Abstract
The paradigm termed circular blue economy has become a concept that is greatly associated with the utilization of marine resources to ensure continued sustainability. Several constraints and limitations related to plants and plant products means human needs to look beyond green economy. The chemical constituents of animals also allow researchers to evaluate their growth and development. This study evaluates the proximate and chemical compositions of Anadara senilis, Crassostrea gasar, and Mytilus edulis, with emphasizes on their calcium and calcium carbonate contents and industrial importance. A total of 270 live bivalve samples were collected from March to May 2021 from Lagos Lagoon harbour. Each bivalve sample collected was opened to separate the flesh from the species’ shell, sun-dried and transported to the laboratory for analysis. One-way analysis of variance was adopted to estimate the significance level at 5% (P < 0.05). Post-HOC LSD test was performed to verify the disparity of mean. The results of this study revealed that Mytilus edulis shells had the highest moisture, crude protein, and crude fat at 1.15 ± 0.05%, 4.29 ± 0.43%, and 0.96 ± 0.15%, respectively and showed significant difference (P < 0.05). Anadara senilis shells had high levels of calcium (51.00 ± 2.87 mg/kg), magnesium (0.59 ± 0.23 mg/kg) and calcium carbonate (60.91 ± 2.50 mg/kg). Due to the high Ca and CaCO3 contents obtained, these shells can be processed alongside other biomaterials into food supplements, animal feeds, dental products, plant nutrient supplements, ornamental purpose, construction, agricultural industries among others. These would enhance the development of cottage industries, promotes farming of shell animals, help to alleviate the unemployment crisis and creation of wealth from substances which would have been regarded as waste.
Highlights
Bivalve’s exoskeletons from Anadara senilis, Crassostrea gasar, and Mytilus edulis are highly utilizable for a circular economy to reduce seafood waste. These shellfishes are high in minerals and chemical compounds and can be highly valorized in various industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Elemental and mineral compositions of mollusk shells are mostly governed by biology and genetics [1, 2]. However, some environmental factors, such as temperature, salinity, and other water quality parameters, also impact the mineral composition of mollusk shells [3]. As a result, the growth histories, metabolisms, and environmental conditions in which the creature calcified are recorded in the shell’s sequentially created calcium carbonate layers [4]. In addition, comparing the chemical constituents of current and petrified shells allows researchers to evaluate the background level of minerals even as trace concentrations and enables monitoring of the development and growth (evolution) of ecological factors in a biological manner [5].
Bivalves form an essential part of aquatic ecosystems, either marine or freshwater ecosystems. After gastropods, they are the most diverse category of mollusks. Several species of clams, oysters, mussels, scallops, and other Molluska members belong to the class Bivalvia. A hard calcareous shell made up of two pieces or ‘valves’ that are usually bilaterally symmetrical distinguishes them. At a hinge, the valves are joined to one another. The soft parts of the creature are contained within the shell.
Numerous studies have linked the chemical constituents of growth-layered mollusk shells to extrinsic environmental factors. Oluwatobi et al. [6] proposed that the ratio of essential minerals such as magnesium to calcium (Mg/Ca) and strontium to calcium (Sr/Ca) in calcite and aragonite influences the balance of seawater temperature. The amount of nonbiogenic manganese (Mn2+) incorporated into calcite is related to the amount of dissolved Mn2+ in seawater. As a result, Mn/Ca serves as a proxy for dissolved Mn2+, reflecting some chemical reactions (redox reactions) required for primary production [7]. The proportions of barium to calcium (Ba/Ca) are known to aid the rapid growth of diatoms [8] or are recommended to be utilized as an indicator in an estuary due to the specific salinity content [3]. The ratio of sodium to calcium (Na/Ca) has also been considered suitable for salinity indicators. However, the chemical bond of Na+ with organic matter and its mobility nature in the crystalline phase of a shell must be considered. During pollution monitoring, heavy metals such as copper (Cu), lead (Pb), cadmium (Cd), nickel (Ni), and vanadium (V) have also been discovered [9, 10].
Only one oceanographic parameter should be used to create a reliable marine geochemical proxy. Recent research has shown that using the chemical constituents, mineral and elemental constituents, of bivalve shells as proxies in marine ecosystems is not an effortless process since the factors responsible for variations in bivalve shell composition are not understood [9]. Multiple factors, such as environmental factors such as temperature and salinity, as well as physiological factors such as body size, growth, and metabolic processes of bivalve shells, can alter this proxy, making it difficult for users. As a result, each prospective proxy must be thoroughly researched.
Bivalve shells are commonly utilized as supplements against inflammation-associated arthritis in humans [11], as flooring materials [12], and as raw materials in the production of jewelry [13]. Other mollusk shells, especially gastropod shells, are used for traditional medical purposes and whitening teeth. Bivalve shells are often discarded since customers are unaware of the mineral and nutritional components of bivalve shells. Despite the amount of research on the bivalve family, little or no research has been done on the mineral and phytochemical composition of the bivalve shell. It becomes imperative to quantify the influence of size and the calcium carbonate polymorph produced on the chemical composition of the bivalve shell [3].
The paradigm termed circular blue economy has become a concept that is greatly associated with the utilization of marine resources to ensure continued sustainability. Several constraints and limitations related to plants and plant products means human needs to look beyond green economy. Intensive attention must be directed towards the marine ecosystems such as seas and oceans due to the readily available and sufficient resources in these ecosystems. Globally, fishery and aquaculture has undergone an exponential growth and development in terms of fish production and is projected to reach over 200 million tonnes by 2030. Unequivocally, one of the major aims of Circular Economy Action Plan of the European Commission project of 2015, upgraded in 2020 is to legitimize minimization of food waste to ensure the developments of sustainable jobs, livelihoods of people, growth, investment and building a zero-waste and resource-efficient economy [14]. The long-shared linear economy model, based on the take–make–dispose concept has been used for several years to ensure non-regulatory collection of the limited natural resources, while mismanaging enormous volumes waste [15]. Valorisation of products for the attainment of zero waste remains the basic and crucial driver towards an effective waste management system either for the green or the blue circular economy. However, Shells, which are the major mollusk by-product, can also be included in this paradigm in several ways [16].
Waste valorization of valuable resources has reported in several papers. For instance, Jovic et al. [17] mentioned the significance of shells and how they can be utilized to reduce the unregulated harnessing of natural limestone. Jovic et al. [17] also compare the ancient usage and application of shells with the modern utilization which includes soil conditioners, bioremediation agents, catalysts, and construction materials. Similarly, Morris et al. [18] also affirmed from an ecological and economic point of view that shell wastes are indispensable resources, pinpointing that inappropriate management of shell represents the major constraint of mollusk aquaculture development. Due to the importance of shells, it becomes imperative to contribute to the limited available scientific literature on shells. Hence, this study evaluates the proximate and chemical compositions of Anadara senilis, Crassostrea gasar, and Mytilus edulis, with emphasizes on their calcium and calcium carbonate contents and industrial importance.
2 Materials and methods
2.1 Study sites
2.1.1 Lagos Lagoon
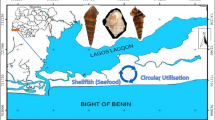
Lagos Lagoon is a system in the Gulf of Guinea. It is within the coordinates of longitudes 3° 22′ E and 3° 4′ E and latitudes 6° 17′ N and 6° 28′ N [6], having a low frequency and alkalinity in nature. It runs 257 km from Cotonou, Benin, to the Niger Delta, Nigeria. It opens into the Gulf of Guinea through Lagos Harbor, which serves as the only seaport for Nigeria’s western lagoons (Fig. 1).
Map of Lagos Lagoon. Source [19]
2.1.2 Lagos Harbor
The area is a hydrographic harbor in Lagos and is situated at an elevation of 35 m above sea level. Its coordinates are 6° 26′ 52″ N and 3° 23′ 9″ E. Lagos Harbor is the only exit available for the outflow and inflow of lagoon waters due to the rise of tides [20].
2.2 Collection of experimental samples
Thirty (30) fresh specimen samples of West African bloody cockles (Anadara senilis), blue mussels (Mytilus edulis), and mangrove oysters (Crassostrea gasar) were collected once monthly in March, April, and May. The reason for collecting the samples on multiple occasions was to increase the reliability of the result, to check the stability or variation of bivalve shells composition across months and for possible statistical tests. Samples of Crassostrea gasar and Mytilus edulis were collected from Lagos Lagoon. Anadara senilis was collected from Lagos Harbor. The samples were collected in bowls, topped with lagoon water, poured out, and frozen for preservation. The oysters were collected from mangrove tree roots and placed in polythene bags. Samples are presented in Plates 1, 2, 3.
2.3 Preservation of samples
Live samples were collected in polythene bags, placed in an ice cooler, and transported to the Marine Sciences Laboratory at the University of Lagos. The bags were adequately labelled for easy identification and stored in the freezer at temperatures below 0 °C as directed by the laboratory assistant. Samples were removed and thawed when needed for analysis.
2.4 Procedure for proximate and mineral analysis
The extracted shell of the samples collected were sun-dried for 2 weeks and transported in insulated polythene bags under cool conditions to Precision Analytical and Research Laboratory, located at Iyana Agbala, Ejikeye, Ibadan, Oyo State, Nigeria, for further analysis. The dried samples were ground upon arrival at the lab.
2.4.1 Determination of crude fat content using the Soxhlet extraction method
A total of 5.00 g of totally ground sample was measured into a thimble, and cotton wool was used to cover the ground sample in the thimble to prevent the sample from pouring out during extraction. A flask with a round bottom was oven-dried at 60 °C and weighed before 80 ml of hexane was poured into it. The thimble containing the ground sample was also fitted to the extractor. The heating mantle was switched on, and water was set to run through the condenser for cooling. The reflux extraction lasted for 2 h, while the flask was then oven-dried to remove the hexane. The crude fat content in the sample was calculated as provided below [21].
2.4.2 Determination of ash
Empty crucibles were oven-dried at 130 ± 150 °C for ½ h to ensure total removal of moisture in the crucibles. The dried crucible was weighed (tagged as the initial weight WO) and then placed in a desiccator for 15 min to allow cooling at room temperature. Pestle and mortar were then used to blend the samples to increase the surface area. An analytical weighing balance was used to weigh 1.0000 g of sample into the empty crucible and recorded as W1. The measured sample was ashed in the furnace at 500 ± 150 °C for 5–6 h. The crucible in the furnace was allowed to cool for 25 min with a crucible tongue before transferring it to a desiccator. It was further allowed to cool at room temperature for 40 min. The final weight of the crucible containing the ashed sample was recorded as W2 [22].
where W0 is the weight of the empty or dried crucible, W1 is the weight of the crucible containing samples, W2 is the final weight of the crucible containing the ashed samples.
2.4.3 Determination of crude protein content according to the Kjeldahl method
A total of 1.00 g of prepared sample was weighed into a 250 ml digestion tube, and 2 kjeltabs Cu 3.5 and 12 ml of concentrated H2SO4 were added and gently shaken to wet the sample with acid. An exhaust system was fitted to the tube placed in the rack, and a water aspirator was set to full effect. The samples in the tube were digested for one hour at 420 °C. The rack of tubes was later placed on a stand for 15 min to ensure cooling. Afterward, the rack was placed in the distillation unit, and the safety door was closed. Eighty milliliters of deionized water was carefully added to the tubes. Then, 25–30 ml of receiver solution was added to the conical flask and placed into the distillation unit; the platform was placed, so the distillate outlet was submerged in the receiver solution. Fifty milliliters of 40% NaOH was dispensed into the tube and distilled for approximately 5 min. The distillate was titrated with standardized HCl (0.1) until the blue‒gray endpoint was achieved. A blank was run through all the steps above (EN ISO 20483:2006).
W1 = Sample weight (mg), T = Titration volume of sample (ml), B = Titration volume of blank (ml), N = Normality of acid to 4 decimal places, F = Conversion factor for nitrogen to protein = 6.25 for food & feed, gN/l = Gram nitrogen per liter, 14.007 = molecular weight of nitrogen.
2.4.4 Determination of crude fiber using fibroscopy
Crude fibre was determined by weighing 1.0 g of the finely ground sample into a round bottom flask, 100 ml of 1.25% sulphuric acid solution was added and the mixture boiled under a reflux for 30 min as described by [23].
2.4.5 Determination of moisture content
The moisture content in the tissue of bivalve samples was quantified using the procedure described by Osibona [24]. The method is based upon the removal of water from the sample and its measurement by loss of weight. A clean crucible was weighed and dried in the oven (W1); 1.0 g of each of the samples was weighed into the crucible (W2) and was dried at 105 °C, for 24 h. The crucible was then transferred from the oven to desicator, cool and reweighed (W3). The % moisture content was calculated from:
where W1 is the weight of the empty dried crucible, W2 is the weight of the crucible containing samples, W3 is the final weight of heated crucible containing samples after cooling.
2.4.6 Determination of minerals
A total of 1.00 g of bivalve sample was weighed into a digestion tube or a conical flask, and 10 ml of H2SO4 and 30 ml of nitric acid were added. The tube was then placed on a hot plate in a fume cupboard and digested until the digest became clear and then diluted to 100 ml, after which it was taken to atomic absorption spectrophotometer (AAS) for metal and heavy metal determination. In the AAS, a corresponding lamp for the corresponding mineral or heavy metal was placed, and the wavelength was explicitly set for the metal to be determined. The AAS siphoning hose was then placed into the digested sample after running. The standards for minerals/metals were determined, and the concentration of minerals/metals in the solution was then displayed on the screen of the AAS machine [25].
2.5 Statistical analysis
Mean ± standard calculated and descriptive analyses were performed using Microsoft Excel. All analyses were performed in replicate. One-way analysis of variance (ANOVA) using SPSS version 17.0 followed by a post hoc LSD test was performed to verify the mean disparity. The significance level was established at 5% (P < 0.05).
3 Results
Table 1 depicts the proximate compositions of the samples, indicating that some of the contents were significantly different (P < 0.05) from each other. Mytilus edulis had the highest moisture level at 1.15 ± 0.05%, while Anadara senilis had the lowest moisture content at 0.21 ± 0.02%. A high crude protein concentration was found in Mytilus edulis at 4.29 ± 0.43%, while the lowest concentration was recorded in Anadara senilis (0.61 ± 0.03%). At 0.05 ± 0.05%, Anadara senilis presented the lowest crude fat level, and Mytilus edulis presented the highest at 0.96 ± 0.15%. Anadara senilis had higher levels of total ash at 98.43 ± 0.22%. The nitrogen-free extract in Mytilus edulis was 0.91 ± 0.54, and the highest level recorded in Crassostrea gasar was 3.59 ± 0.46%.
Table 2 shows the mineral contents of the bivalve shells. A high calcium level was recorded in Anadara senilis at 51.00 ± 2.87 mg/kg and was significantly different (P < 0.05) from others. Mytilus edulis recorded the lowest concentration at 12.44 ± 2.51 mg/kg. Magnesium presented low levels in Mytilus edulis at 0.13 ± 0.07 mg/kg and high levels at 0.59 ± 0.23 mg/kg in Anadara senilis. Potassium appeared higher in Crassostrea gasar at 0.02 ± 0.00 mg/kg and lower in both Anadara senilis and Mytilus edulis at 0.01 ± 0.00 mg/kg. No significant difference (P > 0.05) was established for potassium contents. The phosphorus contents in this study were generally low and led to the concept of the calcium to phosphorus ratio. However, the calcium contents in this study were high. Anadara senilis had the highest calcium carbonate concentration at 60.91 ± 2.50 mg/kg, while Mytilus edulis had the lowest at 20.59 ± 3.18 mg/kg.
Figure 2a shows a graphical representation of variations in the proximate composition of the samples in March. Mytilus edulis was found to have a moisture content of 1.09%, and crude protein was also higher in Mytilus edulis at 4.71% and lowest in Anadara senilis at 0.64%. Crude fat was absent in Anadara senilis but recorded 1% in Mytilus edulis, whereas crude fiber appeared higher in Mytilus edulis at 1.84%.
Figure 2b shows the graphical representation of variations in the proximate composition of the samples in April. During this month, the moisture content in Mytilus edulis was higher at 1.19%, crude fat was more significant at 1.09% in Mytilus edulis, and crude protein also appeared to be high in Mytilus edulis at 4.29%. The crude fiber content was lowest in Anadara senilis at 0% and highest at 1.61% in Mytilus edulis.
Figure 2c also represents the variations in the proximate composition of the samples in May. The moisture content was much higher in Mytilus edulis at 1.16%, and crude protein appeared higher at 3.86%. In Anadara senilis, crude fat was lowest at 0.07% and highest at 0.8% in Mytilus edulis. The crude fiber was best in Anadara senilis at 98.58%, while Crassostrea gasar had the highest nitrogen-free extract value at 3.13%.
Figure 2d shows the graphical representation of monthly variations in the total Ash content of the samples in March, April, and May. Anadara senilis had the highest total Ash content (98.18%) while the lowest was recorded in Mystilus edulis (91.54%) in the month of March. For April, the total ash content obtained was 92.95%, 98.54% and 93.24% in Crassostrea gasar, Anadara senilis, Mytilus edulis respectively. The total ash was high in Anadara senilis at 98.18%, while Crassostrea gasar had the lowest Ash (93.63%) content in month of May. In terms of the parameters evaluated, the total Ash content had the highest content in all samples across the whole months.
Figure 3a shows the variations in the mineral elements of the samples for March. Anadara senilis and Crassostrea gasar had similar magnesium contents 0.37 mg/kg. Potassium and phosphorus were reported to be higher in Crassostrea gasar at 0.03 mg/kg and 0.06 mg/kg, respectively while the lowest potassium and phosphorus content was obtained in Anadara senilis at 0.01 mg/kg and 0.02 mg/kg respectively.
Variations in the mineral elements of Crassostrea gasar, Anadara senilis and Mytilus edulis in a March, b April, c May 2021, d Ca content in Crassostrea gasar, Anadara senilis, and Mytilus edulis in March, April, and May 2021, e CaCO3 content in Crassostrea gasar, Anadara senilis, and Mytilus edulis in March, April, and May 2021
Figure 3b represents the variations in the mineral elements of the samples collected in April. Magnesium was recorded at higher levels in Anadara senilis at 0.55 mg/kg. Crassostrea gasar had the highest values of potassium and phosphorus at 0.024 mg/kg and 0.136 mg/kg respectively, while Anadara senilis had the lowest value of potassium at 0.01 mg/kg.
Figure 3c shows the graphical representation of the variations in the mineral elements for samples collected in May. Crassostrea gasar was reported to have high values of potassium and phosphorus at 0.019 mg/kg and 0.357 mg/kg respectively.
Figure 3d shows the graphical representation of monthly variations in the calcium content of the shell samples in March, April, and May. Anadara senilis had the highest calcium content (53.47 mg/kg) while the lowest was recorded in Mystilus edulis (14.20 mg/kg) in the month of March. For April, the calcium content obtained was 48.97 mg/kg, 51.67 mg/kg and 13.56 mg/kg in Crassostrea gasar, Anadara senilis, Mytilus edulis respectively. The calcium was high in Anadara senilis at 47.86 mg/kg, while Mytilus edulis had the lowest calcium composition (9.57 mg/kg) content in month of May.
Figure 3e shows the graphical representation of monthly variations in the CaCO3 content of the samples in March, April, and May. Anadara senilis had the highest CaCO3 content (63.77 mg/kg) while the lowest was recorded in Mystilus edulis (23.63%) in the month of March. For April, the CaCO3 content obtained was 52.86 mg/kg, 59.85 mg/kg and 20.87 mg/kg in Crassostrea gasar, Anadara senilis, Mytilus edulis respectively. The CaCO3 content was high in Anadara senilis at 59.11 mg/kg, while Mytilus edulis had the lowest CaCO3 (17.28 mg/kg) content in month of May. In terms of the parameters evaluated, the Ca and CaCO3 content were high in all samples across the whole months except the low level of Ca and CaCO3 content in Mystilus edulis.
4 Discussion
The nutritional constituent of bivalves varies depending on the species, individuals of the same species and the part (shell or viscera) evaluated. Variations in chemical composition may also be attributed to the influence of intrinsic and extrinsic factors [26]. Low moisture content prevents the vulnerability of organisms to microbial spoilage and helps species retain their shelf life during preservation, especially for a more extended period. The percentage moisture content obtained in this study ranged from 0.21 ± 0.02% to 1.15 ± 0.05%, with Mytilus edulis having the highest value of % moisture (1.15 ± 0.05) and showed significantly difference (P < 0.05), followed by Crassostrea gasar (0.27 ± 0.06%), with the lowest percentage moisture found in Anadara senilis (0.21 ± 0.02%). This result obtained for moisture content in Mytilus edulis indicated higher chance of vulnerability of organism to microbial spoilage and low shelf life. Gopalsamy et al. [27] reported relatively high moisture content in some bivalve species compared to our result, which was similar to that of Kiin-Kabari et al. [28] on selected bivalve species. Nunes et al. [29] mentioned that moisture content in either bivalve shell or flesh influences exoskeletal strength, nutritive content value, and organoleptic attributes. Generally, molluscs contains protein that is extremely rich in essential amino acids which aids morphological growth, reproduction and those required for synthesizing vitamins in living systems [30]. Okuzumi and Fujii [31] also claimed that protein is a naturally large and important molecule that is required by living organisms for sustainability. Usually, most shellfishes, including bivalves, contain higher protein than most fin fishes [32]. Protein availability aids body growth and helps repair worn-out tissue and other essential body functions [29]. From our results, crude protein ranged from 0.61 ± 0.03 to 4.29 ± 0.43, with the highest percentage of protein found in Mytilus edulis (1.15 ± 0.05), followed by Crassostrea gasar (1.46 ± 0.21), with Anadara senilis having the lowest percentage (0.61 ± 0.03), which was close to the values obtained by Hamed et al. [33]. The reason for low protein content obtained from this study might be due to the fact the bivalve shells rather than the flesh was evaluated. The Mytilus edulis sample showed significant difference (P < 0.05) to other samples. Fat is essential for the development of body morphological structure, aids easy transportation of essential soluble vitamins, helps to retain food protein and play a significant role in biological function of the cells of the body. In this study, Bivalve shell crude fat content ranged from 0.05 to 0.96% with low variability. Fat content of Mytilus edulis showed significant difference (P < 0.05). Few researchers have shown that n-3 long-chain polyunsaturated fatty acids (PUFAs) are bivalve mollusks’ dominant fatty acids [34, 35] but this claim is mostly related to bivalve flesh. Kris-Etherton et al. [36] and Hamed et al. [33] claimed that n-3 PUFAs exhibit some protective effects on bivalves. The absence of crude fiber content in Anadara senilis can be related to low moisture in the species, which subsequently led to the inability of the species to absorb water, leaving it dehydrated before capture. This claim is reflected in the low moisture content in Anadara senilis, as shown in Table 1 above. Krzynowek et al. [37] reported that the amount of water absorbed by any aquatic organism is due to the presence of crude fiber and that crude fiber aids in the transitions of food particles in the alimentary system. Total Ash content helps in the development of the body and also serves as an indicator for the amount of mineral available in the body. The high value of total ash obtained in all bivalve shells is an indication of its high mineral content like magnesium, calcium, potassium, and zinc [38]. The range of ash content recorded in this study indicated that the species are a good source of minerals such as calcium, potassium, sodium, zinc, iron and magnesium. Our results showed that the three samples’ total ash was not significantly different. However, the highest total Ash value (98.43 ± 0.22%) seen in Anadara senilis showed significantly difference (P < 0.05), which implies that Anadara senilis will likely contain more mineral content than Mytilus edulis (92.96 ± 1.31) and Crassostrea gasar (93.15 ± 0.42%). These values are consistent with the report of Silva and Batista [35]. The ash contents in the examined samples show that they are a good source of minerals. High ash contents in all bivalve shell samples may be attributed to high chitin in samples strengthened by high calcium levels [39]. The values of NFE obtained from various samples ranged from 0.69 ± 0.22 to 3.59 ± 0.46, which were lower than the results of other researchers, such as [39]. This difference might be due to seasons.
Minerals are beneficial to human because they form part of the essential component of hormones, enzymes and enzyme activators in their diets [40]. Inadequate minerals in human body or nutrition may lead to morphological dysfunction and biochemical pathologies which depend on several factors, including the duration and degree of mineral deprivation [41].
Surprisingly, relatively low level of P, K and Mg was recorded in the shells which contrast the findings of Dickson [42], and Ivon and Eyo [43] where higher mineral contents were recorded. Davies and Jamabo [4] also claimed that bivalve shell exoskeletons are rich in essential minerals such as magnesium, sodium, potassium, calcium and phosphorus. The mineral contents obtained in the shell samples were not expected because these minerals are the major elements that make up the hard shell of bivalve mollusks. Another essential element (Na/NaCO3) that helps in the structural formation and compactness of bivalve shell was also not detected in our study. The presence of sodium and its chemical derivatives in bivalve shell help to regulate pH necessary for the formation of precipitates. Similarly, Dickson [42], also affirmed that MgCO3 also influences the compactness of bivalve shells. However, the magnesium content showed low levels in Mytilus edulis at 0.13 ± 0.07 mg/kg and high levels at 0.59 ± 0.23 mg/kg in Anadara senilis but no significant difference (P < 0.05) between Anadara senilis and Crassostrea gasar. Enyedi and Czijak [44], claimed that during the formation of shell, derivatives of K provides CO2 and CO3 which enhances the compactness and thickness of the shell. Potassium also affects the thickness of animals shells with reduction in shell thickness linked with potassium deficiency [22]. Phosphorus works in conjunction with calcium and magnesium to build and maintained strong bones and tissues [45]. The potassium content recorded appeared higher in Crassostrea gasar at 0.02 ± 0.00 mg/kg and lower in both Anadara senilis and Mytilus edulis at 0.01 ± 0.00 mg/kg and no significant difference (P > 0.05) observed. Calcium and their compounds are essential for bone formation. Low calcium content may lead to high phosphorus since calcium may be lost through the excremental process, thereby reducing the amount of calcium present in bones. From our results, the percentage composition of calcium was highest in Anadara senilis (51.00 ± 2.87 mg/kg) and showed significant difference (P < 0.05), followed by Crassostrea gasar (48.13 ± 4.01 mg/kg), with Mytilus edulis (12.44 ± 2.51) having the lowest calcium content. High calcium content obtained in shell samples led to low phosphorus content in this study. The result of this study therefore shows that calcium is the major constituent of bivalve shells and contributes to its hardness; thus, the harder the shell of an animal, the greater the amount of calcium content [42]. The bivalve shell is made up of calcium in the form of calcium carbonate. The level of calcium of this study was found to be relatively higher than other parameters. Calcium is essential for the formation of the muscle system and is required for physiological processes such as muscle contraction and blood clotting and is heavily involved in the active development of brain cells [34]. Although, in relatively low quantities but the presence of K, Mg, Ca and P in the shells of these animals offers positive relationship which indicates that these elements and their derivatives are mutually involved in shell formation processes. This has already been reported by Jatto et al. [45]. The result of this study is therefore in agreement with the result of this researcher.
4.1 Potential for circular economy
Shells are natural biomaterials that could partially be incorporated in the currently dominating non-renewable mineral sources in some applications. Bivalve shells are majorly composed of CaCO3, with some potential to become a prominent supplemental raw material for several applications, under the current framework of zero waste circular economy perspective. Studies such as Jatto et al. [45], Effiong et al. [46], and Dickson [42] had reported high levels of calcium in shells of some animals. Unfortunately, these shells are mostly ignored or littered in their habitats especially in coastal areas. Due to their compact and harden nature, they are also not readily degradable which means they are not vulnerable to rapid spoilage. This adverse nature makes them exist unaltered in the environment for a relatively longer period of time. Several comments or attributions on the importance and application of shells in major innovations of products have been made. Yet, these remarks are yet to be fully exploited beyond the microscale in research laboratories [42]. Calcium occurs specifically as calcium carbonate and forms the major composition of calcite rich rock [21]. Furthermore, animal shells have reportedly been used for liming of acidic soils due to its high calcium contents [46]. Calcium is a macro element that is needed to maintain strong and healthy bones and teeth. The medical industries particularly the dental specialist rely mostly on metals, porcelains and plastics or even calcium from expensive source other than bivalve shell for the production of prosthetic teeth. However, due to high calcium content and its compound (CaCO3) present in bivalve shells, availability and cost effective nature of various bivalve shells, they can be processed and used for the production of artificial tooth for dental implant purposes. Calcium also forms an essential part of human nutrition. It aids in the formation of the bone and also enhances growth. Diets where calcium can be derived includes but not limited to Dairy products such as milk, yoghurt and cheese, kelp, broccoli, almonds, sardines eaten with the bones and sesame seeds. Most of these sources are relatively expensive. The high calcium contents of these shells show that, they can be used as a source of calcium supplement in plants, animals and humans nutrition. Addition of grinded shells as premix or supplement in animal feeds e.g. poultry is already well established. Introduction of crushed shells as premix or supplement in fish feeds will further enhance the attainment of the zero waste framework or goal, besides; it will contribute to economic development of aquaculture and also help to reduce feed cost on fish farmers. The application of grinded shells as nutritional supplement for plants either for fertilization in form of inorganic fertilizer has been highlighted. Efforts to effectively use shells of animals for various purposes such as making scrubbing powder, animal feeds, and decoration among others have not been effective. Presently, most people including the Nigerians do not appreciate or fancy these shells for utilization due to poor knowledge of the potentials of these shells. This explains why many litter them in their environments. There is therefore a need to create awareness on the alternative usages for these shells.
5 Conclusion
The result obtained from this study showed that Anadara senilis, Crassostrea gasar, and Mytilus edulis shells contains relatively low protein and crude fat which makes them less important in terms of nutritional utilization. However, high total Ash obtained in all bivalve shell samples indicated their mineral potentials as they contains appreciable amounts of minerals elements such as Ca, CaCO3, Mg, K, and P. People consider only the viscera/fleshy part of bivalve as important thus discarding the shells afterward. Consequently, the disposed shells create a waste disposal problem and environmental stress. Additionally, human and animals tend to be injured by shells if stepped upon. It is therefore advisable to keep safe these shells after detaching from the fleshy parts because these shells can be processed alongside other resources into food supplements, animal feeds, dental products, plant nutrient supplements among others. They can be utilized, especially in medical, construction, and agricultural industries, if these shells are seen beyond their ornamental uses. Ca, K, and Na have been known as coagulants, fillers, pigments, adsorbents. Due to the presence of these substances in appreciable quantities in these shells, it is therefore predicted that, these shells can be sources of some of the mineral elements for use as catalyst, therapeutic agents, coagulants, pigments, absorbents or fillers. These acts would enhance the development cottage industries as well as promote farming in shell animals. It would further help to alleviate the unemployment crisis and creation of wealth from substances which would have been regarded as waste. It is therefore recommended that, the shells of these animals should be properly collected and utilized rather than being discarded as waste.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Wala C, Hart AI, Babatunde BB, Zabbey N (2016) Assessment of human health risk from heavy metal loads in freshwater clam, Ergeria radiata, from the Nun River, Niger Delta, Nigeria. J Environ Earth Sci 6:10–20
Zabbey N, Babatunde BB (2015) Trace metals in intertidal sediment of mangrove-sheltered creeks in Niger Delta, Nigeria: variability before and after crude oil spillage. Afr J Environ Sci Technol 9:371–378
Moniruzzaman M, Lee S, Park Y, Min TS, Bai SC (2021) Evaluation of dietary selenium, vitamin C and E as the multiantioxidants on the methylmercury intoxicated mice based on mercury bioaccumulation, antioxidant enzyme activity, lipid peroxidation and mitochondrial oxidative stress. Chemosphere 273:129673
Davies IC, Jamabo NA (2016) Determination of mineral contents of edible parts of shellfishes from Okpoka Creeks in Rivers State, Nigeria. Int J Fish Aquac Res 2:10–18
Das R, Islam MS, Moniruzzaman M, Ahmed KKU (2020) Natural breeding of freshwater apple snail Pila globosa (Swainson) in pond and aquarium. Bangladesh J Fish 32:45–54
Oluwatobi AA, Mutalib HA, Adeniyi TK, Olabode JO, Adeyemi A (2017) Possible aquaculture development in Nigeria: evidence for commercial prospects. J Agric Sci Technol 7:194–205
Udotong JIR, Udoudo UP, Udotong IR (2017) Effects of oil and gas exploration and production activities on production and management of seafood in Akwa Ibom State, Nigeria. J Environ Chem Ecotoxicol 9:20–42
Thébault J, Chauvaud L, L’Helguen S, Clavier J, Barats A, Jacquet S, Pecheytan C, Amouroux D (2009) Barium and molybdenum records in bivalve shells: geochemical proxies for phytoplankton dynamics in coastal environments? Limnol Oceanogr 54:1002–1014
Yesudhason P, Al-Busaidi M, Al-Rahbi WAK, Al-Waili AS, Al-Nakhaili AK, AlMazrooei NA, Al-Habsi SH (2013) Distribution patterns of toxic metals in the marine oyster Saccostrea cucullata from the Arabian Sea in Oman: spatial, temporal, and size variations. SpringerPlus 2:282
Zhu Y, Li Q, Yu H, Kong L (2018) Biochemical composition and nutritional value of different shell color strains of Pacific oyster Crassostrea gigas. J Ocean Univ China 17:897–904
Busse WW (1998) Leukotrienes and inflammation. Am J Respir Crit Care Med 157(6):210–213
Cobb CS, Ernst E (2006) Systematic review of a marine nutriceutical supplement in clinical traits for arthritis: the effectiveness of the New Zealand green-lipped mussel Perna canaliculus. Clin Rheumatol 25(3):275–284
FAO (2018) Bivalve trade is growing. Globefish—analysis on world fish trade 5(1):30–35
Chiaraluce G, Bentivoglio D, Finco A (2021) Circular economy for a sustainable agri-food supply chain: a review for current trends and future pathways. Sustainability 13:9294
Osorio LLDR, Flórez-López E, Grande-Tovar CD (2021) The potential of selected agri-food loss and waste to contribute to a circular economy: applications in the food, cosmetic and pharmaceutical industries. Molecules 26:515
Summa D, Lanzoni M, Castaldelli G, Fano EA, Tamburini E (2022) Trends and opportunities of bivalve shells’ waste valorization in a prospect of circular blue bioeconomy. Resources 11:48. https://doi.org/10.3390/resources11050048
Jović M, Mandić M, Šljivić-Ivanović M, Smičiklas I (2019) Recent trends in application of shell waste from mariculture. Stud Mar 32:47–62
Morris JP, Backeljau T, Chapelle G (2019) Shells from aquaculture: a valuable biomaterial, not a nuisance waste product. Rev Aquac 11:42–57
Lawal-Are AO, Afolabi JO, Akinwunmi MF (2014) Growth pattern and specificity of attachment of Lagoon crab (Callinectes amnicola) fouled with barnacles (Chelonibiapatula) from Lagos Lagoon, South West, Nigeria. J Fish Aquat Sci 5(9):311–320
Onyema IC, Nwankwo DI, Owolabi KO (2008) Temporal and spatial changes in the phytoplankton dynamics at the Tarkwa-bay jetty in relation to environmental characteristics. Ecol Environ Conserv 14(4):1–9
AOAC (2008) Official methods of analysis of AOAC International, 17th edn. Association of Official Analytical Chemists, Gaithersburg
AOAC (1996) Official methods of analysis of AOAC International, 16th edn. Association of Official Analytical Chemists, Gaithersburg
AOAC 978.10 (2005) Crude fiber. Official methods of analysis of AOAC International. Association of Official Analytical Chemists, Gaithersburg
Osibona AO (2011) Comparative study of proximate composition, amino and fatty acids of some economically important fish species in Lagos, Nigeria. Afr J Food Sci 5:581–588
AOAC (2010) Official methods of analysis of AOAC International, 18th edn. Association of Official Analytical Chemists, Washington, DC
Osibona AO, Kusemiju K, Akande GR (2009) Proximate composition and fatty acids profile of the African Catfish Clariasgariepinus. Acta SATECH 3:85–89
Gopalsamy I, Arumugam M, Saravanan K, Thangavel B (2014) Nutritional value of marine bivalve, Donax cuneatus (Linnaeus, 1758) from Cuddalore coastal waters, southeast coast of India. Inventi Impact Life Style 1:15–19
Kiin-Kabari DB, Hart AD, Nyeche PT (2017) Nutritional composition of selected shellfish consumed in Rivers State, Nigeria. Am J Food Nutr 5:142–146
Nunes ML, Batista I, Bandarra NM, Morais MG, Rodrigues PO (2008) Produtos da pesca: Valornutricional e importância para a saúde e bem-estar dos consumidores. Publicações Avulsas do IPIMAR No. 18, Instituto Português do Mar e Atmosfera (IPIMAR), Lisbon
Rajkumar T (1995) Studies on biology of Rapana rapiformis (Born) (Mollusca: Gastropoda: Rapanidae) from Parangipettai. PhD Thesis, Annamalai University
Okuzumi M, Fujii T (2000) Nutritional and functional properties of squid and cuttle fish. 35th Anniversary Commemorative Publication
Venugopal V, Gopakumar K (2017) Shellfish: nutritive value, health benefits, and consumer safety. Compr Rev Food Sci Food Saf 16:1219–1242
Hamed I, Ozogul F, Ozogul Y, Regenstein JM (2015) Marine bioactive compounds and their health benefits: a review. Compr Rev Food Sci Food Saf 14:446–465
Belitz HD, Grosch W, Schieberle P (2004) Food chemistry. Springer, Berlin
Silva HA, Batista I (2008) Produção, salubridade e comercialização de moluscos bivalves em Portugal. Publicaçõesavulsas do IPIMAR No. 20, Instituto Português do Mar e Atmosfera (IPIMAR), Lisbon
Kris-Etherton PM, Harris WS, Appel LJ (2002) Fish consumption, fish oil, omega-3 fatty acids and cardiovascular disease. Circulation 106:2747–2757
Krzynowek K, Wiggein K, Donahuer P (1982) Cholesterol and fatty acid content in three species of crab found in the Northwest Atlantic. Food Sci 47:1025–1026
Emmanuel BE, Oshionebo C, Aladetohun NF (2011) Comparative analysis of the proximate composition of Tarpon atlanticus and Clarias gariepinus from culture systems in south-western Nigeria. Afr J Food Agric Nutr Dev 11:5344–5359
Karnjanapratum S, Benjakul S, Kishimura H, Tsai YH (2013) Chemical compositions and nutritional value of Asian hard clam (Meretrix lusoria) from the coast of Andaman Sea. Food Chem 141:4138–4145
Khan PA (1992) Biochemical composition, minerals (calcium and iron) and chitin content of two portunid crabs Scylla serrata Forskal and Portunus pelagicus Linnaeus available in and around the coastal regions of Bangladesh. MSc thesis, Institute of Marine Sciences, Chittagong University
Srilatha G, Chamundeeswari K, Ramamoorthy K, Sankar G, Varadharajan D (2013) Proximate, amino acid, fatty acid and mineral analysis of clam, Meretrix casta (Chemnitz) from Cuddalore and Parangipettai coast, south east coast of India. J Mar Biol Oceanogr 2:2
Dickson UJ (2013) Mineral composition of shells of some animals found in the Niger Delta region of Nigeria. Afr J Sci Res 2(6):7–13
Ivon EA, Eyo VO (2018) Proximate composition and mineral contents of edible part of four species of shellfishes from the Calabar River, Nigeria. Annu Res Rev Biol 26(1):1–10
Enyedi P, Czirják G (2010) Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90(2):559–605
Jatto OE, Asia IO, Medjor WE (2010) Proximate and mineral composition of different species of snail shell. Pac J Sci Technol 11(1):416–410.48 67
Effiong G, Ibia TO, Ogban PI, Inyang ND (2009) Evaluation of locally-sourced liming materials for acid soils in AkwaIbom State, Southeastern Nigeria. Am Eurasian J Agron 2(3):144–151
Acknowledgements
The authors express gratitude to the Department of Marine Science, University of Lagos, for all the support to facilitate the research.
Funding
This work was supported by GIZ during the CIM engage of the corresponding author as a diaspora expert from Germany to Nigeria.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. IE, AL-A, OF, TJ performed material preparation, data collection, and analysis. The first draft of the manuscript was written and edited by all authors who commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or nonfinancial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elegbede, I., Lawal-Are, A., Favour, O. et al. Chemical compositions of bivalves shells: Anadara senilis, Crassostrea gasar, and Mytilus edulis and their potential for a sustainable circular economy. SN Appl. Sci. 5, 44 (2023). https://doi.org/10.1007/s42452-022-05267-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05267-7