Abstract
Despite their exceptional mechanical and corrosion properties, duplex stainless steels (DSS) have not found widespread use in high-temperature applications due to concerns over thermal aging and embrittlement at elevated operational temperatures (> 300 °C). The present study investigated the effect of thermal aging time on the electrochemical properties of lean and standard grade DSS that are exposed to a range of pressurized water reactors containing LiOH and H3BO3. The results indicated that the electrolyte chemistry plays a significant role in the corrosion behavior of the DSS alloys. Corrosion resistance decreased with thermal aging time for all DSS alloys; however, standard grade DSS (2205 and 2209-w) alloys showed better corrosion resistance than lean grades (2003, 2101, 2101-w). The presence of dissolved oxygen in the electrolytes resulted in a significant increase in corrosion rate for the DSS alloys, but it did not affect the general trend of corrosion rates with aging time. All DSS alloys became vulnerable to pitting corrosion due to chloride addition, but the pitting resistance decreased with increasing thermal aging time. Increased boron B content resulted in degradation of corrosion resistance of the DSS alloys, while minor changes in pH did not show a significant change in corrosion resistance. Mechanical and metallurgical characterization coupled with electrochemical characterization of the DSS alloys gave a comprehensive insight into the effects of thermal aging on the electrochemical response of the DSS.
Graphical abstract

Article highlights
-
Effect of thermal aging on corrosion behavior of five DSS in PWR water chemistries are studied.
-
Standard grade DSS show higher corrosion resistance than lean grade DSS after thermal aging.
-
Corrosion current density increases with increasing thermal aging time.
-
Increasing boron content increases the corrosion rates of DSS alloys
-
Chlorides makes all alloys vulnerable to pitting at all aging times.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Because of their mechanical properties and corrosion resistance, stainless steels are extensively used as structural alloys in high-temperature applications such as power plants, boilers, steam turbines, heat treatment, chemical processing, and pulp and paper industry. Austenitic stainless steel welds and cast austenitic stainless steel (CASS), which contain ~ 5–30% ferrite, are ubiquitous in these systems. While duplex stainless steels (DSS) are not extensively used in high-temperature applications, their roughly equal phase fractions of austenite (FCC) and ferrite (BCC) phases, and high Cr and Mo contents, make them a good alternative to austenitic stainless steels [1]. The duplex alloys have high strength, good ductility, good weldability, excellent stress corrosion cracking resistance, and outstanding resistance to pitting corrosion [2, 3]. Furthermore, the lower Ni content in DSS makes them less susceptible to price volatility when compared to their austenitic counterparts.
Despite their exceptional mechanical and corrosion properties, DSS has not found widespread use in high-temperature applications due to concerns over thermal aging and embrittlement at elevated operational temperatures (> 300 °C). When exposed to high temperatures for extended periods, DSS might undergo thermal embrittlement due to localized hardening in the chromium-rich ferrite phase. The localized hardening is the result of the formation of Cr-rich (αʹ) regions and Fe-rich (α) regions in the ferrite grains [4,5,6], which is caused by the miscibility gap in the Fe–Cr system. Both α and α′ phases have the same BCC crystal structure, but the lattice mismatch between two phases leads to hardening, embrittlement, and the loss of bulk toughness [7,8,9]. The α–α′ phase separation within the ferrite grains occurs through either spinodal decomposition or nucleation and growth, and these two mechanisms depend on the alloy chemistry and the aging time [5, 10]. However, spinodal decomposition has been reported to be a more thermodynamically favored mechanism than nucleation-growth for α–α′ phase separation [7, 8]. Although α–α′ phase separation is limited to the ferrite grains, the overall dual phase material experiences embrittlement. A reduction of corrosion resistance can also be severe, enhancing the likelihood of leaks or failure in these components. Small changes in alloy composition can alter the rate of phase separation and property degradation.
The metallurgical and mechanical characteristics of thermally aged DSS have been studied extensively [7, 8, 11,12,13,14,15]. The mechanical degradation resulting from elemental segregation is highly dependent on the alloy chemistry, and subtle differences in alloy composition can have a significant effect on the thermal aging behavior, as noted by Tucker et al. [16]. The lean grade DSS, containing lower concentrations of Cr equivalent (Cr, Mo, Nb) and Ni equivalent (Ni, Cu, N, C) elements, show more resistance to thermal embrittlement as compared to the standard grade DSS, which makes them an attractive candidate for high-temperature applications. However, for unaged DSS, the lean grades show higher corrosion susceptibility in the near-neutral environment than standard grade DSS [1, 3]. Zhang et al. [17] reported a decreased pitting potential and lower critical pitting temperature for thermally aged DSS 2101 (700 °C, 3 min to 4 h) when exposed to 1 M NaCl solution. Additionally, thermally aged DSS 2205 (400 °C, 3000–7000 h) showed increasing corrosion current density with thermal aging time when exposed to 0.1 M H2SO4 aqueous solution [18]. Although these studies have shown that thermal aging adversely affects the corrosion performance of DSS, the research is still limited with respect to longer thermal aging periods (e.g., 10,000 h) for a variety of DSS alloys in other exposure water chemistries such as those used in pressurized water reactors (PWR). Globally, various agencies set guidelines for regulating the PWR water chemistry. Balanced coolant water chemistry is indispensable for the optimum performance of nuclear power plants. The role and effect of constituent ions, mainly lithium (Li) and boron (B), in PWR water has been previously studied in detail by numerous researchers [19,20,21,22,23]. The PWR water chemistry greatly deviates from the optimum chemistry during the plant startup and shutdown, resulting in the release of corrosion products. The B in PWR water is introduced by the use of boric acid as a chemical shim to control nuclear reactivity; however, the addition of boric acid lowers the pH of the PWR water. Low pH water poses serious corrosion concerns for the steel at high operating temperatures. Thus, to regulate the pH of the PWR water, lithium hydroxide is added to avoid a continually changing pH through the fuel cycle. Although Rovere et al. [18] and May et al. [11] previously studied the corrosion behavior of thermally aged DSS 2205 (up to 7000 h at 300 °C and 400 °C) in 0.1 M H2SO4, and Silva et al. [12] studied thermally aged DSS 2404 (up to 2000 h in 475 °C) in 0.5 M H2SO4 + 0.01 MKSCN solution, there is a knowledge gap in research on corrosion behavior of thermally aged DSS in the context of PWR water chemistries.
The main objective of this work is to investigate the effects of thermal aging time on the electrochemical and corrosion characteristics of five different DSS alloys (2101, 2101-w, 2003, 2205, and 2209-w) in various simulated PWR water chemistries. In addition to the characterization of wrought alloys, the investigation of corrosion performance of weld metals (2101-w and 2209-w) used to join them is equally important, and they are characterized in this work. The exposure environments used for the electrochemical tests consisted of simulated water chemistries with Li contents of 2.2 ppm and B concentrations ranging from 400 to 2000 ppm in aerated and deaerated conditions. Both the aerated and deaerated conditions were studied to assess the impact on corrosion performance.
Furthermore, for selected experiments, chlorides were added to the studied water chemistries to investigate the effect of contamination, impurities, and thermal aging time on pitting corrosion behavior of the DSS. Although chlorides may not be found under normal operating conditions, they are studied here because the thermal aging is believed to significantly decrease the pitting potential of the DSS alloys due to higher susceptibility resulting from α and αʹ phase formation. Chromium segregation on the nano-scale might increase the pit nucleation sites at the alloy surface, and it would lead to the vulnerability of DSS to corrosion. This work builds on previous work to assess the impact of these microstructural changes on the corrosion performance of these alloys to assess whether or not aging makes these alloys unsuitable for use in high-temperature applications. Dual-phase stainless steel alloys, such as CASS, are in-service for 40 + years in existing power plants, undergoing aging and corrosion, making this study relevant.
2 Materials and methods
2.1 Materials and sample preparation
Five DSS alloys were considered for the present study: three hot-rolled wrought alloys (2003, 2101, and 2205), and two weld alloys (2101-w and 2209-w). The weld alloy specimens were machined from a large weld pad measuring 13 × 11 × 16 cm3 (wxhxl) manufactured via multi-pass gas tungsten arc welding using 2101 and 2209 filler metal on 2003 and 2205 plates, respectively. DSS 2003, 2101, and 2101-w are considered lean grade alloys, whereas 2205 and 2209-w are standard grades. The nominal chemical compositions of all the DSS alloys used for this study are listed in Table 1. Equations 1 and 2 are used to calculate Cr and Ni equivalent (Creq, Nieq) compositions [2]:
Apart from the unaged DSS alloys, the test matrix included aged samples with three different aging times: 100 h, 1000 h, and 10,000 h. The aging cycle consisted of isothermal annealing at 427 °C (800 °F), followed by air cooling. The DSS alloys used for the electrochemical tests were wire cut in square specimens of dimensions 10 × 10 × 3 mm3. For microscopy and electrochemical testing, all the samples were polished using silicon carbide papers with roughness ranging from 180 to 2000 grit size followed by cloth polishing using 0.5 µm and 0.03 µm sized alumina–water suspension. The cloth polishing stage was followed by ultrasonic cleaning of the specimens in an isopropanol bath. For metallographic sample preparation, the ASTM E3-11 (2017) standard guidelines [24] were followed.
2.2 Metallurgical characterization
2.2.1 Optical microscopy (OM)
A Leica DM2700 was used for optical microscopy (OM) to compare the microstructure of the different alloys. To reveal the microstructures, the DSS samples were electrochemically etched in a 10% oxalic acid solution at 5 V for one minute to achieve an optimal contrast between the austenite and ferrite phases.
2.2.2 Scanning electron microscopy (SEM)
Phase fraction analysis was conducted using the SEM images and ImageJ image analysis software. The high contrast between the austenite and ferrite grains in the SEM images enabled segmentation and subsequent measurement of the phase fraction and grain size.
2.2.3 Atom probe tomography (APT)
APT was performed on all five alloys after aging at 427 °C for 1, 100, 1000, and 10,000 h. Specimens were fabricated from the ferrite phase of each alloy by a standard focused-ion-beam-based in situ lift-out and annular milling method [25]. APT of the resulting needle-shaped specimens was performed with a CAMECA Instruments LEAP-4000X HR local electrode atom probe, which is equipped with an energy-compensating reflectron lens for improved mass resolution. The field evaporation of the specimens was performed with the following conditions: 200-kHz pulse repetition rate, 50 K specimen temperature, 20% pulse fraction. Additional details on the APT experiments and results can be found in references [7, 15, 26].
2.3 Corrosion testing
The electrochemical properties of the thermally aged DSS alloys were investigated using various electrochemical techniques. All tests were carried out at room temperature employing a three-electrode setup, with graphite rod and saturated calomel electrode (SCE) as the counter electrode and the reference electrode, respectively, and the DSS alloys as the working electrode. The exposed area of the working electrode was 0.5 cm2. The tests were carried out in the 1 L multiport spherical electrochemical cell. The test sequence started with one-hour open-circuit potential (OCP) monitoring, followed by electrochemical impedance spectroscopy (EIS) measurements. While all the tests were sequentially performed on the DSS specimen, each test probes the various different aspects of corrosion response of the DSS in modified PWR water chemistry. The OCP testing gives an insight into the relative thermodynamic tendencies of the alloy to either passivate or corrode in the exposure medium. The EIS tests the frequency-dependent corrosion response of the alloys and the nature of passive film forming on the alloy. The LPR gives the general corrosion rates of the alloy in the given corrosion environments; lastly, the CP probes the pitting potential of the DSS in exposure medium. The frequency scan range for EIS measurements was 50 kHz to 10 mHz with an applied AC perturbation voltage of 5 × 10–3 V r.m.s. The data acquisition rate was 10 points per decade of frequency. The EIS scans were followed by linear polarization resistance (LPR) and a cyclic polarization (CP) tests.
The potential range for the LPR tests was ± 0.015 V w.r.t. OCP, with a potential scan rate of 0.166 × 10–3 V/s [27]. In the CP test, the potential was swept from − 0.5 V w.r.t. OCP to an apex potential of + 1.0 V w.r.t. OCP. The peak current was set at 20 mA/cm2. The forward and reverse scan rates were 0.166 × 10–3 V/s following ASTM G61-86 [27]. Gamry Reference 3000 potentiostats were used for all the corrosion tests, and the electrochemical data were analyzed using Gamry Echem analyst software. Each corrosion test was repeated three times; for sampled aged for 10,000 h additional tests were conducted to obtain statistical variability data for the measurements. The variations of the results are reported in Tables S1 to S3 of the supporting information file.
The electrochemical studies were performed in simulated PWR environments due to the lack of data in this specific application—little is currently known about the effects of B and Li ions on corrosion of thermally aged DSS alloys. In these systems, at the beginning of the production cycle, B is injected to absorb additional neutrons and reduce the excess reactivity of the fresh fuel. The B ions are added in the form of boric acid (H3BO3), resulting in a pH decrease, which may adversely affect the service life of the exposed DSS. Often LiOH is added as a pH neutralizer to balance the pH. Furthermore, the water chemistry in such systems has a significant degree of variability in different operating environments, especially during startup and shutdown. Typically, the Li/B concentration (ppm) ratio (Li/B) varies from ~ 2.2/1200 to ~ 2.2/400, resulting in a solution pH between ~ 6.9 and ~ 7.4 at operational temperatures (the pH of the same solution would be about 9 to 11.5 at room temperature) within the aforementioned Li/B concentration range [19]. Maintaining Li concentrations at a maximum of 2.2 ppm during startup, however, can result in plants operating with an initial B concentration exceeding ~ 1200 ppm and pH dropping below 6.9. Depending on the cycle length and fuel design, the startup B concentration may also exceed 2000 ppm, which significantly lowers the pH to about ~ 6.4.
Furthermore, the PWR water also contains additional species, including dissolved oxygen, anions (e.g., chloride, fluoride, and sulfate) and cations (e.g., sodium), which may lead to increase in corrosion rates of the exposed DSS. Although such deleterious ionic species are very well controlled and are not present in abundance, the study of chloride exposure to thermally aged DSS is critical, in light of the possibility of internal component failure and the high margins of safety. Potential sources of chlorides could be airborne aerosols, accidental wetting of inappropriate thermal insulation materials, leaks from cooling and fire fighting systems that carry water that are much less pure than reactor coolants, and lastly ingress of cooling water into the secondary circuit as a result of condenser leakage may lead to the exposure of thermally aged steels to chloride-containing species [17].
In this study, the base water chemistry consisted of Li (2.2 ppm) and B (800 ppm) ions, which were added in the form of lithium hydroxide (LiOH) and boric acid (H3BO3). The test solutions included both naturally aerated and deaerated forms; deaeration was achieved by bubbling Ar gas (flow rate: ~ 40 cm3/min) through the solution for 6 h before the tests, followed by continuous Ar bubbling during the tests. Consistent dissolved oxygen content of 0.05 ppm was achieved in all deaerated test solutions, which is marginally higher than actual PWR water chemistry (~ 0.01 ppm). Although DSS alloys show excellent resistance to chloride-induced pitting corrosion, the effect of thermal aging time on the pitting resistance was investigated by adding 0.1 M Cl (NaCl) to the electrolyte for selected experiments. Lastly, to investigate the effect of different water chemistries, especially increasing B content, test solutions were prepared, keeping the Li-ion concentration (2.2 ppm) constant and varying the B ion concentration from 400 to 2000 ppm. All the test solutions were prepared with ASTM Type II deionized water [28]. The exact water chemistries (Li, B, and Cl ion concentrations) and dissolved oxygen content with their respective pH values are reported in Table 2. It should be noted that the pH of water used in PWR reactors at room temperature ranges from 9 and 11.5, which reduces to 6.9–7.4 at elevated operational temperatures, as reported in the literature [29]. In this paper, we have used the reduced pH of PWR water that is expected at high operational temperatures (6.9–7.4) as opposed to the pH of PWR at room temperature (9 to 11.5). With this approach, we intended to simulate the operational PWR chemistry at room temperature.
3 Results and discussion
3.1 Metallurgical characterization
3.1.1 Microscopic analysis
An optical micrograph of each of the five DSS alloys after 10,000 h of thermal aging is presented in Fig. 1. The same austenite-ferrite microstructure is observed in all samples. The three wrought alloys, Fig. 1a–c, exhibit austenite islands within a ferrite matrix and elongation of grains along the rolling direction. The austenite and ferrite phase fractions are reported in Table 3 and are the same for the three wrought alloys within the error of the measurements. Additionally, the average size of the grains is comparable in the three wrought alloys, as was confirmed through image analysis. The average size of the austenite grains in alloys 2003, 2101, and 2205 was 534, 582, and 527 µm2, respectively. Welded DSS alloys (2101-w and 2209-w) after 10,000 h of aging (Fig. 1d, e, respectively) showed finer grain structure with fine austenitic grains in the ferrite matrix. The average grain size of the austenite grains in DSS 2101-w and 2209-w alloys was 218 µm2 and 144 µm2, respectively. This difference in the measured grain size is not unexpected as material within the weld will have had a non-uniform thermal history, and thus spatial variation in the microstructure within and between welded alloys is expected. The phase fraction analysis demonstrated that alloys within the same classification (weld or wrought) had very similar phase fractions, as seen in Table 3. The welded alloys had a higher fraction of austenite and a finer grain size because the welded alloys are cooled more rapidly from the melt than wrought alloys.
The OM micrographs for the as-received (AR) DSS alloys are presented in Figure S1 of the supporting information file; comparison of the scale of the grains shows that little coarsening occurred during aging. This is not unexpected because the ferrite and austenite grains are on the order of 100 µm2, so their grain boundary area-to-volume ratio is small, and thus the driving force for coarsening is low. Besides, the aging temperature was relatively low.
3.1.2 Atom probe tomography
Atom probe tomography (APT) was conducted on samples of each alloy following thermal aging at 427 °C for 1 h, 100 h, 1000 h, and 10,000 h. APT was used to quantify α-αʹ separation through the measurement of the difference in chromium composition (ΔCr) between the α and αʹ regions. More information on the analysis methods can be found elsewhere [8, 9, 26]. Figure 3 shows the change in Cr content (ΔCr) between the Cr-rich and Cr-poor regions of the microstructure based on the APT measurements for each alloy as a function of time. The Langer-Bar-on-Miller (LBM) nonlinear theory of the spinodal decomposition method [30] was used to quantify ΔCr in the early stages of aging/decomposition. The proxigram method was used to quantify the late stages. More information on these methods can be found in Ref. [26]. Trends are in general agreement with those observed in Fig. 2: spinodal decomposition is enhanced with aging, and the weld alloys, along with 2101, demonstrate the highest degree of decomposition. Other trends that are more difficult to see in Fig. 2 become apparent in Fig. 3. For example, in Fig. 2, there is a seemingly negligible difference in aging behavior between 2003 and 2205; however, Fig. 3 demonstrates a significantly larger ΔCr for 2205 than 2003.
3.2 Electrochemical tests: effects of thermal aging
The observations are first made and demonstrated for DSS 2205 using the OCP, EIS, and CP results. Then the observations on effect of thermal aging time on electrochemical response are extended to all the other alloys, but these results are presented in supporting information file for clarity and readability. Since the test methods (OCP, EIS, LPR, and CP) showed similar trends for different alloys, to maintain the brevity of the manuscript, the comparison of all alloys is made using the corrosion rates as obtained from the LPR method. The OCP, EIS, and CP data for all the other alloys are presented in the supporting information file.
3.2.1 Open circuit potential
Figure 4 presents the OCP plots for unaged (as received: AR) and aged DSS 2205, for one-hour exposure to the base electrolyte (2.2 ppm Li, 800 ppm B) in aerated and deaerated forms. When the aged DSS is exposed to the electrolyte, it takes about one hour for the alloy-electrolyte system to stabilize and OCP values to reach a steady value; in all the cases, the OCP measurements were terminated at 1-h mark, when the change of OCP was within 0.002 V over a period of 5 min. Generally, decreasing (more electronegative) OCP is a sign of corroding behavior, whereas increasing (more electropositive) OCP is a sign of passivating behavior.
At one-hour exposure period, the OCP values were progressively more active (more negative OCP) with increasing thermal aging time in both aerated and deaerated electrolyte. The AR alloy showed most noble OCP values (deaerated: − 2.2 × 10–3 V vs. SCE; aerated: − 0.054 V vs. SCE) as compared to active OCP values for 10,000 h aged counterparts (deaerated: − 0.096 V vs. SCE, aerated: − 0.134 V vs. SCE). For aging time less than 10,000 h, the alloys showed an increasing OCP value with exposure time, which indicates a passivating behavior, whereas, for 10,000 h aged alloy, the OCP values decreased with exposure time, which suggests active corrosion. The OCP trends illustrate a decreasing corrosion resistance with increasing thermal aging time. Higher spinodal amplitude (ΔCr) or Cr segregation with longer aging time causes degradation of corrosion resistance. Elemental segregation in ferrite grains causes galvanic corrosion on a micron-scale due to the concentration gradient (ΔCr). Higher ΔCr at longer aging times increases the driving force for galvanic corrosion resulting in decreasing OCP values or active OCP values.
On comparing OCP values for 2205 alloys in aerated and deaerated electrolytes, it was evident that the presence of dissolved oxygen adversely affected the corrosion performance of the alloy. The OCP values for aerated conditions were active/more negative than OCP values in deaerated case, for all aging times. Comparing deaerated and aerated cases, the AR alloy showed OCP drop from − 2.2 × 10–3 to − 0.054 V (vs. SCE); similarly, 10,000 h aged alloy showed a decrease in OCP from − 0.096 × 10–3 to − 0.134 V (vs. SCE). The noble OCP values for the deaerated case could be attributed to the suppression of oxygen reduction reaction due to the absence of dissolved oxygen. Due to a lack of oxygen ions, the oxygen reduction reaction was the rate-limiting step for in the cathodic reaction. Thus, the metal ion dissolution (anodic reaction) significantly slowed down, resulting in relatively noble OCP values and effectively low corrosion rates.
Furthermore, a pH increase was observed as a result of the deaeration process. This increase of solution pH post-deaeration aids passivation of the DSS alloy resulting in higher OCP value, as reflected in Fig. 4. Typical PWR chemistries have dissolved oxygen content less than 0.01 ppm, which significantly reduces the risk of corrosion, and hence, associated corrosion rates. However, the results here indicate that the vulnerability of the alloy increases with increasing thermal aging time.
Similar trends of decreasing OCP with increasing thermal aging time and more noble OCP for deaerated conditions were observed for DSS 2003, DSS 2101, DSS 2101-W, and DSS 2209-W. The OCP plots for all mentioned DSS alloys in aerated and deaerated base water chemistry are presented in Figure S2 in the supporting information file. In all cases, the corrosion resistance went down with increasing aging time with 10,000 h-aged DSS showing the lowest corrosion resistance. This was the case with both the aerated and deaerated base solutions. Furthermore, in general, it was observed that the corrosion resistance of the DSS was higher in the deaerated electrolyte, and the corrosion resistance dropped in aerated water; however, there was no correlation found between the corrosion rate ranking of aging times in deaerated and aerated electrolytes. Welded alloys showed a lower decrease in OCP due to deaeration, and this could be attributed to the higher Ni/Cr ratio of these alloys, but this has not been investigated in detail this study.
3.2.2 Electrochemical impedance spectroscopy
Figure 5 shows the EIS data presented as Nyquist plot (a) and phase angle Bode plots (b) for the DSS 2205 alloy exposed to aerated and deaerated base electrolyte (2.2 ppm Li and 800 ppm B). In order to study the effect of aging time on key corrosion parameters, equivalent circuit modeling was used for comparison purposes. For simplicity, modeling was performed using a simplified Randles circuit, as shown in Fig. 5c-right, which contain circuit elements representing solution resistance, Rs, charge transfer resistance, Rct, and double layer capacitance, Cedl.
EIS data presented as a Nyquist plot, b phase angle Bode plot for DSS 2205 showing decreasing impedance and increasing phase angle with increasing thermal aging time, exposed to base deaerated and aerated electrolyte (2.2 ppm Li, 800 ppm B), c representative Nyquist plot and corresponding simplified Randles circuit used for modeling EIS data. (ω = frequency; θ = phase angle; Z: impedance; Zimg: Imaginary impedance; Zreal: real impedance)
A typical Nyquist plot (Fig. 5c-left) is characterized by a semicircle, with its center on the Zreal axis given by (Rs + Rct/2, 0) and the highest point of the semicircle represented by the impedance at a frequency given by 1/(Rct Cedl). On the Nyquist plot, the lowest Zreal value (high frequency) represents the Rs, and the highest Zreal value represents Rs + Rct values at low AC frequencies. The Rs value represents the test electrolyte/solution resistance, i.e., in the present context aerated (13.7 × 103 Ω cm2) and deaerated (45.4 × 103 Ω cm2) base electrolyte. The Rct is the ohmic resistance to the charge movement offered by the electrical double layer (EDL) forming on the DSS alloy surface upon exposure to the electrolyte. Typically, higher Rct values imply higher corrosion resistance for the alloys.
The Rs + Rct values for the AR alloy (deaerated: 677 × 103 Ω cm2; aerated: 451 × 103 Ω cm2) were significantly higher than impedance values of 10,000 h aged alloy (deaerated: 277 × 103 Ω cm2, aerated: 75 × 103 Ω cm2), confirming a higher corrosion resistance of the AR alloy. Typically, protective oxide films forming on corrosion resistant steels exhibit charge transfer resistance on the order of 100 × 103 Ω cm2 or higher [31]. For the present study, the impedance decreased with increasing aging time, implying an adverse impact of the α−αʹ phase separation on corrosion resistance of the alloys. A similar decreasing trend of impedance with increasing aging time was observed for all the DSS alloys tested. The EIS plots for all mentioned DSS alloys in aerated and deaerated base electrolyte chemistry are presented in Figure S3 and Tables S1-S2 in the supporting information file.
The phase angle between the applied AC potential and the resulting alternating current characterizes the capacitive behavior of the EDL on the alloy surface. Typically, an ideal capacitive behavior of the EDL/ passive film is characterized by a phase angle of − 90°, whereas a pure resistor behavior shows the phase angle of 0°. Generally, highly capacitive EDL protects the alloy surface from corrosion attack. Thus, high corrosion resistance is characterized by negative phase angles approaching − 90° [31].
Figure 5b presents the phase angle Bode plots for AR and aged DSS 2205 alloys, exposed to aerated and deaerated conditions. In the mid-frequency range (100–10−1 Hz), the AR alloy showed more negative peak phase angles (deaerated: − 63°; aerated: − 54°) than aged specimens, which indicates a stronger capacitive behavior of the EDL formed on the AR alloy surface. Typically passivated surfaces show negative phase angles that are close to the ideal capacitor, i.e., − 90° [31]. This suggests that the EDL and chromium oxide film formed on the 10,000 h aged alloy is not as protective as the AR alloys, due to elemental Cr segregation in αʹ regions in the ferrite phase [26]. Guo et al. pointed out that a decrease in Cr concentration in the α regions could result in sensitization at 10,000 h of aging [26]; it is, therefore, likely that Cr segregation can result in inhomogeneous and nonuniform passive chromium oxide film on the alloy surface. Similar to the OCP observations, the deaeration of the test solution resulted in higher impedance and more negative phase angles than their counterparts in aerated electrolyte [31, 32]. Similar trends of impedance and phase angles with increasing aging time and with aeration of test solution were observed for DSS 2003, DSS 2101, DSS 2101-w, and DSS 2209-w. The EIS plots for all mentioned DSS alloys in aerated and deaerated base water chemistry are presented in Figure S3 and Tables S1-S2 in the supporting information file. It should be noted that the effect of thermal aging on impedance measures was sometimes unclear for aging time shorter than 1000 h, and in some cases, minor anomalies were present. An example of this is shown in Figure S2(a) for DSS 2003 alloy, for which the Nyquist plots for 100 h and 1000 h aged samples are similar. Although in most cases, ageing correlated well with reduced impedance, and hence reduced corrosion resistance, these differences became clearer at 10,000 h aging. For DSS 2003, the relatively similar impudence values for 100 h and 1000 h aged samples could be an artifact of the fact that this alloy had the lowest initial impedance among all tested alloy types.
3.2.3 Cyclic polarization
To investigate the effect of α–αʹ phase separation on the localized corrosion (e.g., pitting) characteristics of the thermally aged DSS alloy, cyclic polarization (CP) tests were carried out in the base electrolyte (2.2 ppm Li, 800 ppm B). Figure 6a, b presents the CP curves for DSS 2205 for all thermal aging times in deaerated and aerated test solutions.
No signs of pitting were observed for DSS 2205 in deaerated solution at all aging durations. Furthermore, 2205 alloy showed passivation at all aging times, as reflected by the higher repassivation potential (inflection point in the reverse loop (B)) relative to corrosion potential (Ecorr: grey-dashed arrows) in the forward loop (F). This is a typical sign of the formation of the protective oxide film on the alloy [31]. Despite the passivation of the alloys, the corrosion potential, Ecorr. (V vs. SCE), progressively became more active/negative with increasing aging time. This decreasing Ecorr trend is in good agreement with the OCP and EIS results.
For the aerated test solution, AR, 100, and 1000 h aged alloys did not show any signs of pitting; however, 10,000 h aged alloy showed signs of metastable pitting. The signs of metastable pitting at the highest aging times (10,000 h) were an indicator of decreasing protectiveness of the oxide film on the surface with an increasing extent of spinodal decomposition. Similar to the deaerated test solution, the Ecorr values decreased with increasing aging time in the aerated test solution. Moreover, aerated test solution resulted in a reduced separation between the Ecorr in the forward (F) loop and the repassivation potential in the reverse loop (B), which suggests a decreased protectiveness of the passive film in the presence of dissolved oxygen. This is consistent with the previous observation of decreased corrosion resistance in the presence of dissolved oxygen [33]. Therefore, it is of vital importance that PWRs operate below a well-defined dissolved oxygen level to maximize the service life of the components. The DSS alloys tested in aerated solution did not show distinct pitting potentials; however, 10,000 h aged DSS alloys showed metastable pitting for lean alloys, especially DSS 2003 (Figure S4 (b)). The CP plots for all the DSS alloys in aerated and deaerated solutions are presented in Figure S4 in the supporting information file.
3.2.4 Linear polarization resistance
Figure 7 shows the corrosion current density, icorr [A/cm2], of all DSS alloys plotted as a function of thermal aging time. The polarization resistance (Rp) values from LPR tests were used to calculate the corrosion current density using the Stern–Geary equation [34]. As shown in Fig. 7, all DSS alloys show an increase in the icorr with increasing thermal aging time, with 10,000 h aged alloys showing the highest icorr values, while the AR alloys showed the lowest values. As previously established from APT results, higher ΔCr due to α–α′ segregation at 10,000 h aging time results in a larger number of corrosion-susceptible hot spots at chromium deficient regions, thereby increasing the susceptibility for corrosion attack.
Increasing corrosion current density with increasing thermal aging time for all DSS alloys: a deaerated electrolyte, b aerated electrolyte (2.2 ppm Li, 800 ppm B). Deaeration results in lowering of corrosion current density for all DSS alloys. Note that corrosion current density in aerated conditions is approximately an order of magnitude larger than those in deaerated conditions
On the contrary, AR alloys, due to their homogenous chemical compositions, form uniform passive chromium oxide layers, resulting in lower icorr. Furthermore, spinodal decomposition results in a concentration gradient leading to a galvanic effect on the nanoscale, thereby increasing icorr. values at the longer aging time.
To rank the corrosion performance of all DSS alloys, the standard grade DSS (2205 and 2209-w) with higher Cr, Ni, Mo contents, and Nieq and Creq values performed better than lean grade DSS (2003, 2101, 2201-w). Furthermore, increasing the corrosion resistance of the lean grades was observed to be directly related to increasing Cr and decreasing Fe contents. Notably, the welded alloys (2101-w and 2209-w) showed a finer austenite grain structure, but did not show significantly different corrosion resistance as compared to their as-received counterparts, 2101 and 2205, respectively.
The icorr increased by about one order of magnitude in aerated solutions; this increase was the largest at longer aging times (e.g., at 10,000 h), as presented in Fig. 7b. However, the deaeration of the test solution did not affect the ranking of the DSS alloys. Standard grade DSS consistently performed better than lean grade DSS. The standard errors for icorr. measurements for 10,000 h aged specimens were within 15% of the mean.
Interestingly, the DSS alloys that showed the lowest thermal embrittlement (lean grades) [7,8,9] also showed the worst corrosion resistance, making the material selection for elevated temperatures all the more challenging.
3.3 Effects of chloride contamination of pitting behavior of the aged alloys
To investigate the effects of chloride contamination on the pitting behavior of thermally aged DSS alloys, the base water chemistry (2.2 ppm Li, 800 ppm B) was modified to include 0.1 M chlorides through NaCl addition. Despite high pitting resistance equivalent number (PREN) of the DSS alloys, thermal aging would result in an increased vulnerability toward pitting corrosion in DSS alloy; therefore, the effects of thermal aging on pitting behavior need to be investigated. This section discusses the effect of chloride additions to the base water chemistry on the electrochemical properties of thermally aged DSS alloys in aerated form. However, from the previous comparisons, it could be deduced that the chloride addition to both aerated and deaerated form would have similar effect with increasing aging time. The authors have assumed that, in case of chloride contamination, the contaminated medium would be in the aerated state. In the present section, the EIS, OCP, and CP results are presented only for DSS 2205, while the electrochemical data for other DSS alloys exposed to chloride modified electrolyte are presented in the supporting information file. The comparison of all DSS alloys was made using the LPR data.
3.3.1 Open-circuit potential: base + 0.1 M NaCl
Figure 8 presents the OCP plots for AR and aged DSS 2205 alloys exposed to base electrolyte chemistry (2.2 ppm Li, 800 ppm B) with 0.1 M chlorides. Regardless of the aging time, the DSS showed decreasing OCP trend with increasing exposure time indicative of active corrosion of DSS in the presence of chlorides. Similar to the earlier observations (without chlorides), the OCP decreased with increasing thermal aging time in the presence of chloride ions. The 10,000 h aged alloy showed the most active OCP value (− 0.297 V vs. SCE), while AR alloy showed the most noble OCP value of − 0.1 V vs. SCE. After the chloride additions, the OCP values decreased in the active direction for all aging times, as compared to the base electrolyte chemistry (no chlorides) due to the increased corrosion susceptibility of the alloys. The decrease in OCP upon Cl addition was the largest for the 10,000 h aged alloys, decreasing from − 0.134 (base) to − 0.297 V versus SCE, but smallest for the AR alloy where the OCP only dropped from − 0.054 (base) to − 0.100 V versus SCE. The increased corrosion susceptibility is a combined effect of the presence of chloride ions and increased ΔCr due to increasing α–α′ phase separation with aging time, as shown in Fig. 2.
Similar trends of decreasing OCP trends with increasing thermal aging time were observed in 2003, 2101, 2101-w, and 2209-w. The OCP plots for all mentioned DSS alloys in modified electrolyte chemistry with 0.1 M chlorides are presented in Figure S5 in the supporting information file. After the chloride additions, the OCP values decreased in the active direction for all aging times, compared to the base electrolyte chemistry (no chlorides) due to the increased corrosion susceptibility of the alloys. The decrease in OCP upon Cl addition was largest for the 10,000 h aged alloys. OCP plots of specimen exposed to chlorides showed more fluctuations over the measurement time, which suggests that chlorides interact with the surface. EIS and CP testing provide more information about these interactions and pitting potentials.
3.3.2 Electrochemical impedance spectroscopy: base + 0.1 M NaCl
Figure 9 presents the EIS data for AR and aged DSS 2205 alloy exposed to base electrolyte (2.2 ppm Li, 800 ppm B) with 0.1 M chloride. The Rs value decreased from 13.7 × 103 to 50 Ω cm2 with chloride additions, which is a significant increase (two orders of magnitude) in solution conductivity (Figs. 5a, 9a). Similar to the base case (no chlorides), the low-frequency impedance values (Rct + Rs) decreased with increasing thermal aging time. However, the impedance values significantly decreased with chloride addition for all aging conditions, e.g., for 10,000 h aged alloy, the Rct + Rs value dropped from 75 × 103 to 7.3 × 103 Ω cm2, which is about a tenfold decrease.
On the other hand, the AR alloy showed an impedance drop of about threefold, going from 451 × 103 Ω cm2 to 128 × 103 Ω cm2. After chloride additions, the change in impedance (Δ|Z|) increased with longer thermal aging times. This implies that the presence of chlorides decreases the corrosion resistance of the DSS alloys, especially for highly aged alloys. The phase angle Bode plots for DSS 2205 alloys exposed to chloride contaminated base water chemistry are presented in Fig. 9b. Similar to the results presented in Fig. 5b, the phase angle value increases with increasing thermal aging, implying decreasing protectiveness of the EDL forming with aging time. Due to the conductive or non-ideal capacitive behavior of the EDL, the chloride ions can attack the alloy surface with relative ease, thereby decreasing the corrosion resistance of the alloy. Thus, chloride contamination would have deleterious effects of the service life on the components, particularly at advanced service ages.
Similar trends of impedance and phase angle with increasing thermal aging time were observed for DSS 2003, DSS 2101, DSS 2101-W, and DSS 2209-W. The EIS plots for all mentioned DSS alloys in base water chemistry with 0.1 M chlorides are presented in Figure S6 in the supporting information file.
3.3.3 Cyclic polarization: base + 0.1 M NaCl
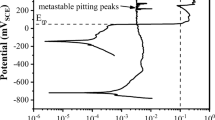
Figure 10a presents the CP results for DSS 2205 exposed to base electrolyte (2.2 ppm Li, 800 ppm B) with 0.1 M chloride addition. Unlike the base case, the addition of chlorides resulted in the pitting of DSS 2205 alloys at all aging times and the pitting potential (Epit) decreases with increasing thermal aging time. Decreasing pitting potentials with increasing aging time was likely due to an increase in widely spaced Cr-deficient regions (α), coupled with the segregation of Ni and Mo with aging time, which made the alloy more susceptible to pitting [35]. Increased spinodal decomposition with thermal aging time increases pit nucleation sites, and since the chromium-depleted regions are susceptible to pit nucleation, the pitting potential of the alloys decreased with aging time. Furthermore, chromium segregation results in the formation of non-homogenous or patchy chromium oxide film on the DSS surface, which makes the alloy vulnerable to pitting and general corrosion at a higher aging time, as compared to uniform and passive protective film on AR DSS alloy. The AR alloy showed the highest pitting potential of 0.277 V (vs. SCE), whereas the 10,000 h aged alloy showed the lowest Epit of − 0.084 V (vs. SCE).
a CP plots for DSS 2205 with increasing thermal aging time exposed to base electrolyte (2.2 ppm Li, 800 ppm B) with chloride, showing a decrease in pitting potential (Ep) values with increasing aging duration. b Pitting potentials of aerated and deaerated DSS 2205 for 10,000 h aged samples. c Optical micrograph of the DSS 2205 aged for 10,000 h (aerated); arrows indicate pits that formed after the CP testing
It is interesting to note that despite high Creq, Nieq, and PREN numbers, all the DSS alloys pit in the presence of Cl ions. Furthermore, the reverse loop (B) for all the CP curves lay below the forward loop (F), which is indicative of the non-protective nature of the rust/oxide product formed on the alloy surface and active corrosion of the alloy. Deaeration of the chloride-containing test solution increased pitting potentials for all 10,000 h aged DSS alloys, as shown in Fig. 10b. An increase in Epit with deaeration would follow the same reasoning as previously mentioned, i.e., sluggish kinetics for the cathodic reaction of oxygen reduction. Figure 10c presents an optical micrograph of the DSS 2205 aged for 10,000 h (aerated), which confirms the formation of pits on the specimen after the CP testing.
All the DSS alloys tested in chloride-containing solution showed pitting and further showed a decrease in pitting potential with increasing thermal aging time. The CP plots for all the DSS alloys are presented in Figure S7 in supporting the information file. In the case of 2209-w, only 10,000 h aged specimens showed distinct pitting potential, whereas specimens at early aging times showed signs of metastable pitting.
3.3.4 Linear polarization resistance: base + 0.1 M NaCl
Figure 11a presents the icorr for all DSS alloys exposed to the chloride-containing base electrolyte chemistry (2.2 ppm Li, 800 ppm B). All DSS alloys showed an increase in corrosion current density with increasing thermal aging time. The 10,000 h aged alloys showed a high corrosion rate, which was manifested by a sharp increase in icorr from 1,000 to 10,000 h of aging. The addition of chlorides did not alter the alloy ranking obtained in the base electrolyte (without chlorides) such that the standard grade DSS showed superior corrosion performance as compared to the lean grades. Similar to the observations made in the base electrolyte, the presence of dissolved oxygen resulted in the decrease of corrosion resistance of the DSS alloys in chloride-containing test solutions, as illustrated by the increase in icorr. as shown in Fig. 11b. The decrease was the largest for the lean grade DSS (e.g., 2003 and 2101) as compared to standard grades (2205 and 2209-w). The alloy ranking in terms of corrosion performance remained unchanged with the addition of chlorides; standard grade (higher Mo, Cr and Ni content, and higher Nieq and Creq) alloys performed better than lean grade alloys.
3.4 Effect of boron (B) concentration
The B content was varied from 400 to 2,000 ppm, and the increased B content resulted in a decrease in pH from 7.2 to 6.4, respectively. Considering a higher corrosion susceptibility for 10,000 h aged alloys, the icorr. values at different B concentrations for 10,000 h of aging are shown in Fig. 12a. The increased B concentration resulted in higher corrosion current density for all DSS alloys, and the most pronounced effect was observed on the lean grade DSS alloys. Changing B concentrations did not alter the ranking of the alloys, with standard grade DSS consistently performing better than lean grades.
a Increase in corrosion current density with increasing B concentration and decreasing pH for 104 h aged DSS alloys, and b effect of boron concentration and pH on icorr for all DSS alloys. Increasing boron concentrations leads to a decrease in corrosion resistance of the DSS alloys. All the test solutions had 0.1 M chloride concentration
The increase in icorr could be attributed either to decreasing pH or increasing B concentration. To discern the two competing effects, the icorr values for 800 and 2000 ppm B were compared to a third modified electrolyte chemistry. This electrolyte had B and Li content similar to the base case (pH = 6.9), but 3.5 ppm HCl was added to bring down the pH to the level of the 2000 ppm B containing test solution (i.e., pH = 6.4), the remaining chlorides were added in the form of NaCl maintaining the total chlorides concentration at 0.1 M Cl. Figure 12b shows the icorr for all the alloys at different pH and different B concentration. The data are presented in three different columns (C1, C2, and C3) for better readability. The first two columns (C1 and C2) reiterate the results presented in Fig. 12a while C2 and C3 show data of water chemistry with the same pH but different B concentration. By comparing C3 and C2, it is evident at the same pH level, increasing B content from 800 ppm to 2000 ppm increases the icorr values for all DSS alloys, showing that the presence of B ions (H3BO3) in the electrolyte has deleterious effects on the service lives of the DSS components. Furthermore, comparing C1 and C3 shows that pH has an inconsequential effect on icorr. The determination of the exact mechanism of the B-induced corrosion is beyond the scope of this paper; however, the study is underway.
3.5 Conclusions
We investigated the effect of thermal aging on the electrochemical properties of DSS 2205, along with DSS 2101, DSS 2003, DSS 2205, DSS 2101-welded, and DSS 2209-welded alloys, which were thermally aged at 427 °C for up to 10,000 h. Electrochemical experiments were performed in a range of water chemistries containing Li (LiOH) and B (H3BO3), and the effects of chloride contamination were considered, and the following conclusions have been drawn:
-
1.
The results indicated decreasing corrosion resistance with thermal aging time for all DSS alloys; however, standard grade DSS (2205 and 2209-w) alloys with higher Cr, Ni, Mo contents showed better corrosion resistance than lean grades (2003, 2101, 2101-w). Furthermore, increasing the corrosion resistance of the lean grades was observed to be directly related to increasing Cr and decreasing Fe contents. The welded alloys (2101-w and 2209-w) showed a finer austenite grain structure, but did not show significantly different corrosion resistance as compared to their as-received counterparts; 2101 and 2205, respectively.
-
2.
The presence of dissolved oxygen in the electrolytes resulted in a significant increase in icorr. for the DSS alloys, but it did not affect the general trend of corrosion rates with aging time.
-
3.
DSS alloys did not show pitting corrosion in the base water chemistry (2.2 ppm Li, 800 ppm B); however, all the DSS alloys became vulnerable to pitting corrosion upon addition of 0.1 M chlorides. The pitting resistance (pitting potential) decreased with increasing thermal aging time. The addition of chlorides did not alter the alloy ranking obtained in the base electrolyte (without chlorides) such that the standard grade DSS showed superior corrosion performance as compared to the lean grades. The deaeration of the chloride-containing test solution increased pitting potentials and decreased corrosion rates. The increase in corrosion vulnerability was the largest for the lean grade DSS (e.g., 2003 and 2101) as compared to standard grades (2205 and 2209-w).
-
4.
The electrolyte chemistry played a significant role in the corrosion behavior of the DSS alloys; increased B content resulted in degradation of corrosion resistance of the DSS alloys, while minor changes in pH did not show a significant change in corrosion resistance. The most pronounced decrease in corrosion performance with increasing B content was observed for the lean grade DSS alloys. Changing B concentrations did not alter the ranking of the alloys, with standard grade DSS consistently performing better than lean grades.
Mechanical and metallurgical characterization coupled with electrochemical characterization of the DSS alloys gave a comprehensive insight into the effects of thermal aging on the electrochemical response of the DSS. Interestingly, the DSS alloys that showed the lowest thermal embrittlement (lean grades) also showed the worst corrosion resistance, making the material selection for elevated temperatures all the more challenging.
References
Francis R (2018) The corrosion of duplex stainless steels: a practical guide for engineers. NACE International, New York
Gunn R (1997) Duplex stainless steels: microstructure, properties and applications. Elsevier Science, New York, p 218
Solomon HD, Devine TM, Metals ASf, (1982) Duplex stainless steels: a tale of two phases. American Society for Metals, New York
Avrami M (1940) Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J Chem Phys 8(2):212–224
Chung HM, Chopra OK (1987) Kinetics and mechanism of thermal aging embrittlement of duplex stainless steels. In: Conference: 3. international symposium on environmental degradation of materials in nuclear power systems: water reactors, Traverse City, MI, USA, 30 Aug 1987; Other Information: Portions of this document are illegible in microfiche products; Argonne National Lab
Tucker JD, Young GA Jr, Eno DR (2011) Thermal embrittlement of a lean grade of duplex stainless steel: alloy 2003. Solid State Phenom 172–174:331–337
Garfinkel DA, Poplawsky JD, Guo W, Young GA, Tucker JD (2018) Influence of alloying on α–αʹ phase separation in duplex stainless steels. In: Jackson JH, Paraventi D, Wright M (eds) Proceedings of the 18th international conference on environmental degradation of materials in nuclear power systems—water reactors, Cham, 2018. Springer, pp 1183–1192
Garfinkel DA, Poplawsky JD, Guo W, Young GA, Tucker JD (2015) Phase separation in lean-grade duplex stainless steel 2101. JOM 67(10):2216–2222
Garfinkel DA, Poplawsky JD, Guo W, Young GA, Tucker JD (2017) Influence of alloying on α–αʹ phase separation in duplex stainless steels. Environmental degradation of materials in nuclear power systems. Springer, pp 1183–1192
Danoix F, Auger P, Blavette D (2004) Hardening of aged duplex stainless steels by spinodal decomposition. Microsc Microanal 10(3):349–354
May JE, Souza CACd, Nascente PAdP, Soares P, Lepienski CM, Kuri SE (2010) Effect of thermal aging conditions on the corrosion properties and hardness of a duplex stainless steel. Mater Res 13:431–436
Silva R, Baroni LFS, Kugelmeier CL, Silva MBR, Kuri SE, Rovere CAD (2017) Thermal aging at 475°C of newly developed lean duplex stainless steel 2404: Mechanical properties and corrosion behavior. Corros Sci 116:66–73
Silva R, Baroni LFS, Silva MBR, Afonso CRM, Kuri SE, Rovere CAD (2016) Effect of thermal aging at 475°C on the properties of lean duplex stainless steel 2101. Mater Charact 114:211–217
Young GA, Tucker JD, Lewis N, Plesko E, Sander P (2016) Assessment of lean grade duplex stainless steels for nuclear power applications. In: Busby JT, Ilevbare G, Andresen PL (eds) Proceedings of the 15th international conference on environmental degradation of materials in nuclear power systems—water reactors, Cham, 2016. Springer, pp 2369–2380
Tucker JD, Miller MK, Young GA (2015) Assessment of thermal embrittlement in duplex stainless steels 2003 and 2205 for nuclear power applications. Acta Mater 87:15–24
Tucker J, Young G, Eno D (2011) Thermal embrittlement of a lean grade of duplex stainless steel: alloy 2003. Solid State Phenom. https://doi.org/10.4028/www.scientific.net/SSP.172-174.331
Zhang L, Jiang Y, Deng B, Zhang W, Xu J, Li J (2009) Effect of aging on the corrosion resistance of 2101 lean duplex stainless steel. Mater Charact 60(12):1522–1528
Rovere CAD, Santos FS, Silva R, Souza CAC, Kuri SE (2013) Influence of long-term low-temperature aging on the microhardness and corrosion properties of duplex stainless steel. Corros Sci 68:84–90
Suganya P, Swaminathan G, Anoop B, Prasad GVRRSGS, Nagarajan J (2020) Assessing the factors affecting the water chemistry parameters in the auxiliary water system of a nuclear power plant. Sn Appl Sci 2:1889. https://doi.org/10.1007/s42452-020-03693-z
Lister D, Uchida S (2015) Determining water chemistry conditions in nuclear reactor coolants. J Nucl Sci Technol 52(4):451–466
Ishigure K, Abe K, Nakajima N, Nagao H, Uchida S (1989) Water chemistry experience of nuclear-power plants in Japan. J Nucl Sci Technol 26(1):145–156
Kawamura H, Shoda Y, Terachi T, Katsumura Y, Uchida S, Mizuno T, Muroya Y, Tsuzuki Y, Umehara R, Hirano H, Nishimura T (2019) PWR secondary water chemistry guidelines in Japan—purpose and technical background. Prog Nucl Energy 114:121–137
Uchida S, Katsumura Y (2013) Water chemistry technology one of the key technologies for safe and reliable nuclear power plant operation. J Nucl Sci Technol 50(4):346–362
ASTM E3 (2017) Standard guide for preparation of metallographic specimens. ASTM International, West Conshohocken
Miller MK, Russell KF, Thompson K, Alvis R, Larson DJ (2007) Review of atom probe FIB-based specimen preparation methods. Microsc Microanal 13(6):428–436
Guo W, Garfinkel DA, Tucker JD, Haley D, Young GA, Poplawsky JD (2016) An atom probe perspective on phase separation and precipitation in duplex stainless steels. Nanotechnology 27(25):254004
ASTM G59-97 A (2014) Standard test method for conducting potentiodynamic polarization resistance measurements. ASTM International, West Conshohocken
ASTM D1193-06 (2018) Standard specification for reagent water. ASTM International, West Conshohocken
Institute EPR (1999) PWR-primary water chemistry guidelines volume-1 (EPRI-1999-TR-105714-V1R4)
Zhou J, Odqvist J, Thuvander M, Hedstrom P (2013) Quantitative evaluation of spinodal decomposition in Fe-Cr by atom probe tomography and radial distribution function analysis. Microsc Microanal 19(3):665–675
Orazem ME, Tribollet B (2017) Electrochemical impedance spectroscopy. Wiley, New York
Fontana MG (2005) Corrosion engineering. Tata McGraw-Hill, New York
Rybalka KV, Beketaeva LA, Davydov AD (2018) Effect of dissolved oxygen on the corrosion rate of stainless steel in a sodium chloride solution. Russ J Electrochem 54(12):1284–1287
Mansfeld F, Oldham KB (1971) A modification of the stern—geary linear polarization equation. Corros Sci 11(10):787–796
Jones DA (1996) Principles and prevention of corrosion. Prentice Hall, Hoboken
Funding
This research is supported by the US Department of Energy, Nuclear Energy University Programs under the Grant DE-NE0008668.
Author information
Authors and Affiliations
Contributions
JDT, LA, OBI, and YZ were involved in conceptualization. PM, OBI, IM, MKS, and JDT were involved in experimental investigations and writing the original draft. All authors were involved in the review and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murkute, P., Oware Sarfo, K., McGieson, I. et al. Effect of thermal aging on corrosion behavior of duplex stainless steels. SN Appl. Sci. 4, 97 (2022). https://doi.org/10.1007/s42452-022-04978-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-04978-1