Abstract
In this paper, four novel kinds of triazine-trione based tri-imidazole derivatives (IM-TT, 2MI-TT, 2EI-TT and EMI-TT) were synthesized through the addition reaction between triglycidyl isocyanurate (TGIC) and imidazole (IM), 2-methylimidazole (2MI), 2-ethylimidazole (2EI) and 2-ethyl-4-methylimidazole (EMI), respectively. The triazine-trione based tri-imidazole derivatives were blended with epoxy resin and the reactivity, thermal latency and thermal property were investigated. The results on curing behaviors indicated that the curing exothermic peaks of the blends with triazine-trione based tri-imidazole derivatives shifted to higher temperatures compared with those with commercial imidazoles. The curing exothermic peak temperatures (Tps) of the synthesized tri-imidazole derivatives were increased by 23–32 ℃ compared with the unmodified imidazoles. In addition, Rheological behavior results indicated that the EP blends with tri-imidazole derivatives also exhibited excellent storage stability which was as long as 38 days under room temperature. Last but not the least, the EP blends with triazine-trione based tri-imidazole derivatives also exhibited high glass transition temperatures due to introducing of triazine-trione structures with high crosslinking density. The glass transition temperatures (Tgs) of the prepared thermosets ranged from 128 to 152 ℃. The triazine-trione based tri-imidazole derivatives provide a way to prepare latency epoxy resin with high high glass transition temperature and long storage stability.
Article Highlights
-
Four novel kinds of triazine-trione based tri-imidazole derivatives were synthesized.
-
The EP cured with the tri-imidazole derivatives displayed great thermostability.
-
The EP cured with the tri-imidazole derivatives exhibited long storage stability.
Similar content being viewed by others
1 Introduction
For several decades, epoxy resin (EP) has been used as coating or adhesive, and developed as fiber reinforced composite material due to its excellent comprehensive performances [1,2,3,4,5]. The properties of EP systems especially curing behaviors depend on the curing agents. The commercial curing agents are amines, acid anhydrides, phenols, imidazoles and others [6,7,8,9]. EP with latent curing agents exhibit long shelf life at the room temperature and fast curing ability at high temperature. Those EP which is called one-component epoxy resin system is widely developed as the prepreg, due to its long shelf life, less volatile organic compounds and stable product quality. Therefore, latent curing agents have been investigated by researchers.
Compared with amine-cured EP, imidazole cured EP exhibits wider curing temperature, lower tensile elongation, higher modulus and higher heat resistance [10,11,12]. EP mixed with imidazole or its derivatives can form crosslinked networks within minutes, through anionic chain polymerization of epoxy groups [13,14,15]. The high curing activity of common imidazole derivatives prevent it from forming one-component epoxy resin system. The previous studies indicated that 1-position and 3-position nitrogen atoms of imidazole ring react with other functional groups can effectively reduce the curing activity of imidazole derivatives. As one kind of lewis base, 3-position nitrogen atoms of imidazole ring could react with some protonic acid or lewis acid, such as carboxylic acids and metal cations, to form imidazolium salts or imidazolium ionic liquids [16,17,18,19]. Besides, some researchers utilized the active hydrogen atom of 1-position nitrogen atoms on imidazole ring to prepared a series of latent imidazole curing agents by reacting common imidazoles with epoxy group, acyl chloride, lewis acid, carbon–carbon double bond and phosphoryl chloride [20,21,22,23,24].
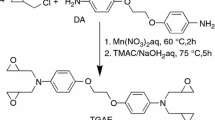
In this paper, the novel imidazole derivatives with triazine-trione structure are synthesis through the addition reaction between triglycidyl isocyanurate (TGIC) and imidazole (IM), 2-methylimidazole (2MI), 2-ethylimidazole (2EI) and 2-ethyl-4-methylimidazole (EMI), respectively. Due to the incorporation of triazine-trione structure with electron withdrawing effect, the curing activity of imidazole derivatives are reduced, and the one-component epoxy resin system with those imidazole derivatives exhibit better thermal latency. The curing behaviors, thermal property and storage stability of the EP blends with triazine-trione based tri-imidazole derivatives were investigated.
2 Experimental
2.1 Materials
Diglycidyl ether of bisphenol-A (DGEBA, epoxide equivalent weight: ~ 188 g/equiv) was supported by Yueyang Baling Huaxing Petrochemical Co., Ltd. Triglycidyl isocyanurate (TGIC, purity ≥ 98%) was supported by Jinan Zian Chemicals Co., Ltd. Imidazole (IM, purity ≥ 98%), 2-Methylimidazole (2MI, purity ≥ 98%), 2-Ethylimidazole (2EI, purity ≥ 98%), 2-Ethyl-4-methylimidazole (EMI, purity ≥ 98%) and dimethylformamide (DMF, purity ≥ 99.9%) were supported from Aladdin Reagents (Shanghai) Co., Ltd. All the reagents were used as received.
2.2 Preparation of triazine-trione based tri-imidazole derivatives
2.2.1 Preparation of IM-TT
20.4 g (0.3 mol) IM and 500 mL DMFwas added into a three-neck glass flask. The temperature was heated to 80 °C. Then 29.7 g (0.1 mol) TGIC was introduced within 1 h. The blend was further heated to 120 °C and stirred at the temperature for 3 h. Then, the solution was distilled to remove DMF, and viscous liquid product was obtained. Yield: 92%.
2.2.2 Preparation of 2MI-TT
24.6 g (0.3 mol) 2MI and 500 mL DMF was added into flask, which was similar to the synthesis of IM-TT, differently when the 29.7 g (0.1 mol) TGIC was introduced within 1 h. The blend was further heated to 120 °C and stirred at the temperature for 5 h. Then, the solution was distilled to remove DMF, and viscous liquid product was obtained. Yield: 94%.
2.2.3 Preparation of 2EI-TT
28.8 g (0.3 mol) 2EI and 500 mL DMF was added into flask, which was similar to the synthesis of IM-TT, differently the 29.7 g(0.1 mol) TGIC was introduced within 1.5 h, when the temperature was heated to 90 °C. The blend was further heated to 130 °C and stirred at the temperature for 5 h. Then, the solution was distilled to remove DMF, and viscous liquid product was obtained. Yield: 96%.
2.2.4 Preparation of EMI-TT
33 g (0.3 mol) EMI and 500 mL DMF was added into flask, which was similar to the synthesis of IM-TT, differently the 29.7 g(0.1 mol) TGIC was introduced within 2 h, when the temperature was heated to 100 °C. The blend was further heated to 140 °C and stirred at the temperature for 8 h. Then, the solution was distilled to remove DMF, and viscous liquid product was obtained. Yield: 91%.
The reaction is summarized in Scheme 1.
2.3 Preparation of EP blends
2.3.1 Preparation of EP/EMI, EP/IM-TT, EP/2MI-TT, EP/2EI-TT and EP/EMI-TT blends
For the viscous liquid imidazole derivatives, EMI, IM-TT, 2MI-TT, 2EI-TT and EMI-TT were respectively blended with DGEBA immediately by mechanical stirring with different ratios.
2.3.2 Preparation of EP/IM, EP/2MI and EP/2EI blends
For the crystal curing agent, IM, 2MI and 2EI were respectively dispersed in DGEBA at different ratio by milling.
2.4 Characterization
Fourier Transform Infrared (FTIR) was tested by a Nicolet 6700 infrared spectrometer. The sample was blended with KBr and pressed into pellets.
-
1H NMR spectra was tested by Bruker AV400 NMR with DMSO-d6 as the solvent.
-
Differential scanning calorimetry (DSC) was tested by Perkin-Elmer DSC 4000 with N2 atmosphere.
-
Dynamic mechanical analysis (DMA) was determined using a PerkinElmer Diamond from 30 to 250 \(^\circ \mathrm{C}\) with a heating rate 10 \(^\circ \mathrm{C}\)/min with the frequency 1 Hz.
-
Viscosity data were tested by ARES dynamic rotational rheometer.
3 Results and discussion
3.1 Characterization of triazine-trione based tri-imidazole derivatives
To confirm the structure of the triazine-trione based tri-imidazole derivatives, FTIR and NMR were tested. The FTIR characteristic absorption peaks were shown in Fig. 1. The peaks at 1740 and 1670 cm−1 were assigned to the stretching vibration for C=O [23]; the peak appeared at 1435 cm−1 was assigned to the stretching vibration for C–N [22]; those characteristic absorption peaks indicated that the presence of triazine-trione structures in tri-imidazole derivatives. The peaks at 3000–3500 cm−1 were attributed to the presence of hydroxyl [24]; the peaks at 2900–3000 cm−1 were attributed to the stretching of the imidazole ring and the aliphatic chain for C–H [17]; those characteristic absorption peaks indicated that IM, 2MI, 2EI and EMI were reacted with epoxy group of TGIC through nucleophilic addition reaction.
1H NMR spectra of IM-TT, 2MI-TT, 2EI-TT and EMI-TT were shown in Figs. 2 and 3 to further confirm the structure of tri-imidazole derivatives. The chemical shifts at about 11–12 ppm disappeared, which were attributed to the shifts of the N–H for IM, 2MI, 2EI and EMI, that was due to the addition reaction of N–H with epoxy group. Owing to the effect of triazine-trione structure, the chemical shifts of aromatic hydrogen of imidazole ring moved to lower chemical shift.
For IM-TT, 7.64, 7.57 and 7.18 ppm are attributed to the imidazole ring; 6.92 ppm is attributed to the hydroxyl group; 4.72–5.43 ppm is attributed to the CH linked with hydroxyl group; 4.14–4.38 ppm is attributed to the CH2 linked with imidazole moiety; 3.13–3.20 and 3.43–3.59 ppm are attributed to the CH2 linked with triazine-trione ring.
For 2MI-TT, 7.61 and 7.06 ppm are attributed to the imidazole ring; 6.75 ppm is attributed to the hydroxyl group; 4.45–5.27 ppm is attributed to the chemical shift of CH linked with hydroxyl group; 4.02–4.29 ppm is attributed to the CH2 linked with imidazole moiety; 3.11–3.23 and 3.42–3.63 ppm are attributed to the CH2 linked with triazine-trione ring. 2.29 ppm is attributed to the chemical shift of CH3 linked with imidazole ring.
For 2EI-TT, 7.60 and 7.07 ppm are attributed to the imidazole ring; 6.78 ppm is attributed to the c hydroxyl group; 4.45–5.25 ppm is attributed to the CH linked with hydroxyl group; 4.02–4.34 ppm is attributed to the CH2 linked with imidazole moiety; 3.07–3.27 and 3.41–3.62 ppm are attributed to the CH2 linked with triazine-trione ring; 2.63 ppm is attributed to the CH2 in ethyl linked with imidazole ring; 1.20 ppm is attributed to the CH3 in ethyl linked with imidazole ring.
For EMI-TT, 7.60 ppm is attributed to the imidazole ring; 6.74 ppm is attributed to the chemical shift of hydroxyl group; 4.48–5.23 ppm is attributed to the CH linked with hydroxyl group; 4.02–4.28 ppm is attributed to the CH2 linked with imidazole moiety; 3.05–3.29 and 3.42–3.58 ppm are attributed to the CH2 linked with triazine-trione ring; 2.59 ppm is attributed to the CH2 in ethyl linked with imidazole ring; 2.03 ppm is attributed to the methyl linked with imidazole ring; 1.18 ppm is assigned to the CH3 in ethyl linked with imidazole ring.
3.2 Curing behavior
The EP blends with different ratio of imidazole or tri-imidazole derivatives as curing agents are heat from 60 to 200 °C with the heating rate of 10 °C/min. The DSC curves of the EP blends are shown in Figs. 4 and 5, and the exothermic parameters are shown in Table 1.
The EP blended with commercial imidazoles exhibited two exothermic peaks. The first exothermic peak was attributed to the addition reaction between the N–H of imidazole ring and epoxy group. The second exothermic peak was attributed to the anionic polymerizations of epoxy groups initiated by the imidazole structures [25, 26].
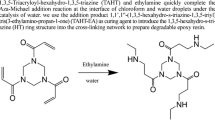
Differently, the EP blends with triazine-trione based tri-imidazole derivatives exhibited only one exothermic peak, which was attributed to the anionic polymerizations of epoxy groups. In addition, the curing exothermic peaks shifted to lower temperature ranges and became narrower and sharper with the promotion of imidazole curing agents. The total exothermic heat was increased with the promotion of imidazole curing agents, which indicated that the epoxy groups were not consumed end at low concentration of imidazole curing agents. Compared with the EP blends with commercial imidazoles, it was found that the curing exothermic peaks moved to higher temperature ranges for the EP blends with triazine-trione based tri-imidazole derivatives. The electron withdrawing effect of introduced triazine-trione structure reduced the nucleophilicity of imidazole moiety, and thus restrained the curing activity. Hence, higher temperature and more activation energy were needed to initiate the curing reaction.
The DSC curves of the EP blends were tested with the heating rate of 5, 10, 15, and 20 °C/min, respectively, to investigate the curing kinetic parameters of the EP blends with the highest content of imidazole curing agents. The curing kinetic parameters were calculated through Kissinger method. The curing kinetic parameters of the prepared EP blends are shown in Table 2. As shown in Figs. 6 and 7, the exothermic peaks of all the EP blends moved to higher temperatures with the promoting of heating rate. The activation energy values of the EP blends with triazine-trione based tri-imidazole derivatives were much higher than the EP blends with commercial imidazoles, which was indicated that the triazine-trione based tri-imidazole derivatives exhibited lower curing activity which was due to the curing reaction energy barrier of triazine-trione structure.
The reactivity of triazine-trione based tri-imidazole derivatives were further investigated by isothermal DSC method with different curing temperatures. The Isothermal DSC curves of the EP blends were summarized in Fig. 8. First of all, when the curing temperature was 70 °C, no exothermic peak was observed within 60 min for the tests, which indicated that triazine-trione based tri-imidazole derivatives exhibited good thermal latency within 70 °C. While the curing temperature raised form 120 to 140 °C, the exothermic peaks were observed and became flat within 10–15 min, which indicated that triazine-trione based tri-imidazole derivatives exhibited fast curing ability at high temperature.
To further investigate the curing speed of the EP blends with triazine-trione based tri-imidazole derivatives, the curses of conversion rate of the EP blends were summarized in Fig. 9, which were tested through integral of total curing exothermic energy for DSC test. The EP blends with IM-TT cured within 1.5 min at 140 °C, and the EP blends with 2EI-TT cured within 3 min. As the curing temperature lower to 135 °C, the curing time get longer. For all of the EP blends with triazine-trione based tri-imidazole derivatives cured with complete conversion of EP group among 135 °C and 140 °C, which indicated that triazine-trione based tri-imidazole derivatives exhibited higher fast curing ability at higher temperatures.
3.3 Storage stability of EP blends
To evaluate the storage stability of the EP blends, the promotion of the viscosity for the EP blends at room temperature were tested. When the viscosity for the EP blends doubled as the initial viscosity, the shelf life was identified as end. As shown in Fig. 10a, b, the viscosity values of the EP blends with triazine-trione based tri-imidazole derivatives were almost unchanged over one week and the shelf life were reached as 22 days for EP/IM-TT, 25 days for EP/2MI-TT, 38 days for EP/2EI-TT, 32 days for EP/EMI-TT. The results indicated that the EP blends with triazine-trione based tri-imidazole derivatives exhibited long shelf life at room temperature, which was an ideal way to prepare epoxy resin based prepregs.
3.4 Thermal property
The glass transition temperatures (Tgs) of the EP blends with triazine-trione based tri-imidazole derivatives were measured by DMA and the curves of storage modulus and tan D were summarized in Figs. 11 and 12. The Tg was characterized by the peak temperature of tan \(\mathrm{D}\). The EP/IM-TT reached the highest Tg as 152 °C, while EP/2MI-TT get the Tg as 128 °C. The Tgs of triazine-trione based tri-imidazole derivatives cured EP blends were higher than those EP blends cured by methyhetrahydrophthalic anhydride or m-xylylenediamine [27, 28]. The results indicated that triazine-trione based tri-imidazole derivatives led to forming of EP crosslink network during the curing processing. The crosslink density of the EP blends reduced, which was due to the ester exchanging reaction between triazine-trione structure and epoxy group, and resulted in Tgs reduced to lower temperature.
4 Conclusions
The synthesis of novel triazine-trione based tri-imidazole derivatives were successful. The curing activity of triazine-trione based tri-imidazole derivatives reduced for the incorporation of the electrophilic triazine-trione structure. The triazine-trione based tri-imidazole derivatives in the EP blends exhibited good thermal latency within 70 °C, and the EP blends exhibited long shelf life at room temperature. In addition, the EP blends also exhibited fast curing ability under heating condition, due to the triazine-trione based tri-imidazole derivatives overcame curing reaction energy barrier at high temperature. What’s more, the Tgs of cured EP blends with triazine-trione based tri-imidazole derivatives were among 128–152 °C. Those properties indicated that the EP blends with triazine-trione based tri-imidazole provided an ideal way to prepare epoxy resin based prepregs with high glass transition temperature.
Availability of data materials
All the reagents were used as received.
References
Morancho JM, Ramis X, Fernández-Francos X, Konuray O (2020) Dual curing of an epoxy resin with dicarboxylic acids. Thermal Anal Calorim. 142(2):1
Siddiqi HM, Siraj A, Khalid N, Akhtar Z, Muhammad Z (2018) Thermally stable epoxy polymers from new tetraglycidyl amine-based resin. Thermal Anal Calorim 132:205–214
Jiao E, Wu K, Qu Z, Liu Y, Lu M, Na NB et al (2020) Preparation and curing kinetics of intrinsic flameretardant epoxy resin system based on polyoxometalates. Thermal Anal Calorim. 2020:1–14
Lee H, Neville K (1967) Book review-handbook of epoxy resins. Ind Eng Chem 59(9):16–17
Maggana C, Pissis P (1999) Water sorption and diffusion studies in an epoxy resin system. Polym Sci Part B Polym Phys 37(11):1165–1182
Gerami G, Bagheri R, Darvishi R (2019) Investigation of isothermal and dynamic cure kinetics of epoxy resin/nadic methyl anhydride/dicyandiamide by differential scanning calorimetry (DSC). Thermal Anal Calorim 137:575–582
Morancho JM, Ramis X, Fernández-Francos X, Salla JM, Konuray AO, Serra A (2018) Curing of off-stoichiometric amine–epoxy thermosets. Thermal Anal Calorim. 2:519–527
Monteserín C, Blanco M, Laza JM, Aranzabe E, Vilas JL (2018) Thickness effect on the generation of temperature and curing degree gradients in epoxy–amine thermoset systems. Thermal Anal Calorim 132(3):1–15
Kumar S, Samal SK, Mohanty S, Nayak SK (2019) Curing kinetics of bio-based epoxy resin-toughened DGEBA epoxy resin blend. Thermal Analy Calorim 137:1567–1578
Leena K, Soumyamol PB, Baby M, Suraj S, Mohan DS (2017) Non-isothermal cure and decomposition kinetics of epoxy–imidazole systems. Thermal Anal Calorim 130(3):1–9
Seidi F, Jouyandeh M, Akbari V, Paran SMR, Livi S, Ducos F et al (2020) Super-crosslinked ionic liquid-intercalated montmorillonite/epoxy nanocomposites: cure kinetics, viscoelastic behavior and thermal degradation mechanism. Polym Eng Sci 60(8):1940–1957
Jouyandeh M, Akbari V, Paran SMR, Livi S, Lins L, Vahabi H et al (2021) Epoxy/ionic liquid-modified mica nanocomposites: network formation–network degradation correlation. Nanomaterials 11(8):1990
Furutani M, Kakinuma A, Arimitsu K (2018) A dismantlable photoadhesion system fabricated by an anionic UV curing of epoxy resins with a base amplifier having a disulfide bond. Polym Sci Part A Polym Chem 56(2):237–241
Kudo K, Furutani M, Arimitsu K (2015) Imidazole derivatives with an intramolecular hydrogen bond as thermal latent curing agents for thermosetting resins. ACS Macro Lett 4(10):1085–1088
Guo CG, Zhou JX (2013) Effects of expandable graphite and modified ammonium polyphosphate on the flame-retardant and mechanical properties of wood flour-polypropylene composites. Polym Polym Compos 21(7):449–456
Kaplan ML, Wayda AL, Lyons AM (1990) Lanthanide–imidazole complexes as latent curing agents for epoxy resins. Polym Sci Part A Polym Chem 28(4):731–740
Li L, Ming L (2010) Curing mechanisms and kinetic analysis of DGEBA cured with a novel imidazole derivative curing agent using DSC techniques. Appl Polym Sci 117(6):3220–3227
Jang ES, Khan SB, Seo J, Akhtar K, Nam YH, Seo KW et al (2011) Preparation of cationic latent initiators containing imidazole group and their effects on the properties of DGEBA epoxy resin. Macromol Res 19(10):989
Maka H, Spychaj T, Pilawka R (2012) Epoxy resin/ionic liquid systems: the influence of imidazolium cation size and anion type on reactivity and thermomechanical properties. Ind Eng Chem Res 51:5197–5206
Fang W, Xiao J, Wang JW, Li SQ (2010) A novel imidazole derivative curing agent for epoxy resin: synthesis, characterization, and cure kinetic. Appl Polym Sci 107(1):223–227
Shuang Y, Zhang Q, Hu Y, Ding G, Cheng J (2017) Synthesis of s-triazine based tri-imidazole derivatives and their application as thermal latent curing agents for epoxy resin. Mater Lett 216:127–130
Arimitsu K, Tomota K, Fuse S, Kudo K, Furutani M (2016) A non-linear organic reaction of malonate derivative as a base amplifier to generate imidazoles without producing gas. RSC Adv 6(44):38388–38390
Huo S, Wang J, Shuang Y, Wang J, Zhang B, Bo Z et al (2016) Synthesis of a novel phosphorus-nitrogen type flame retardant composed of maleimide, triazine-trione, and phosphaphenanthrene and its flame retardant effect on epoxy resin. Polym Degrad Stab 131:106–113
Shuang Y, Zhang Q, Hu Y (2016) Synthesis of a novel flame retardant containing phosphorus, nitrogen and boron and its application in flame-retardant epoxy resin. Polym Degrad Stab 133:358–366
Jouyandeh M, Paran S, Jannesari A, Puglia D, Saeb MR (2019) Protocol for nonisothermal cure analysis of thermoset composites. Prog Org Coat 131:333–339
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda L, Sbirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520(1–2):1–19
Tan SG, Chow WS (2010) Thermal properties of anhydride-cured bio-based epoxy blends. Thermal Anal Calorim 101(3):1051–1058
Antoon MK, Koenig JL (1981) Crosslinking mechanism of an anhydride-cured epoxy resin as studied by Fourier transform infrared spectroscopy. Polym Sci Polym Chem Ed 19(2):549–570
Acknowledgements
The research is support by “Open Fund of State Key Laboratory of Power Grid Environmental Protection” (No. GYW51201901306) are gratefully acknowledged.
Funding
The research is support by “Open Fund of State Key Laboratory of Power Grid Environmental Protection” (No. GYW51201901306) are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
XW is the lead of data curation, JL is the lead of investigation, XC is the lead of writing, JW is the supporting of writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wan, X., Liu, J., Chen, X. et al. Study on tri-imidazole derivatives modified with triazine-trione structure as latent curing agents for epoxy resin. SN Appl. Sci. 4, 24 (2022). https://doi.org/10.1007/s42452-021-04905-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04905-w