaria? PLoS Biol 14(3):e1002380. https://doi.org/10.1371/journal.pbio.1002380
Hwang J, Cullen KA, Kachur SP, Arguin PM, Baird JK (2014) Severe morbidity and mortality risk from malaria in the United States, 1985–2011. Open Forum Infect Dis. https://doi.org/10.1093/ofid/ofu034
WHO (2016) Test procedures for insecticide resistance monitoring in malaria vector mosquitoes Second edition
Blasco B, Leroy Di, Fidock DA (2017) Antimalarial drug resistance: linking Plasmodium falciparum parasite biology to the clinic. Nat Med 23(8):917–928. https://doi.org/10.1038/nm.4381
WHO (2022) Malaria report 2022
Gallup JL, Sachs JD (2001) The economic burden of malaria. Am J Trop Med Hyg 64(1–2):85–96. https://doi.org/10.4269/ajtmh.2001.64.85
CDC (2022) Center for Global Health Division of Parasitic Diseases and Malaria. http://reference.medscape
Shretta R, Liu J, Cotter C, Cohen J, Dolenz C, Makomva K, Newby G, Ménard D, Phillips A, Tatarsky A, Gosling R, Feachem R (2017) Malaria elimination and eradication. In: Holmes KK, Bertozzi S, Bloom BR, Jha P (eds) Disease control priorities, third edition (volume 6): major infectious diseases. The World Bank, Washington DC, pp 315–346
Jaswal P (2018) Surface of modification of fibre an aspect of comfort properties fabric. Latest Trends Text Fash Des. https://doi.org/10.32474/lttfd.2018.02.000137
Monllor P, Bonet MA, Cases F (2007) Characterization of the behaviour of flavour microcapsules in cotton fabrics. Eur Polymer J 43(6):2481–2490. https://doi.org/10.1016/j.eurpolymj.2007.04.004
Bezerra FM, Carmona OG, Carmona CG, Lis MJ, de Moraes FF (2016) Controlled release of microencapsulated citronella essential oil on cotton and polyester matrices. Cellulose 23(2):1459–1470. https://doi.org/10.1007/s10570-016-0882-5
Perinelli DR, Palmieri GF, Cespi M, Bonacucina G (2020) Encapsulation of flavours and fragrances into polymeric capsules and cyclodextrins inclusion complexes: an update. Molecules 25(24):5878. https://doi.org/10.3390/MOLECULES25245878
Bender ML, Komiyama M (1978) Cyclodextrin chemistry. Springer, New York
Buschmann HJ, Schollmeyer E (2002) Applications of cyclodextrins in cosmetic products: a review. J Cosmet Sci 53(3):185–191
Saenger W (1980) Cyclodextrin inclusion compounds in research and industry. Angew Chem Int Ed Engl 19(5):344–362. https://doi.org/10.1002/anie.198003441
Hebeish A, El-Hilw ZH (2001) Chemical finishing of cotton using reactive cyclodextrin. Color Technol 117(2):104–110. https://doi.org/10.1111/j.1478-4408.2001.tb00343.x
Sricharussin W, Sopajaree C, Maneerung T, Sangsuriya N (2009) Modification of cotton fabrics with β-cyclodextrin derivative for aroma finishing. J Text Inst 100(8):682–687. https://doi.org/10.1080/00405000802158999
Andrade B, Song Z, Li J, Zimmerman SC, Cheng J, Moore JS, Harris K, Katz JS (2015) New frontiers for encapsulation in the chemical industry. ACS Appl Mater Interfaces 7(12):6359–6368. https://doi.org/10.1021/acsami.5b00484
Govindarajan M, Mathivanan T, Elumalai K, Krishnappa K, Anandan A (2011) Mosquito larvicidal, ovicidal, and repellent properties of botanical extracts against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 109(2):353–367. https://doi.org/10.1007/s00436-011-2263-1
Hazarika S, Dhiman S, Rabha B, Bhola RK, Singh L (2012) Repellent activity of some essential oils against simulium species in India. J Insect Sci 12:5. https://doi.org/10.1673/031.012.0501
Okoli BJ, Ladan Z, Mtunzi F, Hosea YC (2021) Vitex negundo L. Essential oil: odorant binding protein efficiency using molecular docking approach and studies of the mosquito repellent. Insects 12(12):98–100. https://doi.org/10.3390/insects12121061
Okoli BJ, Eltayb WA, Gyebi GA, Ghanam AR, Ladan Z, Oguegbulu JC, Abdalla M (2022) In silico study and excito-repellent activity of Vitex negundo L. Essential oil against Anopheles gambiae. Appl Sci (Switzerland) 12(15):7500. https://doi.org/10.3390/app12157500
Navayan A, Moghimipour E, Khodayar MJ, Vazirianzadeh B, Siahpoosh A, Valizadeh M, Mansourzadeh Z (2017) Evaluation of the mosquito repellent activity of nano-sized microemulsion of Eucalyptus globulus essential oil against Culicinae. Jundishapur J Nat Pharm Prod. https://doi.org/10.5812/jjnpp.55626
Pavela R, Benelli G, Pavoni L, Bonacucina G, Cespi M, Cianfaglione K, Bajalan I, Morshedloo MR, Lupidi G, Romano D, Canale A, Maggi F (2019) Microemulsions for delivery of Apiaceae essential oils: towards highly effective and eco-friendly mosquito larvicides? Ind Crops Prod 129:631–640. https://doi.org/10.1016/j.indcrop.2018.11.073
Sangsuriya WSSMMS (2009) Modification of cotton fabrics with β-cyclodextrin derivative for aroma finishing. J Text Inst 100(8):682–687
Nelson G (2002) Application of microencapsulation in textiles. Int J Pharm 242(1–2):55–62. https://doi.org/10.1016/S0378-5173(02)00141-2
Wen P, Zhu D, Feng K, Liu F, Lou W, Li M, Zong M, Wu H (2016) Fabrication of electro_spun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem 196:996–1004
Bhaskara-Amrit UR, Agrawal PB (2011) Applications of β-cyclodextrins in textiles. Autex Res J 11(4):94–101
Abdel-Halim ES, Abdel-Mohdy FA, Al-Deyab SS, El-Newehy MH (2010) Chitosan and monochlorotriazinyl-β-cyclodextrin finishes improve antistatic properties of cotton/polyester blend and polyester fabrics. Carbohydr Polym 82(1):202–208. https://doi.org/10.1016/j.carbpol.2010.04.077
Cabrales L, Abidi N, Hammond A, Hamood A (2012) Cotton fabric functionalization with cyclodextrins. J Mater Environ Sci 3(3):561–574
Ali MA, Amr A, Abou-Okeil A, Aly NM (2013) Comfort properties of the inner padding layer for motorcycle helmet. Life Sci J 10(4):1386–1399
Coetzee M (2020) Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J 19(1):70. https://doi.org/10.1186/s12936-020-3144-9
Wang J, Wang S (2020) Reactive species in advanced oxidation processes: formation, identification and reaction mechanism. Chem Eng J 401:126158. https://doi.org/10.1016/j.cej.2020.126158
Khokra S, Prakash O, Jain S, Aneja K, Dhingra Y (2008) Essential oil composition and antibacterial studies of Vitex negundo Linn. extracts. Indian J Pharm Sci 70(4):522–526. https://doi.org/10.4103/0250-474X.44610
Padalia RC, Verma RS, Chauhan A, Chanotiya CS, Thul S (2016) Phytochemical diversity in essential oil of Vitex negundo L. populations from India. Rec Nat Prod 10(4):452–464
Menezes PP, Serafini MR, Santana BV, Nunes RS, Quintans LJ, Silva GF, Medeiros IA, Marchioro M, Fraga BP, Santos MRV, Araújo AAS (2012) Solid-state β-cyclodextrin complexes containing geraniol. Thermochim Acta 548:45–50. https://doi.org/10.1016/j.tca.2012.08.023
Tao F, Hill LE, Peng Y, Gomes CL (2014) Synthesis and characterization of b -cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT Food Sci Technol 59(1):247–255. https://doi.org/10.1016/j.lwt.2014.05.037
Tao F, Hill LE, Peng Y, Gomes CL (2014) Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT 59(1):247–255. https://doi.org/10.1016/j.lwt.2014.05.037
Renscher H, Hinsenkorn R (1996) BETA W7 MCT-new ways and, in surface modification. J Incl Phenomen Macrocycl Chem 25:191–196
Nielsen ML (1957) Fire-resistant treatment of cotton using phosphorylamide. Text Res J 27(8):603–610. https://doi.org/10.1177/004051755702700802
Costantini C, Badolo A, Ilboudo-Sanogo E, Ouédraogo AP (2004) Evaluation of the sensitivity of Aedes aegypti and Anopheles gambiae complex mosquitoes to two insect repellents: DEET and KBR 3023. Trop Med Int Health 9(3):330–334. https://doi.org/10.1111/j.1365-3156.2004.01206.x
Dung NT, Hoa VT, Liên ĐT, Trung PV, Ngọc PH (2018) Chemical composition of essential oil extracted from leaves of vitex negundo linn from Binh Thuan province by hydrodistillation and microwave hydrodistillation. J Sci Technol 6:1–3
Conti B, Benelli G, Flamini G, Cioni PL, Profeti R, Ceccarini L, Macchia M, Canale A (2012) Larvicidal and repellent activity of Hyptis suaveolens (Lamiaceae) essential oil against the mosquito Aedes albopictus Skuse (Diptera: Culicidae). Parasitol Res 110(5):2013–2021. https://doi.org/10.1007/s00436-011-2730-8
Kadir FA, Kassim NM, Abdulla MA, Yehye WA (2013) Effect of oral administration of ethanolic extract of Vitex negundo on thioacetamide-induced nephrotoxicity in rats. BMC Complement Altern Med. https://doi.org/10.1186/1472-6882-13-294
Kamimura JA, Santos EH, Hill LE, Gomes CL (2014) Antimicrobial and antioxidant activities of carvacrol microencapsulated in hydroxypropyl-beta-cyclodextrin. LWT 57(2):701–709. https://doi.org/10.1016/j.lwt.2014.02.014
Kfoury M, Auezova L, Ruellan S, Greige-Gerges H, Fourmentin S (2015) Complexation of estragole as pure compound and as main component of basil and tarragon essential oils with cyclodextrins. Carbohydr Polym 118:156–164. https://doi.org/10.1016/j.carbpol.2014.10.073
dos Passos Menezes P, dos Santos PBP, Dória GAA, de Sousa BMH, Serafini MR, Nunes PS, Quintans-Júnior LJ, de Matos IL, Alves PB, Bezerra DP, Mendonça Júnior FJB, da Silva GF, de Aquino TM, de Souza Bento E, Scotti MT, Scotti L, de Souza Araujo AA (2017) Molecular modeling and physicochemical properties of supramolecular complexes of limonene with α- and β-cyclodextrins. AAPS PharmSciTech 18(1):49–57. https://doi.org/10.1208/s12249-016-0516-0
Wang J, Cao Y, Sun B, Wang C (2011) Physicochemical and release characterisation of garlic oil-β- cyclodextrin inclusion complexes. Food Chem 127(4):1680–1685. https://doi.org/10.1016/j.foodchem.2011.02.036
Santos EH, Kamimura JA, Hill LE, Gomes CL (2015) Characterization of carvacrol beta-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT 60(1):583–592. https://doi.org/10.1016/j.lwt.2014.08.046
Hill LE, Gomes C, Taylor TM (2013) Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT 51(1):86–93. https://doi.org/10.1016/j.lwt.2012.11.011
Torres-Alvarez C, Castillo S, Sánchez-García E, González CA, Galindo-Rodríguez SA, Gabaldón-Hernández JA, Báez-González JG (2020) Inclusion complexes of concentrated orange oils and β-cyclodextrin: physicochemical and biological characterizations. Molecules 25(21):5109. https://doi.org/10.3390/molecules25215109
Cid-Samamed A, Rakmai J, Mejuto JC, Simal-Gandara J, Astray G (2022) Cyclodextrins inclusion complex: preparation methods, analytical techniques and food industry applications. Food Chem 384:132467. https://doi.org/10.1016/j.foodchem.2022.132467
Saikosin R, Limpaseni T, Pongsawasdi P (2002) Formation of inclusion complexes between cyclodextrins and carbaryl and characterization of the complexes. J Incl Phenom 44(1–4):191–196. https://doi.org/10.1023/A:1023099925658
El-Sayed Saeed S, Al-Harbi TM, Abdel-Mottaleb MSA, Al-Hakimi AN, Albadria AEAE, Abd El-Hady MM (2022) Novel Schiff base transition metal complexes for imparting UV protecting and antibacterial cellulose fabric: experimental and computational investigations. Appl Organomet Chem 36(12):E6889. https://doi.org/10.1002/aoc.6889
Lis MJ, Carmona ÓG, Carmona CG, Bezerra FM (2018) Inclusion complexes of citronella oil with β-cyclodextrin for controlled release in biofunctional textiles. Polymers 10(12):1324. https://doi.org/10.3390/polym10121324
Medronho B, Valente JM, Costa P, Romano A (2014) Inclusion complexes of rosmarinic acid and cyclodextrins: Stoichiometry, association constants, and antioxidant potential. Colloid Polym Sci 292(4):885–894. https://doi.org/10.1007/s00396-013-3124-5
Vigneshwaran N (2009) Modification of textile surfaces using nanoparticles. Surf Modif Text. https://doi.org/10.1533/9781845696689.164
Abdul Karim MR, Tahir D, Hussain A, Ul Haq E, Khan KI (2020) Sodium carbonate treatment of fibres to improve mechanical and water absorption characteristics of short bamboo natural fibres reinforced polyester composite. Plast Rubber Compos 49(10):425–433. https://doi.org/10.1080/14658011.2020.1768336
Bruckmann FD, Rossato Viana A, Tonel MZ, Fagan SB, Garcia WJ, Oliveira AH, Dorneles LS, Roberto Mortari S, Silva WL, Silva IZ, Rhoden CR (2022) Influence of magnetite incorporation into chitosan on the adsorption of the methotrexate and in vitro cytotoxicity. Environ Sci Pollut Res 29(46):70413–70434. https://doi.org/10.1007/s11356-022-20786-x
Kringel DH, Antunes MD, Klein B, Crizel RL, Wagner R, de Oliveira RP, Dias ARG, da Zavareze ER (2017) Production, characterization, and stability of orange or eucalyptus essential oil/β-cyclodextrin inclusion complex. J Food Sci 82(11):2598–2605. https://doi.org/10.1111/1750-3841.13923
Galvão JG, Silva VF, Ferreira SG, França FRM, Santos DA, Freitas LS, Alves PB, Araújo AAS, Cavalcanti SCH, Nunes RS (2015) β-cyclodextrin inclusion complexes containing Citrus sinensis (L.) Osbeck essential oil: an alternative to control Aedes aegypti larvae. Thermochim Acta 608:14–19. https://doi.org/10.1016/j.tca.2015.04.001
da Silva Bruckmann F, Viana AR, Lopes LQS et al (2022) Characterization, and biological activity evaluation of magnetite-functionalized Eugenol. J Inorg Organomet Polym 32:1459–1472
Sodsangchan C, Setthayanond J, Suwanruji P (2014) Influence of monochlorotriazine-β-cyclodextrin treatment on dyeing and fastness properties of the hot-dyeing reactive dye on cotton. Appl Mech Mater 535:662–665. https://doi.org/10.4028/www.scientific.net/AMM.535.662
Hassan M, Attia MK, Attia RM (2022) Improving of thermal stability and water repellency of cotton fabrics via coating with silicone rubber under the effect of electron beam irradiation. J Ind Text 51(3):4899S-4912S. https://doi.org/10.1177/15280837211046622
Babu KF, Senthilkumar R, Noel M, Kulandainathan MA (2009) Polypyrrole microstructure deposited by chemical and electrochemical methods on cotton fabrics. Synth Met 159(13):1353–1358. https://doi.org/10.1016/j.synthmet.2009.03.005
Liu W, Zhang S, Chen X, Yu L, Zhu X, Feng Q (2010) Thermal behavior and fire performance of nylon-6,6 fabric modified with acrylamide by photografting. Polym Degrad Stab 95(9):1842–1848. https://doi.org/10.1016/j.polymdegradstab.2010.04.023
Ho TM, Truong T, Bhandari BR (2017) Methods to characterize the structure of food powders: a review. Biosci Biotechnol Biochem 81(4):651–671. https://doi.org/10.1080/09168451.2016.1274643
Khanna S, Chakraborty JN (2017) Optimization of monochlorotriazine β-cyclodextrin grafting on cotton and assessment of release behavior of essential oils from functionalized fabric. Fash Text. https://doi.org/10.1186/s40691-017-0089-x
Ambaye TG, Vaccari M, van Hullebusch ED, Amrane A, Rtimi S (2021) Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int J Environ Sci Technol 18(10):3273–3294. https://doi.org/10.1007/s13762-020-03060-w
Drobota M, Ursache S, Aflori M (2022) Surface functionalities of polymers for biomaterial applications. Polymers (Basel) 14(12):2307
Cimino C, Maurel OM, Musumeci T, Bonaccorso A, Drago F, Souto EMB, Pignatello R, Carbone C (2021) Essential oils: pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 13(3):1–35. https://doi.org/10.3390/pharmaceutics13030327
Hebbalkar DS, Hebbalkar GD, Sharma RN, Joshi VS, Bhat VS (1992) Mosquito repellent activity of oils from Vitex negundo Linn. leaves. Indian J Med Res 95:200–203
Maes C, Bouquillon S, Fauconnier ML (2019) Encapsulation of essential oils for the development of biosourced pesticides with controlled release: a review. Molecules 24(14):2539. https://doi.org/10.3390/molecules24142539
Acknowledgements
We thank Vaal University of Technology in South Africa, Kaduna State University, and Bingham University, Nigeria for facilitating this joint research.
Funding
Tertiary Education Trust Fund supported this study with Grant Number TETFund/DR&D/CE/NRF/UNI/CC/22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okoli, B., Ladan, Z. & Mtunzi, F. Fabric pre-treated with Vitex negundo L essential oil as a preventive tool against mosquito bite. SN Appl. Sci. 5, 230 (2023). https://doi.org/10.1007/s42452-023-05425-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05425-5