Abstract
Anaerobic co-digestion is widely adopted to enhance process efficacy by balancing the C/N ratio of the feedstock while converting organic wastes to biomethane. Goat manure (GM) and cotton gin trash (CGT) were anaerobically co-digested in triplicate batch bioreactors. The process was optimized and evaluated utilizing mathematical equations. The liquid fraction of the digestate was analyzed for nitrate and phosphate. The co-digestions with 10 and 20% CGT having the C/N ratios of 17.7 and 19.8 yielded the highest and statistically similar 261.4 ± 4.8 and 262.6 ± 4.2 mL/gvs biomethane, respectively. The biodegradability (BD) of GM and CGT was 94.5 ± 2.7 and 37.6 ± 0.8%, respectively. The BD decreased proportionally with an increase in CGT percentage. The co-digestion having 10% CGT yielded 80–90% of biomethane in 26–39 d. The modified Gompertz model-predicted and experimental biomethane values were similar. The highest synergistic effect index of 15.6 ± 4.7% was observed in GM/CGT; 30:70 co-digestion. The concentration of nitrate and phosphate was lower in the liquid fraction of digestate than the feedstocks, indicating that these nutrients stay in the solid fraction. The results provide important insights in agro-waste management, further studies determining the effects of effluent application on plants need to be conducted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The systems where animals are raised under confined situations for more than 45 days a year are defined as animal feeding operations (AFO). Concentrated animal feeding operations (CAFO) is a term given by EPA to the large-sized AFOs and/or AFOs where animal wastewater (manure etc.) is stored in open ditches (manmade or natural) or released to the environment via water streams [1]. The animal manures stored in tanks or lagoons release large amounts of methane and other air pollutants (hydrogen sulfide, ammonia, and other particulate matter) in the atmosphere during the summer months [2,3,4,5]. Consequently, CAFOs are regulated under the National Pollutant Discharge Elimination System of EPA [1]. Anaerobic digestion (AD) is widely adopted to convert organic matter into biomethane or refined natural gas (a form of bioenergy and potential alternative to fossil fuels) at such farms [6, 7]. As of October 2019, a total of 287 anaerobic digesters were operating at animal farms across the US [1, 6, 7].
The AD process relies upon the intricate balance of various bacterial groups which carry out the four distinct phases of hydrolysis (substrate break down to simple organic and amino acids), acidogenesis (conversion of simple organic and amino acids to volatile fatty acids, H2 and CO2), acetogenesis (volatile fatty acids are converted to CH3COOH) and finally methanogenesis [8]. The microbial degradation is impacted by various factors such as inoculum, substrate, experimental and operational conditions [9, 10]. The anaerobic co-digestion is a process of treating more than one organic wastes in a suitable proportion and is known to improve process efficacy by balancing nutrients, diversifying microbial population and lowering toxins, balancing alkalinity, etc., and has been studied extensively [10,11,12,13]. The mono-digestion of animal manures is sometimes prone to process failures due to nutrient imbalance for the microbes owing to their low C/N ratios. The high C/N ratio of lignocellulosic feedstocks makes them feedstocks of choice as a co-substrate for AD with manures [14].
Texas is one of the major cotton (Gossypium hirsutum, G. barbadense) producing states in the US. Once picked from the farm cotton is further processed in gins to separate fiber, leaving behind cotton gin trash (CGT) as agro-industrial waste. According to Thomasson [15], ginning one bale (217–218 kg) of cotton yields about 37–147 kg of CGT [16], thus annually generating millions of tons of CGT in the states of Texas, Georgia, Arkansas, and Mississippi. The CGT, a byproduct of the cotton industry, mainly composed of clean lint (8–15%), hulls (18.5–32.9), stem (5.2–5.9), grass (0.1–1.1), seed (0–2.9%), motes (20.4–21.6%), small leaves (19.4–34.9%) [17] is abundantly available in cotton-growing regions of the US. Roughly two million tons of CGT are annually produced in the US. Being a major cotton producer, 99,473 tons of CGT is produced annually in Texas [18]. The traditional management strategies have been to use it for feeding and bedding for the animals or composting, followed by addition to the soil as humus. The presence of microbial pests (verticillium wilt in particular) and pesticide residues, low heating value [19] in energy production limit the recycling options of CGT [12, 13]. However, the urgency to lower fossil fuel consumption had the scientists investigating agro-industrial wastes such as CGT as potential renewable energy sources [22]. Consequently, CGT has been investigated as a bioenergy source through ethanol production, gasification, and anaerobic degradation [20]. The high chemical oxygen demand (COD) and volatile solid (VS) contents in CGT can be recovered as biomethane through anaerobic degradation [18, 20].
Many reports indicate that the co-digestion of organic wastes with crop residues improves the biochemical methane potential (BMP) over mono-digestion [11, 23,24,25]. Findings from previous studies indicate that having animal manures along with crop residues (or more than one crop residue) in an anaerobic digester meets the microbial nutritional requirements better, making the process more efficient [23, 25]. Zhang et al. [24] reported that the co-digestion of GM with wheat, rice, and corn straw yielded higher biomethane compared with GM mono-digestion.
Few studies have reported the effects of co-digesting animal manures (e.g., swine and cow manure) with cotton industry byproducts on the biomethane recovery during anaerobic digestion [23, 26, 27]. Funk et al. [27] co-digested CGT with swine manure (mixing ratios varying from 1:1–10:1) in a two-stage bioreactor. The optimum percentage for anaerobic co-digestion of different feedstocks is not universal and needs to be investigated in each case [13, 28].
The bio-solids left after the anaerobic digestion process may be of high bio-fertilizer value, depending upon the organic wastes and other digester components [29]. Nitrate, ammonium, and orthophosphate are the main plant-available forms of nitrogen and phosphorus in warm aerobic soils under normal pH conditions [30]. The effluents left after AD are well-studied for the plant available nutrients [31,32,33]. Kafle and Chen [34] analyzed the influents and effluents of five animal manures and compared mineral nitrogen, phosphorus, the total ammonical nitrogen (TAN), total nitrogen (TN), total Kjeldahl nitrogen (TKN), and total phosphate (PO4−3). The biochemical methane potential (BMP) tests are used to determine the suitability of a given substrate for anaerobic co-digestion [4, 35]. To the best of our knowledge, no previous studies are reporting the BMP of GM–CGT co-digestion in different mixing ratios.
The objectives of this study were to investigate the BMP, biodegradability, and synergistic effect index of GM–CGT co-digestion at different mixing ratios (compared with mono-digestion of each substrate). The process was simulated utilizing the modified Gompertz equation. The liquid fraction effluents left after AD was evaluated for their bio-fertilizer quality by determining the available N and P forms.
2 Materials and methods
2.1 Chemicals, substrates, and inoculum
All the analytical grade chemicals were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA) or HACH Company (Loveland, Colorado, USA).
Goat manure was collected from the International Goat Research Center, Prairie View A&M University. It was dried at 80 °C passed through Willy mill, sieved (2 mm), and stored at 4 °C. The weathered CGT (from three months of cotton ginning) was collected from Varisco-Court Gin Co. (5354 Steel Store Rd, Bryan, TX, 77807). The samples were collected from weathered, transition, and core layers and well-mixed to obtain a uniform gin waste. It was passed through Willy mill, sieved (2 mm) to obtain uniform-sized particles, and stored at 4 °C until further analysis. The fresh inoculum obtained from the Prairie View wastewater treatment plant (operating at 36 ± 1 °C) was de-gassed [36,37,38] for a week and used for the assays.
2.2 Substrate characterization
The proximate analysis of well-mixed feedstocks and inoculum in triplicates was carried out per method 2540 [39] using a Lindberg Blue M electric furnace (Fisher Scientific, Pittsburgh, PA).
The ultimate analysis/elemental composition (C, H, N, and S) of the feedstocks was determined with the Flash 2000 elemental analyzer (Elementar Americas Inc., Ronkonkoma, NY) utilizing BBOT (C, H, N, and S; 72.5%, 6.1, 6.5 and 7.5%, respectively) as a standard compound. In compositional analysis lignin, cellulose, and hemicellulose contents were determined by thermogravimetric analysis in the PerkinElmer Diamond TG/DTG system.
For chemical analysis, triplicates of 1.0 g of each feedstock (wet weight w/w) were diluted 100 fold with deionized (DI) water in 250-mL Erlenmeyer flasks, stirred (hot plate and magnetic stir bars) for 30 min, and centrifuged (Thermo Scientific, Waltham, MA, USA) for 15 min at 5000 rpm. The pH of the supernatant was determined, appropriately diluted, and analyzed utilizing HACH kits; TNT 870, TNT 836, TNT 846, TNT 872, molybdovandate test N tube (Method 10127), and TNT880 for alkalinity, nitrate, orthophosphate, total phosphorus, and volatile acids and total Kjeldahl nitrogen (TKN), respectively, utilizing DRB 200 reactor and DR 3900 spectrophotometer (Loveland, Colorado). The pure compounds, 500 mg/L PO4, 1000 mg/L CH3COOH, 25,000 mg/L CaCO3, 500 mg/L NO3−, and 10 mg/L NH4–N, were diluted as needed and used as standards to determine total phosphorus, volatile acids, alkalinity, nitrate, ammonium, and TKN, respectively. Blank was adjusted by deionized (DI) water. To equate with treatments, the triplicate concentrations (mg/L) were multiplied by 10 and reported as mean ± SD (Table 1).
2.3 Biochemical methane potential tests
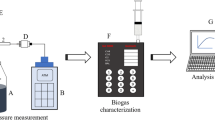
The effect of co-digesting GM with CGT on the biomethane recovery was determined by running BMP tests for 62 days in 250-mL serum bottles (Fisher Scientific, Pittsburgh, PA). The tests were conducted as described by Zhang et al. [40]. There were total 36 batch reactors, having blank, GM and CGT mono-digestions and their co-digestion with varied ratios (GM/CGT; 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80 and 10:90) in triplicates. The organic loading rate was TS at 10%. Blank bioreactors contained all ingredients except the substrate. Inoculum to substrate ratio (ISR) of 0.03–0.04 (VS basis) was maintained in all mono- and co-digestions. The ISRs of 0.005, 0.007, 0.01, 0.02 and 0.04 (on VS basis) were tested on GM (unpublished data), and statistically similar yields were observed at ISRs of 0.005, 0.007, 0.01, 0.02 and 0.04. In our previous study [41], we reported that CGT yielded similar values of BMP at 0.02 and 0.03 ISRs. An equal (as that of inoculum) volume of trace medium [35] was added to each bioreactor. The working volume of 100 mL in each bioreactor was achieved by adding DI water. The pH of the bioreactors was in the optimum range [9]. The bioreactors were purged with N2 (99.9% purity) for five minutes, sealed with straight plug stoppers, and secured using aluminum crimp seals (Fisher Scientific, Pittsburgh, PA) for biomethane sampling and maintaining the anaerobic conditions, respectively. The bioreactors were incubated at 36 °C ± 1.
The biomethane volume was measured with one of the volumetric techniques or liquid displacement method. According to Holliger et al. [36], if the volumetric method involves absorption of CO2 in an alkaline solution, direct methane values are obtained, compositional analysis of biogas is not required. The pH of DI water was adjusted to 10:30 with 0.5 M NaOH solution and added to a large container, holding an inverted cylinder filled with the same solution. The biomethane was read by recording the initial and final volumes of the inverted graduated cylinder. The batch reactors were manually shaken once a day before collecting data to mix the contents to avoid the formation of dead zones inside [36]. The daily gas measurements were continued until the total biomethane production during three consecutive days was less than 1% of the previously accumulated [36].
2.4 Theoretical maximum biomethane yield
From the chemical formula of the feedstocks (Table 1), the theoretical maximum biomethane yields (TMYs) of GM and CGT were determined by Boyle’s equation [42] (Eq. 1), which is a modification of the original Buswell Muller’s [43] original equation after including N and S to obtain NH3 and SO2 fractions.
2.5 Modeling methane production
The anaerobic degradation process or bacterial growth in the bioreactors can be described by fitting the modified Gompertz equation. The original equation (based on the exponential relationship between specific growth rate and population density) was developed by Causton [44], and further modified by Gibson et al. [45] to describe bacterial growth rate in terms of exponential and lag phase duration. Lay et al. [46] further assumed that the specific growth rate of methanogenic bacteria is directly proportional to the methane production rate in its current version as described below:
P(t) = The accumulated methane (mL/gvs, minus the blank) at digestion time t (days); P0 = Maximum cumulative methane production (mL/gvs); Rm = Maximum daily rate of biomethane production (mL/gvs/days), or specific growth rate of bacteria; λ = lag phase (days), minimum time to produce biomethane or time for bacterial acclimatization; e = Mathematical constant 2.718.
2.6 Biodegradability
The anaerobic biodegradability (BD) of a substrate is its volatile fraction that is converted to biomethane during AD. The BD of a co-digestion (at each mixing ratio) was determined using cumulative biomethane yield (EMY) from the experimental and theoretical biomethane (TMY, Eq. 1) employing Eq. (4) as described by Li et al.[47].
EMYco = The biomethane potential of co-digestion; TMY1 = Theoretical biomethane potential of GM; TMY2 = Theoretical biomethane potential of CGT; X1 = Volatile solid fraction of GM; X2 = Volatile solid fraction of CGT.
2.7 Synergistic effect index
The synergistic effect index (SEI) for the anaerobic co-digestions was determined as described by Li et al. [47] in equation below.
EMYco = The biomethane potential of co-digestion; X1 = The VS fractions of GM in the co-digestion; X2 = The VS fraction of CGT in the co-digestion; EMY1 = The biomethane potential of GM; EMY2 = The biomethane potential of CGT.
2.8 Data analysis
The experimental data were processed in Microsoft Excel 2010 (Microsoft, USA). The biomethane volume was converted to dry gas volume at STP by multiplying with a dry biomethane factor of 0.838, as described by Richards et al. [48]. The blank (d-1; 20 ± 1 and d-2; 3.7 ± 0.6 mL) value was subtracted from treatments to neutralize the inoculum.
The model fit was evaluated by calculating the relative root-mean-square error (rRMSE) as described by Kafle et al. [34] utilizing Eq. 6.
where m is the number of data pairs, j is jth values, Y is measured methane yield (mL/gvs), and d is the deviations between measured and predicted methane yield.
All of the data were analyzed using the general linear model (GLM) and analysis of variance procedure of Statistical Analysis System (SAS® 9.2, SAS Institute Inc., Cary, NC, USA), and statistically significant treatment means were separated using the least significant difference (LSD) test at 5% probability.
3 Results and discussion
3.1 Properties of goat manure and cotton gin trash
The proximate, ultimate, and compositional characteristics of feedstocks and inoculum are presented in (Table 1). The data values confirm that these agro wastes and inoculum are suitable for anaerobic degradation [9]. The C/N ratio of GM; 15.7 ± 0.7 is in the reported range of 13–20 [34, 40, 49]. The C/N ratio of CGT 36.3 ± 0.9 is also in the range of 28.0–51.3 in previous studies [20, 50, 51].
3.2 Daily biomethane production
The daily biomethane yields for mono- and the co-digestions of GM and CGT peaked at d-6 (Fig. 1a & b). The daily peak values of 17.6 ± 0.8, 18.9 ± 1.7, 17.3 ± 3.0, 12.8 ± 6.3, 12.5 ± 0.2, 11.8 ± 1.3, 10.2 ± 1.7, 10.7 ± 1.1, and 9.1 ± 3.7 mL/gvs, respectively, were observed on d-6 for the anaerobic digesters containing 100, 90, 80, 70, 60, 50, 40, 30, and 20% GM, respectively (Fig. 1a & b). The co-digestion with 10% GM yielded maximum daily biomethane on d-9. The maximum daily biomethane yield of 9.0 ± 0.9 mL/gvs was observed on d-13 from the CGT mono-digestion. The daily peaks signify that 6.1, 6.0, 5.2, 3.6, 4.0, 3.5, 2.9, 2.8, 2.2, 3.1, and 2.0% of the added VS was degraded in anaerobic digestions having GM and CGT mixing ratios of 100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, and 10:90 and 0:100. The second daily peaks of 12.3 ± 4.6 and 10.2 ± 0.8 mL/gvs were observed on d-8 and d-9 for co-digestions with 70 and 80% CGT. After the second peak, the biogas yield in all co-digestions declined steadily until 15–17 d. After 17 d, no additional peaks were observed in co-digestions. Our findings corroborate with those of Kafle and Chen [34], who reported the biomethane peak value of 18 mL/gvs at d-7 in GM during 55 d anaerobic mono-digestion. Zhang et al. [52] reported two daily peaks of 8 and 11 mL/gvs at 13 and 25 d in GM mono-digestion.
Daily biomethane yields (mL/gvs) for goat manure (GM) and cotton gin trash (CGT) mixing ratios of 90:10, 80:20, 70:30, 60:40, 50:50 (a); and 40:60, 30:70, 20:80 and 10:90 (b). The error bars represent the standard deviation. Each dot on the chart represents the mean of three values. Cumulative biomethane yields (mL/gvs) for goat manure (GM) and cotton gin trash (CGT) mixing ratios of 100:0, 90:10, 80:20, 70:30, 60:40 and 50:50 (c); and 40:60, 30:70, 20:80, 10:90 and 0:100 (d). The error bars represent the standard deviation. Each dot on the chart represents the mean of three values
3.3 Cumulative biomethane production and biodegradability
The cumulative biomethane yield from mono- and-co-digestions of GM and CGT at the end of BMP assays is shown in Fig. 1. The biomethane yield of 274.1 ± 7.8 mL/gvs was observed in the mono-digestion of GM. The BMP values of 261.4 ± 4.8 and 262.6 ± 4.2 mL/gvs were observed in GM–CGT co-digestions at the mixing ratios of 10 and 20% CGT, respectively. The co-digestion of GM and CGT with 10 and 20% CGT yielded similar (P ˂ 0.0001) biomethane as that of GM mono-digestion. The co-digestions having GM and CGT in proportions of 70:30, 60:40, 50:50, 40:60, 30:70, 20:80 and 10:90 yielded 236.2 ± 17.8, 203.5 ± 1.1, 197.4 ± 3.8, 204.0 ± 14.2, 206.0 ± 8.4, 186.7 ± 1.9 and 189.0 ± 7.1 2 mL/gvs biomethane, respectively. As a general rule, an increase in the CGT percentage in a co-digestion significantly lowered the biomethane yield. Statistically similar (P ˂ 0.0001) biomethane yields of 203.5 ± 1.1, 197.4 ± 3.8, 204.0 ± 14.2, and 206.0 ± 8.4 mL/gvs were observed for the co-digestion of GM with 40–70% of CGT (Fig. 1c & d).
The technical digestion time (T80–90) is defined as the time taken to obtain 80–90% of the BMP of a given substrate [34]. An increase in CGT in co-digestion increased the T80–90 and was between those of the GM and CGT mono-digestions. The T80–90 of mono-digestions of GM and CGT was 24–39, and 41–50 d, respectively. Kafle and Chen [34] reported T80–90 of 31–37 d for GM mono-digestion. The T80–90 for the co-digestions with 10, 20, 30, 40, 50, 60, 70, 80, and 90% GM was found to be 36–47, 33–47, 30–44, 34–44, 31–43, 30–42, 30–43, 28–41, and 26–39 d, respectively.
There are no reports of anaerobic digestion of GM with CGT to date. However, previous studies have reported the biomethane potential of GM with other agricultural wastes such as wheat straw (WS), corn stalks (CS), and rice straw (RS) [40, 53] swine, and dairy cow manure [23, 27]. Zhang et al. [52] reported biomethane yields of 225.5, 261.6, 331.2, and 324.3 mL/gvs from 55 days of GM mono-digestion and co-digestions having missing ratios of GM/WS 30:70, GM/CS; 70:30, and GM/RS; 50:50, respectively, under mesophilic conditions. Funk et al. [27] reported that CGT and cow manure yielded maximum biomethane at mixing ratios below the value of 5:1 in a two-stage bioreactor. Macias-Corral et al. [23] stated that the co-digestion of CGT with cow manure in 5:1 ratio enhanced biomethane yield to 87 m3/ton than the value of 62 m3/ton in cow manure mono-digestion by utilizing the microbial diversity and nutrients in each substrate. Zhang et al. [52] reported that co-digesting GM with WS, RS, and CS improved the biomethane over GM mono-digestion by improving the C/N ratio. The C/N ratios of GM and CGT were 15.7 ± 0.7 and 36.3 ± 0.9, respectively (Table 1). The biomethane yield in the GM mono-digestion and co-digestions having 10 and 20% CGT was 274 ± 7.8, 261.4 ± 4.8, and 262.6 ± 4.2 mL/gvs, respectively, and statistically similar. The optimum C/N ratio to support anaerobic digestion for biomethane production was 15.7–19.8 in this study. Siddiqui et al. [54] reported that the optimum C/N ratio for biogas production is 15, while other researchers [55, 56] reported it to be between 16 and 25. Some studies reported the optimum value of the C/N ratio for anaerobic degradation to be in the range of 20–35 [57,58,59].
The biodegradability and synergistic effect index (SEI) of the anaerobic digestions are provided in Table 2. The BDs of GM and CGT mono-digestions were 94.5 ± 2.7 and 37.6 ± 0.8%, respectively. As expected, the biodegradability decreased as the proportion of CGT in the co-digestion was increased. The highest SEI of 15.6 ± 4.7% was observed in the GM co-digestion with 70% CGT.
Goat manure and CGT used in the BMP assays have high lignin contents of 17.6 and 32.7% of TS, respectively (Table 1.). Lignin is one of the most recalcitrant components of plant-based agro-wastes and is not easily degraded during anaerobic digestion [47]. Triolo et al. [60] also stated that the lignin content of organic manures and energy crops is negatively correlated to their BMP. The acid-insoluble fraction of CGT is reported to be 21–25% [61,62,63]; thus, low biodegradability of the co-digestions containing a higher proportion of CGT may be attributed to the high lignin content of the substrates. However, the EMY of 169.6 ± 3.5 mL/gvs (Table 2) in CGT mono-digestion is within the range of 80–242 mL/gvs in pretreated cotton stalks reported by Adl et al. [64].
Although, Kafle and Chen [34] reported that the BMP of animal manures is negatively correlated with the manure lignin content, we report a higher BD value of 94.5 ± 2.7%. It may be because the GM for this study was thermally treated at 80 °C, which may have further softened the lignin, thus contributing to higher biodegradability. Lignocellulosic biomass of agricultural origin is subjected to various pre-treatments such as hot water, weak alkali, or acid treatment, along with a mild increase in temperature [64,65,66]. These pre-treatments aim to loosen the cell walls of the recalcitrant plant components, such as lignin. In a previous study [41] we reported similar BMP values in control, hot water, and alkali pretreated CGT. The CGT in our study was collected from a pile outside the gin mill (weathered for more than 3 months). During the period it was piled up in the open, it had been exposed to environmental factors, and it was considered as a pretreatment before the BMP assays. As mentioned before the BMP of 169.6 ± 3.5 mL/gvs in CGT are in the range observed by Adl et al. [64] after pretreatment of cotton residues. The storage of CGT may have enhanced BMP yield.
3.4 Modeling biomethane production
The mathematical models are integral to experimental research to optimize the process and evaluate digester performance. The models are classified as white-box, grey-box, and black box depending upon the amount of prior information required [67]. The algebraic equations (e.g., Buswell equation) which describe the biochemical reactions utilizing known information (eg chemical composition) are non-dynamic white-box models. The black-box models link input to output without requiring any knowledge of the biochemical reactions. The grey-box or mechanistically driven models require physical interpretation of the parameters by estimation, which is adjustable. Most of the dynamic models describing anaerobic digestion are in this category [67]. In this study, the anaerobic digestion process was optimized and evaluated by the non-dynamic white-box model; Boyle’s equation (Eq. 1), and by fitting the cumulative data to the modified Gompertz equation (Eq. 3.). The modified Gompertz model was validated by calculating the coefficient of determination (R2) and rRMSE. The parameters obtained by fitting the data are given in Table 3. The predicted, and experimental biomethane values were similar as described in Fig. 2. The 20, 50, and 60% additions of CGT (to GM) in co-digestion significantly lowered the lag phase compared to GM mono-digestion. These observations are consistent with the feedstock properties, with GM having a higher proportion of soluble/readily available fraction of volatile solids for microbial consumption while CGT provided carbon (being a high C/N ratio of the two substrates). The maximum and similar daily rates of biomethane production (Rm) were observed in GM mono-digestion and a 10% co-digestion with CGT. As the proportion of CGT in a co-digestion rose, the maximum daily rate decreased. In the co-digestions of 10, 20, 40, and 50% CGT, the Rm values were 5.9, 6.1, 6.4, and 6.7 (Table 3.), respectively, and statistically similar (P ˂ 0.0001). The high R2 values of 0.986–0.990 prove that the modified Gompertz equation fits very well with the cumulative data and hence the anaerobic digestion of GM–CGT. The least R2 value of 0.986 was observed in CGT mono-digestion and GM co-digestion with 10% CGT. The rRMSE values varied between 0.487 and 1.663. Similar rRMSE values for observed and modified Gompertz model predicted biomethane from the anaerobic digestion of organic wastes had earlier been reported by Srivastava et al. [68].
3.5 Phosphate and nitrate in liquid fraction of effluents
The PO4− concentration in the liquid fraction of the animal manure digestates varies between 110–125 mg/L [69]. The observed PO4− concentration of digestate from GM’s mono-digestion was 178.7 ± 37.2 mg/L. However, the PO4− and NO3− concentration in the liquid fraction of the digestate (Table 4) was lower than feedstocks in all treatments. The concentrations were in proportion to the quantity of feedstocks in each mixing ratio. The animal manures are known to have high organic N and P contents, which usually are not in the available forms but during the anaerobic degradation process, the unavailable forms of N and P are solubilized to available forms by the microbial populations carrying out the anaerobic digestion process [70]. The availability of nutrients in the digestate and animal slurries depends upon their pH which is affected by the concentration of various ions (released during organic matter degradation or added in the form of micronutrients) in the solution [29]. The pH of the digestates was between 6.8 and 7.0. Moller and Sommer [71] reported that most of the phosphorus and organic nitrogen stays in the solid fraction of the digestate, while the liquid fraction contains most of the inorganic nitrogen. The inorganic nitrogen may be in the NH4+ form, while PO4− may be in the solid fraction of the digestate.
4 Conclusion
This research was aimed at improving the performance of digesters at goat CAFOs by incorporating cotton gin trash; another agro-waste. The GM and CGT were anaerobically digested, the process performance was optimized and evaluated utilizing suitable models. Despite high non-degradable contents, the GM co-digestion with 10 and 20% CGT yielded statistically similar biomethane as that of GM. The high experimental biomethane yield (94% of the theoretical value) in GM mono-digestion indicates high performance without process inhibition, which might be aided by its thermal treatment. The farms operating GM mono-digestions may incorporate CGT (10–20%) and mitigate the twin problems of environmental pollution from GM and CGT disposal at goat farms and cotton gins, respectively. Although CGT incorporation did not improve biomethane yield over GM mono-digestions, the values observed were in the range reported by other researchers after pretreatment of cotton residues. The liquid fraction of the digestate had negligible nitrate and phosphate concentrations. Thus, this study may provide valuable information to the researchers in this field as there are no reports investigating these feedstocks together. Future studies will focus on pre-treatments to soften the lignin content of the CGT before co-digesting with GM and evaluating effluents on crops as potential biofertilizers.
References
USEPA (2019) Livestock anaerobic digester database AgSTAR: Biogas recovery in the agriculture sector. https://www.epa.gov/agstar/livestock-anaerobic-digester-database. Accessed 5 Jan 2020
He Z, Pagliari PH, Waldrip HM (2016) Applied and environmental chemistry of animal manure: a review. Pedosphere 26:779–816
Arndt C, Leytem AB, Hristov AN, Zavala-Araiza D, Cativiela JP, Conley S, Daube C, Faloona I, Herndon SC (2018) Short-term methane emissions from 2 dairy farms in California estimated by different measurement techniques and US Environmental Protection Agency inventory methodology: a case study. J Dairy Sci 101:11461–11479. https://doi.org/10.3168/jds.2017-13881
Howard A, Botlaguduru VSV, Du H, Kommalapati RR, Huque Z (2019) Measurements and comparative air quality analysis of a goat farm operation. Trans ASABE 62:1723–1733. https://doi.org/10.13031/trans.13344
Ramírez-Restrepo CA, Vera-Infanzón RR, Rao IM (2020) Predicting methane emissions, animal-environmental metrics and carbon footprint from Brahman (Bos indicus) breeding herd systems based on long-term research on grazing of neotropical savanna and Brachiaria decumbens pastures. Agric Syst 184:102892. https://doi.org/10.1016/j.agsy.2020.102892
Guo M, Song W, Buhain J (2015) Bioenergy and biofuels: history, status, and perspective. Renew Sustain Energy Rev 42:712–725
Moriarty K, Milbrandt A, Lewis J, Schwab A (2017) Bioenergy industry status report
Meegoda JN, Li B, Patel K, Wang LB (2018) A review of the processes, parameters, and optimization of anaerobic digestion. Int J Environ Res Public Health 15:2224. https://doi.org/10.3390/ijerph15102224
Raposo F, Fernández-Cegrí V, De la Rubia MA, Borja R, Béline F, Cavinato C, Demirer G, Fernández B, Fernández-Polanco M, Frigon JC, Ganesh R, Kaparaju P, Koubova J, Méndez R, Menin G, Peene A, Scherer P, Torrijos M, Uellendahl H, Wierinck I, de Wilde V (2011) Biochemical methane potential (BMP) of solid organic substrates: evaluation of anaerobic biodegradability using data from an international interlaboratory study. J Chem Technol Biotechnol 86:1088–1098. https://doi.org/10.1002/jctb.2622
Esposito G, Frunzo L, Giordano A, Liotta F, Panico A, Pirozzi F (2012) Anaerobic co-digestion of organic wastes. Rev Environ Sci Bio Technol. https://doi.org/10.1007/s11157-012-9277-8
Rabii A, Aldin S, Dahman Y, Elbeshbishy E (2019) A review on anaerobic co-digestion with a focus on the microbial populations and the effect of multi-stage digester configuration. Energies. https://doi.org/10.3390/en12061106
Karki R, Chuenchart W, Surendra KC, Shrestha S, Raskin L, Sung S, Hashimoto A, Kumar Khanal S (2021) Anaerobic co-digestion: current status and perspectives. Bioresour Technol 330:125001
Orangun A, Kaur H, Kommalapati RR (2021) Batch anaerobic co-digestion and biochemical methane potential analysis of goat manure and food waste. Energies 14:1952. https://doi.org/10.3390/en14071952
Neshat SA, Mohammadi M, Najafpour GD, Lahijani P (2017) Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew Sustain Energy Rev 79:308–322
Thomasson JA (1990) A review of cotton gin trash disposal and utilization. In: Proceedings—beltwide cotton production research conferences. USDA, ARS, Cotton Ginning Laboratory, Stoneville, MS
Wanjura JD, Armijo CB, Delhom CD, Boman RK, Faulkner WB, Holt GA, Pelletier MG (2019) Effects of harvesting and ginning practices on southern high plains cotton: fiber quality. Text Res J 89:4938–4958. https://doi.org/10.1177/0040517519844215
Agblevor FA, Batz S, Trumbo J (2003) Composition and ethanol production potential of cotton gin residues. Appl Biochem Biotechnol 105:219–230
Wilde C, Johnson J, Farmer M (2010) Inventory of cotton gin trash on the Texas high plains and bio-energy feedstock potentials. Tex J Agric Nat Resour 23:42–49
Holt GA, Barker GL, Baker RV, Brashears A (2000) Characterization of cotton gin byproducts produced by various machinary groups used in the ginning operation. Trans ASAE 43:1393–1400. https://doi.org/10.13031/2013.3036
Hamawand I, Sandell G, Pittaway P, Chakrabarty S, Yusaf T, Chen G, Seneweera S, Al-Lwayzy S, Bennett J, Hopf J (2016) Bioenergy from cotton industry wastes: a review and potential. Renew Sustain Energy Rev 66:435–448
Haque ANMA, Remadevi R, Naebe M (2021) A review on cotton gin trash: sustainable commodity for material fabrication. J Clean Prod 281:125300
Zabaniotou A, Andreou K (2010) Development of alternative energy sources for GHG emissions reduction in the textile industry by energy recovery from cotton ginning waste. J Clean Prod 18:784–790. https://doi.org/10.1016/j.jclepro.2010.01.006
Macias-Corral M, Samani Z, Hanson A, Smith G, Funk P, Yu H, Longworth J (2008) Anaerobic digestion of municipal solid waste and agricultural waste and the effect of co-digestion with dairy cow manure. Bioresour Technol 99:8288–8293. https://doi.org/10.1016/j.biortech.2008.03.057
Gu Y, Chen X, Liu Z, Zhou X, Zhang Y (2014) Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour Technol 158:149–155. https://doi.org/10.1016/j.biortech.2014.02.011
Hartmann H, Ahring BK (2005) Anaerobic digestion of the organic fraction of municipal solid waste: influence of co-digestion with manure. Water Res 39:1543–1552. https://doi.org/10.1016/j.watres.2005.02.001
Cheng X-Y, Zhong C (2014) Effects of feed to inoculum ratio, co-digestion, and pretreatment on biogas production from anaerobic digestion of cotton stalk. Energy Fuels 28:3157–3166. https://doi.org/10.1021/ef402562z
Funk PA, Armijo CB, Hanson AT, Samani ZA, Macias-Corral MA, Smith GB, Riordan JT (2005) Converting gin and dairy wastes to methane. Trans Am Soc Agric Eng 48:1197–1201. https://doi.org/10.13031/2013.18502
Ponsá S, Gea T, Sánchez A (2011) Anaerobic co-digestion of the organic fraction of municipal solid waste with several pure organic co-substrates. Biosyst Eng 108:352–360. https://doi.org/10.1016/j.biosystemseng.2011.01.007
Möller K, Müller T (2012) Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng Life Sci 12:242–257. https://doi.org/10.1002/elsc.201100085
Brady NC, Weil R (2013) Nature and properties of soils, 15th edn. Pearson Education, London
Fuente C, Alburquerque JA, Clemente R, Bernal MP (2013) Soil C and N mineralisation and agricultural value of the products of an anaerobic digestion system. Biol Fertil Soils 49:313–322. https://doi.org/10.1007/s00374-012-0719-9
Akhiar A, Battimelli A, Torrijos M, Carrere H (2017) Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag 59:118–128. https://doi.org/10.1016/j.wasman.2016.11.005
Sigurnjak I, Vaneeckhaute C, Michels E, Ryckaert B, Ghekiere G, Tack FMG, Meers E (2017) Fertilizer performance of liquid fraction of digestate as synthetic nitrogen substitute in silage maize cultivation for three consecutive years. Sci Total Environ 599–600:1885–1894. https://doi.org/10.1016/j.scitotenv.2017.05.120
Kafle GK, Chen L (2016) Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag 48:492–502
Moody L, Burns R, Wu-haan W, Spajic R (2009) Use of biochemical methane potential (BMP) assays for predicting and enhancing anaerobic digester performance. In: Proceedings of the 44th Croatian and the 4th international symposium on agriculture, Opatija, Croatia, 16–20 February 2009
Holliger C, Alves M, Andrade D, Angelidaki I, Astals S, Baier U, Bougrier C, Al E (2016) Towards a standardization of biomethane potential tests. Water Sci Technol 74:2515–2522. https://doi.org/10.2166/wst.2016.336
Angelidaki I, Sanders W (2004) Assessment of the anaerobic biodegradability of macropollutants. Rev Environ Sci Biotechnol 3:117–129
Zhang C, Su H, Baeyens J, Tan T (2014) Reviewing the anaerobic digestion of food waste for biogas production. Renew Sustain Energy Rev 38:383–392. https://doi.org/10.1016/j.rser.2014.05.038
APHA (2005) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Zhang T, Liu L, Song Z, Ren G, Feng Y, Han X, Yang G (2013) Biogas production by co-digestion of goat manure with three crop residues. PLoS ONE 8:e66845. https://doi.org/10.1371/journal.pone.0066845
Kaur H, Kommalapati RR (2021) Effect of inoculum concentration and pretreatment on biomethane recovery from cotton gin trash. J Agric Sci 13:15. https://doi.org/10.5539/jas.v13n4p15
Boyle WC (1977) Energy recovery from sanitary landfills—a review. Pergamon Press, New York
Buswell AM, Mueller HF (1952) Mechanism of methane fermentation. Ind Eng Chem 44:550–552. https://doi.org/10.1021/ie50507a033
Causton D (1977) A biologist’s mathematics. Edward Arnold, London
Gibson AM, Bratchell N, Roberts TA (1987) The effect of sodium chloride and temperature on the rate and extent of growth of Clostridium botulinum type A in pasteurized pork slurry. J Appl Bacteriol 62:479–490. https://doi.org/10.1111/j.1365-2672.1987.tb02680.x
Lay J-J, Li Y-Y, Tatsuya N (1997) Influences of pH and moisture content on the methane production in high-solids sludge digestion. Water Res 31:1518–1524. https://doi.org/10.1016/S0043-1354(96)00413-7
Li W, Khalid H, Zhu Z, Zhang R, Liu G, Chen C, Thorin E (2018) Methane production through anaerobic digestion: participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl Energy 226:1219–1228. https://doi.org/10.1016/j.apenergy.2018.05.055
Richards BK, Cummings RJ, White TE, Jewell WJ (1991) Methods for kinetic analysis of methane fermentation in high solids biomass digesters. Biomass Bioenergy 1:65–73. https://doi.org/10.1016/0961-9534(91)90028-B
Mupondi LT, Mnkeni PNS, Brutsch MO (2006) The effects of goat manure, sewage sludge and effective microorganisms on the composting of pine bark. Compost Sci Util 14:201–210. https://doi.org/10.1080/1065657X.2006.10702284
Santos A, Bustamante MA, Tortosa G, Moral R, Bernal MP (2016) Gaseous emissions and process development during composting of pig slurry: the influence of the proportion of cotton gin waste. J Clean Prod 112:81–90. https://doi.org/10.1016/j.jclepro.2015.08.084
Maglinao AL, Capareda SC, Nam H (2015) Fluidized bed gasification of high tonnage sorghum, cotton gin trash and beef cattle manure: evaluation of synthesis gas production. Energy Convers Manag 105:578–587. https://doi.org/10.1016/j.enconman.2015.08.005
Zhang C, Xiao G, Peng L, Su H, Tan T (2013) The anaerobic co-digestion of food waste and cattle manure. Bioresour Technol 129:170–176. https://doi.org/10.1016/j.biortech.2012.10.138
Ndubuisi-Nnaji UU, Ofon UA, Asamudo NU, Ekong VM (2020) Enhanced biogas and biofertilizer production from anaerobic codigestion of harvest residues and goat manure. J Sci Res Rep. https://doi.org/10.9734/jsrr/2020/v26i330231
Siddiqui Z, Horan NJ, Anaman K (2011) Optimisation of C:N ratio for co-digested processed industrial food waste and sewage sludge using the BMP test. Int J Chem React Eng. https://doi.org/10.1515/1542-6580.2327
Bouallagui H, Lahdheb H, Ben Romdan E, Rachdi B, Hamdi M (2009) Improvement of fruit and vegetable waste anaerobic digestion performance and stability with co-substrates addition. J Environ Manag 90:1844–1849. https://doi.org/10.1016/j.jenvman.2008.12.002
Luostarinen S, Luste S, Sillanpää M (2009) Increased biogas production at wastewater treatment plants through co-digestion of sewage sludge with grease trap sludge from a meat processing plant. Bioresour Technol 100:79–85. https://doi.org/10.1016/j.biortech.2008.06.029
Dai X, Duan N, Dong B, Dai L (2013) High-solids anaerobic co-digestion of sewage sludge and food waste in comparison with mono digestions: stability and performance. Waste Manag 33:308–316. https://doi.org/10.1016/j.wasman.2012.10.018
Creamer KS, Chen Y, Williams CM, Cheng JJ (2010) Stable thermophilic anaerobic digestion of dissolved air flotation (DAF) sludge by co-digestion with swine manure. Bioresour Technol 101:3020–3024. https://doi.org/10.1016/j.biortech.2009.12.029
Kanwar SS, Kalia AK (1993) Anaerobic fermentation of sheep droppings for biogas production. World J Microbiol Biotechnol 9:174–175. https://doi.org/10.1007/BF00327830
Triolo JM, Sommer SG, Møller HB, Weisbjerg MR, Jiang XY (2011) A new algorithm to characterize biodegradability of biomass during anaerobic digestion: influence of lignin concentration on methane production potential. Bioresour Technol 102:9395–9402. https://doi.org/10.1016/j.biortech.2011.07.026
Agblevor FA, Cundiff JS, Mingle C, Li W (2006) Storage and characterization of cotton gin waste for ethanol production. Resour Conserv Recycl 46:198–216. https://doi.org/10.1016/j.resconrec.2005.07.002
Plácido J, Capareda S (2014) Analysis of alkali ultrasonication pretreatment in bioethanol production from cotton gin trash using FT-IR spectroscopy and principal component analysis. Bioresour Bioprocess 1:23. https://doi.org/10.1186/s40643-014-0023-7
McIntosh S, Vancov T, Palmer J, Morris S (2014) Ethanol production from cotton gin trash using optimised dilute acid pretreatment and whole slurry fermentation processes. Bioresour Technol 173:42–51. https://doi.org/10.1016/j.biortech.2014.09.063
Adl M, Sheng K, Gharibi A (2012) Technical assessment of bioenergy recovery from cotton stalks through anaerobic digestion process and the effects of inexpensive pre-treatments. Appl Energy 93:251–260. https://doi.org/10.1016/j.apenergy.2011.11.065
Kim JY, Lee HW, Lee SM, Jae J, Park YK (2019) Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour Technol 279:373–384
Pang YZ, Liu YP, Li XJ, Wang KS, Yuan HR (2008) Improving biodegradability and biogas production of corn stover through sodium hydroxide solid state pretreatment. Energy Fuels 22:2761–2766. https://doi.org/10.1021/ef800001n
Lauwers J, Appels L, Thompson IP, Degrève J, Van Impe JF, Dewil R (2013) Mathematical modeling of anaerobic digestion of biomass and waste: power and limitations. Prog Energy Combust Sci 39:383–402
Srivastava AN, Chakma S (2021) Investigating leachate decontamination and biomethane augmentation through co-disposal of paper mill sludge with municipal solid waste in simulated anaerobic landfill bioreactors. Bioresour Technol 329:124889. https://doi.org/10.1016/j.biortech.2021.124889
Kocatürk-Schumacher NP, Bruun S, Zwart K, Jensen LS (2017) Nutrient recovery from the liquid fraction of digestate by clinoptilolite. Clean: Soil, Air, Water 45:1500153. https://doi.org/10.1002/clen.201500153
Alfa MI, Adie DB, Igboro SB, Oranusi US, Dahunsi SO, Akali DM (2014) Assessment of biofertilizer quality and health implications of anaerobic digestion effluent of cow dung and chicken droppings. Renew Energy 63:681–686. https://doi.org/10.1016/j.renene.2013.09.049
Moller HB, Sommer SG, Ahring BK (2002) Separation efficiency and particle size distribution in relation to manure type and storage conditions. Bioresour Technol 85:189–196. https://doi.org/10.1016/S0960-8524(02)00047-0
Acknowledgements
This work is supported by the USDA-CBG program grant (Award No. 2018-38821-27750) and partial support by the NSF CREST Center for Energy and Environmental Sustainability (CEES) at Prairie View A&M University (NSF Award #1914692), Prairie View, Texas. Dr. Kommalapati would like to thank Dr. Vamsi Botlaguduru, Assistant Professor, IIT Bombay, India, who immensely contributed to this project in the initial stages as a post-doctoral researcher at PVAMU. The contributions of CEES Research Scientist Dr. Hongbo Du are also highly appreciated. The authors would also like to acknowledge the assistance from CEES graduate student, Ayobami Orangun.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaur, H., Kommalapati, R.R. Optimizing anaerobic co-digestion of goat manure and cotton gin trash using biochemical methane potential (BMP) test and mathematical modeling. SN Appl. Sci. 3, 724 (2021). https://doi.org/10.1007/s42452-021-04706-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04706-1