Abstract
Senna fistula (commonly called golden shower tree) is a tropical plant renowned for its established medicinal properties. Additionally, it serves as a popular ornamental tree for homesteads and roadside plantings. But previous phytochemical studies on the plant adopted the conventional methods of extraction without optimal yield of the plant extract. This study investigated on the phytochemistry of S. fistula leaf, stem bark and flower using ethanol, aqueous and palm wine extracts. Also, optimization of extraction with the aid of design expert was carried out to determine the extraction condition that would give the optimum yield using different solvent. Result of qualitative analysis on phytochemicals showed presence of anthraquinones, flavonoids, saponins, terpenoids, phenols, cardiac glycosides and tannins. An extraction of 10 g of S. fistula for 24 h at 300 rpm, with an orbital shaker time of 30 min, gave an optimal yield of 3.6 g. The bio-active compounds found in S. fistula are subjects of more exploratory research with usable products that can benefit mankind as the focus.
Highlights
-
1.
Phytochemical analysis of S. fistula flower revealed the present of bioactive phytochemicals which can be further harnessed for pharmaceutical purposes.
-
2.
Orbital shaking time of 29 min at 300 rpm gave an optimum yield of 3.4 g in S. fistula.
-
3.
Dichloromethane and ethyl acetate solvents produced the best retention factor for S. fistula.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The right extraction technique is crucial for qualitative and quantitative research of bioactive chemicals from plant materials [1, 2]. The initial step in every study of a medicinal plant is extraction, which has a substantial impact on the outcome. “Sample preparation techniques” is another name for extraction methods [3]. This area of research has received limited attention and is often conducted by researchers with limited experience [4].
The success of the analysis of any bioactive component depends largely on the extraction procedures, input parameters, and precise nature of plant parts [5]. It is true that development of contemporary chromatographic and spectrometric techniques has made the analysis of bioactive compounds easier. The matrix qualities of the plant portion, the solvent, the temperature, the pressure, and the duration are the most frequent variables influencing the extraction processes [6].
All plants contain phytopharmaceuticals, that have discovered widespread use in human, agriculture and veterinary medicine, and other fields. Senna fistula (syn. Cassia fistula) a legume commonly called the golden shower tree is a plant with reported medicinal efficacy [7, 8]. Senna fistula (Fabaceae, Caesalpinioideae) is a semi-wild leguminous plant that is highly renowned for its established medicinal effects. It is locally called “Aidan tooro” in Yoruba language, south western Nigeria [9]. It can be found in Asia, Africa, the West Indies, and Brazil, among other places [6, 10]. It is a popular ornamental tree for homesteads and roadside plantings. Many physiologically active chemicals (i.e. phytochemicals) can be extracted and identified from various sections of the plant. The plant parts have been reported to possess analgesic, antifungal, antibacterial, antipyretic, anti-inflammatory, larvicidal, anti-tumor, antioxidant, hepatoprotective, hypoglycemic activities, and laxative properties [11, 12].
But there are limitations in previous extraction techniques, such as choice of solvents, alteration in temperature, pH of plant extract, etc. [13, 14]. Therefore, the need for cheminformatic guided way of extracting bioactive compounds from plants.
The word “cheminformatics” has only been in usage since the beginning of the 1990s, regardless of the fact that computers have been used in chemistry since their inception, when the field sprung out of previous work in the domains of computation, chemistry, information science, and drug discovery, to mention a few. Chemoinformatics (most common in Europe), chemical informatics, and sometimes even chemi-informatics are all terms that have been used to describe the field as a result of these various influences. The use of this techniques guides extraction which maximizes resources, yield, and reduces environmental losses [15,16,17,18].
Several literature surveys revealed that S. fistula is an important medicinal plant [9]. This plant is used by traditional medical practitioners for the treatment of various diseases [19, 20]. Therefore, optimizing the extraction of the phytochemicals from the plant parts will provide more valuable information which will assist scientists in obtaining maximum resources from plant species for the improvement of human and animal health and wellbeing.
The main purpose of this study was to use chemistry informatics (cheminformatics) to improve the extraction of phytochemicals from S. fistula plant parts. To know what extraction condition will give the optimum yield using different solvent.
2 Materials and methods
2.1 Sample collection
The S. fistula samples were collected from Bowen University, Iwo, Nigeria (Latitude 7° 38′ N, 4° 11′ E) during its flowering period. The plant was authenticated, and assigned herbarium number BUH032. The collected leaves, stem and flowers were dried at room temperature, ground to smaller particulates and stored in airtight containers.
2.2 Maceration
Senna fistula powder weighting 100 g (leaves, stembark, flower respectively) was put in 1000 ml glass jars and then soaked with three different solvents (distilled water, palm wine and ethanol respectively) of about 800 ml volume. The glass jars were placed on an orbital shaker at about 253–300 rpm for about 10–20 min. The extracts were filtered out and concentrated on a rotary evaporator under the reduced pressure conditions at 60 to 85 °C.
2.3 Thin layer chromatography
A capillary tube was used to take sample extract and create a spot on the chromatography plate. The chromatography plate was labeled at the very top with pencil for ease of noting the point of dropping. The developing chromatography plate was then dipped in a 250 ml beaker containing solvents (e.g., n-hexane, ethyl acetate, dichloromethane, ethanol) ratios. After this process, the plates were checked under an ultraviolet (UV) light and the various retention factor (rf) taken.
2.4 Phytochemical tests
Tests were carried out to discover the presence or absence of the following phytochemicals: flavonoids, saponins, phenols, alkaloids, tannins, cardiac glucosides, proteins, steroids, terpenoids, anthraquinones and carbohydrates. Method as described by Banu and Cathrine [21] and Harborne [22] were adopted for the phytochemical analysis.
2.5 Experimental design
A 23 factorial table was chosen in the Design Expert Software v12.0 (Stat-Ease Free Download for Windows), and the relevant factors were inputted into the table given. The factors were extraction time (h), orbital shaking time (min) and shaking (rpm). These factors would then give result as yield of extract (g).
Variations in the extraction time, shaking time and speed of shaking for S. fistula stem bark was made and also 10 g of the mass of dry sample of S. fistula stem bark and a constant volume of 100 ml distilled water, while extraction time, shaking time and speed of shaking was varied. The results that gave the best yields were selected out of the pool of results given.
3 Results and discussion
Qualitative analysis done on the distilled water, ethanol and palm wine extracts of S. fistula leaves, stem bark and flowers revealed the presence of anthraquinones, flavonoids, saponins, terpenoids, phenols, cardiac glycosides and tannin. While alkaloids, proteins, carbohydrates and steroids were absent in all extracts (Table 1). Previous reports on phytochemical constituents of S. fistula have been extracts from the leaf [23, 24], stem bark [25] and seed pulp [26, 27]. Bioactive compounds from S. fistula were reported to be more in polar solvents than in nonpolar solvents [27]. This research further highlighted the bioactive phytochemicals present in S. fistula flower, such as anthraquinones, flavonoids, saponins, tannins, cardiac glycosides, phenols, and terpenoids. The benefit of which can be further harnessed for pharmaceutical purposes.
An increase in extraction time (24 h), orbital shaking time (30 min) and speed of shaking (300 rpm) resulted in the highest yield of 3.6 g (Table 2). Time of orbital shaking was reported to have positive influence on the yield plant extract [28, 29]. In this study increase in time of orbital shaking at room temperature of increased yield of extracts.
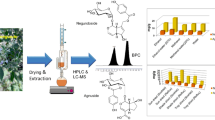
Figure 1 shows that the two best yields 3.6 g and 3.5 g occur at 300 and 257 rpm respectively. While Fig. 2 shows the best two yields 3.6 g and 3.5 g occur at 30 min of orbital shaker time also Fig. 3 shows the optimum yields 3.6 g and 3.5 g to occur at 24 h of extraction time. Therefore, we can conclude that carrying out an extraction of 10 g of S. fistula for 24 h at 300 rpm, with an orbital shaker time of 30 min, an optimal yield of 3.6 g would be obtained.
From the graphs in Figs. 1, 2, 3, 4, 5, and 6, we could predict and derive the values below by assigning and estimating using the graphs.
Table 3 is useful as a model for extraction process of phytocompounds from S. fistula stem bark and it can also be used to optimize an existing model’s yield. From the run table, the highest yield 3.6 g occurred as a result of extraction time (24 h), orbital shaking time (30 min) and shaking (300 rpm) while the lowest yield 2 g occurred as a result of extraction time (24 h), orbital shaking time (10 min) and shaking (300 rpm). From the predictive table, the highest yield 3.4 g was gotten as a result of extraction time (22 h), orbital shaking time (29 min) and shaking (300 rpm) while the lowest yield 2.4 g occurred as a result of extraction time (24 h), orbital shaking time (11 min) and shaking (298 rpm).
In this study, the crude extract of S. fistula resulted in good separation from the TLC analysis. The mobilities of all compounds were calculated and recorded after separation from the various S. fistula extracts in an appropriate composition solvent system, as shown in Tables 4 and 5. Using their rf values as a guide, Fig. 7 illustrates the separation of the mixture compounds. A sample's chemical components can be separated using the TLC technique to establish each component's chemical identity [30]. Using visible and ultra violet light, five distinct photographs of the mobile phases on TLC plates revealed colors with the calculated rf value as tabulated. The UV 254 nm light showed colours ranging from black to red and some kind of florescence; evidence that S. fistula is rich in phytocompounds.
Out of all the mean rf values calculated, it was found that dichloromethane:ethyl acetate at ratio 8:2 produced the best separation of retention mean at 0.0677. When comparing two different compounds under identical conditions, the compound with the large rf value is less polar because it does not adhere to the stationary phase as much as the polar compound does. Rf can be used to identify compounds based on their characteristics compared to other compounds which has a lower rf value.
Visible light, UV light at 245 nm and UV light at 365 nm identified various color spots on the TLC plates. The UV light identified the fluorescent spots. Evidence for identifying the features of the samples included rf values, TLC plate color, size, and shape of detection zones under visible, 245 nm, and 365 nm UV light [31, 32].
The use of solid phase extraction technique such as TLC plate helps in the isolation of bioactive compounds by their retention properties on solid phase. This method is effective for sampling, extracts preservation and as an indicator of bioactive compounds present in plant extract [33]. This method is inexpensive, non-laborious and an improved precision and accuracy methods of solvent extraction [34]. The TLC plates obtained for S. fistula in this study can be stored and later used as a reference for extraction of bioactive compounds based on their retention properties.
Previous publications documented the phytochemical constituents of S. fistula using polar solvents such as methanol [24], ethanol [28], and water [28] for the extraction process and its absorption properties [35]. There are no reports on the use of palm wine (indigenous solvent) as an extraction solvent for the phytochemical analysis of S. fistula. Although in this study, the palm wine extract showed the same phytochemical constituents as with aqueous extract. The use of S. fistula flowers in this study is also an additional raw material for medicinal use of this plant.
4 Conclusion
Senna fistula is a plant that is abundant in popular phytochemical components like flavonoids, saponins, tannins, phenols, cardiac glycosides, and terpenoids, according to the phytochemical analysis. This study also demonstrated the use of design expert in the extraction procedure, thus furthering into the area of computational chemistry. The bio-active substances found in S. fistula should be the subject of more exploratory research with usable products that can benefit mankind as the focus. Additionally, more research should be done on how to increase the use of cheminformatics in chemical extraction procedures and other fields so as to optimize the extraction procedures.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sasidharan S, Chen Y, Saravanan SKM, Latha YL (2011) Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med 8(1):1–10
Smith RM (2003) Before the injection—modern methods of sample preparation for separation techniques. J Chromatogr A 1000(1–2):3–27
Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117(4):426–436. https://doi.org/10.1016/j.jfoodeng.2013.01.014
Hennion MC, Cau-Dit-Coumes C, Pichon V (1998) Trace analysis of polar organic pollutants in aqueous samples: tools for the rapid prediction and optimization of the solid-phase extraction parameters. J Chromatogr A 823(1–2):147–161
Poole SK, Dean TA, Oudsema JW, Poole CF (1990) Sample preparation for chromatographic separations: an overview. Anal Chim Acta 236(1):3–42
Prashanth Kumar V, Chauhan NS, Padh H, Rajani M (2006) Search for antibacterial antifungal agents from selected. International Science Congress Association. Indian medicinal plants. J Ethnopharmacol 107:182–188
Ayinla TM, Owoyele BV, Yakubu MT (2015) Effect of ethanolic leaf extract of Senna fistula on some haematological parameters, lipid profile and oxidative stress in alloxan-induced diabetic rats. Niger J Physiol Sci 30(1–2):87–93. https://www.ajol.info/index.php/njps/article/view/145521. Accessed 12 Apr 2023
Barnaby GA, Reid R, Warren D (2016) Antioxidant activity, total phenolics and fatty acid profile of Delonix regia, Cassia fistula, Spathodea campanulata, Senna siamea and Tibouchina granulosa. J Anal Pharm Res 3(2):00056. https://doi.org/10.15406/japlr.2016.03.00056
Ajao AA, Mukaila YO, Sabiu S (2022) Wandering through southwestern Nigeria: an inventory of Yoruba useful angiosperm plants. Heliyon 8(1):e08668. https://doi.org/10.1016/j.heliyon.2021.e08668
Hernández Y, Lobo MG, González M (2009) Factors affecting sample extraction in the liquid chromatographic determination of organic acids in papaya and pineapple. Food Chem 114(2):734–741
Bahorun T, Neergheen VS, Aruoma OI (2005) Phytochemical constituents of Cassia fistula: review. Afr J Biotechnol 4(13):1530–1540
Duraipandiyan V, Ignacimuthu S (2007) Antibacterial and antifungal activity of Cassia fistula L.: an ethnomedicinal plant. J Ethnopharmacol 112(3):590–594. https://doi.org/10.1016/j.jep.2007.04.008
Yung OH, Maskat MY, Wan Mustapha WA (2010) Effect of extraction on polyphenol content, antioxidant activity and pH in pegaga (Centella asiatica). Sains Malays 39:747–752
Azwanida NN (2015) A Review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants 4:196. https://doi.org/10.4172/2167-0412.1000196
Belwal T, Devkota HP, Ramola S, Andola HC, Bhatt ID (2020) Chapter Ten—optimization of extraction methodologies and purification technologies to recover phytonutrients from food. In: Woodhead Publishing series in food science, technology and nutrition, phytonutrients in food. Woodhead Publishing, pp 217–235. https://doi.org/10.1016/B978-0-12-815354-3.00007-1
Jurischk C, Dorneanu B, Stollberg C, Arellano-Garcia H (2022) A novel approach to continuous extraction of active ingredients from essential oils through combined chromatography. Comput Aided Chem Eng 51:769–774. https://doi.org/10.1016/B978-0-323-95879-0.50129-6
Maher T, Kabbashi NA, Mirghani MES, Alam MZ, Daddiouaissa D, Abdulhafiz F, Reduan MFH, Omran JI, Abdul Razab MKA, Mohammed A (2021) Optimization of ultrasound-assisted extraction of bioactive compounds from Acacia seyal gum using response surface methodology and their chemical content identification by Raman, FTIR, and GC–TOF-MS. Antioxidants 10(10):1612. https://doi.org/10.3390/antiox10101612
Zhang H, Birch J, Xie YH, Bekhit AE (2019) Optimization of ultrasound assisted extraction method for phytochemical compounds and in vitro antioxidant activity of New Zealand and China Asparagus cultivars (officinalis L.) roots extracts. Food Chem 294:276–284. https://doi.org/10.1016/j.foodchem.2019.03.012
Oladeji OS, Adelowo FE, Oluyori AP (2021) The genus Senna (Fabaceae): a review on its traditional uses, botany, phytochemistry, pharmacology and toxicology. S Afr J Bot 138:1–32. https://doi.org/10.1016/j.sajb.2020.11.017
Sutjaritjai N, Panyadee P, Phumthum M, Inta A, Balslev H (2022) High diversity of medicinal uses of Thai legumes (Fabaceae) and their potential in public herbal medicine. Diversity 14:588. https://doi.org/10.3390/d14080588
Banu KS, Cathrine L (2015) General techniques involved in phytochemical analysis. Int J Adv Res Chem Sci 2(4):25–32
Harborne JB (1984) Phytochemical methods: a guide to modern techniques of plant analysis. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-5570-7
Panda SK, Padhi LP, Mohanty G (2011) Antibacterial activities and phytochemical analysis of Cassia fistula (Linn.) leaf. J Adv Pharm Technol Res 2(1):62–67. https://doi.org/10.4103/2231-4040.79814
Nagpal MA, Nagpal N, Rahar S, Shah G, Swami G, Kapoor R (2011) Phytochemical investigation of methanolic extract of Cassia fistula leaves. Pharmacogn J 3(26):61–69. https://doi.org/10.5530/pj.2011.26.11
Meena AK, Ilavarasan R, Perumal A, Singh R, Ojha V, Srikanth N, Dhiman KS (2022) Evaluation for substitution of stem bark with small branches of Cassia fistula Linn. for traditional medicinal uses: a comparative chemical profiling studies by HPLC, LC–MS, GC–MS. Heliyon 8(8):e10251. https://doi.org/10.1016/j.heliyon.2022.e10251
Bhalodia NR, Acharya RN, Shukla VJ (2011) Evaluation of in vitro antioxidant activity of hydroalcoholic seed extracts of Cassia fistula Linn. Antioxidants 1(1):68–76. https://doi.org/10.5530/ax.2011.1.11
Irshad M, Mehdi SJ, Al-Fatlawi AA, Zafaryab M, Ali A, Ahmad I, Singh M, Moshahid M, Rizvi A (2014) Phytochemical composition of Cassia fistula fruit extracts and its anticancer activity against human cancer cell lines. J Biol Act Prod Nat 4(3):158–170. https://doi.org/10.1080/22311866.2014.933084
Upadhya V, Pai SR, Hegde HV (2015) Effect of method and time of extraction on total phenolic content in comparison with antioxidant activities in different parts of Achyranthes aspera. J King Saud Univ Sci 27(3):204–208. https://doi.org/10.1016/j.jksus.2015.04.004
Younas U, Iqbal S, Bashir R, Sajjad N, Saeed Z, Pervaiz M, Hassan F, Ali F, Ibrahim S, Batool F, Hussain I, Iqbal M (2021) An eco-friendly approach for the extraction of antioxidant components from Artemisia annua leaves using response surface methodology. Pol J Environ Stud 30(5):4827–4833. https://doi.org/10.15244/pjoes/132787
Wang J, Rio A, Lisova K, Slavik R, Chatziioannou AF, vanDam RM (2020) High-throughput radio-TLC analysis. Nucl Med Biol 82–83:41–48
Aloisi JD, Sherma J, Fried B (1990) Comparison of mobile phases for separation and quantification of lipids by one-dimensional TLC on pre-adsorbent high performance silica gel plates. J Liq Chromatogr 13(20):3949–3961
Mayasari D, Islami D, Oktariani E, Wulandari P (2023) Phytochemical, antioxidant and antibacterial evaluations of Ipomoea batatas L. from Riau, Sumatera Island, Indonesia. Trop J Nat Prod Res 7(1):2157–2162
Poole CF (2002) Chapter 12: principles and practice of solid-phase extraction, In: Comprehensive analytical chemistry, vol 37, pp 341–387. https://doi.org/10.1016/S0166-526X(02)80049-6
Armenta S, Esteve-Turrillas FA, Garrigues S, de la Guardia M (2021) Smart materials for sample preparation in bioanalysis: a green overview. Sustain Chem Pharm. https://doi.org/10.1016/j.scp.2021.100411
Ajaelu CJ, Oyedele O, Ikotun AA, Faboro EO (2022) Safranin O dye removal using Senna fistula activated biomass: kinetic, equilibrium and thermodynamic studies. J Niger Soc Phys Sci 5(1):951. https://doi.org/10.46481/jnsps.2023.951
Acknowledgements
The authors wish to acknowledge Bowen University for providing the fund for this research under the Second Tier Bowen Refund, 2022 through the Directorate of Research and Strategic Partnership.
Funding
This research was carried out with the financial support from Bowen University, Iwo, Osun State, Nigeria under the Second Tier, Bowen University Research Grant Programme, 2022.
Author information
Authors and Affiliations
Contributions
D.O., I.O. and E.F. conceptualized the research, D.O. and T.O. carried out the experiment. D.O. and I.O. drafted the manuscript and wrote the final manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest with regards to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Faboro, E.O., Adekunle, D.O., Obisesan, I.A. et al. Optimization of extraction conditions for phytochemicals from Senna fistula using cheminformatics. SN Appl. Sci. 5, 209 (2023). https://doi.org/10.1007/s42452-023-05421-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05421-9