Abstract
We report on the immobilization of carbon nitride (CN) materials and β-Bi2O3 on electrospun polyvinyl acetate (PVAc) fiber substrates using a dispersion based dip coating process. The spinning process was optimized by variation of several parameters to finally obtain continuous droplet-free fibers at 15 kV and a flow rate of 50 µL min−1 using a needle with 1.2 mm diameter. The polymer substrates were coated with the β-Bi2O3 and CN materials, which were characterized using SEM and applied in the photocatalytic degradation of organic pollutants such as Rhodamine B (RhB), ethinyl estradiol (EE2) and triclosan using visible light irradiation. The pollutants were degraded with up to 50% of the initial concentration within 8 h. Different amounts of CN material were deposited to evaluate the photocatalytic activity per mass. Immobilized CN materials were shown to be of higher activity (2.0 × 10−10 mol mg−1 min−1) than β-Bi2O3 (1.3 × 10−10 mol mg−1 min−1) and the mixture CN/β-Bi2O3 (1.6 × 10−10 mol mg−1 min−1). Reference samples with CN particles partially embedded in the polymer fleece showed minor degradaton rates (18% RhB degradation within 8 h) as compared to coated fiber substrates (47% RhB degradation within 8 h). Minor leaching of the carbon nitride material and no leaching of β-Bi2O3 occurs as shown by NPOC (non purgeable organic carbon) and ICP-MS measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the last decades an increased usage of dyes, biocides and pharmaceuticals is recorded, and especially the use of antibiotics increased significantly. For example, in Brazil, Russia, India, China and South Africa an increase was accounted for 76% between 2000 and 2010 [1]. The use and careless disposal of these organic compounds causes environmental pollution and drinking water contamination worldwide. Around 150 different pharmaceutical substances were detected in sewage effluents and surface water to date [2]. Studies showed that the amount of these substances released into the environment is much higher than previously assumed [3,4,5,6]. It is important to note that due to their high persistence and bioaccumulation pharmaceuticals, drugs and dyes, which are harmful to human and aquatic living beings, become increasingly part of the food chain [7, 8]. Dyes such as RhB, methyl orange and orange G are used in the fabrication of dye textiles, paper, cosmetics and food. They are suspected for being carcinogenic and mutagenic and show ecotoxic properties. It is estimated that between 1% and 20% of the dyes used worldwide are released into the environment during the synthesis and dyeing processes [6, 9]. The disinfectant triclosan and the contraceptive agent EE2 were detected in urine samples of respondents in the USA and China. Triclosan is suspected to increase the risk of asthma, allergies and food intolerances and affects human fertility [10, 11]. In addition to further regulation for the use of these substances new solutions are needed to purify drinking water and remove these organic contaminations. Different strategies like the treatment of sewage effluents with ozone, UV light, hydrogen peroxide and combined processes are currently under investigation. Their potential is widely recognized, but the costs limit their application [5, 12,13,14,15,16]. Therefore, the photocatalytic process is also examined to degrade organic compounds. TiO2 is a commonly used photocatalyst material [17,18,19] which requires UV-light for activation and which is under suspicion to be carcinogenic [20]. Visible-light active photocatalysts like CN materials and environmentally benign β-Bi2O3 attracted increased attention in the last years [21,22,23,24,25,26]. They provide a suitable band gap for exposure with visible light, they are non-toxic and therefore particularly suitable for photocatalytic water purification of drinking water [16, 25, 27]. Due to the different band gap structure of β-Bi2O3 (CB +0.33 V, VB +3.17 V vs. NHE, Eg = 2.3 eV) and CN materials (CB 1.13 V, VB 1.60 V vs. NHE, Eg = 2.8 eV) [25, 28, 29] the usage of both photocatalyst materials in a combined application might increase the visible light response and therefore was expected to be beneficial for photocatalytic pollutant degradation processes. Reactors for waste water treatment using photocatalysts are already in operation and they make use of immobilized photocatalyst materials to avoid a costly separation step of the catalyst material from the cleaned water feed [6, 15, 25, 30,31,32]. A strategy to immobilize photocatalysts is based on an electrospinning process. Electrospun fibers offer a range of advantages compared to traditional substrates composed of metal or glass, namely a higher surface area, flexibility and the potential application as filter medium [33,34,35]. The electrospinning process allows the formation of continuous fibers with a controlled diameter and orientation and provides access even to large area fleeces [34]. Electrospinning of fibers and their functionalization during or after the spinning process with a photocatalyst material have been described in the literature [33, 36,37,38]. For example, CN particles were spun together with a titanium dioxide precursor from a PVAc solution into fibers. Subsequent drying and annealing decomposed the titanium dioxide precursor which lead to the formation of TiO2 and CN fibers with lengths of several micrometers. However, the fleece-like structure collapsed as a result of the annealing process. For the photocatalytic investigations, the fiber pieces were dispersed in the organic dye solution and the degradation of RhB was investigated. Composites of TiO2 and CN materials on PVAc fibers were also investigated. The photocatalytic activity was studied as a function of different CN contents; a composite with 5 wt% CN exhibited the highest RhB degradation rate [39]. Bi2O3 nanofibers were obtained by electrospinning of Bi(NO3)3 and polyacrylonitrile in N,N-dimethyl formamide and thermal treatment of the fibers. They were successfully used for the degradation of RhB [40]. It should be emphasized, however, that in most cases large-area substrates were not investigated, but only particulate fiber pieces [41,42,43]. Studies on coated fabrics and fleeces of larger dimensions (substrate sizes of several square centimeters) are rare. A representative example consists of larger fleeces resulting from electrospinning of polyvinylidene fluoride with a maximum loading of 2 wt% CN. For this purpose the CN particles were added to the polymer solution and embedded in the polymer substrate [44, 45]. A fleece made of carbon fibers, which was subsequently coated with CN, was successfully used to degrade RhB and 4-chlorophenol. The fleece was functionalized via a dip-coating process using a CN precursor, which was decomposed to a CN material in a furnace at 550 °C [46].
Here we present a preparation method of PVAc fleeces, which were used as support for the photocatalysts CN and β-Bi2O3. Based on previous studies [25], the highly soluble bismuth-containing molecular precursor [Bi38O45(OMc)24(EtOH)13] [47] was used to synthesize β-Bi2O3 particles, which are deposited on the fiber substrates by a dispersion based dip coating process. CN particles were obtained via a thermal decomposition of dicyandiamide according to a previous study [29] and deposited on the substrates. The degradation rates of RhB using either CN- or β-Bi2O3-coated fleeces, fleeces coated with a mixture composed of β-Bi2O3 and CN, and polymer fleeces with partially embedded CN particles are compared. CN coated polymer substrates showed the highest activity and were furthermore tested to degrade EE2 and triclosan.
2 Results and discussion
2.1 Optimization of spinning parameters of PVAc fibers
First, to obtain droplet-free aligned fibers spinning parameters such as the polymer solution flow rate and the speed of the rotary collector drum were optimized (Fig. 1). In order to produce uniform and aligned fibers, the use of a rotating collector proved to be essential. The fiber quality increases with increasing rotation speed. With a flow rate of 100 µL min−1 the shape of the polymer droplets changes from spheres to ellipsoids with increasing rotation speed until droplet-free fibers are formed at 700 rpm (Fig. 1a–d). A similar trend but even better results were observed for flow rates of 50 µL min−1 (Fig. 1e–h). Further reduction of the volume flow to 30 µL min−1 does not lead to an improvement of the fiber quality with increasing rotation speed. The reason is that the fiber is pulled out of the Taylor cone too quickly, which is formed on the tip of the needle during spinning. This may cause the fibers to break and deposit irregularly on the collector (Fig. 1k, l). In general, lower flow rates should be used for electrospinning, as the fibers are stretched and faster drying is observed. Formation of droplets and agglomeration of the fibers into thick fiber strands is thus prevented [48, 49]. Further variation of the spinning parameters such as the applied voltage or the diameter of the used needle did not improve the fiber morphology.
For the full parameter set investigated, the most promising result was obtained using a rotation speed of 700 rpm, a flow rate of 50 µL min−1, a voltage of 15 kV, a needle with 1.2 mm diameter and a distance of 5 cm between collector and needle. Approximately equally thick polymer fibers were obtained. Fleeces produced with these parameters were used as support (substrate area 5.75 cm2) for CN and β-Bi2O3 particles, which were immobilized via a dip coating process.
2.2 Coating of PVAc fleeces with CN and β-Bi2O3
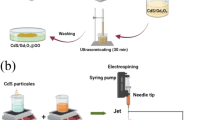
The as-prepared fleeces are used as substrates for the photocatalyst material. The preparation process, the characterization (SI 1; UV-vis spectra, PXRD, photoluminescence spectra, SEM) and the evaluation of the photocatalytic activity of the used CN [50] and β-Bi2O3 [26, 51] particles is described in the literature. To coat the fleeces with these catalyst materials, the electrospun fleeces are dipped into a solution of ethanol (EtOH) and water, which causes the polymer to swell. The higher the EtOH content, the more the fibers swell, which is essential for adhesive coating of the photocatalyst particles. Thus, different EtOH/water ratios (1 vol% to 10 vol% of EtOH/water) were used for the pretreatment of the fibers in order to obtain different loadings of the substrate. The fleeces are then immersed in the particle dispersion and coated by ultrasonication for 30 s. Different masses of the photocatalysts were applied using this process. Substrates loaded with 1.7 mg β-Bi2O3 (β-Bi2O3-ES) and 2.1 mg of a 1:1 mixture of β-Bi2O3 and CN (β-Bi2O3/CN-ES) are obtained. The applied CN catalyst masses are 1.0 mg (CN-ES1), 1.7 mg (CN-ES2) and 2.4 mg (CN-ES3). The surface structures of the coated and blank substrates were examined by SEM showing that the fiber structure changes during coating in ethanolic aqueous solutions (Fig. 2, SI 2). With an increasing amount of EtOH in the coating solution and the associated increase in the mass of catalyst applied, the fibers swell increasingly and adhere together. A difference in morphology between CN coated fibers (Fig. 2b–d), β-Bi2O3 coated fibers (Fig. 2e) and β-Bi2O3/CN (Fig. 2f) is detected. The CN particles cover larger areas of the substrate as a result of smaller size (SI 1), which is beneficial for the photocatalytic performance.
For comparison, a polymer fleece with embedded CN particles was prepared (CN-IF). The implementation of CN particles in the fiber is an already described method in the literature to functionalize electrospun fibers with a photocatalyst [39]. The CN particles were added to the polymer solution, which was used in the electrospinning process. To prevent sedimentation of the CN particles in the polymer solution during the spinning process a maximal loading of 0.3 wt% CN was possible which is equivalent to a loading of 0.23 mg of the catalyst per substrate area of 5.75 cm2. Due to the viscosity change by adding CN particles into the polymer solution the rotation speed of the collector drum had to be adjusted to 1000 rpm. SEM images (Fig. 3a) show a dense fleece structure with aligned fibers. The CN catalyst particles are visible on the fiber surface as grains with approximate size of 10–50 μm and thus their size did not change upon electrospinning (SI 1). Light microscope images under polarized light show regularly distributed catalyst particles as bright spots within the fiber matrix (Fig. 3b).
The influence of the photocatalyst coating on the absorption of visible light is demonstrated on the basis of diffuse UV–vis spectra (Fig. 4). In comparison to the uncoated fiber substrate the substrate CN-IF shows only a slight change in the absorption band at 370 nm compared to the band at 275 nm. A significant change of this absorption band is given for CN-ES2, β-Bi2O3-ES and β-Bi2O3/CN-ES caused by coating with the visible light photocatalyst. In addition samples containing β-Bi2O3 exhibit an additional broad absorption band in the region of 425 nm to 525 nm, which is in accordance to the UV–Vis spectrum of bulk β-Bi2O3 (SI 1).
2.3 Photocatalytic degradation of organic pollutants
The as prepared substrates were used for degradation experiments using RhB as model substance and CN materials (CN-IF, CN-ES1, CN-ES2, CN-ES3), β-Bi2O3 (β-Bi2O3-ES) and CN/β-Bi2O3 (β-Bi2O3/CN-ES) as photocatalysts (Fig. 5a). CN-ES2 and β-Bi2O3-ES, which show a comparable amount of deposited catalyst (1.7 mg), differ significantly in their photocatalytic behavior. While CN-ES2 degades 47% RhB within 8 h, β-Bi2O3-ES degrads only 31%, which demonstrates that the immobilized CN material shows higher photocatalytic activity compared to the immobilized β-Bi2O3. The better photocatalytic performance of CN materials compared to β-Bi2O3 is also verified for degradation experiments with particle dispersions (SI 3). The material combination of β-Bi2O3 and CN enhances the photocatalytic performance compared to pure β-Bi2O3 with a degrdation rate of 45%, but does not exceed that of CN-ES2. For β-Bi2O3/CN-ES a larger amount of RhB as compared to all other substrates is adsorbed in the dark phase, but this does not enhance the overall degradation rate. Noteworthy, CN-ES2 (1.7 mg) and CN-ES3 (2.4 mg) show similar degradatio rates with up to 47% RhB degradation while lowering the amount of catalyst for CN-ES1 (1.0 mg) shows a reduced dgradation rate of 28%. The substrate containing partly embedded CN (CN-IF) degrades only 18% RhB within 480 min irradiation time as a result of a lower number of active sites at the surface. A photosensitive degradation mechanism of the RhB molecule is detected for all samples by the hypsochromic shift of the absorption maximum of RhB. The shift is due to the stepwise de-ethylation of the RhB molecule. To initiate the photosensitive degradation mechanism, the RhB molecule has to be adsorbed onto the catalyst surface. By additional light-induced excitation of the RhB molecule, electrons are transferred from the HOMO to the LUMO of the dye and then to the conduction band of the photocatalyst material [52, 53]. The excitation and transfer of the charge carriers contribute significantly to degradation of RhB, which was outlined in a previous study [29]. The corresponding time-dependent RhB absorption diagram as a result of illumination is presented for CN-ES2 exemplarily (Fig. 5b). Thus, we conclude that in accordance with previous studies for CN and β-Bi2O3 particles, RhB degradation results from a combination of indirect photocatalytic oxidation and photosensitive decomposition [29, 36].
a Time-dependent photodegradation of an aqueous RhB solution (1 × 10−5 M) under visible light irradiation with a 300 W Xe-lamp (t > 0 min) without catalyst and in the presence of the CN-ES1, CN-ES2, CN-ES3, β-Bi2O3-ES, β-Bi2O3/CN-ES and CN-IF, b visible light spectra of RhB during the photodegradation process with CN-ES2
To compare the different samples and to select which substrate is best suited for further applications, the photocatalytic activity per applied catalyst mass and the photocatalytic activity per substrate area were evaluated (Fig. 6). The highest photocatalytic activity of all samples is determined for CN-IF (5.6 × 10−10 mol mg−1 min−1), but the overall degradation rate of CN-IF is low (Fig. 5). The photocatalytic activities of the CN-ES samples are lower with 2.0 × 10−10 mol mg−1 min−1 for CN-ES1 and CN-ES2, 1.4 × 10−10 mol mg−1 min−1 and for CN-ES3, but overall degradation is higher. For β-Bi2O3/CN-ES an activity of 1.6 × 10−10 mol mg−1 min−1 is calculated and for β-Bi2O3-ES the photocatalytic activity per catalyst mass is 1.3 × 10−10 mol mg−1 min−1. For the dip coated fleece substrates, the photocatalytic activity per mass decreases almost linearly with the increase of catalyst loading. However, the photocatalytic activity in relation to the substrate area (5.75 cm2) must also be taken into account, as it describes the area of potential activity of the coatings. Here, the lowest photocatalytic activity per substrate area is obtained for CN-IF with 2.2 × 10−11 mol cm−2 min−1. For CN-ES1 a value of 3.5 × 10−11 mol cm−2 min−1 is obtained, which is, however, lower than the value of 5.9 × 10−11 mol cm−2 min−1 as determined for CN-ES2 and CN-ES3. The value of β-Bi2O3/CN-ES is similar to CN-ES2 with 5.7∙10−11 mol∙cm−2∙min−1, while for β-Bi2O3-ES an activity per surface area of 3.9 × 10−11 mol cm−2 min−1 is calculated.
For further photocatalytic investigations, CN-ES2 was used, as the coated substrate represents the best compromise between photocatalytic activity per catalyst mass and area of activity of the substrate and shows one of the highest RhB degradation rates (Figs. 5 and 6).
A comparison with literature data is limited to rare examples, which e.g. show that a CN material, immobilized on a PVDF membrane, enabled RhB degradation of 80% within 24 h [54]. Bi2O3 fibers obtained after calcination at 500 °C were shown to degrade ca. 60% RhB after 120 min irradiation time (adsorption in the dark withdrawn) [40]. Both are of higher activity compared to the presented data. However, comparison is of limited value, because light source, reactor setup, measurements conditions, concentration of pollutant solutions and catalyst loadings are not identical but influence the photocatalytic performance significantly. In addition, the applied catalyst masses during immobilization process are not given in the references, which hinders the evaluation of photocatalytic activity (per mass or area).
In order to determine whether leaching of the photocatalysts may occur during catalysis, the substrates were stirred in the reactor for 8 h in water, analogously to the conditions used for the catalytic experiments. The non purgeable carbon content (NPOC) of the solution was determined. For comparison, the NPOC value of a water sample (118.7 µg L−1) and from a water sample which was in contact with an uncoated fleece was determined (814.5 ± 88 µg L−1, triple determination). The increase of the carbon content most probably results from the detachment of small fiber fragments from the fleece. The NPOC values for the CN modified fleeces were slightly higher with values between 865.0 and 958.2 µg L−1 (CN-ES1, 865.0 µg L−1; CN-ES2, 958.2 µg L−1; CN-ES3 873.7 µg L−1; CN-IF, 867.2 µg L−1) and it thus can be concluded that minor leaching cannot be excluded. To determine possible leaching for the β-Bi2O3 containing substrates (β-Bi2O3-ES, β-Bi2O3/CN-ES), the RhB solutions after catalysis were used to determine the bismuth content using ICP-MS. Bismuth was not detected. To investigate the cycle stability and to evaluate if further leaching of the coated fleeces occurs, several measurements (RhB degradation) were carried out with the same fleece (CN-ES2) (Fig. 7a). The degradation rate and rate constants decrease after the first cycle from intially 47% to 33% and from 2.2 × 10−5 s−1 to 1.4 × 10−5 s−1, respectively, in the second cycle, but remain almost unchanged and stable in the third cycle (Fig. 7b). The slight decrease of photocatalytic activity might be due to some minor detachment of fiber and catalyst fragments from the fleece substrate.
a Time-dependent cycling experiment for the photodegradation of an aqueous RhB solution (1 × 10-5 M) under visible light irradiation with a 300 W Xe-lamp (t > 0 min) without catalyst and in the presence of the CN-ES2, b semi logarithmic plots for the cycling experiment of the photodegradation of RhB with CN-ES2
Finally, the photocatalytic degradation efficiency for other pollutants such as triclosan and EE2 using CN-ES2 was evaluated (Fig. 8). EE2 is degraded to 29% within 480 min irradiation time, which corresponds to a photocatalytic activity of 5.0 × 10−10 mol mg−1 min−1, and triclosan is degraded to 50%, which corresponds to a photocatalytic activity of the coated fleece for triclosan of 7.1 × 10−10 mol mg−1 min−1. It should be emphasized, that the adsorption behavior of triclosan on the CN-ES2 substrate differs significantly compared to the adsorption behavior of RhB and EE2. During the dark phse, 34% of the triclosan adsorbs to the substrate surface, which indicates a strong interaction of the triclosan with the substrate. We assume that adsorption takes place at the polymer matrix, which contributes to the reduction of the amount of the pollutant in solution significantly.
3 Conclusions
Here we present a method to prepare fleeces with a size of 150 cm2 via electrospinning of PVAc fibers, which show potential as substrates for the immobilization of photocatalyst materials such as CN, β-Bi2O3 and a mixture of both. During the dip coating process, the fibers adhere to each other, which might offer the additional application as a filter medium. Commonly, such electrospun fibers are converted to inorganic fibers by thermal treatment. We have shown that the as-prepared PVAc mats show potential for photocatalysis applications in water without additional complex treatment. The observed minor leaching of organic products might require further adaption, but noteworthy leaching of bismuth oxide is not observed.
Embedding of CN particles directly into the fiber structure (CN-IF) during the spinning process was successful, but in comparison to fleeces with dip coated photocatalyst material (CN-ES) a low RhB degradation rate and photocatalytic activity per substrate area is observed (CN-ES2: 5.9 × 10−11 mol cm−2 min−1; CN-IF: 2.2 × 10−10 mol mg−1 min−1). Compared to CN-ES2 samples (2.0 × 10−10 mol mg−1 min−1), a lower photocatalytic activity for substrates coated with β-Bi2O3 (β-Bi2O3-ES 1.3 × 10−10 mol mg−1 min−1) is determined, which is as expected since degradation experiments with catalyst dispersions using the same reactor setup showed higher degradation rates for CN materials compared to β-Bi2O3 (SI 3) [29, 51]. The use of the mixture of β-Bi2O3 and CN (β-Bi2O3/CN-ES; 1.6 × 10−10 mol mg−1 min−1) did not enhance the degradation rates as expected, thus better absorption capability of the mixture or formation of a heterojunction, as reported earlier for this combination of materials, is not observed [28]. The most promising sample (CN-ES2) with high photocatalytic activity, high surface efficiency and high degradation rate was selected for the degradation experiments with EE2 and triclosan. Both substances were degraded substantially, whereby triclosan was degraded more efficiently.
The presented simple immobilization method offers the opportunity for the implementation of diverse photocatalyst materials on flexible PVAc substrates, which might be used as catalyst support in waste water treatment and other fields of applications such as CO2 conversion, water splitting or disinfection.
4 Experimental section
Catalyst preparation
Dicyandiamide (99% Co. Alfa Aesar) was dried with P2O5 for 5 days under vacuum before use. The dicyandiamide (1 g) is decomposed in a crucible (covered with a lid) at 550 °C for 4 h using a heating rate of 5 K min−1. The obtained yellow powder is grinded in a mortar and sieved to a particle size smaller than 100 μm. Detailed information on synthesis and characterization of the CN photocatalyst materials was reported in reference [50]. To obtain β-Bi2O3, the [Bi38O45(OMc)24(EtOH)13] cluster was synthesized according to reference [47] and hydrolyzed to obtain pure β-Bi2O3 according to [26, 51]. Detailed information about the characterization of these materials are given in [26, 51] and SI 1.
Electrospinning of PVAc fibers and coating with CN particles: An electrospinner (Co. Spraybase®) was used for the production of polymer fiber fleeces. A polymer solution of PVAc (MW = 55,000–70,000, Carl Roth) in a mass ratio of 1:2 was dissolved in ethyl acetate and spun into fibers on a rotating collector drum (80–1000 rpm), covered with aluminum foil. The distance between the collector and the needle with a diameter of 1.2 mm was 5 cm. Due to the horizontal movement of the needle during the spinning process, a large area was covered with statistically distributed polymer fibers. For spinning the large area fleece (8 cm × 30 cm), 15 kV was applied, the flow rate ranged between 30 and 100 µL min−1 and 8 mL of the polymer solution were spun. To ensure reproducibility and a sufficient thickness of the polymer fleece, only a central strip of 5 cm × 30 cm was used to cut the substrates (23 mm × 25 mm) for easier handling during the photocatalytic degradation measurements.
To coat the fleece substrates with the CN particles and β-Bi2O3, the substrates were dipped into 10 mL of an EtOH/water mixture for 5 min to allow swelling of the polymer fibers. To obtain a deposition of 1 mg CN onto the fleece substrate, a mixture or 1 vol% EtOH/water was used (CN-ES1). To deposit 2 mg CN or β-Bi2O3 (CN-ES2, β-Bi2O3-ES) a mixture of 5 vol% EtOH/water was used, and to deposit between 3 and 4 mg (CN-ES3) a mixture of 10 vol% EtOH/water was used. 100 mg of the CN particles or β-Bi2O3 were dispersed in the corresponding EtOH/water mixture for 5 min in an ultrasonic bath. Then, the fleece substrates were dipped into the CN dispersion, sonicated for 30 s and dried in air for 3 days. In order to remove only loosely adhering particles, the substrates were placed in water and subsequently in an ultrasonic bath for a few seconds. This is followed by a further drying step under ambient conditions. To coat a mixture of β-Bi2O3 and CN (β-Bi2O3 /CN-ES), 50 mg of β-Bi2O3 and 50 mg of CN were added to the 5 vol% EtOH/water mixture. To obtain PVAc fibers with embedded CN particles, 50 mg of sieved (<32 μm) CN particles were dispersed in 30 g ethyl acetate and sonicated for one hour in an ultrasonic bath. Then, 15 g PVAc were added and dissolved. The spinning process was carried out with a needle of 1.2 mm in diameter, a flow rate of 50 µL min−1 at 15 kV, a spinning distance of 5 cm and a rotation speed of 1000 rpm (CN-IF). The as prepared substrate includes 0.3 wt% CN material, which corresponds to a mass of 0.23 mg per substrate. For detailed electrospinning and deposition conditions see SI 4.
Evaluation of photocatalytic activity
The photocatalytic activity of the as prepared fleece coatings was evaluated in a water cooled glass reactor (T = 15 °C) using the coated fleece material and 35 mL of an aqueous solution of 1 × 10− 5 M RhB (triclosan and EE2 with 4 × 10− 5 M). The reactor is equipped with a 300 W xenon lamp (type Cermax® VQTM ME300BF, Co. Perkin Elmer, intensity of ~ 1839 W m− 2). A hot mirror filter (λ ≤ 700 nm) is located within a distance of 12.3 cm at one side of the reactor and directly illuminates an area of 4.5 cm2. A cut-off filter (λc (τi = 0.50) = 420 ± 6 nm, GG420, Co. Schott) is used to remove the UV light. Before illumination, the solutions are stirred for a minimum of 30 min in the dark to establish the adsorption-desorption equilibrium of the dye at the catalyst surface. The progress of photodegredation and the adjustment of the adsorption-desorption equilibrium is studied by in situ UV–Vis spectroscopy. The illumination process is interrupted by stopping to stir and darkening the light beam by a cover prior to the UV–Vis measurement. The interval of measurement is timed every 10 min for the first 30 min, every 15 min between 30 and 90 min, every 30 min between 90 and 180 min and every 60 min to the last measurement after 480 min. The concentration of the pollutant is determined by calculating the area under the UV–Vis curve from 450 to 600 nm for RhB, for the triclosan and EE2 in the area between 200 and 300 nm. The photodegradation is plotted as a function of the irradiation time. The photocatalytic activity is calculated according to Eq. (1).
Here, c0 is the initial concentration of the degradation solution used, V is the volume of degradation solution used, XPC is the conversion in moles during photocatalysis at the time tPC and mcat is the catalyst mass used. The determination of the photocatalytic activity for coatings is carried out by means of the conversion after 480 min irradiation time. The photocatalytic activity per substrate area is calculated in a similar way, but the catalyst mass mcat is substituted to the substrate area of 5.75 cm2.
Material characterization
The light microscope images are obtained using an Axio Scope.A1 (Co. Zeiss), equipped with a HBO 100 illuminator and halogen lamp Hal 100. SEM images were obtained using a XL 30 (Co. Philips) with a working distance between 10 and 11 cm and an acceleration voltage of 5–30 kV. The samples were sputtered with gold to enhance the conductivity during the SEM measurements. Diffuse reflectance UV-Vis spectroscopy was performed using a Carry 60 UV–Vis (Co. Agilent Technologies) equipped with a Barrelino™ (Co. Harrick Scientific Products) remote diffuse reflection probe. The band gap Eg of the semiconductor was estimated according to the equation \( (\alpha h \nu)^{1/n} = A \cdot (h \nu - E_g)\) where α is the absorption coefficient of the material, hν is the photon energy and A represents a proportionality constant. For a direct band gap n = 0.5, for determination of an indirect band gap n = 2 [55]. In accordance to literature data [25, 56] direct band gaps were assumed for all materials. In order to detect leaching of the immobilized CN particles on different fleeces (CN-ES1, CN-ES2, CN-ES3, CN-IF), corresponding substrates and an uncoated substrate are stirred as a reference sample in 35 mL ultrapure water for 8 h (analogous to catalysis conditions). In addition, a sample of ultrapure water is stirred under the same conditions to obtain a comparative value for the carbon content of the ultrapure water and organic impurities that diffuse in during stirring. 20 mL of the respective water samples are then transferred to a measuring vessel and acidified with 100 µL of a 2 N HCl. The subsequent analysis of the NPOC content is carried out using a TOC analyzer (Co. Analytik Jena). The ICP-MS measurements to analyze if bismuth oxide leaching occurs were carried out by adding 1 mL of 63% HNO3 to 35 mL o the degraded solution (RhB; 1 × 10− 5 M) after an irradiation time of 480 min and usage of β-Bi2O3/CN and β-Bi2O3 as catalysts in order to convert leached out bismuth oxide into soluble compounds. The samples were analyzed by Berghof Analytik + Umweltengineering GmbH. A sample after a photolysis measurement was also analyzed. Photoluminescence measurements were carried out using a FluorMax 4 (Co. Hobira), equipped with a 150 W Xe lamp and an excitation wavelength of 350 nm. Powder X-ray diffraction (XRD) was carried out with a STOE-STADI-P diffractometer equipped with a Ge(111)-monochromator. The X-ray source was CuKα1-radiation (40 kV, 40 mA).
References
Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R (2014) Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infec Dis 14:742–750. https://doi.org/10.1016/s1473-3099(14)70780-7
Fick J, Söderström H, Lindberg RH, Phan C, Tysklind DG, Larsson DGJ (2009) Contamination of surface, ground, and drinking water from pharmaceutical production. Environ Toxicol Chem 28:2522–2527. https://doi.org/10.1897/09-073.1
Ahmed S, Rasul MG, Martens WN, Brown R, Hashib MA (2010) Heterogeneous photocatalytic degradation of phenols in wastewater: a review on current status and developments. Desalination 261:3–18. https://doi.org/10.1016/j.desal.2010.04.062
Rao N (2003) Photocatalytic degradation of 2-chlorophenol: a study of kinetics, intermediates and biodegradability. J Hazard Mater 101:301–314. https://doi.org/10.1016/s0304-3894(03)00180-8
Madima N, Mishra SB, Inamuddin I, Mishra AK (2020) Carbon-based nanomaterials for remediation of organic and inorganic pollutants from wastewater. A review. Environ Chem Lett 18:1169–1191. https://doi.org/10.1007/s10311-020-01001-0
Mahlambi MM, Ngila CJ, Mamba BB (2015) Recent developments in environmental photocatalytic degradation of organic pollutants: the case of titanium dioxide nanoparticles—a review. J Nanomater 2015:1–29. https://doi.org/10.1155/2015/790173
Snyder SA, Westerhoff P, Yoon Y, Sedlak DL (2004) Pharamceuticals, personal care products, and endocrine disruptors in water: implications for the water industry. Environ Eng Sci 20:449–469. https://doi.org/10.1089/109287503768335931
Wang R, Ma X, Liu T et al (2020) Degradation aspects of endocrine disrupting chemicals: a review on photocatalytic processes and photocatalysts. Appl Catal A 597:117547. https://doi.org/10.1016/j.apcata.2020.117547
Hong W, Li C, Tang T et al (2021) The photocatalytic activity of the SnO2/TiO2/PVDF composite membrane in rhodamine B degradation. New J Chem 45:2631–2642. https://doi.org/10.1039/d0nj04764c
Weatherly LM, Gosse JA (2017) Triclosan exposure, transformation, and human health effects. J Toxicol Environ Health B Crit Rev 20:447–469. https://doi.org/10.1080/10937404.2017.1399306
Adeel M, Song X, Wang Y, Francis D, Yang Y (2017) Environmental impact of estrogens on human, animal and plant life: a critical review. Environ Int 99:107–119. https://doi.org/10.1016/j.envint.2016.12.010
Malato S, Blanco J, Vidal A, Alarcón D, Maldonado MI, Cáceres J, Gernjak W (2003) Applied studies in solar photocatalytic detoxification: an overview. Sol Energy 75:329–336. https://doi.org/10.1016/j.solener.2003.07.017
Beydoun D, Amal R, Low, G, McEvoy G, S (1999) Role of nanoparticles in photocatalysis. J Nanopart Res 1:439–458. https://doi.org/10.1023/A:1010044830871
Wankhade AV, Gaikwad, GS, Dhonde MG, Khaty NT, Thakare SR (2013) Removal of organic pollutant from water by heterogenous photocatalysis: a review. Res J Chem Environ 17:84–94
Alharbi NS, Hu B, Hayat T, Rabah SO, Alsaedi A, Zhuang L, Wang X (2020) Efficient elimination of environmental pollutants through sorption-reduction and photocatalytic degradation using nanomaterials. Front Chem Sci Eng 14:1124–1135. https://doi.org/10.1007/s11705-020-1923-z
Qi K, Liu S-y, Zada A (2020) Graphitic carbon nitride, a polymer photocatalyst. J Taiwan Inst Chem Eng 109:111–123. https://doi.org/10.1016/j.jtice.2020.02.012
Jack RS, Ayoko GA, Adebajo MO, Frost RL (2015) A review of iron species for visible-light photocatalytic water purification. Environ Sci Pollut Res 22:7439–7449. Doi:https://doi.org/10.1007/s11356-015-4346-5
Kumar SG, Devi LG (2011) Review on modified TiO2 photocatalysis under UV/visible light: selected results and related mechanisms on interfacial charge carrier transfer dynamics. J Phys Chem A 115:13211–13241. https://doi.org/10.1021/jp204364a
Ohtani B (2010) Photocatalysis A to Z: what we know and what we do not know in a scientific sense. J Photochem Photobiol C 11:157–178. https://doi.org/10.1016/j.jphotochemrev.2011.02.001
Saber AT, Mortensen A, Szarek J et al (2019) Toxicity of pristine and paint-embedded TiO2 nanomaterials. Hum Exp Toxicol 38:11–24. https://doi.org/10.1177/0960327118774910
Fu J, Yu J, Jiang C, Cheng B (2018) g-C3N4-based heterostructured photocatalysts. Adv Energy Mater 8:1701503. https://doi.org/10.1002/aenm.201701503
Kumar S, Karthikeyan S, Lee A (2018) g-C3N4-based nanomaterials for visible light-driven photocatalysis. Catalysts 8:74. https://doi.org/10.3390/catal8020074
Saleem Z, Pervaiz E, Usman Yousaf M, Bilal Khan Niazi M (2020) Two-dimensional materials and composites as potential water splitting photocatalysts: a review. Catalysts 10:464. https://doi.org/10.3390/catal10050464
Chebanenko MI, Zakharova NV, Popkov VI (2020) Synthesis and visible-light photocatalytic activity of graphite-like carbon nitride nanopowders. Russ J Appl Chem 93:494–501. https://doi.org/10.1134/S1070427220040035
Hofmann M, Rößner L, Armbrüster M, Mehring M (2020) Thin coatings of α- and β‐Bi2O3 by ultrasonic spray coating of a molecular bismuth oxido cluster and their application for photocatalytic water purification under visible light. ChemistryOpen 9:277–284. https://doi.org/10.1002/open.201900323
Schlesinger M, Weber M, Schulze S, Hietschold M, Mehring M (2013) Metastable beta-Bi2O3 nanoparticles with potential for photocatalytic water purification using visible light irradiation. ChemistryOpen 2:146–155. https://doi.org/10.1002/open.201300013
Li Y, Li X, Zhang H, Xiang Q (2020) Porous graphitic carbon nitride for solar photocatalytic applications. Nanoscale Horiz 5:765–786. https://doi.org/10.1039/d0nh00046a
Liu S, Chen J, Xu D, Zhang X, Shen M (2018) Enhanced photocatalytic activity of direct Z-scheme Bi2O3/g-C3N4composites via facile one-step fabrication. J Mater Res 33:1391–1400. https://doi.org/10.1557/jmr.2018.67
Köwitsch I, Mehring M (2021) Carbon nitride materials: impact of synthetic method on photocatalysis and immobilization for photocatalytic pollutant degradation. J Mater Sci 56:18608–18624. https://doi.org/10.1007/s10853-021-06405-z
Bharathi S, Nataraj D, Mangalaraj D, Masuda Y, Senthil K, Yong K (2010) Highly mesoporous α-Fe2O3 nanostructures: preparation, characterization and improved photocatalytic performance towards Rhodamine B (RhB). J Phy D Appl Phys 43:015501. https://doi.org/10.1088/0022-3727/43/1/015501
Barrio J, Volokh M, Shalom M (2020) Polymeric carbon nitrides and related metal-free materials for energy and environmental applications. J Mater Chem A 8:11075–11116. https://doi.org/10.1039/d0ta01973a
Kreuzinger N, Liebmann B, Fürhacker M (2016) Synthetische Nanopartikel in der Abwasserreinigung. Österr Wasserund Abfallw 69:34–42. https://doi.org/10.1007/s00506-016-0363-y
Chen H, Huang M, Liu Y, Meng L, Ma M (2020) Functionalized electrospun nanofiber membranes for water treatment: a review. Sci Total Environ 739:139944. https://doi.org/10.1016/j.scitotenv.2020.139944
Deitzel JM, Kleinmeyer J, Harris D, Beck Tan NC (2001) The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 42:261–272. https://doi.org/10.1016/S0032-3861(00)00250-0
Ratshiedana R, Kuvarega AT, Mishra AK (2021) Titanium dioxide and graphitic carbon nitride-based nanocomposites and nanofibres for the degradation of organic pollutants in water: a review. Environ Sci Pollut Res 10357–10374. https://doi.org/10.1007/s11356-020-11987-3
Ray SS, Chen S-S, Li C-W, Nguyen NC, Nguyen HT (2016) A comprehensive review: electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv 6:85495–85514. https://doi.org/10.1039/c6ra14952a
Panthi G, Park M, Kim H-Y, Lee S-Y, Park S-J (2015) Electrospun ZnO hybrid nanofibers for photodegradation of wastewater containing organic dyes: a review. J Ind Eng Chem 21:26–35. https://doi.org/10.1016/j.jiec.2014.03.044
Cui Y, Jiang Z, Xu C, Zhu M, Li W, Wang C (2021) Preparation, filtration, and photocatalytic properties of PAN@g-C3N4 fibrous membranes by electrospinning. RSC Adv 11:19579–19586. https://doi.org/10.1039/d1ra03234h
Adhikari SP, Awasthi GP, Kim HJ, Park CH, Kim CS (2016) Electrospinning directly synthesized porous TiO2 nanofibers modified by graphitic carbon nitride sheets for enhanced photocatalytic degradation activity under solar light irradiation. Langmuir 32:6163–6175. https://doi.org/10.1021/acs.langmuir.6b01085
Wang C, Shao C, Wang L, Zhang L, Li X, Liu Y (2009) Electrospinning preparation, characterization and photocatalytic properties of Bi2O3 nanofibers. J Colloid Interface Sci 333:242–248. https://doi.org/10.1016/j.jcis.2008.12.077
Wang M, Liu Z, Fang M et al (2016) Enhancement in the photocatalytic activity of TiO2 nanofibers hybridized with g-C3N4 via electrospinning. Solid State Sci 55:1–7. https://doi.org/10.1016/j.solidstatesciences.2016.02.002
Tang Q, Meng X, Wang Z, Zhou J, Tang H (2018) One-step electrospinning synthesis of TiO2 /g-C3N4 nanofibers with enhanced photocatalytic properties. Appl Surf Sci 430:253–262. https://doi.org/10.1016/j.apsusc.2017.07.288
Wang Y, Sun J, Li J, Zhao X (2017) Electrospinning preparation of nanostructured g-C3N4/BiVO4 composite films with an enhanced photoelectrochemical performance. Langmuir 33:4694–4701. https://doi.org/10.1021/acs.langmuir.7b00893
Khalifa M, Mahendran A, Anandhan S (2019) Synergism of graphitic-carbon nitride and electrospinning on the physico-chemical characteristics and piezoelectric properties of flexible poly(vinylidene fluoride) based nanogenerator. J Polym Res 26:73. https://doi.org/10.1007/s10965-019-1738-0
Xu T, Wu F, Gu Y et al (2015) Visible-light responsive electrospun nanofibers based on polyacrylonitrile-dispersed graphitic carbon nitride. RSC Adv 5:86505–86512. https://doi.org/10.1039/c5ra15973c
Shen X, Zhang T, Xu P, Zhang L, Liu J, Chen Z (2017) Growth of C3N4 nanosheets on carbon-fiber cloth as flexible and macroscale filter-membrane-shaped photocatalyst for degrading the flowing wastewater. Appl Catal B 219:425–431. https://doi.org/10.1016/j.apcatb.2017.07.059
Weber M, Thiele G, Dornsiepen E, Weimann DP, Schalley CA, Dehnen S, Mehring M (2018) Impact of the exchange of the coordinating solvent shell in [Bi38O45(OMc)24(dmso)9] by alcohols: crystal structure, gas phase stability, and thermoanalysis. Z Anorg Allg Chem 644:1796–1804. https://doi.org/10.1002/zaac.201800350
Haider A, Haider S, Kang I-K (2018) A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab J Chem 11:1165–1188. https://doi.org/10.1016/j.arabjc.2015.11.015
Pillay V, Dott C, Choonara YE et al (2013) A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J Nanomater 2013:1–22. https://doi.org/10.1155/2013/789289
Köwitsch I, Mehring M (2021) Coatings of magnetic composites of iron oxide and carbon nitride for photocatalytic water purification. RSC Adv 11:14053–14062. https://doi.org/10.1039/D1RA00790D
Schlesinger M, Schulze S, Hietschold M, Mehring M (2013) Metastable beta-Bi2O3 nanoparticles with high photocatalytic activity from polynuclear bismuth oxido clusters. Dalton Trans 42:1047–1056. https://doi.org/10.1039/c2dt32119j
Wang Q, Chen C, Zhao D, Ma W, Zhao J (2008) Change of adsorption modes of dyes on fluorinated TiO2 and its effect on photocatalytic degradation of dyes under visible irradiation. Langmuir 24:7338–7345. https://doi.org/10.1021/la800313s
Watanabe T, Takizawa T, Honda K (1977) Photocatalysis through excitation of adsorbates. 1. Highly efficient N-deethylation of rhodamine B adsorbed to cadmium sulfide. J Phys Chem 81:1845–1851. https://doi.org/10.1021/j100534a012
Kolesnyk I, Kujawa J, Bubela H, Konovalova V, Burban A, Cyganiuk A, Kujawski W (2020) Photocatalytic properties of PVDF membranes modified with g-C3N4 in the process of Rhodamines decomposition. Sep Purif Technol 250:117231. https://doi.org/10.1016/j.seppur.2020.117231
Tauc J, Grigorovici R, Vanou A (1966) Optical properties and electronic structure of amorphous germanium. Phys Stat Sol 15:627–637. https://doi.org/10.1002/pssb.19660150224
Algara-Siller G, Severin N, Chong SY et al (2014) Triazine-based graphitic carbon nitride: a two-dimensional semiconductor. Angew Chem Int Ed 53:7450–7455. https://doi.org/10.1002/anie.201402191
Acknowledgements
We are grateful to the Europäische Sozialfonds (ESF) for financial support (Isabel Köwitsch; Project Number 100 327 773). We also thank Prof. Dr. Marc Armbrüster for access to the light microscope and Nicolas Köwitsch and Judith Streif for SEM measurements. We thank Prof. Dr. Werner Goedel, Nicole Behme and Helen Hintersatz for sputtering the samples and Rico Thomas for photoluminescence measurements and PXRD.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Köwitsch, I., Schäfer, A., Hornig, D. et al. Photocatalytic water purification under visible light using carbon nitride materials and β-Bi2O3 immobilized on electrospun polyvinyl acetate fibers. SN Appl. Sci. 4, 64 (2022). https://doi.org/10.1007/s42452-022-04945-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-04945-w