Abstract
Hydrometallurgical and pyrometallurgical processing of ores and concentrates generates large amount of various solid residues. These may contain significant amount of heavy metals, particularly lead is of great importance. It is present in form of anglesite, cerrusite or lead oxides. Hydrometallurgical processing of leaching residues, dusts, and fly ashes is used to recover valuable metals from these streams. Acid leaching in sulfuric acid solutions or spent electrolyte results in producing high amount of lead sulfate that is still very attractive material to fulfill demand of lead acid battery market. Aliphatic linear polyamines are solvent extracting agents very selective towards lead sulfate. Here we report the comparative tests of four different linear aliphatic homologs, namely ethylenediamine, diethylenetriamine, triethylenetetramine, and tetraethylenepentamine as potential extracting agents of lead sulfate. The core research was devoted to the possibility of direct electrodeposition of lead from various polyamines solutions. Electrochemical tests performed at current density 200 A/m2, 60 °C, with an addition of selected inhibitor showed the possibility to produce metal layer with 97.2% purity for triethylenetetramine.
Graphical abstract

Similar content being viewed by others
1 Introduction
Although eliminated by international restrictions and regulations from different industrial branches, lead is still use in lead-acid batteries. In terms of occurance it’s relatively rare metal, uniformly distributed throughout the world. The most important lead ores are galena (PbS), anglesite (PbSO4) and cerussite (PbCO3). Low melting point, ease of casting, high density, softness, malleability, acid resistance and excellent corrosion resistance to air, water, and soil are the main properties [1]. Lead can be produced either from primary or secondary sources. Primary lead processing involves ores mining with subsequent crushing, grinding, and beneficiation, then smelting, drossing, and finally refining. Secondary sources deal with scraps such as battery plates and paste, drosses, skimmings, and industrial wastes solders, babbitts, cable sheathing. In spite of large increase in lithium ion batteries applications and production, the lead acid batteries will remain the principal automotive energy storage devices in combustion engines for the next two decades. This is consistent with the increase in sale value of lead acid batteries in the USA and Europe [1]. The main uses of lead include lead acid batteries, cables heating, lead-antimony anodes and ammunition. The global trend recently observed is to increase lead recovery from various secondary feedstocks and wastes. Environmental legislation concerning lead has strenghtened recycling of tailings and wastes to recover lead. The potential industrial source of lead are leaching residues from zinc and copper industries as well as flue dusts. These materials may contain from a few to tenths percents of lead, in form sulfates, oxides or hydroxysulfates [2]. High selectivity and lower gas emissions are the main features of hydrometallurgical and/or electrochemical processing of industrial wastes [3]. One example of hydrometallurgical processing of lead is the FAST process, developed for desulphurized paste. Lead sulfate is leached with ammonium chloride electrolyte. After removal of excessive sulfate ions and cementation of metal impurities, the electrolyte is fed to electrowinning [4]. The produced lead is in form of flakes rather than well-adhered strips. Lead can be also found in fume dust generated in many industrial processes. A clean and competitive to pyrometallurgy, method of lead recovery is hydrometallurgical treatment in alkaline solution [5]. Elevated temperature and high concentration of sodium hydroxide are able to convert lead oxide (PbO), lead sulfate (PbSO4) and any lead silicate (PbSiO3), into NaPb(OH)3 which can be then leached into aqueous phase. It allows to leach out of the dust over 90% lead, however, the drawback is high liquid to solid ratio, namely 25:1 required. Lead can be efficiently extracted from anglesite-containing residues using brine leaching [6], with a sodium chloride concentration from 100 to 150 g/L, and pulp density (solid/liquid) ratio) up to 100 g/L. Then, addition of sodium sulfide results in precipitation of lead sulfide from the solution. Acidic calcium chloride solution is a good candidate for lead leaching from materials composed of sparingly soluble lead jarosite [7]. Low pH in the range 0.5–2, set by hydrochloric acid is responsible for lead jarosite decomposition into lead sulfate, while calcium and chloride ions are required for extraction of lead into chloride anions (PbCl42−) and produce calcium sulfate precipitate. Another method uses triethylenetetramine solution as selective extracting agent of lead sulfate. This method has been tested in a pilot scale for battery paste desulphurisation as well as in laboratory scale for copper leaching residues [8, 9]. The activity of polyamines towards metals extraction was many times proven in the past, however, most of reports deals with amines embedded on a solid carrier. Silica gel modified with diethylenetriamine showed good adsorptive capability towards mercury, copper, nickel and lead [10]. The observation that the higher nitrogen content of amines did not satisfy bigger metal extraction was important. Comparison made for silica functionalized with ethylenediamine, diethylenetriamine and tetraethylenepentamine showed that nitrogen content is 9.8, 12 and 23%, respectively [11], however, metal uptake (including copper, zinc and cadmium) was significantly higher for the shortest chain. Triethylenetetramine (TETA) intercalated into montmorillonite showed high leaching ability towards manganese, copper, cadmium, zinc and lead [12]. A popular lixivant for lead recovery from solid residue is methanesulfonic acid, which can be used for zinc leach residues valorisation [13]. However, lead recovery from anglesite-contaning residues is relatively poor. An increase in leachability requires conversion of anglesite into cerussite by a carbonation reaction. Consequently, methanesulfonic acid present high selectivity towards lead carbonate, with a leaching efficiency of lead around 80%. Ethylenediamine and diethylenetriamine have proven to be promising leaching and electrowinning candidates for nickel and copper production from hydroxide sludges [14]. It was showed that lower current density was required to electrowin metals from EDA (40A/m2) than DETA (200A/m2).
2 Experimental
2.1 Procedure
Electrodeposition trials were performed using 5% w/w amines solutions saturated by lead. Namely, a proper amount of ethylenediamine (EDA, 99%, alfa aesar), diethylenetriamine (DETA, 99%, alfa aesar), triethylenetetramine (TETA, 97%, acros organics) and tetraethylenepentamine (TEPA, p.a., acros organics) were mixed with deionized water to obtained 5% w/w concentration of each polyamine. Then lead sulfate (p.a., chempur) was added to each solution in such amount to obtain supersaturation. Polyamines are selective towards lead sulfate and are able to extract it into aqueous phase. Solutions were thouroughly mixed, excees of salt was separated by vacuum filtration and lead concentration was quantitatively analyzed. As prepared solutions were used in electrodeposition tests. The electrochemical cell was configured as follows: processes were carried out at constant charge, using acid-resistant steel electrodes (2 anodes, 1 cathode), with an electrode distance 2.5 cm, active surface area of cathode (immersed in electrolyte) was 16 cm2, and current density 200 A/m2. Power was supplied by SPD3303X programmable DC power supplies (Siglent, Helmond, The Netherlands).
2.2 Characterization
Lead concentration in polyamines solutions were analyzed by chemical precipitation. Lead, and any contaminations in electrodeposited metal were analyzed using flame atomic absorption spectroscopy (FAAS) Panalytical Axios Max with iCE3300 from Thermo Fischer Scientific (Schwerte, Germany). Additionally, visual observation was performed to define any cracks, discoloration, compactness, dendritic lead, and smoothness of metal layer. X-ray powder diffraction was performed using Rigaku MiniFlex 600 with Cu Kα of wave length 1.5406 Å, to qualitatively evaluate the structure of electrodeposited metal. High resolution images of the deposited metal surface were registered by scanning electron microscope, Zeiss Leo Gemini 1525 equipped with Bruker Quantax XFlash® 6 Bruker Nano SDD microanalyzer. Quantitative analysis of lead was performed dissolving electrodeposited solid samples in nitric acid solution. Then Pb was determined by volumetric method after precipitation of PbSO4, dissolution of the precipitate in ammonium acetate and titration.
3 Results
Linear aliphatic polyamines are generally considered as hazardous materials. This includes flammability, harmfulness to skin, respiratory system, and their toxicity. On the other hand polyamines show high affinity to heavy metals salts and are able to extract specific metal species from solid materials. This makes them perspective extracting/leaching agents for metals recovered from various industrial residues. It was showed that polyamines solution after metal extraction and product separation can be regenerated to achieve their initial activity [9]. This feature is very important as makes polyamines very perspective and competitive with respect to other hydromateallurgical techniques of solid wastes recycling. This study reports the possibility to recover lead from various linear polyamines solutions using the electrochemical method instead of chemical precipitation. The initial concentration of lead in EDA, DETA, TETA and TEPA was 9.5, 40.8, 41.9, and 38.7 g/L, respectively. These results showed bigger number of amine groups which can complex metals, are able to extract more lead. However, this increase in lead concentration is not proportional – similar to results presented by [11] to %N. All electrodeposition tests were carried out at uniform conditions, with a current density 200 A/m2. The optimum current density was chosed based previous experience with lead electrowinning from triethylenetetramine solutions [15]. It was found that at higher current density dendritic lead was formed causing shortcut. Detailed data were presented in Table 1.
Lead electrodeposition tests were performed at typical and low current density 200A/m2. Any increase to value as high as 400 A/m2 and more resulted in dendrites formation and short circuit. Temperature of electrolytes was increased, as polyamines are viscous liquids, which at ambient temperature causes nonhomogenous electrolyte concentration, even at extensive mixing. Additionally polyvinyl pyrolidone (PVP) was added to all baths in concentration 1 g/L to improve the quality of cathodic lead and inhibit dendrites formation. During electrochemical deposition EDA and TEPA solutions presented different behaviour. In EDA solution there appeared white deposit at the bottom of cell as well as voltage changes were observed. TEPA solution changed its colour during progress of electrolysis from pale blue, first to greenish, then to yellow. Visual observation of cathodic deposits were presented in a Fig. 1.
The lowest quality of lead was reported for EDA solution with a very rough, nonhomogenous surface of deposited metal. Satisfactory compact layer were found for DETA, TETA, and TEPA deposits, however, in case of DETA and TEPA dendritic lead was formed. For DETA deposit these dendrites were uniformly generated at the electrode edges and were relatively short. While for TEPA a long braids with a risk of short circuit were found. Consequently, a quick look at electrode surface, priviledged TETA as the most perspective polyamine in terms of electrochemical lead deposition. Produced deposits were then stripped (torn with a knife) from the electrodes. Quantitative analysis revealed lead content 92.7, 91.1, 97.2, and 81% for EDA, DETA, TETA, and TEPA, respectively, while oxygen content was as high as 7.3, 8.8, 2.7, and 18.5%, respectively. There was about 0.01% Ni in all analyzed deposits as well. Additionally, lead electrodeposited from TEPA solution showed 0.05% S, which may be attributted to sulfate anions trapped between metal atoms.
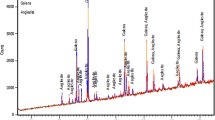
The XRD patterns showed similarity in DETA, TETA and TEPA lead deposits with intensive signals attributted to lead around 31.6°, 36.3°, 52.2°, 62.1°, 65.4°, 85.5° and 88.2°. These peaks were also found in EDA pattern but the intensity was significantly lower. Additionally, low intensity signals belonging to lead oxide (massicot, PbO) were found in EDA and TETA lead deposits, as well as plumbonacrite (Pb5(CO3)3O(OH)2) were detected in deposits from EDA, DETA and TEPA solutions. Structure of EDA deposit was the most complex with trilead dihydroxide (Pb3(OH)2O2) and hydrocerrusite (Pb3(CO3)2(OH)2), as well (Fig. 2).
Morphology of deposits was examined using scanning electron microscopy at different magnifications. It showed that structure of lead deposited from DETA and TETA solutions produced compact layers with tightly-packed beads of a regular size around 50 mm. It was found that the surface of TETA-produced lead was more porous with well developed surface area, i.e. process of metal layer formation is less uniform. Layer formation is triggered by flake like porous domains rather than well-organized packed crystals (Fig. 3).
The opposite can be observed for metal produced from EDA and TEPA solution. EDA solution produces highly disordered metal deposit with large amount of voids. Lead is deposited predominantly in form of long chains, resemble to fabric woven. Intringuing are images took for deposit obtained from TEPA solution. As „eye view” revelaed relatively high compactness with some dendrites at the edges, while SEM images showed that lead structure is like a matrix of EDA and TETA layers. It is composed of uniform beads with excresences on their surface, and the space between beads is filled by a woven-like structure. Unfortunately this complicated layer morphology, which partially contains lead oxide, is responsible for high amount of embedded contaminations (merely 82% Pb).
4 Conclusions
This paper showed that linear aliphatic polyamines are very attractive solvent extracting agent for lead. Metal can be directly electrowon from the extracting solution. This also showed that the electrochemical treatment of lead containing leaching solutions may be considered competitive for precipitation of lead salt. Four linear amines including ethylenediamine, diethylenetriamine, triethylenetetramine, and tetraethylenepentamine were examined. They are able to complex varied amount of lead metal, and consequently extract metal cation as well as accompanying anion into liquid phase. Based on this capacity amines can be arranged in order TETA, DETA, TEPA, and EDA, starting from the highest lead extraction capacity. Relation between lead extraction and number of nitrogen atoms in particular amine is not so direct, as saturation of DETA and TETA with lead were higher than for TEPA, while EDA showed significantly lower lead content. Structural and chemical analysis as well as visual observation of electrodeposited lead suggest that TETA is the most promising agent for electrochemical deposition of lead.
References
King M, Ramachandran V, Prengaman RD, DeVito SC, Breen J, Updated by Staff (2014) Kirk‐Othmer encyclopedia of chemical technology. Wiley, New Jersey
Cui W, Chen M, Zhao B (2020) Pyrometallurgical recovery of valuable metals from flue dusts of copper smelter through lead alloy. In: Siegmund A, Alam S, Grogan J, Kerney U, Shibata E (eds) PbZn 2020: 9th International symposium on lead and zinc processing the minerals metals & materials Series. Springer, Berlin
Kim K, Candeago R, Rim G, Raymond D, Alissa Park A-H, Su X (2021) Electrochemical approaches for selective recovery of critical elements in hydrometallurgical processes of complex feedstocks. iScience 24(5):102374
Maccagni M, Guerrini E (2020) The FAST Pb Process and Its Impact on Secondary Lead Production. In: Siegmund A, Alam S, Grogan J, Kerney U, Shibata E (eds) PbZn 2020: 9th International symposium on lead and zinc processing the minerals, metals & materials series. Springer, Berlin
Youcai Z, Chenglong Z (2017) Leaching of zinc and lead hazardous wastes in alkaline solutions. In: Pollution control and resource reuse for alkaline hydrometallurgy of amphoteric metal hazardous wastes. Handbook of environmental engineering, vol 18. Springer, Cham
Ye M, Li G, Yan P, Zheng L, Sun S, Huang S, Li H, Chen Y, Yang L, Huang J (2017) Production of lead concentrate from bioleached residue tailings by brine leaching followed by sulfide precipitation. Sep Purif Technol 183(7):366–372
Wang L, Mu W, Shen H et al (2015) Leaching of lead from zinc leach residue in acidic calcium chloride aqueous solution. Int J Miner Metall Mater 22:460–466
Chmielarz A, Szołomicki Z, Mrozowski J, Prajsnar R, Wolarek J, Skawiński L, Celarek A, Henzel M (2010) Piloting amine battery paste desulphurization process, Lead‐Zinc 2010, edited by A. Siegmund, L. Centomo, C. Greenen, N. Piret, G. Richards and R. Stephens, TMS (The Mineral, Metals and Materials Society), pp. 747–756, 2010, ISBN978‐0‐47094‐315‐1, Wiley, New Jersey
Ciszewski M, Chmielarz A, Szołomicki Z, Drzazga M, Leszczyńska-Sejd K (2021) Lead recovery from solid residues of copper industry using triethylenetetramine solution. Minerals 11(5):546
Zhang Y, Qu R, Sun C, Chen H, Wang C, Ji C, Yin P, Sun Y, Zhang H, Niu Y (2009) Comparison of synthesis of chelating resin silica-gel-supported diethylenetriamine and its removal properties for transition metal ions. J Hazard Mater 163:127–135
Alothmana ZA, Apblett AW (2010) Metal ion adsorption using polyamine-functionalized mesoporous materials prepared from bromopropyl-functionalized mesoporous silica. J Hazard Mater 182:581–590
Huang Z, Jiang L, Wu P, Dang Z, Zhu N, Liu Z, Luo H (2020) Leaching characteristics of heavy metals in tailings and their simultaneous immobilization with triethylenetetramine functioned montmorillonite (TETA-Mt) against simulated acid rain. Environ Pollut 266:115236
Rodriguez Rodriguez N, Onghena B, Binnemans K (2019) Recovery of lead and silver from zinc leaching residue using methanesulfonic acid. ACS Sustain Chem Eng 7(24):19807–19815
Gélinas S, Finch JA, Rao SR (2002) Electrowinning of nickel and copper from ethylenediamine complexes. Can Metall Q 41(3):319–325
Ciszewski M, Orda S, Drzazga M, Kowalik P, Hawełek Ł, Malec W, Leszczynska-Sejda K (2021) Lead electrodeposition from triethylenetetramine solution containing inhibitors. Metals 11(8):1330
Funding
No funding was received for these research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ciszewski, M., Drzazga, M., Kowalik, P. et al. Lead electrodeposition from aliphatic polyamines solutions. SN Appl. Sci. 4, 130 (2022). https://doi.org/10.1007/s42452-022-05020-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05020-0