Abstract
This study is to determine some physicochemical parameters and potentially toxic elements (PTE) in water samples collected from a borehole, a stream and a lake in Okposi, Nigeria, for their quality status and the human health risk of exposure to the waters. The physico-chemical properties: pH, electrical conductivity (EC), salinity, nitrate and sulphate, were determined by standard methods, while the level of PTE contamination was analysed by Flame Atomic Absorption Spectroscopy (FAAS). The results show that the levels of physicochemical parameters in the water samples are within the World Health Organisation (WHO) permissible limit. The PTE concentrations (mg/L) in the samples are in the ranges of 0.12–0.35 (Cr); 0.06–0.19 (Cu); 0.24–2.45 (Fe); 0.0–0.06 (Mn); 0.00–0.75 (Pb); and 0.0–02 (Zn). The levels of Cr and Pb in all the samples, except Pb in the downstream water, are above the WHO permissible limits, indicating that the waters are contaminated and unsafe for drinking. PTE form complexes with anions in water at high EC and salinity which reduces availability of toxic metals to organisms. Therefore, toxicity of PTE in the samples is in decreasing order: Upstream > Downstream > Lake water > borehole water. Calculations of hazard quotients shows possible adverse effects on adults and children on dermal contact with the waters due to Cr. Children might get cancer due to Pb on ingestion of water from the studied borehole, the Lake and the Upstream waters. Statistical analysis suggests that the geology of the area influenced PTE contamination of the water bodies.
Article highlights
-
Evaluation of the pollution status of the major water sources (borehole, lake, upstream and downstream) in the study area was conducted since the information will be useful for making decisions that will protect human health by the policy makers and the public.
-
The samples from all the water bodies in the study area were contaminated by chromium. The borehole water samples were contaminated by Iron and Lead; the downstream samples were contaminated by Iron; while samples from the lake and the upstream were contaminated by Lead. They are contaminated because the concentrations of these metals in the water bodies were above the World Health Organisation’s permissible limit for those metals in drinking water. Human exposure to the level of these metals in the water bodies may lead to severe health problems.
-
Based on the bioavailability studies of the metals and the physicochemical parameters of the major water bodies in the study area, the borehole water (ground water) is the safest (best) for drinking and domestic purposes, then, the lake water (safer), followed by the downstream water (safe), while the upstream water has the highest toxicity potential (not safe).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Access to safe water is crucial to sustenance of living organisms. Human beings need water for domestic, industrial, agricultural, recreational and laboratory activities. Potable water is scarce in so many areas especially in developing countries. According to UNICEF [1], 30 per cent of Nigerians lacked access to basic water services and less than 10 per cent had access to safe water. The various sources of water are prone to pollution by natural and anthropogenic activities, which will have impact on the quality of the water bodies.
Water pollutants include pathogens, organic materials, macroscopic pollutants and inorganic constituents such as heavy metals and salts. The PTE are of major environmental concern worldwide [2,3,4]. This has influenced several studies on the characteristics of different water bodies across the world. These studies include a risk assessment of dissolved trace metals in drinking water of Karachi, Pakistan which was high for some metals [5]. Naveedullah et al. [6] provided preliminary information on different heavy metals’ status in Siling reservoir while Lu et al. [7] assessed drinking water from Shenzhen, China and noted the levels of these metals. In addition, Bortey-Sam et al. [8] published a report on the health risk assessment of heavy metals and metalloid in drinking water from communities near gold mines in Tarkwa, Ghana while Suthar et al. assessed metals in water and sediments of Hindon River, India. Their study described the impact of industrial and urban discharges [9].
Researchers have published several articles on the chemical analysis of different water sources across Nigerian states. Ilechukwu et al. [10] studied water samples of a dam water in Abuja, Nigeria’s capital city, while Ogbueke et al. assessed metals in drinking water in different parts of Lagos, a coastal city with high volume of commercial and industrial activities [11]. Also, the quality of water from different sources in the Niger Delta Region of Nigeria, which is a coastal area with oil exploration activities, have been reported [12,13,14]. These studies gave results on the levels of PTE in peculiar environments in which pollutants in water occur from varying feeding sources. The quality of these waters are affected by the geology of where the water flows and also from anthropogenic activities [15]. Okposi town has a peculiar environment. The town is located upland, over 300 km away from the Atlantic Ocean, with no sign of sea water intrusion, but its waters have salty taste, and leaves white substances on the skin due to evaporation after bathing with it. Besides, Okposi waters require using of much soap to form lather while washing. Data on various water parameters of the distinct Okposi waters are lacking. Therefore, this study is necessary to assess some physicochemical parameters and levels of some heavy metals in the major water sources at Okposi. The results provide useful information for consumers and policy makers on the quality of the waters and the toxicity of the metals in the studied waters.
It has been reported that the particular behaviour of metals in an environment is influenced by their physicochemical forms as against the total concentration of the metals in a matrix [16, 17]. The solubility of PTE is enhanced at low pH [18]. Acidic medium aids the conversion of PTE to stable biotoxic complexes in the body, which has adverse effect to human health [19]. Dissolved salts, measured as conductivity and salinity, usually form metal complexes with anions in water. The formation of these complexes reduces availability of the metals in the medium, and since the organism absorbs less amount of the PTE, the toxicity to the organism will be reduced [20]..
The objectives of the present study are to (i) determine the physico-chemical characteristics (salinity, electrical conductivity, pH, nitrate, sulphate and chloride) of water samples obtained from a borehole (ground water), a lake and a stream (surface water) in the waters, (ii) determine selected PTE (Cr, Cu, Fe, Mn, Pb and Zn) in the ground and surface water samples obtained at a naturally salty water environment, (iii) discuss the possible effects of the physico-chemical properties of the waters on the toxicity of the PTE through the bioavailability relationship, (iv) assess the health risk on exposure to the PTE in the waters through oral ingestion and dermal contact of children and adults, (v) study the correlation to evaluate similarity of sources of the PTE.
In the next section, we consider the methodology, Sect. 3 shows the results of the various analysis carried out. In Sect. 4, we discuss the results, while in Sect. 5, we present the recommendations with conclusions.
2 Materials and methods
2.1 Study area
The study area is Okposi, in Ebonyi State, Southeast region of Nigeria, which has a salt lake. The research interest in Okposi is borne out of the environmental peculiarity of the area because its natural waters have salty taste. The inhabitants depend mostly on lakes, streams, and hand pump boreholes for their water need, as the availability of rain water is seasonal and there is no treated water supply in the community. The present study assesses water collected from a borehole, a lake, and a stream, which are the major sources of water during the dry season in the study area. These sources differ in their characteristics and are grouped into surface water and ground water..
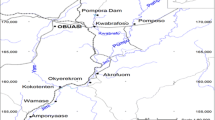
Figure 1 shows the study area in the map of Ebonyi State, Nigeria with the sampling locations, while Table 1 shows the coordinates of the sampling sites and their environmental characteristics.
2.2 Sample collection
Samples were collected from nineteen sampling points which comprised eighteen sampling points from different surface water sources and from the only functional ground water source in the area. Standard sample collection and preservation methods of the American Public Health Association (APHA) were followed [20].
Polyethylene bottles used for water sample collection were washed, rinsed with clean water and dried. The bottles were soaked in 15% nitric acid for 12 h before the acid was rinsed off with deionized water. The bottles were dried at room temperature after they were rinsed several times with the water from each sampling point to ensure that they were free from any contamination.
Hand pump was used to get water from the borehole at Akpugo. About 1 L was collected into a washed bottle (SA 1), another 1 L was pumped out after 10 min into a second bottle (SA 2), and then the third 1L was pumped out after another 10 min into a third bottle (SA 3).
About 1 L of water was collected from the Enwu lake at six different sampling points into six different properly washed bottles and labeled SB (1–6). Each bottle had been rinsed with the water from the sampling points.
From the Ogwunoti stream (downstream), about 1 L of water was collected at six different sampling points into six different properly washed bottles and labeled SC (1–6). Each bottle had been rinsed with the water from the sampling points.
About two kilometers from the Downstream is the Upstream where 1 L of water was collected at six different sampling points into six different labeled bottles SD (1–6).
After sample collection, the pH value of each of the samples is measured with a Jenway 3510 pH meter (USA). The samples were stored in a refrigerator at the laboratory of the National Center for Energy Research and Development, University of Nigeria, Nsukka. A portion of water from each sampling point was designated for heavy metal analysis while the remainder was retained for other chemical analysis. The samples for metal analysis were acidified with concentrated HNO3, to limit degradation of the water samples. Sample collections were carried out twice between March and November, 2019.
2.3 Determination of selected physicochemical parameters
Samples of water from the various sources are analyzed for the physicochemical parameters with the methods approved by the American Public Health Association [21].
Electrical conductivity and pH are determined after sample collection with a WTW Conductivity Meter, LF 90 model (Germany) and a pH meter (Jenway 3510) respectively.
Nitrate (NO3−) and sulphate (SO42−) levels in the water samples are determined by colorimetric method with a UV–Visible spectrophotometer (UV 1800, Shimadzu Corporation, Japan). Chloride ion is determined by argentometric titration with silver nitrate while salinity is determined as described by Radojevic and Bashkin [22].
2.4 Analysis for potentially toxic elements
The water samples were filtered through Whatman filter paper, No. 42 (Whatman, Germany) into prewashed plastic bottles and adjusted to pH value of 3.5 with analytical grade 1 M HCl. The concentrations of Cr, Cu, Fe, Mn, Pb and Zn in the water samples are determined with a Flame atomic absorption spectrophotometer (Model AA-6800, Shimadzu Corporation, Japan) connected to air-acetylene flame and a digital read-out system. The instrument is calibrated using different standard solutions of each of the metals. The hollow cathode lamp for each metal is used for each determination. Calibration curve is generated from the standard solutions. The minimum detection limit of the equipment for each metal is established.
2.5 Quality control protocols of the PTE analysis
In line with quality control requirements, the following protocols are carried out: (1) Washing of the glassware and other containers as recommended by Radojevic and Bashkin [21] before dipping them into a HNO3 solution of 10% concentration where they remain for 24 h. They are dried in an oven for 5 h at 80 °C after washing with deionized water. (2) Ensuring that the FAAS remain calibrated through the analysis by analyzing the calibration standards after ten sample analysis. (3) Recovery experiment by spiking method using standard solutions of the analytes. In this method, the standard solutions are spiked/added to samples and the metal concentrations in the samples are determined with the FAAS. Concentrations of metals in the samples that were not spiked are similarly determined using FAAS. Equation (1) is used to calculate the recovery in percent [23]:
where Css = concentration of the metal in the spiked sample, Cus = concentration of the metal in the unspiked sample, Cso = concentration of the metal in the spiked solution.
The results for the recovery experiments ranged between 97.4 and 102% for chromium; 96.2–98% for copper; 98–99.7% for iron; 100–102% for zinc; and 89–98% for lead. (4) Blank determination is carried out to make corrections arising from the glassware, reagents and other materials (5) Analysis of samples from the sampling point is carried out in duplicate (6) The Limit of detection (LOD) and the Limit of Quantitation (LOQ) are established from the calibration curves of the standard solutions of the elements being determined. The LOD is recorded from the signal to noise ratio of 3 and the LOQ is determined from the signal to noise ratio of 10. The LoD values (mg/L) for the metals were 0.00001 (Cr), 0.00001 (Fe), 0.00001 (Cu), 0.00001 (Zn), and 0.0001 (Pb) while the LoQ values (mg/L) were 0.0001 (Cu), 0.0001 (Cr), 0.0001 (Zn), 0.0001 (Ni), 0.0001 (Fe) and 0.001 (Pb).
Precise and accurate results are obtained from the determination as the instrument is skillfully operated, and correct calibration procedure is followed.
2.6 Assessment of the health risk of the PTE in the water bodies
The potential risk of exposure of children and adults to the water samples is estimated from (1) the concentrations of the selected PTE in the surface and ground water samples; (2) exposure pathways (ingestion and dermal contact); (3) the population category for the study, adults (> 60 years old) and children (6 years), representing the most vulnerable group; (4) the exposure factors including frequency and duration of exposure; (5) the average daily dose (ADD), and (6) the Hazard Quotient (HQ).
The ADD for non-carcinogenic health risk was calculated separately for ingestion and dermal contact from Eqs. (2) and (3).
Thus, the ADD through ingestion for non-chronic non-cancer effects, was calculated using Eq. (2) [24].
ADD through dermal contact was calculated using Eq. (3), [6]
Definitions of the parameters and adopted values are given in Table 2.
The ADD for cancer effects (ADD−ce) was calculated in terms of lifetime ADDs (LADDs). In calculating for cancer effects due to carcinogens, the duration of a lifetime has traditionally been assigned the nominal value of 70 years [24].
The non-cancer risk assessment of exposure to the selected PTE in the water bodies was estimated by interpreting the values on calculation of the Hazard Quotient (HQ) as given in Eq. (5), [27].
where, HQdermal/ingestion is the hazard quotient on ingestion or dermal contact; RfDdermal/ingestion is the oral/dermal reference dose of the elements. The recommended RfDingestion and RfDdermal values are obtained from the references as presented in Table 3.where HQ > 1, there may be significant non-carcinogenic health risk, while HQ < 1 implies that exposure may not lead to non-carcinogenic health risk [25].
The cancer effects of exposure to the selected PTEs in the water bodies are assessed from the calculation of the carcinogenic risk index (CR), [28].
where CR is Carcinogenic Risk; SF is the carcinogenic Slope Factor.
Carcinogenic Risk is the probability of an individual developing cancer over lifetime as a result of exposure to a potential carcinogen or a mixture of contaminants. In the United States of America, the range of values of CR acceptable or tolerable is 1.0E-06 to 1.0E-04 [29].
2.7 Data analysis
All statistical data were processed with Microsoft Excel and IBM SPSS Statistics 20.0 (SPSS Inc., USA. The IBM SPSS Statistics is used for one-way analysis of variance (ANOVA), pearson correlation, and the hierarchical cluster analysis (CA). ANOVA indicate significant differences in average values of the concentration of PTE across the sampling sites. Cluster analysis is performed by means of Ward’s method with squared Euclidean distances which identify similarities between metal contents, the physicochemical parameters and the samples.
3 Results
The mean and standard deviations of the physico-chemical properties and the PTE concentrations for each sampling site (Borehole, lake, downstream and Upstream) are presented. Physicochemical properties of the water samples are shown on Table 4, while the results of analysis for PTE in the water samples are presented on Table 5. The results of calculations for Average Daily Dose via ingestion and dermal contact of the PTE in the water samples for adults and children are shown on Table 6. On Table 7, calculations of Hazard Quotient for the PTE in the water bodies for adults and children are presented, while on Table 8, cancer risk calculations on carcinogens (Cr and Pb) in the water bodies for adults and children are presented. On Table 9, Pearson’s correlation analysis between the metals in water samples from the 4 locations are presented. The Dendrogram showing 48 cases from physicochemical parameters measured in the water sources is shown in Fig. 2.
4 Discussion
4.1 Physicochemical properties of the water samples
The characteristics of parameters in the matrix influences the biological availability of the PTE. The results of the determination of these parameters are presented on Table 4.
The pH is a measure of hydrogen ion concentration. pH of water indicates the degree of acidity or alkalinity of a water body. From the results on Table 4, the Enwu Lake and the downstream water have the highest mean pH value of 8.4. The pH value of the Upstream water is lowest at 7.7 while the pH value of the borehole water at Akpugo is 8.3. WHO permissible range of pH values for drinking water is 6.5–8.5 while the NSDWQ limit is 6.5- 9. This implies that the water samples have tolerable pH values. According to the US EPA, pH may be the single most important factor that influences metals’ behaviour in the environment [34]. A study revealed that solubility of some toxic metals increases with lower pH [35]. Generally, matrix with low pH will induce metal dissociation thereby increasing the solubility of the metals and consequently their toxicity. However, in water with high alkalinity, metals, with few exceptions, precipitate as oxides hydroxides and carbonates making the metals less bioavailable and less toxic [36]. From the perspective that low pH value induces higher metal solubility, and hence more availability, the metals are more toxic in the Ogwunoti Upstream water and less toxic with the Lake and the Downstream waters. The level of acidity in the waters may be influenced by anthropogenic activities within the vicinity of the water bodies. The range of pH values in the present study is higher than the values reported for similar waters in Shagamu, Southwest Nigeria [30], and Lagos, Nigeria [37] and, but on the same range with values obtained in Fasha Town, Ethiopia [31].
Electrical conductivity measures the ability of any medium to conduct an electric current. It estimates the amount of dissolved salts because dissolved solids carry electric current. Higher EC value implies higher levels of dissolved salt in the medium. The water samples in the study area have EC values which are greater than 16 dSm−1. This level is within the class of very strongly saline medium [38]. The maximum tolerable level of EC in drinking water by WHO and the NDWQS is 1000 μS/cm. The results as presented in Table 4 show that the water samples have values which are less than 1000 μS/cm. The borehole has the highest value of 630 μS/cm, then 370 μS/cm for the Lake, and the Upstream has the least value of 167.3 μS/cm. These salts usually form metal complexes with anions in water thereby reducing availability of potentially toxic metals. With reduction of the amount of PTE through formation of complexes, potential toxicity will reduce. From this study, the borehole water has the least propensity of having toxic metals, then the Lake, followed by the Downstream water, while the Upstream water has the highest toxicity potential. The EC level in the Okposi borehole water is far higher than the level in Shagamu borehole water and that in Fasha town (Ethiopia) borehole water (Table 4).
Similarly, there is reduction in toxicity of metals where the salinity levels is high compared to where it is low. This is due to formation of metal complexes with anions, thereby reducing toxicity [36]. The results of the present study show in descending order that the borehole water, with mean salinity level of 231.76 mg/L, has the least available metals that may be toxic, then the Lake water, with mean salinity level of 151.97 mg/L. The Downstream water (134.87 mg/L mean salinity level) and the Upstream water with mean salinity level of 95.52 mg/L may have the highest available metals that can cause toxicity. Water salinity is due to the geological strata where water flows and also anthropogenic contribution [16].
The levels of nitrate, sulphate and chloride in the present study are below the tolerable level for drinking water in Nigeria [32]. These levels in the samples follow the same trend with the EC and the salinity levels. Chloride levels in the studied water samples are higher than the levels in Shagamu and Fasha town waters (Table 4). The range of values for chloride, nitrate and sulphate in the water samples are 87.54–230.64 mg/L, 1.87–7.71 mg/L and 7.93–19.13 mg/L respectively. A comparison with the range of values for chloride, nitrate and sulphate in Lagos waters which are 84.89–192.37 mg/L, 0.29–2.16 and 9.88–22.30 mg/L respectively, indicates that the average of both chloride and nitrate levels are higher than in Lagos waters while the average for sulphate is lower than that of Lagos waters [37].
4.2 PTE analysis
Results on Table 5 show the concentrations of the investigated metals in the water samples. Chromium concentration in the water samples ranges from 0.12 mg/L to 0.35 mg/L. It is highest in (Ogwunoti) Downstream water then in (Ogwunoti) Upstream water, followed by Borehole (ground water) and least in the (Enwu) Lake water. The levels may cause adverse health impact as they are above the NSDWQ and the WHO permissible level in drinking water (0.05 mg/L) (Table 5). Chromium levels in Okposi borehole and the stream water samples are higher than the values obtained in similar waters in Lagos [11], but lower than the chromium level in a surface water in China [6]. Sources of chromium are majorly from anthropogenic sources and runoff water.
The concentration of copper is highest in the lake water sample compared to the other water bodies in the study area. The level of Cu in the sampled waters, in descending order, is: Lake water > Upstream > Downstream > Borehole. Though Cu is an essential element, its consumption beyond the maximum allowable limit may cause gastrointestinal disorder. Cu levels in the studied samples are within the safe limit for drinking water [32, 33]. The level is within the same range within similar water sources in Lagos (Table 5). The source of Cu in the study area may be from anthropogenic sources such as domestic waste water, dust, and combustion of fossil fuels.
Downstream water has the highest concentration of Fe compared to the other water sources in the study area as presented on Table 5. Borehole water has higher Fe level than the Lake and Upstream samples which have equal concentration of Fe. Fe in the waters may be attributed to the runoff water over the natural iron-rich soil of the study area. The mean level of Fe in the downstream and borehole water samples are above the value recommended for drinking water [32, 33]. Fe level in the surface waters is higher than the level that was obtained in Lagos but lower than the values recorded in China (Table 5).
The level of Mn is not detectable in the water samples collected from Upstream and the lake but borehole has a concentration of 0.03 mg/L which is less than the amount in the Downstream (0.06 mg/L). The presence of Mn may be due to anthropogenic activities. The average concentration of Mn is within the permissible limit for drinking water (Table 5). Exposure to Mn outside the permissible limit may cause neurological disorder.
The Lake has the highest concentration of Pb, then the borehole water sample, followed by the Upstream water. It is not detectable in the Downstream water. Probable sources of Pb include exhaust emissions and atmospheric lead deposition [36]. The Lake is situated within 150 m from the busy Okposi–Amasiri road. Deposition on the roadside of products of fuel combustion in vehicles eventually wash into nearby water bodies and affect the level of the element in the water body. Human exposure to Pb above the permissible level may cause cancer and can also interfere with Vitamin D metabolism. It may affect mental development in infants. It may also be toxic to the central and peripheral nervous system. Pb content in Okposi water samples (except in the Downstream) is above the safe limit [32, 33] and higher than the level recorded in similar waters in Lagos [11].
Zn is present in the Upstream water at 0.02 mg/L level, and in the borehole water (0.01 mg/L) but not in the Downstream and the Lake waters. Sources of Zn are usually from anthropogenic activities.. The levels of Zn in the studied samples are within the permissible level for drinking water [32, 33].
From the bioavailability perspective, toxicity of PTE will be highest in the Upstream water, then the Downstream, followed by the Lake, while the borehole water will be the least in potential toxicity of the studied waters because of the propensity to form metal complexes due to the highest values of EC and salinity in the borehole water. Therefore, the borehole water (ground water) is the safest (best) for drinking and domestic purposes, then, the lake water (safer), followed by the downstream water (safe), while the upstream water has the highest toxicity potential (not safe).
Liu and Ma reported that the toxicity associated with chromium include headache, diarrhea, nausea, vomiting and carcinogenic. Notwithstanding, copper toxicity may lead to liver damage, Wilson disease and insomnia. On the other hand, Zinc toxicity leads to depression, lethargy, neurological signs with increase in thirst, while lead toxicity may result in damage to fetal brain, diseases of the kidneys, circulatory system and nervous systems [39]. Therefore, investigation of factors that affect toxicity of PTE in water bodies is imperative in environmental studies. Mean values are in use in this study since they are higher than the median and the standard deviations..
4.3 Assessment of potential health risk of oral ingestion and skin contact with the waters
The results of the calculations of Average Daily Dose via ingestion (ADDing) and the Average Daily Dose via dermal contact (ADDder) of the PTEs in the water bodies for adults and children are presented in Table 6.
On Table 7, calculation results for HQ are presented. Where HQ value is greater than 1, it implies that exposure to PTE may cause adverse health effect. Cr is greater than one on dermal contact with the waters by adults or children. Therefore, it may cause adverse health effect on dermal contact. The worst condition due to Cr is expected in the Upstream, then the Lake and the Downstream being the least for adverse effect on adults. The situation differs for children as Downstream water is most perilous due to Cr, while Upstream and the Lake water being the least dangerous.
Ingestion of the waters by children, based on the HQ, will cause most adverse effect, especially by Downstream water, followed by Upstream, but the least tendency to cause health problem is in the Lake, Adults have potential health problems on ingestion of water in Downstream with less health problem with Upstream, No health problem is expected on adults on ingestion of the lake and borehole waters due to Cr.
Ingestion of ground and surface waters from the study area do not pose any adverse health effect due to Cu. This is inferred from the HQ calculations which was less than 1 in all the samples. Dermal contact with the waters pose potential adverse health effect for children and for adults with only the Lake water.
There is no potential adverse health due to Fe on ingestion of the waters but dermal contact with the waters poses potential adverse health effect to children. Dermal contact with the Downstream water by adults poses a health risk due to Fe.
No adverse health risk due to Mn is expected on ingestion of the waters by children and adults but health risk to children and adult is expected on dermal contact with the borehole and downstream waters. The risk is higher for children especially with the Downstream water.
Ingestion and dermal contact with all but Downstream water pose health risk to children and adults due to Pb. Children are at more risk especially with the borehole water. Dermal contact poses greater risk than ingestion. Pb will cause more health problems to adults on dermal contact with the Lake water.
The HQ is less than 1 which implies that there is no adverse health problems due to Zn which is posed by ingestion or dermal contact with the waters.
4.4 Cancer risk
Table 8 shows the calculations for cancer risk assessment of the water samples in this study. It is shows that ingestion of water from the borehole, Lake and Upstream might lead to cancer in children. This is attributable to the Cr values which were outside the range of l0−4 and 10–6. With such values, there will be propensity to develop cancer [40].
4.5 Pearson correlation analysis
The result of analysis of correlation is presented in Table 9.
Correlation coefficient is done to ascertain the level of relationship in the sources of the elements. The results are presented in Table 9. There is significant positive correlation between Cr and Fe (0.722) and Cu and Pb (0.754). On the other hand, there is significant negative correlation between Fe and Pb (−0.773) and Mn/Fe (−0.773) at 0.05 level (2-tailed). A negative correlation is significant at the 0.01 level between Pb and Cr (−0.989). The sources of Cr/Fe and Cu/Pb are closely related as indicated by the high positive figures (possibly anthropogenic) while Fe/Pb, Mn/Fe and Pb/Cr are not related.
4.6 Cluster analysis
The result of cluster analysis is shown as a dendrogram (Fig. 2), grouping 12 parameters at 4 water sources (48 cases) into 2 clusters. Cases 1 to 24 represent occurrences of 6 metals in the 4 water bodies, while cases 25 to 48 show the results of 6 physicochemical parameters (pH, EC, Nitrate, Sulphate, Chloride and Salinity) in the 4 water sources. One cluster has the metals and the EC in lake water, the sulphate and the chloride in the Upstream samples, while the remaining physicochemical parameters clustered separately. It implies that though the concentrations of the metals and the physicochemical parameters vary in the waters, the geology of the study area is similar and this contributes to the chemistry of the waters.
5 Conclusion
The quality of water from the major water sources in Okposi community was evaluated from the levels of physicochemical parameters (pH, electrical conductivity (EC), salinity, nitrate and sulphate), and potentially toxic elements (Cr, Cu, Fe, Mn, Pb and Zn) in the waters. The physico-chemical characteristics of the waters varied and followed the same pattern with salinity, electrical conductivity, nitrate, sulphate and chloride in the waters. This indicated a decrease in this order: Borehole > Lake > Downstream > Upstream. The concentrations of detected PTE varied.. The samples from all the water bodies were contaminated by chromium. The borehole water samples were contaminated by Iron and Lead; the downstream samples were contaminated by Iron; while samples from the lake and the upstream were contaminated by Lead since the concentrations of these metals were above the World Health Organisation’s permissible limit for those metals in drinking water. Human exposure to the these metals at levels above the permissible limit may lead to severe health problems. Higher values of the physic chemical properties indicate less propensity for the metals to be toxic (bioavailable). This study presented borehole water as the safest from the adverse effect of the selected PTE than the Lake water while the Upstream water was the most deleterious water source in the study area. With the information from this study on the contamination of the water sources, the public are aware of the water quality, while of the policy makers can see the need to provide functional borehole water sources in the study area to reduce exposure to potentially toxic elements. A limitation to this study was the lack of background data on the quality of water in the study area. Regular environmental monitoring and reporting of the water quality is planned while relevant authorities should regulate anthropogenic activities that pollute the existing water bodies in the study area.
Data availability
Not Applicable.
Code availability
Not Applicable. All authors read and approved the final manuscript.
References
UNICEF (2020), National Outcome Routine Mapping of WASH services. Report by FMWR, NBS and UNICEF. www.unicef.org/nigeria/reports/water-sanitation-hygiene-national-outcome-routine-mapping-2019. Accessed 20 May, 2021
Fallahzadeh RA, Ghaneian MT, Miri M, Dashti MM (2017) Spatial analysis and health risk assessment of heavy metals, concentration in drinking water resources. Environ Sci Pollut Res 24(32):24790–24802
Krishna AK, Satyanarayanan M, Govil PK (2009) Assessment of heavy metal pollution in water using multivariate statistical techniques in an industrial area: a case study from Patancheru, Medak District, Andhra Pradesh, India. J Hazard Mater 167(1):366
Batayne HA (2012) Toxic (aluminum, beryllium, boron, chromium and zinc) in ground water: health risk assessment. Int J Environ Sci Technol 9(1):153
Karim Z (2011) Risk assessment of dissolved trace metals in drinking water of Karachi, Pakistan. Bull Environ Contam Toxicol 86(6):676
Naveedullah M, Zaffar H, Chunna Y, Hui S, Dechao D, Chaofeng S, Liping L, Yingxu C (2014) Concentrations and human health risk assessment of selected heavy metals in surface water of the siling reservoir watershed in Zhejiang Province, China. Po J Environ Stud 23(3):801–811
Lu SY, Zhang HM, Sojinu SO, Liu GH, Zhang JQ, Ni HG (2015) Trace elements contamination and human health risk assessment in drinking water from Shenzhen, China. Environ Monit Assess 187(1):4220. https://doi.org/10.1007/s10661-014-4220-9
Bortey-Sam N, Nakayama SMM, Ikenaka Y, Akoto O, Baidoo E, Mizukawa H, Ishizuka M (2015) Health risk assessment of heavy metals and metalloid in drinking water from communities near gold mines in Tarkwa. Ghana. Environ Monit Assess 187:397. https://doi.org/10.1007/s10661-015-4630-3
Suthar S, Nema AK, Chabukdhara M, Gupta SK (2009) Assessment of metals in water and sediments of Hindon River, India: impact of industrial and urban discharges. J Hazard Mater 171(1):1088
Ilechukwu I, Olusina TA, Echeta OC (2020) Physicochemical analysis of water and sediments of Usuma Dam, Abuja, Nigeria. Sciendo Ovidius Univ Ann Chem 31(2):80–87. https://doi.org/10.2478/auoc-2020-0015
Ogbuneke CC, Ezeibeanu AP (2020) Comparative assessment of trace and heavy metals in available drinking water from different Sources in the centre of Lagos and off town (Ikorodu LGA) of Lagos State, Nigeria. AJCA 3(1):94–104
Etim EE, Odoh R, Itodo AU, Umoh SD, Lawal U (2013) Water quality index for the assessment of water quality from different sources in the Niger Delta Region of Nigeria. Front Sci 3(3):89–95. https://doi.org/10.5923/j.fs.20130303.02
Edet AE, Okereke CS (2001) A regional study of saltwater intrusion in southeastern Nigeria based on analysis of geoelectrical and hydrochemical data. Environ Geol 40(10):1278–1289
Edet A (2017) Hydrogeology and ground water evaluation of a shallow coastal aquifer, southern Akwa Ibom State (Nigeria) App. Water Sci 7(5):2397–2412. https://doi.org/10.1007/s13201-016-0432-1
Storelli MM, Storelli A, D’ddabbo R, Morano C, Bruno R, Marcotrigiano GO (2005) Trace elements in loggerhead turtles (Caretta caretta) from the Eastern Mediterranean: overview and evaluation. Environ Pollut 135:163–170
Ihedioha JN, Ogili EO, Ekere NR, Ezeofor CC (2019) Risk assessment of heavy metal contamination of paddy soil and rice (Oryza sativa) from Abakalikin, Nigeria. Environ Monit Assess 191:350. https://doi.org/10.1007/s10661-019-7491-3
Landonin DV (2002) Heavy metal compounds in soils: problems and methods of study. Eurasian Soil Sci 35(6):605–613
WHO (2004) Guideline for drinking water quality, 3rd edn. WHO, Geneva
Khaled SB, Muhammad AA (2016) Field accumulation risks of hms in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J Bio Sci 23:32–44
Onuwa OP, Eneji I, Sha’Ato R (2012) Analysis of heavy metals in human hair using atomic absorption spectrometry (AAS). Am J Anal Chem 3:770–773
American Public Health Association (APHA) (2012) Standard methods for the examination of water and waste water, 22nd edn. American Water Works Association, Washington DC
Radojevic M, Bashkin VN (1999) Practical environmental analysis. The Royal Society of Chemistry, Cambridge, p p466
Ugwu KE, Ofomatah AC (2021) Concentration and risk assessment of toxic metals in indoor dust in selected schools in Southeast, Nigeria. SN Appl Sci 3:43. https://doi.org/10.1007/s42452-020-04099-7
EPA, U.S. Environmental Protection Agency (EPA) (2011) Exposure Factors Handbook: 2011 Edition.; EPA/600/R-09/052F. National Center for Environmental Assessment, Washington, DC
UN (United Nations) (2019). World Population Prospects, 2019 : ST/ESA/SER.A/423, United Nations, Department of Economics and Social Affairs, Population Division (2019)
Tay CK, Dorleku M, Doamekpor LK (2019) Human exposure risks assessment of heavy metals in ground water within the Amansie and Adansi districts in Ghana using pollution evaluation indices. West Afri J Appl Ecol 27(1):23–41
US EPA (1989). Report. EPA/540/1- 89/002.1989. Risk Assessment Guidance for Superfund. United States Environmental Protection Agency, Vol. 1, Human Health Evaluation Manual (Part A). Washington, DC, USA
Harmanescu M, Alda LM, Bordean DM, Gogoasa I, Gergen I (2011) Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem Cent J 5:64. https://doi.org/10.1186/1752-153X-5-64
Hsu CH, Jeng WL, Chang RM, Chien LC, Han BC (2001) Estimation of potential lifetime cancer risks for trihalomethanes from consuming chlorinated drinking water in Taiwan. Environ Res 85(2):77–82. https://doi.org/10.1006/enrs.2000.4102
Ojekunle ZO, Adeyemi AA, Taiwo AM, Ganiyu SA, Balogun MA (2017) Assessment of physicochemical characteristics of groundwater within selected industrial areas in Ogun State, Nigeria. Env Pollut Bioavail 32(1):100–113. https://doi.org/10.1080/26395940.2020.1780157
Behailu TW, Badessa TS, Tewodros BA (2017) Analysis of physical and chemical parameters in ground water used for drinking around Konso Area, Southwestern Ethiopia. J Anal Bioanal Tech 8(5):379. https://doi.org/10.4172/2155-9872.1000379
NSDWQ (2007), Nigerian Standard for Drinking Water Quality, Nigerian Industrial Standard, NIS 554: 2007, Standards Organisation of Nigeria, Abuja, Nigeria
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organisation, Geneva
USEPA (United States Environmental Protection Agency) (2007) Framework formetals risk assessment. Office of the Science Advisor, Risk Assessment Forum. Washington, DC: EPA/120/R-07/001)
Wilde KL, Stauber JL, Markich SJ, Franklin NM, Brown PL (2006) The effect of pH on the uptake and toxicity of copper and zinc in a tropical freshwater alga (Chlorella sp.). Arch Environ Con Tox 51:174–185
Cornelis R, Nordberg M (2007) General chemistry, sampling, analytical methods, and speciation. In: Gunnar FN, Bruce AF, Monica N, Lars TF (eds) Handbook on the toxicology of metals, 3rd edn. Academic Press, Burlington, pp 197–208
Osibanjo O, Majolagbe AO (2012) Physicochemical quality assessment of groundwater based on land use in Lagos city, Southwest, Nigeria. Chem J 2(2):79–86
FAO (1988) Salt-affected soils and their management. FAO Soils Bull. 39. FAO, Rome, Italy. 131 pp
Liu Y, Ma R (2020) Human health risk assessment of heavy metals in ground water in the Luan River catchment within the North China Plain. Geofluids 2020:8391793
USEPA (1997) Exposure factors handbook, EPA/600/p-95/002 Fa,b, c. Office of research and development. Environmental Protection Agency: Washington
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ugwu, K.E., Ofomatah, A.C. Bioavailability and health risk assessment of potentially toxic elements in salty water environment of Okposi, Southeastern Nigeria. SN Appl. Sci. 4, 204 (2022). https://doi.org/10.1007/s42452-022-05080-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05080-2