Abstract
The main aim of the present work is to see the performance of “Al–Beryl” composites processed via stir casting route with or without CeO2 on their mechanical, wear and corrosive properties for structural applications in heavy machinery and watercrafts. Hardness and ultimate tensile strength increased with 36% and 43%, respectively, with 9% Beryl addition. Further, effect of addition of constant 0.5 wt.% CeO2 in “Al–Beryl” composite was evaluated and remarkably improvement in corrosion resistance was observed. Tribological performance of the composites was investigated by conducting sliding wear tests against steel at different loads in dry and wet conditions. Characteristic features of adhesion, fracture and delamination were observed in SEM micrograph of the worn composite having low beryl content slid in air whereas abrasive wear predominates in water or oil. Addition of increased wt.% of beryl particles led in reduced wear of the composite at all loads. Increase in corrosion resistance by 45% decrease in weight loss with 0.5% CeO2 addition was observed. “Al6061–9% Beryl–0.5% CeO2” composite with high hardness, wear and corrosion resistance is found most promising for structural applications.
Article Highlights
-
(1)
An increase of 36% in hardness and 43% in ultimate tensile strength was found in Al composites with 9% Beryl addition;
-
(2)
Coefficient of friction was found least for “Beryl–CeO2” added composites in wet sliding conditions;
-
(3)
A transition in wear mechanism occurred with adhesion, fracture and delamination of the worn composite with low beryl content slid in air;
-
(4)
Corrosion resistance increased by 45% with 0.5% CeO2 addition in Al composites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since the last century, aluminum metal matrix composites (MMC’s) are a matter of interest for researchers because of their easy and economical processing with vast structural industrial use in defense, aerospace, marine and automobile components [1,2,3,4,5,6,7,8,9]. To obtain desired properties, Al is reinforced with varying percentage of different metallic and ceramic reinforcements having limitations of poor wettability between matrix and reinforcements and their instability at higher temperature [10,11,12,13,14]. With time, different processing routes were studied and liquid metallurgy technique was found suitable for processing large size components [15,16,17,18,19]. Stir casting technique is generally preferred because of its simplicity, cost-effectiveness and applicability for mass production. Stir casting with increase in holding time, temperature and periodic stirring results in homogenized mixing and reduction in porosity [19,20,21,22]. Reinforcements like MnO2 addition in Al MMC resulted in high hardness and strength with increased holding time during casting and Al–Mn precipitate formation further strengthened the composite [23,24,25]. It was found that Al MMC processed via fly ash addition resulted in effective casting with increased wettability [18, 26]. Jokhio et al. [27] observed Mg addition in Al matrix imparted good bonding between reinforcement and matrix. It has been observed that addition of AlN or ceramic particles like SiC increases the composite strength significantly [28, 29]. Optimum wt.% addition of reinforcement in Al matrix results in less agglomeration and clustering of particles. Increase in strength with decrease in ductility was found with increase in volume % of oxide or ceramic particles in Al composite [30,31,32,33]. McDanels [34] reported that addition of more harder discontinuous SiC in Al MMC led to increase in yield and tensile strength by 60% in comparison with unreinforced matrix. Strengthening of Al composite with strong discontinuous SiC fibers is confirmed by Arsenault and Fischer [35], Arsenault [36] and Taya et al. [37]. Reddappa et al. [38] studied the effect of quenching on Al6061 composite with increasing beryl addition (2–12 wt.%) and found improved hardness and strength in heat treated and aged samples. Beryl particle additions have given rise to large residual compressive stress developed during solidification due to difference in coefficient of expansion between ductile matrix and brittle ceramic particles. Al MMC’s are generally used in making of piston, connecting rod, castings for railways, brake drum, equipment of mining, etc., where wear has a great role in long life functioning of these components; excessive wear of the mating components sometimes lead to catastrophic failure [39, 40]. So, the study for tribological properties of Al MMC’s has become the need of time. Wear properties of many MMC’s having fly ash, Al2O3, glass, graphite, mica and SiC, as reinforcements have been reported [39,40,41,42,43,44,45,46,47,48,49,50,51,52].
However, literature survey revealed that very little is reported about effect of beryllium aluminum silicate (Be3Al2(SiO3)6) (beryl) and cerium oxide (CeO2) addition on properties of Al composites. Cerium oxide has a unique property of having stability in chemical attack and high temperature [53, 54]. Al MMCs are used in different areas of industries for making different machine components which are prone to corrosion [52, 55,56,57,58,59,60]. To protect them from corrosion anti-corrosive coatings of zinc chromate is done which is in general toxic in nature and hazardous to the environment. In the present investigation focus is on effect of CeO2 which is less toxic than zinc chromate on “Al–Beryl” MMC to have an optimized Al MMC’s composition having superior wear and corrosive properties.
2 Materials and methodology
2.1 Materials
For the fabrication of hybrid “Al6061–Beryl–CeO2” composites, “as casted” Al6061 was used, having following chemical composition in weight percent, as determined by ED-XRF spectrophotometer — Table 1:
High-purity commercially available beryl and CeO2 powders (particle size: 20–30 μm) were taken as starting materials. Powders were ball milled in zirconia vial with zirconia balls (ball to powder ratio of 10:1) for 4 h at 250 rpm to obtain uniform size of particles. The powders were, however, heated at 850 °C in order to dehydroxylate the surface to minimize H2 porosity [25]. Following batch compositions (in wt.%) were prepared — Table 2:
2.2 Methodology
2.2.1 Processing
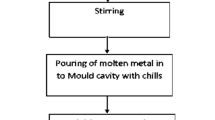
Al6061 MMC’s were prepared by melting half kg of “as casted” Al6061 ingot in a 2.2 kW capacity kanthal wound resistance heating furnace. Varying beryl and constant CeO2 particles were added during stirring. Ingot is put in the crucible made up of very high refractory material and after superheating aluminum melts at 850 °C, preheated beryl, CeO2 powders were gradually added. Beryl does not contain carbon, so formation of Al4C3 is restricted at high temperature which otherwise deteriorate the properties of the composite. The stirred slurry was poured in alloy steel die at a temperature of 750 °C. Stirring speed was kept at 800±50 rpm to increase wettability and good bonding between reinforcement with the matrix. Stirring temperature was kept at 620 °C where Al is in semisolid state to have effective wettability. Porosity is generally the prime defect in casting so while pouring the molten metal the pouring rate was kept constant and the bubble formation was avoided. Prepared composites were investigated for the phases evolved during casting using an X-ray diffractometer with CuKα radiation of 1.54 Å wavelength and the polished surfaces of cast composites were subjected to microstructural characterization.
2.2.2 Hardness and strength tests
Cast components of “Al6061–Beryl–CeO2” composites were machined and samples for tensile, hardness and wear tests were prepared. Samples were polished using emery papers of different grit sizes, and subsequently by 1 μm alumina powder suspension so as to obtain finished surfaces. The polished samples were ultrasonically cleaned in acetone and then dried in a hot air stream.
Hardness was measured using Brinell hardness testing machine at 500 kgf with 10 mm diameter hardened steel ball as penetrator. Five indentation marks were taken, and their average is reported. Tensile tests were done on H75KS Hounsfield testing machine at an extension rate of 1 mm/min. Tensile specimens were prepared on lathe machine according to ASTM E8M standard with diameter 5 mm and gauge length 25 mm. The specimens were tested and average of five readings is reported. Schematic diagram of tensile specimen is shown in Fig. 1.
2.2.3 Tribology tests
Sliding wear tests were conducted in dry (air) and wet (distilled water or automotive oil) conditions. Polished composite samples were subjected to sliding tests using a ball-on-disk wear tester (TR-201EM2-DUCOM). Commercially available bearing grade steel balls (Hardness: 140–160 HB) of 10 mm diameter were used as counter body.
Sample disk was rotated at 500 rpm (0.261 m/s) with a load of 5–20 N and the wear tests were done in different medium in ambient conditions for 20 min (total sliding distance of 314 m). Wear tests were repeated three times for each sample and average is reported. Coefficient of friction (COF) was estimated during the test. For lubricated contacts, commercially available automotive oil and distilled water were used. During test runs, frictional forces were recorded using a force sensor to generate real-time COF data. A stylus profilometer was used to trace surface profiles of worn disk specimen to measure the depth, width of the wear tracks of the specimens after each test.
2.2.4 Corrosion test
Corrosion tests were performed by placing the samples in 3.5% hydrochloric acid — HCl — solution for 48 h, 96 h and 144 h. Tests were repeated four times, initial and final weight of the samples was recorded and gravity weight loss in grams was measured. Hydrochloric acid (HCl) is an important inorganic acid known for its high corrosivity and has been extensively used as industrial cleaning solution [61].
Al6061 having poor mechanical properties was discarded and “Al6061–Beryl” and “Al6061–Beryl–CeO2” composites were selected for wear and corrosion study.
3 Results and Discussion
3.1 XRD and microstructure study of processed “Al6061–Beryl–CeO2” MMC’s
Typical XRD spectra of “Al6061–9% Beryl” and “Al6061–9% Beryl–0.5% CeO2” composites obtained from X-ray diffractometer (XRD) are shown in Fig. 2a and 2b, respectively. Lattice points of Al, Al2O3 and beryl peaks are shown in Fig. 2a and lattice points of CeO2 peaks are shown in Fig. 2b. XRD pattern represents the presence of Al2O3, beryl and CeO2 phases in Al composites evolved during casting. Major beryl peaks were identified till 40° and further they were found unnoticeable. Al2O3 and CeO2 peaks were identified till 90°. Any other unwanted phase was not observed in XRD pattern. XRD spectra of Al6061 after stir casting were also studied which revealed the presence of major phase Al2O3 which evolved due to oxidation.
SEM analysis of processed composites was done and evolution of dendrite structure was observed which extended as branches on the whole surface area upon solidification. Figure 3 shows representative microscopic images of “as casted” Al6061 (Fig. 3a), “Al6061–6% Beryl” (Fig. 3b), “Al6061–9% Beryl” (Fig. 3c) and “Al6061–9% Beryl–0.5% CeO2” (Fig. 3d) composites.
Figure 3a shows the irregular oxide layer particles distributed in the Al matrix. It was observed that there was formation of oxide layer on the surface of Al melt due to oxidation which broke on stirring and gets distributed in the matrix. Figure 3b represents the micrographs of Al composites with 6 wt.% beryl addition which show formation of dendrite structure with clear interface between beryl particle and Al matrix. Dendrite structure prevented cracks and their propagation. Figure 3c and 3d show developed dendrite structure and good visual bonding evolved due to the presence of Al2O3, beryl and CeO2 particles. Micrograph also shows the presence of particles distributed in the matrix which were believed as oxide and reinforcement particles. In general, dendrite structure breaks on stirring resulting in globular morphology and breaks in dendrite branches. Addition of 0.5% CeO2 due to its metallic behavior increased the wettability between ceramic particles in Al matrix during casting. No segregation of the particulates was observed due to the efficient mixing of reinforced particles in base matrix. Summarizing, perfect grain boundaries with the absence of voids resulted in processed composites which show strong adhesive and cohesive bonding between particles and molten matrix.
3.2 Mechanical properties of “Al6061–Beryl–CeO2” MMC’s
The hardness and ultimate tensile strength of the processed composites are shown in Fig. 4a and 4b. With 3–9 wt.% Beryl addition, hardness is increased to 75 BHN from 55 BHN of “as casted” Al6061. With 0.5% CeO2 addition in “Al6061–9% Beryl” further increased the hardness to 78 BHN. Uniform dispersion of hard beryl particles and wettability effect of CeO2 led to increase in hardness and strength. Formation of hard oxide phase Al2O3 in all the composites also resulted in increase in hardness. The ultimate tensile strength (UTS) of “as casted” Al6061 alloy is 76.4 MPa which increased to 110 MPa with 3–9 wt.% beryl addition. It was found that addition of 0.5 wt.% CeO2 in Al-beryl composites resulted in negligible effect on hardness or strength of the composite. Close packing of reinforcement resulted in small interparticle spacing in the matrix [38, 53, 54]. The ductility of the composite is measured in terms of percent elongation and it was found that with increase in tensile strength, ductility decreased. Ductility (21.58%±2% elongation) was found highest in Al6061 (as casted composite) and lower ductility (17.35%±2% elongation) was found in “Al6061–Beryl” with and without CeO2 composites. This behavior may be due to increase in brittleness of the composite with increase in hardness.
3.3 Friction analysis
The investigated composites subjected to sliding wear reflected identical evolution of friction. Generally, COF in its initial stage increased (50–200 s) and further attained a steady state due to the breakage of mechanical bonds between the asperities of two mating faces. Average steady-state COF value is high for the tests conducted in dry conditions, it decreased with increasing load.
Typical plots of COF versus time for “Al6061–Beryl” composites worn against steel ball in air with and without CeO2 is shown in Fig. 5a and 5b. COF value was found less for “Al6061–9% Beryl” composite due to its superior mechanical properties. COF graphs show fluctuations in the COF because of variation in contact between sample and disk due to debris.
Figure 6a–d shows the vertical bar charts of average COF value. COF varied from 0.85 to 0.16 under different conditions of load in dry and lubricated wear tests. A decrease in COF values was observed with increase weight % addition of beryl particles. Generally, during the initial stages, the surfaces of both the composite specimens and the steel counterpart were rough and thus strong interlocking took place, resulting in high friction coefficient. COF decreased in air to oil medium with increasing load with respect to increasing beryl content. This is because at heavy load continuous sliding led to the compaction of debris as lubricated layer due to water or oil which prevented the two-body direct contact resulting in reduced COF in comparison with dry conditions. The compacted debris washes off during continuous sliding. It was observed that 0.5% CeO2 addition has not affected the wear of the composite significantly.
3.4 Wear analysis
Wear is a complex process that depends not only on the material properties but also on the testing parameters and environmental conditions. In the present work, the phenomenal effects of composition, lubrication and load on wear of “Al6061–Beryl” and “Al6061–Beryl–CeO2” composites are discussed. Typical SEM micrographs of wear track of “Al6061–9% Beryl–0.5% CeO2” composite is shown in Fig. 7.
Wear track width and depth were measured at eight locations on the wear track of each worn sample. The average of wear track width and depth was used in computing the wear volume (V) and subsequently specific wear rate (k) was calculated from wear volume normalized for total sliding distance and load of the disk specimens, according to Eqs. 1 and 2, respectively.
where “V” is the wear volume (in [mm3]), “tr” is the track radius (in [mm]), “tw” is the track width (in [mm]) and “td” is the track depth (in [mm]);
where “k” is the specific wear rate (in [mm3/N.m]), “F” is the load (in [N]) and “S” is the sliding distance (in [m]).
Depth and width of wear track were measured from profiles obtained from surface profilometer. It was observed that the depth and width of wear tracks was more in profile of “Al6061–3% Beryl” composite and it reduced with “9% Beryl–0.5% CeO2” addition. Comparing with respect to medium it was observed that high hardness of the composite and presence of oil during sliding has significantly reduced the width and depth of wear track. In general wear volume increased with increase in load but is found less in higher beryl content containing composites in wet conditions. No significant change was observed in wear with 0.5% CeO2 addition in “Al6061–Beryl” composites. Specific wear rate of the processed composites is shown in Fig. 8a–d. Specific wear rate ranged from ≈3.8 × 10− 8 mm3/N.m to ≈5 × 10− 7 mm3/N.m in dry to wet condition. A decrease of at least one order of magnitude in wear rate was observed in composite with higher beryl content in wet conditions. Increased material transfer and formation of oxide-rich tribo-chemical layer were also responsible for the reduction in friction and wear at heavy load in dry conditions and due to lubrication in wet conditions. Summarizing, increased wt.% beryl addition with CeO2 lowered the coefficient of friction and wear attaining a sustainable wear resistance.
3.5 Microstructure analysis of worn surfaces
Worn surface images of casted “Al6061–Beryl–CeO2” MMC’s were studied to understand the dominant wear mechanisms under different sliding conditions. Micrographs were compared and representative SEM images of the worn surfaces of “Al6061–Beryl” and “Al6061–Beryl–CeO2” in air and oil at 20 N load are shown in Fig. 9a–c and Fig. 10a–b.
Figure 9a shows characteristic features of adhesion, fracture and delamination with 3% of beryl addition. Generally during sliding wear, wear debris gets generated as grains gets pulled out with repeated sliding and in course of time wear debris gets compacted and adhere on the worn surface. The adhered layer subsequently gets deformed and the layer gets fractured and delaminated from the surface due to course of sliding. With 9% Beryl and 0.5% CeO2 addition, composite showed consistent behavior of less wear (Fig. 9b and 9c). Wear is accompanied with mild adhesion and abrasion of the surface. Addition of beryl and CeO2 particles resisted the further fracture and delamination process. In general abrasion is the dominant wear mechanism observed when sliding wear tests were conducted in water or oil at varying loads.
Figure 10a and 10b shows cracking and fracture in “Al6061–3% Beryl” composite due to its poor mechanical properties whereas in “Al6061–9% Beryl” and “Al6061–9% Beryl–0.5% CeO2” the dominant presence of mild abrasion wear mechanism was observed. Hardness of the composite restricted further crack and fracture. As wear process continued, the rough profiles of the steel rotating disk and the composite specimens were smoothened as a result of abrasion and debris is washed in water or oil. Worn surface study has been conducted by other researchers on Al composites [39,40,41,42,43,44,45,46, 49,50,51,52, 55,56,57,58,59,60]. It was found that transfer layer of compacted wear debris along with the wear tracks form over the sliding surface with continuous sliding. The reason for lower wear rate in composites is also their high hardness as compared to base alloy. Yuvaraja and Sharma [55] in their wear study found that with increase in load from lower to higher values, the morphology of the worn surface gradually changed from fine scratches to distinct grooves and flake craters. The specimen shows a mixed mechanism of fine scratches and larger grooves with indications of severe deformation and fracture resulting in more material loss.
Worn surfaces of counter steel ball were also studied to understand the wear mechanism and material transfer. It was observed that the elemental iron, chromium or nickel ejected out of the steel ball gets oxidized and abrade contacting surfaces during sliding against Al composites, forming hard oxide debris [40]. In case of lubricated sliding conditions, the friction and wear were considerably decreased due to the formation of lubricating film between contacting surfaces which continuously form and flow during sliding. Steel balls abraded more in air then in water or oil condition. Wear scar on ball is more in diameter when slid against “Al6061–9% Beryl” composite then in “Al6061–3% Beryl” composite as high hardness of the composite led to more wear of the ball.
3.6 Relationship between wear volume and cumulative dissipated energy
In general, there is rise in contact temperature of two sliding bodies and generated energy spent in deformation or wear. The relation between the wear volumes of processed composite against steel ball was studied in terms of dissipated energy under the given loading conditions. The cumulative dissipated energy can be calculated for such sliding conditions, using the following relation (Eq. 3):
where the unit of “Ed” is [J], “W” is the normal load (in [N]), “µ” is the average COF, “v” is the sliding velocity (in [m/s]) and “ttime” is the total testing duration (in [s]) [57,58,59].
The linear increasing trend was observed in wear volume against the dissipated energy for the investigated composites in all medium as shown in Fig. 11a and 11b.
3.7 Corrosion test
Processed Al MMC was further tested for corrosion resistance property by placing the samples in 3.5% HCl solution for 2, 4 and 6 days. Initial and final weight of the samples was recorded and gravity weight loss in grams was measured as shown in Fig. 12a and 12b.
It was observed that corrosion resistance increased with 0.5% CeO2 addition in “Al–Beryl” composite which facilitated formation of protective layer on the composite surface ultimately suppressing the corrosion.
Figure 13 shows microscopic image of A7 composite after exposure in 3.5% HCl solution for 6 days. It is observed that CeO2 particles surrounded by aluminum solid solutions were intact even after continuous exposure in HCl solution. As reported in the literature, these CeO2 particles could probably act as cathodic zones suppressing corrosion of the composite and remain stable in aggressive environment forming stable protective oxide film on the surface [53]. Also increase in beryl content increased corrosion resistance in “Al–Beryl” composites due to the fact that oxides being anti-corrosive in nature corrode less in corrosive environment. The present results lay strong emphasis on the potential scope of use of CeO2 for protection of Al composite in marine environment as these composites have vast application in structural material of boats and ships [53, 54].
Summarizing the present research work shows that mechanical, wear and corrosive properties of Al MMC’s are improved with optimizing its composition with beryl and CeO2 addition. The results obtained with previous research conducted as discussed in literature review section clearly reflects that Al is reinforced with varying percentage of different metallic and ceramic reinforcements, which can be in particulate, whisker or fiber form to impart high strength and stiffness to the composite and distribute the applied load [10,11,12,13], where Al6061 with beryl and ceria is found to be structurally stronger material and wear resistant in the present investigation in comparison with other compositions of Al/A356/A380-MnO2/graphite/SiCp/SiC fibers/fly ash/Mg2Si [17, 18, 23, 29, 34,35,36,37, 49] or against other composites as carbon matrix composites reinforced with carbon nanotubes which is an excellent candidate for lightweight structural materials having potential use in aerospace, military and defense fields [62,63,64]. Further corrosion study shows CeO2 as substitute of zinc chromate which is as reported in general toxic in nature and hazardous to the environment [52, 56]. Therefore, it can be summarized that beryl-CeO2 added Al MMC’s are more suitable in different areas of industry, aerospace and marine for structural applications.
4 Conclusions
The main aim of the present work is to see the performance of “Al–Beryl” composites with or without CeO2 on their mechanical, wear and corrosive properties. Following are the findings:
-
(1)
An increase of 36% in hardness and 43% in ultimate tensile strength was found in Al composites with 9% Beryl addition whereas no significant change in mechanical properties was observed with 0.5% CeO2 addition;
-
(2)
Coefficient of friction was found least for “Beryl–CeO2” added composites in wet sliding conditions. COF decreased with increasing load; continuous sliding at heavy load led to the compaction of debris which restricted wear and resulted in low friction in dry condition;
-
(3)
A transition in wear mechanism occurred with adhesion, fracture and delamination in SEM micrograph of the worn composite with low beryl content slid in air whereas abrasion predominates in water or oil with higher beryl and CeO2 addition;
-
(4)
Reduced wear was observed in “Al6061–9% Beryl–0.5% CeO2” composite at all load. Corrosion resistance increased by 45% with 0.5% CeO2 addition in Al composites;
-
(5)
The corrosion of Al composites, in marine environments, can be suppressed significantly by reinforcing with cerium oxide. “Al6061–9% Beryl–0.5% CeO2” composite with high hardness, wear and corrosion resistance has promising potential future for marine use.
References
Tiryakioǧlu M, Campbell J (2007) Guidelines for designing metal casting research: application to aluminium alloy castings. Int J Cast Met Res 20(1):25–29. https://doi.org/10.1179/136404607X186509
Clyne TW, Withers PJ (1993) An introduction to metal matrix composites. Cambridge University Press. on-line ISBN: 9780511623080. https://doi.org/10.1017/CBO9780511623080
Satyanarayana KG, Pillai RM, Pai CB, Kestursatya M, Rohatgi PK, Kim JK (2002) Development in cast metal matrix composites over the last three and a half decades. In: Proceedings of the third international conference on advances in composites: pp. 753–763.
Venkateshwar Reddy P, Suresh Kumar G, Mohana Krishnudu D, Raghavendra Rao H (2020) Mechanical and wear performances of aluminium-based metal matrix composites: a review. J Bio- Tribo-Corros. https://doi.org/10.1007/s40735-020-00379-2
Prakash T, Sasikumar P, Sivasankaran S (2014) Investigations on the microstructure and mechanical property of friction stir processed AA6061 aluminium sheet metal reinforced with Al2O3 surface composite. Int J Mater Eng Innov 5(3):192–204. https://doi.org/10.1504/IJMATEI.2014.064277
Ray S (1993) Synthesis of cast metal matrix particulate composites. J Mater Sci 28:5397–5413. https://doi.org/10.1007/BF00367809
Sajjadi SA, Ezatpour HR, Beygi H (2011) Microstructure and mechanical properties of Al-Al2O3 micro and nano composites fabricated by stir casting. Mater Sci Eng, A 528(29–30):8765–8771. https://doi.org/10.1016/j.msea.2011.08.052
Miller WS, Zhuang L, Bottema J, Wittebrood AJ, De Smet P, Haszler A, Vieregge A (2000) Recent development in aluminium alloys for the automotive industry. Mater Sci Eng, A 280(1):37–49. https://doi.org/10.1016/S0921-5093(99)00653-X
Brown KR, Venie MS, Woods RA (1995) The increasing use of aluminium in automotive applications. J Miner Metals Mater 47:20–23. https://doi.org/10.1007/BF03221224
Davis JR (1998) Metal-matrix composites. Metals handbook: ASM International. ISBN Electronic: 978-1-62708-199-3. https://doi.org/10.31399/asm.hb.mhde2.9781627081993
Xiu Z-y, Chen G-q, Wang X-f, Wu G-h, Liu Y-m, Yang W-s (2010) Microstructure and performance of Al-Si alloy with high Si content by high temperature diffusion treatment. Trans Nonferrous Metals Soc China 20(11):2134–2138. https://doi.org/10.1016/S1003-6326(09)60430-1
Kok M (2005) Production and mechanical properties of Al2O3 particle-reinforced 2024 aluminium alloy composites. J Mater Process Technol 161(3):381–387. https://doi.org/10.1016/j.jmatprotec.2004.07.068
Rosso M (2006) Ceramic and metal matrix composites: routes and properties. J Mater Process Technol 175(1–3):364–375. https://doi.org/10.1016/j.jmatprotec.2005.04.038
Wang X, Jha A, Brydson R (2004) In situ fabrication of Al3Ti particle reinforced aluminium alloy metal-matrix composites. Mater Sci Eng, A 364(1–2):339–345. https://doi.org/10.1016/j.msea.2003.08.049
Koczak MJ, Premkumar MK (1993) Emerging technologies for the in-situ production of MMCs. J Mater Sci 45:44–48. https://doi.org/10.1007/BF03223365
Verma V, Manoj Kumar BV (2017) Processing of alumina-based composites via conventional sintering and their characterization. Mater Manuf Processes 32(1):21–26. https://doi.org/10.1080/10426914.2016.1198023
Verma V, Kumar P, Mittal KK, Chauhan S, Tewari PC (2016) Microstructure and mechanical behaviour characterisation of Al-Al2O3 MMC processed by DIMOX and Al-Al2O3/MnO2 MMC processed via stir casting route. Int J Mater Eng Innov 7(3–4):219–235. https://doi.org/10.1504/IJMATEI.2016.084626
Verma V, Tewari PC, Ahamed RZ, Ahmed ST (2019) Effect of addition of fly ash and Al2O3 particles on mechanical and tribological behavior of Al MMC at varying load, time and speed. Procedia Struct Integr 14:68–77. https://doi.org/10.1016/j.prostr.2019.05.010
Kumar B, Menghani JV (2016) Aluminium-based metal matrix composites by stir casting: a literature review. Int J Mater Eng Innov 7(1):1–14. https://doi.org/10.1504/IJMATEI.2016.077310
Surappa MK, Rohatgi PK (1981) Preparation and properties of cast aluminium-ceramic particle composites. J Mater Sci 16:983–993. https://doi.org/10.1007/BF00542743
Bhushan RK, Kumar S (2010) Effect of SiC particles on Young’s modulus, peak frequency and porosity of 7075 Al alloy composite. Int J Mater Eng Innov 1(3/4):389–397. https://doi.org/10.1504/IJMATEI.2010.035164
Bhushan RK, Kumar S, Das S (2009) Optimisation of porosity of 7075 Al alloy 10% SiC composite produced by stir casting process through Taguchi method. Int J Mater Eng Innov 1(1):116–129. https://doi.org/10.1504/IJMATEI.2009.024031
Hamid AA, Ghosh PK, Jain SC, Ray S (2005) Processing, microstructure, and mechanical properties of cast In-Situ Al(Mg, Mn)-Al2O3(MnO2) composite. Metall Mater Trans A 36:2211–2223. https://doi.org/10.1007/s11661-005-0340-8
Rahimian M, Ehsani N, Parvin N, Baharvandi HR (2009) The effect of particle size, sintering temperature and sintering time on the properties of Al-Al2O3 composites, made by powder metallurgy. J Mater Process Technol 209(14):5387–5393. https://doi.org/10.1016/j.jmatprotec.2009.04.007
Erkalfa H, Misirli Z, Demirci M, Toy Ç, Baykara T (1995) The densification and microstructural development of A12O3 with manganese oxide addition. J Eur Ceram Soc 15(2):165–171. https://doi.org/10.1016/0955-2219(95)93062-8
Surappa MK (2003) Aluminium matrix composites: challenges and opportunities. Sādhanā 28(1 & 2):319–334. https://doi.org/10.1007/BF02717141
Jokhio MH, Panhwer MI, Unar MA (2011) Manufacturing of aluminum composite material using stir casting process. Mehran Univ Res J Eng Technol 30 (1): 53–64. ISSN: 0254–7821
Wahab MN, Daud AR, Ghazali MJ (2009) Preparation and characterization of stir cast-aluminum nitride reinforced aluminum metal matrix composites. Int J Mech Mater Eng 4(2):115–117 (Corpus ID: 138549635)
Saheb DA (2011) Aluminum silicon carbide and aluminum graphite particulate composites. ARPN J Eng Appl Sci 6 (10): 41–46. Asian Research Publishing Network. ISSN: 1819-6608
Tzamtzis S, Barekar NS, Hari Babu H, Patel J, Dhindaw BK, Fan Z (2009) Processing of advanced Al/SiC particulate metal matrix composites under intensive shearing: a novel Rheo-process. Compos A Appl Sci Manuf 40(2):144–151. https://doi.org/10.1016/j.compositesa.2008.10.017
Kamat SV, Hirth JP, Mehrabian R (1989) Mechanical properties of particulate-reinforced aluminum-matrix composites. Acta Metall 37(9):2395–2402. https://doi.org/10.1016/0001-6160(89)90037-0
Abdel-Azim AN, Shash Y, Mostafa SF, Younan A (1995) Casting of 2024-Al alloy reinforced with Al2O3 particles. J Mater Process Technol 55(3–4):199–205. https://doi.org/10.1016/0924-0136(95)01954-5
Ezatpour HR, Torabi-Parizi M, Sajjadi SA (2013) Microstructure and mechanical properties of extruded Al/Al2O3 composites fabricated by stir-casting process. Trans Nonferrous Metals Soc China 23(5):1262–1268. https://doi.org/10.1016/S1003-6326(13)62591-1
McDanels DL (1985) Analysis of stress-strain, fracture, and ductility behavior of aluminum matrix composites containing discontinuous silicon carbide reinforcement. Metall Trans A 16:1105–1115. https://doi.org/10.1007/BF02811679
Arsenault RJ, Fisher RM (1983) Microstructure of fiber and particulate SiC in 6061 Al composites. Scr Metall 17(1):67–71. https://doi.org/10.1016/0036-9748(83)90072-8
Arsenault RJ (1984) The strengthening of aluminum alloy 6061 by fiber and platelet silicon carbide. Mater Sci Eng 64(2):171–181. https://doi.org/10.1016/0025-5416(84)90101-0
Taya M, Lulay KE, Lloyd DJ (1991) Strengthening of a particulate metal matrix composite by quenching. Acta Metall Mater 39(1):73–87. https://doi.org/10.1016/0956-7151(91)90329-Y
Reddappa HN, Niranjan HB, Suresh KR, Satyanarayana KG (2012) Effect of quenching media and ageing time on Al6061–Beryl composites. Appl Mech Mater 110–116:1374–1379.https://doi.org/10.4028/www.scientific.net/AMM.110-116.1374
Ramesh CS, Seshadri SK, Iyer KJL (1991) A survey of aspects of wear of metals. Indian J Technol 29 (4): 179–185. ISSN: 0019–5669
Stott FH (1998) The role of oxidation in the wear of alloys. Tribol Int 31(1–3):61–71. https://doi.org/10.1016/S0301-679X(98)00008-5
Ramesh CS, Noor Ahmed R, Mujeebu MA, Abdullah MZ (2009) Fabrication and study on tribological characteristics of cast copper-TiO2-boric acid hybrid composites. Mater Des 30(5):1632–1637. https://doi.org/10.1016/j.matdes.2008.07.039
Ramesh CS, Anwar Khan AR, Ravikumar N, Savanprabhu P (2005) Prediction of wear coefficient of Al6061-TiO2 composites. Wear 259(1–6):602–608. https://doi.org/10.1016/j.wear.2005.02.115
Venkatesh R, Srinivas S (2019) Effect of heat treatment on hardness, tensile strength and microstructure of hot and cold forged Al6061 metal matrix composites reinforced with silicon carbide particles. Mater Res Exp 6 (10): 1065g3. https://doi.org/10.1088/2053-1591/ab4297
Hosking FM, Folgar Portillo F, Wunderlin R, Mehrabian R (1982) Composites of aluminium alloys: fabrication and wear behaviour. J Mater Sci 17:477–498. https://doi.org/10.1007/BF00591483
Wang AG, Hutchings IM (1989) Wear of alumina fibre-aluminium metal matrix composites by two-body abrasion. Mater Sci Technol 5:71–76. https://doi.org/10.1179/mst.1989.5.1.71
Wang A, Rack HJ (1991) Transition wear behavior of SiC-particulate- and SiC-whisker-reinforced 7091 Al metal matrix composites. Mater Sci Eng, A 147(2):211–224. https://doi.org/10.1016/0921-5093(91)90848-H
Hillary JJM, Ramamoorthi R, Chelladurai SJS (2020) Dry sliding wear behaviour of Al6061-5%SiC-TiB2 hybrid metal matrix composites synthesized by stir casting process. Mater Res Exp 7(12):126519. https://doi.org/10.1088/2053-1591/abd19b
Dinaharan I, Murugan N (2014) Microstructure and some properties of aluminium alloy AA6061 reinforced in situ formed zirconium diboride particulate stir cast composite. Int J Cast Met Res 27(2):115–121. https://doi.org/10.1179/1743133613Y.0000000097
Murat Lus H, Ozer G, Altug Guler K, Erzi E, Dispinar D (2015) Wear properties of squeeze cast in situ Mg2Si-A380 alloy. Int J Cast Metals Res 28(1):59–64. https://doi.org/10.1179/1743133614Y.0000000131
Ünal N, Çamurlu HE, Koçak S, Düztepe G (2012) Effect of external ultrasonic treatment on hypereutectic cast aluminium-silicon alloy. Int J Cast Met Res 25(4):246–250. https://doi.org/10.1179/1743133612Y.0000000011
Yamanoglu R, Karakulak E, Zeren M, Koç FG (2013) Effect of nickel on microstructure and wear behaviour of pure aluminium against steel and alumina counterfaces. Int J Cast Met Res 26(5):289–295. https://doi.org/10.1179/1743133613Y.0000000066
Culliton D, Betts AJ, Kennedy D (2013) Impact of intermetallic precipitates on the tribological and/or corrosion performance of cast aluminium alloys: a short review. Int J Cast Met Res 26(2):65–71. https://doi.org/10.1179/1743133612Y.0000000038
Muhamed Ashraf P, Shibli SMA (2007) Reinforcing aluminium with cerium oxide: a new and effective technique to prevent corrosion in marine environments. Electrochem Commun 9(3):443–448. https://doi.org/10.1016/j.elecom.2006.09.010
Arenas MA, Conde A, de Damborenea JJ (2002) Cerium: a suitable green corrosion inhibitor for tinplate. Corros Sci 44(3):511–520. https://doi.org/10.1016/S0010-938X(01)00053-1
Yuvaraja C, Sharma KV (2009) A study of transition wear behavior of alumina particle reinforced Al-6061 MMCs. J Reinf Plast Compos 28(23):2903–2909. https://doi.org/10.1177/0731684408095051
Fu Y, Batchelor AW, Loh NL, Tan KW (1998) Effect of lubrication by mineral and synthetic oils on the sliding wear of plasma nitrided AISI 4l0 stainless steel. Wear 219(2):169–176. https://doi.org/10.1016/S0043-1648(98)00184-7
Marui E, Hashimoto M, Kato S, Kojima W (1992) Dissipation of kinematic energy by slip at the interface of mating surfaces. Wear 159(1):141–150. https://doi.org/10.1016/0043-1648(92)90296-K
Huq MZ, Celis JP (1997) Reproducibility of friction and wear results in ball-on-disc unidirectional sliding tests of TiN-alumina pairings. Wear 212(2):151–159. https://doi.org/10.1016/S0043-1648(97)00167-1
Czichos H, Klaffke D, Santner E, Woydt M (1995) Advances in tribology: the materials point of view. Wear 190(2):155–161. https://doi.org/10.1016/0043-1648(96)80014-7
Verma V, Khvan A (2019) A short review on Al MMC with reinforcement addition effect on their mechanical and wear behaviour. IntechOpen. https://doi.org/10.5772/intechopen.83584
Ituen E, Singh A, Yuanhua L, Akaranta O (2021) Green synthesis and anticorrosion effect of Allium cepa peels extract-silver nanoparticles composite in simulated oilfield pickling solution. SN Appl. Sci. 3:679. https://doi.org/10.1007/s42452-021-04670-w
Zhang S, Ma Y, Suresh L, Hao A, Bick M, Tan SC, Chen J (2020) Carbon nanotube reinforced strong carbon matrix composites. ACS Nano 14:9282–9319. https://doi.org/10.1021/acsnano.0c03268
Zhang S, Hao A, Nguyen N, Oluwalowo A, Liu Z, Dessureault Y, Park JG, Liang R (2019) Carbon nanotube/carbon composite fiber with improved strength and electrical conductivity via interface engineering. Carbon 144:628–638. https://doi.org/10.1016/j.carbon.2018.12.091
Zhang S, Nguyen N, Leonhardt B, Jolowsky C, Hao A, Park JG, Liang R (2019) Carbon-nanotube-based electrical conductors: fabrication, optimization, and applications. Adv Electron Mater 1800811:1–36. https://doi.org/10.1002/aelm.201800811
Acknowledgments
The Authors gratefully acknowledge the financial support of the Ministry of Science and Higher Education of the Russian Federation in the framework of Increase Competitiveness Program of NUST «MISiS» (№ K4-2018-020), implemented by a governmental decree dated 16th of March 2013, N 211. Prof. Dr. Ronaldo Câmara Cozza acknowledges Prof. Dr. Marko Ackermann and Prof. Dr. Gustavo Henrique Bolognesi Donato – from University Center FEI – for their help and support with bureaucratic issues related to the publication of this Research Article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Verma, V., Cozza, R.C., Cheverikin, V. et al. Mechanical and tribological behavior of Al composites containing varying beryllium aluminum silicate and constant CeO2. SN Appl. Sci. 3, 821 (2021). https://doi.org/10.1007/s42452-021-04790-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04790-3