Abstract
The health risk of students’ exposure to some potentially toxic metals in classroom dusts in Southeast, Nigeria was assessed. Dust particles were collected from classrooms in some public high schools and digested with aqua regia before analysis for selected metals by atomic absorption spectrophotometry. The geoaccumulation index, contamination factor and the pollution load index were assessed from the metal concentrations of the dust. Hazard quotient and cancer risk index were used to estimate the potential health risk of students’ exposure to the metals in the dust. The metal concentrations (mg/kg) were in the ranges of 1.57–175.38 (Cr); 0.93–463.28 (Cu); 31.94.76–6623.41 (Fe); 4.96–143.98 (Ni); 2.64–375.27 (Zn); and 2.35–53.96 (Pb).The geo-accumulation index values showed that all the dust samples were polluted with Fe and Cr; and unpolluted with other metals with few exemptions. The contamination factor values showed that all the schools but one had a low contamination status due to Ni and Cu. There was moderate contamination by Pb at all the schools but two. All the schools had high contamination of Cr and Fe. The pollution load index indicated that the quality of all the classrooms was deteriorated. The calculated values of hazard quotient indicated that ingestion of dust at most of the classrooms would have no significant risk of non-carcinogenic effects on the health of the students. Dermal contact with the dust at all the classrooms would expose students to adverse effects of Fe. There will be adverse effect due to Pb for dermal contact with dust at most of the schools. Ingestion of dust particles at classrooms in all the schools would have carcinogenic effect due to Ni. Correlation analysis indicated that the sources of the metals varied. This study provided baseline data for relevant bodies to use in monitoring and controlling pollution so as to protect students from toxic metals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dusts are airborne or settled small, dry, solid particles. They are generated naturally in sandstorms, volcanoes, wind, or from anthropogenic activities including bagging, demolition, crushing, conveying, grinding, shoveling, screening, sweeping and piling [1].
Dust particles released into the air will slowly settle due to the earth’s gravitational force [2]. The sizes of dust particles vary; some may be less than 1 μm and up to about 100 μm in diameter. Dust particles can be found indoors and outdoors. The airborne dust particles may be inhaled or may settle on human skin on contact. Dust particles may be dissolved by sweat and eventually enter the bloodstream through the skin pores. Human beings and animals may ingest dusty materials into their mouth or drink water with dust contamination. Human exposure to dust that occurs through oral intake, inhalation, and skin contact is of environmental concern because of the toxic constituents of the dust [3,4,5].
Different types of dust are generated from environmental processes due to their different sources. Therefore, the chemical constituents of dust particles vary. Among the chemical components of dust are metals. Human exposure to high levels of some metals leads to adverse health effects [6].
Reports of several researches on the concentrations of metals in dusts present in some environmental compartments indicate the extent of interests on this subject across the continents. Huang et al. investigated the amount of selected metals in indoor and outdoor dust particles found in cities of Guangzhou for contamination and risk assessment [7]. Taiwo et al. assessed road dust in Abeokuta, Nigeria, while Zheng et al. and Al-Khashman investigated the levels of heavy metals in the streets of Huludao, China and Amman, Jordan, respectively [8,9,10]. The results showed different levels of heavy metals in the areas. Nkansah et al. worked on the levels of metal in school classrooms at Kumasi, Ghana, where it was observed that geographical variation correlated with heavy metal load. Health risk assessment from this study showed that exposure through ingestion was more than exposure through dermal contact [11]. Popoola et al. [12] studied heavy metal concentrations in primary schools in Lagos, Nigeria, and revealed that the amount of the metals detected in classroom dust were lower than the levels observed in street dust of some other cities in the world.
Iniaghe et al. studied heavy metals indoor dust of primary schools in Delta State, Nigeria and opined that location of the schools as well as opened windows for ventilation could be possible routes for heavy metals contamination of classroom dust [13].
In many tropical and developing countries like Nigeria, dust particles are usually seen on most surfaces due to several uncontrolled human activities and natural factors. It is worrisome to notice the amount of settled indoor dusts at some public high school classrooms in Southeast Nigeria. It is common to see dust particles on various surfaces in the classrooms such as the windows, floors, tables, benches, chairs, walls, ceilings, books and shelves. Some of the buildings are dilapidated, lacking proper doors and windows to limit entry of door dust particles into the classrooms. In some schools, the windows and doors are always open without inhibition to entry of dust particles into the classrooms. Wind effect disperses particles from outdoors to indoor where these particles settle as dust. The dust may become resuspended thereby polluting the environment. Most classrooms also lack ceiling boards and are not painted, while in the few painted ones, the paints are peeling off. From literature, there are no prior studies of the pollution indices based on the levels of metals in public high classroom dusts in SE Nigeria. Also, the risk associated with major pathways of students’ exposure to dust in the region has not been investigated. There is a possibility that health implications of exposure to metals in classroom dust may have been ignored or underestimated. There is no policy to regulate or control activities that may affect dust generation and dispersal in the schools. The World Health Organization (WHO) listed various health effects that may result from exposure to dust which included allergic responses, cancer, infection, inflammatory lung injuries, irritation, pneumoconioses and systemic poisoning [14].
Therefore, the aims of this study were: (1) to determine levels of toxic metals (Zn, Pb, Ni, Fe, Cu, and Cr) in some SE Nigeria classrooms’ dust; (2) to assess the pollution indices (geoaccumulation index (Igeo), contamination factors (CF) and pollution load index (PLI)) of the dust in the classrooms due to metals contamination, and (3) to assess the possible adverse health effects as a result of students exposure to the metals from ingestion, or dermal contact with the dust particles in their classrooms.
2 Materials and methods
2.1 Sample collection and sampling sites
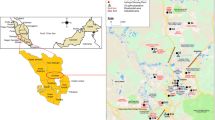
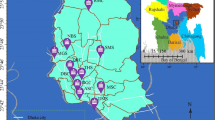
Dust particles were collected from 72 sampling points in classrooms at selected public high schools in Enugu and Ebonyi States in Southeast, Nigeria. The map of the study area is presented in Fig. 1. The schools were chosen based on the student population, willingness of the schools to allow access and consideration of policies from separate state authorities.
Schools A, B, C and D are in rural areas and surrounded by farmlands where different crops were planted. The volume of vehicular movement around the schools was low. There was no industry with associated activities around the schools. Schools A and B are under a separate state environmental management authority from the other schools. Schools E, F, G and H are in urban environments where the volume of traffic due to vehicles was high. Lots of commercial activities with some industrial output were observed.
The samples were collected between August, 2019, and February, 2020, at three points in each classroom and nine classrooms from each school. At every sampling point, different hand gloves were worn and hard plastic brushes were used to gently sweep dust from the bookshelves, cemented floors, and tables in the classrooms into plastic bags. Samples were collected from the visible areas in the classrooms with easy accessibility and where students can easily have contact with dust.
Dust particles collected from each classroom were mixed together as a composite sample which was then placed in sealed bags for each classroom.
2.2 Sample preparation and analysis
The dust samples in different labeled bags were put in a solar dryer at the National Center for Energy Research, University of Nigeria, Nsukka. After four days, the samples were screened with a standard test sieve (Rupson Industries, India) to remove pebbles and other objects larger than 100 μm which were collected with the dust particles. The samples (about 5 g from each classroom) were stored for laboratory analysis.
About 1.0 g of the consolidated dust particles from each classroom was digested separately in a beaker using aqua regia (3:1 HCl and HNO3). This was done by adding 30 mL of the aqua regia to each of the samples in the 250 mL beaker. They were covered and heated on a hot plate, in a fume cupboard for 2.5 h at 120 °C. The digested mixture was then left to cool before it was filtered into a 50 mL volumetric flask, where it was made up to the mark with deionized water. Samples from each sampling point were digested in triplicates. Blanks were prepared and digested using the same procedure applied to the sample solutions.
After digestion, the concentrations of Zn, Ni, Pb, Fe, Cu and Cr in the digested samples and blanks were determined using an atomic absorption spectrophotometer (Model AA-7000, Shimadzu Corporation, Japan) which was connected to an air-acetylene flame burner. The equipment was calibrated with standard solutions of the metals to be determined.
2.3 Quality control
The analytical procedures were subjected to quality control check in the following ways: (1) Recovery experiments, in which mixed standard solution was spiked into the samples. A measured volume of the mixed standard solution was added to a dust sample. The spiked sample was digested and the concentrations of the metals in the spiked sample solutions were determined using the AAS. The percent recovery was calculated as
where Css = concentration of the metal in the spiked sample, Cus = concentration of the metal in the unspiked sample, Cso = concentration of the metal in the spiked solution, (2) Triplicate digestions and analysis of the samples collected from each sampling point, (3) Use of metal concentrations in the blanks for corrections due to reagents, glassware and other materials, (4) The limit of detection (LOD) and the limit of quantitation (LOQ) for each element were determined. The determination was done to ascertain the working performance of the instrument, following a standard method [15]. The LOD and the LOQ were established from the calibration curves obtained when the standard solutions of each of the target metals were analysed in the instrument. The LOD was determined from the signal to noise ratio of 3 and the LOQ was based on the signal to noise ratio of 10.
The results for the recovery experiments ranged between 80 and 98% for chromium, 84–97% for copper, 97–99% for iron, 86–97% for nickel, 92–99% for zinc, and 87–98% for lead.
The LoD values (mg/kg) for the metals were 0.00001 (Cr), 0.00001 (Fe), 0.00001 (Cu), 0.00001 (Ni), 0.0001 (Pb) and 0.00001 (Zn). The LoQ values (mg/kg) were 0.0001 (Cu), 0.0001 (Cr), 0.0001 (Zn), 0.0001 (Ni), 0.0001 (Fe) and 0.001 (Pb).
2.4 Pollution indices of the potential toxic metals in dust
In assessing the levels of contamination due to metals in the classroom dusts, the following pollution indices were investigated: (a) the geo-accumulation index, Igeo (b) the contamination factor, also known as the single factor pollution index (PI) and (c) the pollution load index.
The geo-accumulation index values were assessed using the Muller’s expression [16] as given in Eq. 2:
The crustal abundance values of the metals: Cu = 45, Fe = 4.7, Ni = 68, Zn = 95, Cr = 90, and Pb = 20 were adopted as their background concentrations [17], while the background constant for lithogenic correction is 1.5.
The contamination factors, which interpret the values on the range of < 1 to > 6 were calculated using Eq. 3 by Forstner and Wittmann [18].
where Cs = concentration of the metal in the dust; Cb = concentration of the metal in the background sample [17]
The pollution load index was calculated from the contamination factor of each metal by using Eq. 4 [19, 20].
where n is the number of metals assessed; and CF is the contamination factor of each toxic metal in the dust particles.
2.5 Assessment of risk of exposure to some metals in classroom dust samples
According to Luo et al. health risk assessment is the characterization of possible adverse health effects when humans are exposed to contaminants [21].
The non-cancer risk assessment of exposure to the heavy metals in the classroom dust particles was estimated from the values of the Hazard Quotient (HQ).
The cancer effects of students’ exposure to some metals in their classroom dusts were assessed from the calculation of the carcinogenic risk index (CR).
Hazard Quotient is calculated from the ratio of the average daily dose (ADD) of the metal taken by a person to the reference dose (RfD). It gives an indication of the average daily exposure that is safe to human population [22].
This study used recommended RfD for the metals are presented in Table 1.
The major ways of students’ exposure to toxic metals in dusts in their classroom are through oral intake or ingestion; inhalation; and by dermal contact with the dust particles.
Equation (6) [5] was used to calculate the \({\mathrm{ADD}}_{\mathrm{ing}-\mathrm{nc}}\)(mg/kg/day) for non-cancer metals when dust particles in the classrooms are ingested:
Equation (7) [5] was used to calculate the daily dose of metal absorbed through dermal contact (\({\mathrm{ADD}}_{\mathrm{der}})\) (mg/kg/day) for non-carcinogenic (nc) metals:
Equation (8) [17] was used to calculate the daily dose of metal absorbed through ingestion (\({\mathrm{ADD}}_{\mathrm{ing}-\mathrm{c}})\) (mg/kg/day) for carcinogenic metals:
Equation (7) was also used to calculate the daily dose of metal absorbed through dermal contact (\({\mathrm{ADD}}_{\mathrm{der}-\mathrm{c}})\) (mg/kg/day) for carcinogenic metals.
Where C is the average heavy metal concentration (mg/kg); IgR is the dust ingestion rate (30 mg/day) [26]; ED is the exposure duration (6 years) and EF is the exposure frequency (240 days/year). BW is the average body weight of the students (20.3 kg) [27] and AT is the averaging time (1440 days in 6 years) and SA is the exposed skin area (2800 cm2) [28], SL is the skin adherence factor (0.2 mg/cm2/day for children) [28], and ABS is the dermal absorption factor (0.001) [29].
HQ was calculated through dermal contact and ingestion was calculated for each metal.
Carcinogenic risk is an indication of the probability of an individual developing cancer on exposure to potential carcinogen over a lifetime. Carcinogenic risk (CR) index was calculated for each of these metals using Eq. 9 [30].
where CR is carcinogenic risks and SF stands for Slope factor. The slope factors used for this study are presented in Table 1.
2.6 Statistical analysis
Correlation coefficients were calculated and used to determine the relationship between the metals based on their concentrations. Microsoft Excel version 2013 was used for the statistical calculations.
3 Results and discussion
3.1 Metal concentrations
The results of analysis for some toxic metals in samples of dust collected from the high schools classrooms (A-H) in SE, Nigeria are shown in Table 2. Also, included are in the Table 2 are some results from schools outside SE Nigeria. The mean levels of the metals in the study area in decreasing order were Fe > Zn > Cr > Cu > Ni > Pb. It was observed that the average amount of the metals at the schools in decreasing order was B > G > H > C > E > A > F > D. Schools G and H are in an urban environment where a lot of commercial activities that generate pollution take place. However, School B, which is located in a rural area, had the most elevated toxic metal content. It implies that the extent of pollution of an environment is not determined by only the commercial activities. The level of sanitation awareness and control, state of classroom infrastructure and local environmental policy may contribute to the pollution status. Schools E and F are under a state administration with a different environmental policy, while the others belong to one state administration. Many factors are involved in the complexity of environmental studies. Some factors may be specific to an area which would account for a result of analyte composition in the environmental compartments.
There are no official limits for metals allowable in dust in the Nigerian environment. But permissible limits exist for metals in soil. Therefore, this study used the allowable limits for metals in soils in line with previous published studies on dust in Nigerian environment [13, 17].
Among the heavy metals studied, Fe concentrations were the highest at all the classrooms with a range of 3194.76 to 6623.41 mg/kg. Fe can occur from natural origin and anthropogenic sources. Studies have shown that Fe is the predominant metal in the soil of Southeast ecological region of Nigeria [31]. Activities of welders and the wind effect in the dispersion of the ferralitic soil to several locations including the classrooms can contribute to Fe pollution in the environment. Iron is one of the essential metals for humans. Iron consumption in high dosage over a long term may cause hemosiderosis. This condition is characterized when hemosiderin is deposited in large quantity in the liver and other body tissues [32]. It has been discovered that chronic toxicity can lead to aggressive behaviour, fatigue, anorexia, arthritis, gut damage, headaches, heart disease, histamine, hyperactivity, liver damage, increased oxidative stress and blood levels of serotonin, metabolic acidosis, and loss of weight [33].
The average level of Zn in the study ranged from 2.64 to 375.27 mg/kg. This is less than the 1353.0 mg/kg observed in Toronto classrooms [34]. The sources of Zn vary from paints, roofing materials and soil to infiltration of traffic-contaminated outdoor dust [35]. Zn is classified as an essential metal. However, exposure to very high concentration of Zn or its deficiency may enhance susceptibility to carcinogens [36]. The level of Zn at Schools A and B were higher than the permissible limit for Zn in Nigeria which is 140 mg/kg [37].
The range of concentrations of Cr in the samples was 1.57–175.38 mg/kg. The sources may probably be due to activities involving steel works at the vicinity of some of the schools. Schools C and H had levels higher than 100 mg/kg [37] which was the permissible safe limit for Cr in Nigeria. The average level for the study was 74.47 mg/kg which is higher than in Toronto schools (52.3 mg/kg) and Lagos high density schools (10.53 mg/kg). It is higher than the mean concentration of chromium in the earth's crust which is 0.2 μg/g [38]. This suggests that the chromium was introduced from anthropogenic sources. The carcinogenicity is linked with inhalation of Cr(VI) compounds [39]. Chromium is also an essential element which is important for normal glucose metabolism but chromium(VI) is highly toxic [40] because of their oxidizing properties through the cell membranes [41]. Skin contact with high level of chromium has been known to produce contact dermatitis [42].
The mean concentration range of Cu was 0.93–463.28. Levels above the permissible limit of 20 mg/kg [37] were observed in Schools B and E. The average for this study is 40.01 mg/kg which is lower than the level at Toronto schools (246.0 mg/kg).
The International Agency for Research on Cancer (IARC) has reported that nickel compounds are carcinogenic to humans and metallic nickel is probably carcinogenic to humans [43]. Nickel was among the trace metals detected in the classroom dusts. It ranged from 4.96 to 143.98 mg/kg in the samples studied, with Schools A and G exceeding the permissible limit of 35 mg/kg [37]. The average for this study is 34.17 mg/kg. This is near to the levels at Southsouth, Nigeria schools at 35 mg/kg [13] but lower than the level at Toronto schools at 47.3 mg/kg. One of the sources of Ni in classrooms is from infiltration from outdoor contaminated particulate matter [44].
The average level of Pb in this study is 27.74 mg/kg, from a range 2.35–53.96 mg/kg. The sources of Pb include paint and vehicular emissions from the use of leaded petroleum products. Pb may have accumulated in the classroom dusts due to the paints used in some of the schools. Paint flakes were observed on the floors in some of the classrooms. Adebamowo et al. [45] reported that most emulsion and gloss paints produced in Nigeria had high levels of lead. At 85 mg/kg permissible limit [37], the observed level of Pb at all the studied schools was below the limit. This level may be attributed to the location of some of the schools which were with low vehicular movement that would have emitted lead from fuel. Some of the classrooms were not painted or the paint flakes may have peeled off over time. The levels at Toronto schools (49.0 mg/kg), Lagos high density schools (23.89 mg/kg) and Ghana schools (0.0482 mg/kg) were also low. Lead is a non-volatile toxic metal that affects various systems of the body, such as the cardiovascular, gastrointestinal, hematologic, neurologic and renal systems. Young people, especially children are mostly at higher risk on exposure to lead even at low levels as this can cause serious learning disabilities, seizures, or even death. [46].
The mean, median and standard deviations of concentrations of the metals as shown in Table 2 did not follow a pattern. The mean was used for the discussion as it was observed however that the median and the standard deviations were lower than the mean values in most of the study areas.
The degree of toxicity of toxic metals to humans depends on considerations like the type of metal, dosage of the metal absorbed, length of time and route of exposure, and age of the individual [47].
3.2 Contamination status
The levels of contamination based on the calculation of the Igeo and the CF for the studied schools are given in Tables 3 and 4.
3.2.1 Geoaccumulation index
Thegeo-accumulation index values for the studied sites are presented in Table 3. The interpretation of Igeo values by Muller [16] was applied in this study. The values of Igeo for Cu are negative at all schools except School C. The values for Ni are negative at all the schools except School E, while they are also negative for Pb and Zn except at Schools A and B which were unpolluted to moderately polluted (0 ≤ Igeo < 1). Based on the classification by Muller [16], these negative values imply that the environment is unpolluted with the metals. All the schools were very strongly polluted with Fe (Igeo ≥ 5, Very strongly polluted), strongly polluted to very strongly polluted with Cr, since they had positive Igeo values.
3.2.2 Contamination factor
The pollution intensity or contamination status was assessed using the proposal by Forstner and Wittmann [18]. This was based on the calculated CF values as presented in Table 4. At all the schools, the average concentrations indicated that Ni had a low contamination status (CF < 1) except at School E (moderate contamination), while Cu had a low contamination status except at School C. There was moderate contamination by Pb at all the schools except at Schools G and H (low contamination). All the schools are highly contaminated with Cr and Fe (CF > 6).Fe had the maximum value of CF at School G (1307.24), while the lowest CF value observed was by Cu at School H (− 4.08). The mean CF values for the investigated metals in this study were in the order: Fe > Cr > Pb > Zn > Cu > Ni.
3.2.3 Pollution load index
The pollution load index based on the proposal by Tomllinson et al. [20], show that the PLI was greater than unity in all the studied schools as presented in Table 4. The PLI values ranged from 9555.69 to 718,324.8. This indicates that the dusts were polluted by the heavy metals. It also implies that the quality of the classrooms was deteriorated. The deterioration of site quality, in descending order, was B > C > E > A > F > G > D > H. Thus, School B had the worst deterioration of site quality.
3.3 Assessment of potential health risk
The results of calculated Average Daily Dose (ADD), HQing, and HQder are presented in Tables 5 and 6 respectively. The calculated values of HQing for each of the selected metals except Pb at School B were less than one. This indicates that ingestion of dust at the classrooms except at School B would have no significant non-carcinogenic harmful effects on the health of the students [48, 49]. However, dermal contact with the dust at all the classrooms would expose students to adverse effects of Fe. There will be adverse effect due to Pb for dermal contact with dust at Schools A, B, D, F and H since the Hder is greater than one. Dermal contact with dust at Schools C, E and G would have no significant risk of non-carcinogenic harmful effects on the health of the students.
The calculation for carcinogenic risk assessment is presented in Table 7. Ingestion of dust particles at classrooms in all the schools would have no carcinogenic effect due to Cr and Pbas the cancer risk values were within the cancer tolerable range of 10–6 to 10–4 [50]. But ingestion of dust particles at classrooms in all the studied schools would pose a carcinogenic risk due to Ni. This is due to the values from the calculation for cancer risk, which were outside the cancer tolerable range of 10–6 to 10–4 [50].
From the above results, there is pollution of the dusts in the classrooms by heavy metals at different levels raising health concerns.
3.4 Correlation analysis
Table 8 shows the correlation coefficients between metal concentrations in the dust samples. Significant correlation may be used to indicate heavy metals that originated from the same source [51]. There were positive coefficients observed for Cr/Fe (0.218), Cr/Pb (0.129), Cu/Zn (0.139), Fe/Ni (0.4), Fe/Zn (0.169), Fe/Pb (0.254), Ni/Zn (0.188) and Zn/Pb (0.426). Negative correlations existed between the following metal pairs: Cr/Cu (− 0.515), Cr/Ni (− 0.256), Cr/Cu (− 0.218), Cu/Ni (− 0.157), Cu/Pb (− 0.296), and Ni/Pb (− 0.103). No significant correlation existed between the metals at 0.01 levels (two tailed). The indication is that the metals in the dusts may have originated from different pollution sources.
This study used elemental concentrations in the dust samples to obtain a first and precautionary estimate of the health risk associated with the dermal and oral intake of dusts. Using total elemental concentrations as exposure indicators can be regarded as a limitation of this study because in the digestive system, the degree of solubilization aries from metal to metal, and therefore an estimate of the bioavailable or bioaccessible fraction should provide a better metric in the investigation of its potential health impact than its total concentration. Moreover, there are other methods such as mechanistic models that should provide better estimates of dust and soil ingestion rates.
Adverse health effects from exposure to dusts make it imperative for policies that would reduce its entry into classrooms. These would include construction of the school buildings with appropriate doors and windows, fixing of dust filters on classroom doors and windows as well as regular cleaning and repainting of the classrooms. Some activities that may generate dusts may be restricted to reasonable distances from schools. Proper maintenance of the school buildings, including fixing of damaged doors, ceilings and windows are also recommended. Healthy environmental practices should be imbibed including wearing of protective cloths and cleaning of food before ingestion.
4 Conclusions
The mean levels of the metals in the study area in decreasing order were Fe > Zn > Cr > Cu > Ni > Pb. It was observed that the average amount of the metals at the schools in decreasing order was B > G > H > C > E > A > F > D. School B is in a rural area, while schools G and H are in an urban environment. The findings indicated that all the classrooms were contaminated at different levels with the selected metals. The degree of pollution of the classrooms ranged from low contamination due to Cu and strong contamination due to Fe. It was found that ingestion of dust in the classrooms posed no significant non-carcinogenic risk on the students’ health. There was carcinogenic risk for ingestion of the dusts due to Ni. Dermal contact posed significant non-carcinogenic health risk at some schools. Exposure to these toxic metals may cause adverse health effects depending on the total dose absorbed, level, route of exposure and age of the person. The sources of the metals varied. Measures to monitor and reduce exposure to dust in the classrooms are required. These may include constructing classrooms with appropriate dust traps, regular cleaning and maintenance of the school environment and regulation of activities in the vicinity of the schools to improve the quality of the environment.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
IUPAC (1990) Glossary of atmospheric chemistry terms. International Union of Pure and Applied Chemistry, Applied Chemistry Division, Commission on Atmospheric Chemistry. Pure Appl Chem 62(11):2167–2219
Harris MA (2016) Geobiotechnological solutions to anthropogenic disturbances: a Caribbean perspective. Environ Earth Sci Switz. https://doi.org/10.1007/978-3-319-30465-6_1
Pope CA III, Burnett RT, ThunMJ CEE, KrewskiD IK, Thruston GD (2002) Lung cancer, cardiopulmonary mortality and long-term exposure to fine particulate air pollution. J Am Med Assoc 287:1132–1141
Zhou Y, Levy JI, Hammitt JK, Evans JS (2003) Estimating population exposure to power plant emissions using CALPUFF: a case study in Beijing, China. Atmos Environ 37:815–826. https://doi.org/10.1016/S1352-231(02)00937-8
Xu X, Lu X, Han X, Zhao N (2015) Ecological and health risk assessment of metal in resuspended particles of urban street dust from an industrial city in China. Curr Sci 108(1):72–79
Tong ST, Lam KC (2000) Home sweet home? A case study of household dust contamination in Hong Kong. Sci Total Environ 256(2–3):115–123. https://doi.org/10.1016/s0048-9697(00)00471-X
Huang M, Wang W, Chan CY et al (2014) Contamination and risk assessment (based on bio accessibility via ingestion and inhalation) of metal (loid)s in outdoor and indoor particles from urban centers of Guangzhou, China. Sci Total Environ 480:117–124. https://doi.org/10.1016/j.scitotenv.2014.01.115
Taiwo AM, Awomeso JA, Taiwo OT, Oremodu BD et al (2017) Assessment of health risks associated with road dusts in major traffic hotspots in Abeokuta metropolis, Ogun state, southwestern Nigeria. Stoch Environ Res Risk Assess 31:431–447. https://doi.org/10.1007/s00477-016-1302-y
Zheng N, Liu J, Wang Q, Liang Z (2010) Health risk assessment of heavy metal exposure to street dust in the zinc smelting district, Northeast of China. Sci Total Environ 408(4):726–733
Al-Khashman OA (2007) Determination of metal accumulation in deposited street dusts in Amman, Jordan. Environ Geochem Health 29(1):1–10. https://doi.org/10.1007/s10653-006-9067-8
Nkansah MA, Fianko JR, Mensah S et al (2015) Determination of heavy metals in dust from selected nursery and kindergarten classrooms within the Kumasi metropolis of Ghana. Cogent Chem. https://doi.org/10.1080/23312009.2015.1119005
Popoola O, Bamgbose O, Okonkwo O et al (2012) Heavy metals content in classroom dust of some public primary schools in metropolitan Lagos, Nigeria. Res J Environ Earth Sci 4:460–465
Agbaire PO, Odali EW, Iniaghe PO (2019) Concentrations and risk assessment of selected heavy metals in indoor dust of primary schools in delta state. Niger Niger J Sci Res (NJSR) 18(3):187–194
WHO (1999) Hazard prevention and control in the work environment: airborne dust. World Health Organization. https://apps.who.int/iris/handle/10665/66147. Accessed 21 Mar 2020.
Iqbal J, Shah MH (2011) Distribution, correlation and risk assessment of selected metals in urban soils from Islamabad. Pak J Hazard Mater 192(2):887–898. https://doi.org/10.1016/j.jhazmat.2011.05.105
Muller G (1979) Schwermetalle in den sediment des Rheins, Veranderungem Seit. Umschau 79:778–783
Iwegbue CMA, Obi G, Emoyan OO, Odali EW et al (2018) Characterization of metals in indoor dusts from electronic workshops, cybercafés and offices in southern Nigeria: implications for on-site humanexposure. Ecotoxicol Environ Saf 159:342–353. https://doi.org/10.1016/j.ecoenv.2018.04.070
Forstner U, Wittmann GTW (1983) Metal pollution in aquatic environment. Springer, New York
Angulo E (1996) The Tomlinson pollution load index applied to heavy metals “mussel–watch” data: a useful index to assess coastal pollution. Sci Total Environ 187:19–56. https://doi.org/10.1016/0048-9697(96)05128-5
Tomllinson DC, Wilson JG, Harris CR, Jeffery DW (1980). Problems in the Assessment of Heavy metals levels in Estuaries and the formation of pollution index. HelgolanderWissenschaftlicheMeeresuntersUchunge 33: 566–569.
Luo XS, Ding J, Xu B, Wang YJ, Li HB (2012) Incorporating bio accessibility into human health risk assessments of heavy metals in urban park soils. Sci Total Environ 424:88–96. https://doi.org/10.1016/j.scitotenv.2012.02.053
Kavcar P, Sofuoglu S (2009) A health risk assessment for exposure to tracemetals via drinking water ingestion pathway. Int J Hyg Environ Health 212(2):216–227. https://doi.org/10.1016/j.ijheh.2008.05.002
Harmanescu M, Alda LM, Bordean DM et al (2011) Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem Cent J 5:64. https://doi.org/10.1186/1752-153X-5-64
Kamunda C, Mathuthu M, Madhuku M (2016) Health risk assessment of heavy metals in soils from Witwatersrand gold mining basin, South Africa. Int J Environ Res Public Health 13(7):663. https://doi.org/10.3390/ijerph13070663
Ogbo AB, Patrick-Iwuanyanwu K (2019) Heavy metals contamination and potential human health risk via consumption of vegetables from selected communities in ONELGA, Rivers State, Nigeria. Eur J Nutr Food Saf 9(2):134–151
USEPA (2017) Soil and dust ingestion, update for chapter 5 of the exposure factors handbook, EPA/600/R-17/384F. National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency, Washington, p 20460
De Onis M, Onyango A, Borghi E (2009) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85:660–667. https://doi.org/10.2471/blt.07.043497
Durowoju OS, Edokpayi JN, Popoola OE, Odujo JO (2018) Health risk assessment of heavy metals on primary school learners from dust and soil within school premises in Lagos State, Nigeria. In: Saleh HE, Aglan R (eds) Heavy metals. IntechOpen, London, pp 319–336. https://doi.org/10.5772/intechopen.74741
Ferreira-Baptista L, De Miguel E (2005) Geochemistry and risk assessment of streetdust in Luanda, Angola: a tropical urban environment. Atmos Environ 39:4501–4512. https://doi.org/10.1016/j.atmosenv.2005.03.026
US EPA (1989) Risk assessment guidance for Superfund. Vol. I: human health evaluation manual. EPA/540/1- 89/002. Office of Solid Waste and Emergency Response, Washington DC.
Okoye COB, Agbo KE (2011) Dispersion pattern of trace metals in soils surrounding solid waste dumps in Nsukka. J Chem Soc Nigeria 36(2):112–119
Hoffman Center Staff. Iron: deficiency and toxicity. http://www.drhoffman.com/page.cfm/120. Accessed 24 Jan 2020
Vital health zone (2020) All about minerals: Iron. http://www.vitalhealthzone.com. Accessed 3 Mar 2020.
AlHejami A (1995) Heavy metals in indoor settled dusts in Toronto, Canada. Thesis, Al-Nahrain University, Baghdad, Iraq
Iwegbue CMA (2014) Impact of land use types on the concentrations of metals in soils of urban environment in Nigeria. Environ Earth Sci 72:4567–4585. https://doi.org/10.1007/s12665-014-3355-x
Bellies RP (1994) The metals. In: Clayton G, Clayton FE (eds) Patty’s industrial hygiene and toxicology, vol 2, part C, 4th edn. Wiley, New York
Department of Petroleum Resources (DPR) (2002) Environmental guidelines and standards for the petroleum industry in Nigeria, revised. Ministry of Petroleum and Mineral Resources, Abuja
Katz SA, Salem H (1993) The toxicology of chromium with respect to its chemical speciation: a review. J Appl Toxicol 13(3):217–224. https://doi.org/10.1002/jat.2550130314
Wilbur S, AbadinH, Fay M et al. Toxicological Profile for Chromium. Agency for Toxic Substances and Disease Registry (US). Health effects. http://www.ncbi.nlm.nih.gov/books/NBK158851/. Accessed 3 Mar 2020
Costa M, Klein CB (1997) Toxicity and carcinogenity of Cr(VI) in animal models and humans. Crit Rev Toxicol 27(5):431–442. https://doi.org/10.1080/10408440500534032
Codex Alimentarius Commission (1995) Joint FAO/WHO Food Standards Programme. Codex general standard for contaminants and toxins in foods. Doc. no. CX/FAC 96/17. Codex Alimentarius Commission, Geneva.
Bruynzeel DP, Hennipman G (1988) Irritant contact dermatitis and chrome-passivatedmetal. Contact Derm 19(3):175–179. https://doi.org/10.1111/j.1600-0536-1988.tbo2889.X
IARC (1990) Chromium, nickel, and welding. IARC monographs on the evaluation of carcinogenic risks to humans, vol 49. International Agency for Research on Cancer, Lyon, France
Hassan SKM (2012) Metal concentrations and distribution in the household, stairs and entry way dust of some Egyptian homes. Atmos Environ 54:207–215. https://doi.org/10.1016/j.atmosenv.2012.02.013
Adebamowo EO, Agbede OA, Sridhar MKC, Adebamowo CA (2006) An evaluation of lead levels in residential paints sold in Nigeria markets. Indoor Built Environ 15:551–554. https://doi.org/10.1177/1420326x06072992
WHO (2010) Preventing diseases through healthy environment. Action is needed on chemicals of major public health concern, public health and environment. World Health Organization, Geneva
Ihedioha JN, Okoye COB, Onyechi UA (2014) Health risk assessment of zinc, chromium and nickel from cow meat consumption in an urban Nigerian population. Int J Occup Environ Health 20(4):281–288. https://doi.org/10.1179/2049396714Y.0000000075
Iqbal J, Shah MH (2013) Health risk assessment of metals in surface water from freshwater source lakes. Pak Hum Ecol Risk Assess 19(6):1530–1543. https://doi.org/10.1080/10807039.2012.71681
Huang M, Zhou S (2008) Heavy metals in vegetables: assessment of potential health risk for inhabitants in Khunshan China. Sci Total Environ 405:54–61
USEPA (1997) Exposure factors handbook, EPA/600/p-95/002 Fa, b, c. Office of research and development. Environmental Protection Agency, Washington
Ahamad MI, Song J, Sun H et al (2020) Contamination level, ecological risk, and source identification of heavy metals in the hyporheic zone of the Weihe River, China. Int J Environ Res Public Health 17(3):1070; doi: https://doi.org/10.3390/ijerph 17031070
Acknowledgements
The assistance of Mrs. Ngozi Alumona and Mr. Wilson Kenechukwu in sample collection is acknowledged. Dr. Cynthia Ibeto is hereby acknowledged for proofreading this manuscript
Funding
The research was not funded by any institution. It was funded by the researchers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ugwu, K.E., Ofomatah, A.C. Concentration and risk assessment of toxic metals in indoor dust in selected schools in Southeast, Nigeria. SN Appl. Sci. 3, 43 (2021). https://doi.org/10.1007/s42452-020-04099-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-04099-7