Abstract
Human enteric viruses, such as enteric adenoviruses (HAdV), are known to be involved with gastrointestinal disorders, especially acute gastroenteritis. Several studies have used HAdV as an indicator of water quality, since they are considered highly stable and widely distributed viruses in water matrices. The aim of this study was to detect and genotype HAdVs in water matrices impacted by discharges of treated effluents from wastewater treatment plants (WWTPs). Wastewater treatment plants from the sanitary system of the Brazilian Federal District were assessed in 2018 and 2019. Samples were collected upstream and downstream from discharge points for each WWTP. Viral concentration based on adsorption-elution and conventional PCR was used for molecular detection, and positive samples were sequenced for phylogenetic analysis. Pluviosity data for the period in which the samples were collected were obtained. Our results demonstrated the presence of HAdVs in 27.2% (61/224) of the samples. The positivity was significantly higher in downstream samples compared to upstream. Moreover, the HAdV positivity was higher in downstream samples collected from receiving water bodies impacted by secondary-level WWTPs in comparison with those impacted by tertiary-level WWTPs. Phylogenetic analysis demonstrated the presence of genotypes 40 and 41, with prevalence of HAdV genotype 41. Despite the predominance of HAdV-41, an increasing frequency of the HAdV-40 was associated with higher pluviosity. In conclusion, this study is the first documentation in the Brazilian Federal District dealing with the prevalence and diversity of HAdVs in several WWTP, along with their correlation with rainfall index.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Agents associated with acute gastroenteritis are, in most cases, waterborne pathogens [1]. Among these pathogens, adenovirus (HAdV) is important as it is considered the third most common cause of viral gastroenteritis in children. The main symptoms are diarrhea and vomiting, with respiratory tract infections being common for some species [2]. For most patients the infection is asymptomatic or mild and self-limited, but in immunocompromised patients, the infection can be quite severe and mortality rates high due to a dysfunctional immune system and inability to eliminate the pathogen [3].
HAdV are non-enveloped, linear double-stranded DNA viruses. The mature virions are approximately 90 nm in diameter and consist of a complex of DNA and protein surrounded by the capsid proteins. The capsid is formed by three main proteins—hexons, penton-bases, and fibers—the latter two being responsible for the interaction of the virus with the surface of the host cell [4]. They are considered genetically diverse pathogens, belong to the adenoviridae family, genus Mastadenovirus, and are divided into seven species (A–G) comprising 103 genotypes [5].
The species and genotypes present diverse tissue tropism, and it is for this reason viruses can be associated with a wide range of clinical manifestations, including upper and lower respiratory diseases, conjunctivitis, cystitis, and gastroenteritis. All subgroups of adenovirus infect the respiratory tract, while genotypes 40 and 41, belonging to species F, have enteric tropisms and are regularly responsible for infections in the gastrointestinal tract [6]. On the other hand, under immunosuppression, other non-enteric types of HAdVs are associated with the occurrence of severe gastroenteritis [6]. Also, studies worldwide have described the association of non-enteric HAdV types (such as A, B, C and D) and acute gastroenteritis. Unlike, the HAdVs can be shed in feces after previous infections in other organs and their association with diarrhea is still questionable [7].
Viral particles can be shed in the feces of infected individuals, ranging from 105 to 1013 viral particles per gram of stool. In addition, they are excreted for a long time after infection, even if diarrhea is no longer present [8, 9]. Viral particles can therefore reach the aquatic environment via sewage discharge and their presence in water resources is considered a public health concern, especially in places where basic sanitation coverage is insufficient, such as Brazil [10,11,12].
In general, enteric viruses are more stable in the environment and more resistant to current wastewater treatment methods compared to enteric bacteria. Infectious viral particles have been reported for up to 130 days in seawater, for up to 120 days in freshwater and sewage, and for up to 100 days in soil at 20 to 30 °C. These periods surpass those reported for fecal coliform and other indicator bacteria in these environments [13]. In addition, several studies show that despite treatment, enteric viruses persist at high levels, which is why the use of a viral contamination indicator is proposed. HAdV is among the viruses suggested as an indicator [14, 15].
Considering the high stability of these pathogens in the environment, their resistance to the treatment processes currently applied to the effluents by the wastewater treatment plants (WWTP), and the lack of indicators of viral contamination to ensure the quality of the effluents discharged into the environment [16, 17], the aim of the present study was to evaluate the quality of water bodies in the Brazilian Federal District that accept treated effluents from WWTPs, looking for viral contamination that could threaten the public health.
2 Material and methods
2.1 Wastewater samples
In total, 224 water samples were analyzed from the 14 wastewater treatment plants (WWTP) that are part of the sewage system in the Brazilian Federal District. Brazilian Federal District is divided into 31 administrative regions. Three out of 14 WWTPs serve administrative regions with predominantly rural economic activity: Brazlândia, Planaltina, and Paranoá. The sampling was performed in the following months: July, October, and November 2018, and June, July, August, October, and November 2019. In each WWTP sampling, one sample was collected upstream and another was collected downstream from the discharge point of treated wastewater. It is important to note that WWTPs 5 and 13 discharge the treated wastewater into a lake (Paranoá Lake), and therefore has no downstream or upstream points. For both WWTPs, two samples were collected at different points in each collection cycle, and all samples were considered as downstream.
Sample collection was performed using a polyethylene bucket. Approximately, one liter of surface water from each sample site was collected in sterile bottles and transported to the laboratory in thermal boxes kept at room temperature and protected from sunlight. After processing, the samples were stored at – 30 °C.
2.2 Virus concentration
The viral concentration of the samples was performed using the adsorption-elution method with negatively charged membranes [18, 19]. In order to avoid the contamination with humic acid, besides the negatively charged membranes adopted for viral adsorption, a previous clarifying step using filters with a high capacity of binding dissolved organic materials was done [19, 20]. First, 0.6 mg of MgCl2 was mixed with the water samples (1 L) and the pH was adjusted to 5.0 with 10% HCl. The samples were then filtered twice using a vacuum system. The first filtration was done with a thick, 47-mm-diameter membrane to remove impurities. The second filtration was performed using a negatively charged membrane (Nihon Millipore®, Tokyo, Japan) with a pore size of 0.45 µm and a diameter of 47 mm. In the next step, still in the vacuum filtration system, the membrane was rinsed with 87.5 mL of 0.5 mM H2SO4 solution (pH 3.0); this procedure makes the virus polarity predominantly positive, allowing greater adsorption. The membrane was then removed from the system and placed in a petri dish. For viral elution, 2.5 mL of 1 mM NaOH (pH 10.5) was added to the plate and homogenized for 10 min using the Coleman VDRL TS 2000A multifunctional shaker. Alkaline elution makes the virus charge negative, allowing it to be eluted from the membrane. The eluate was then neutralized by the addition of 50 μL of H2SO4 solution (50 mM) and 50 µL of 1 mM Tris EDTA buffer solution (10X) (pH8). Concentrated samples with a final volume of approximately 2 mL were aliquoted and stored at – 80 °C until further analysis.
Qualitative controls for the virus concentration method were performed in each sample processing round. Three hundred microliters of bronchoalveolar lavage, previously tested positive for HAdV in the Central Laboratory for Public Health, were used to inoculate water samples (V = 1L) prior to the virus concentration procedure. These positive-control samples served as control for the genome viral extraction and PCR assays.
2.3 Viral nucleic acid purification
The viral DNA was extracted from 250 μL of concentrated samples of wastewater using a Maxwell® 16 device with the Maxwell® 16 Viral Total Nucleic Acid Purification Kit Promega (Madison, WI, USA), following the manufacturer's instructions. Nucleic acids were eluted in 50 μL Nuclease-Free Water from Promega (Madison, WI, USA) and stored at − 80 °C until the detection. This magnetic silica bead-based RNA extraction was adopted once it effectively removed inhibitors that interfere with viral nucleic acid extraction and RT-PCR assays [20].
2.4 Detection of HAdV
HAdV was detected by conventional PCR, using primers specific for the hexon gene of all species (A to F) of the HAdV genome [21]. Briefly, amplification was performed using 3 µL of extracted DNA, 1 µL of each primer (forward Ad1 (TTC CCC ATG GCI CAY AAC AC) and reverse Ad2 (CCC TGG TAK CCR ATR TTG TA)), 2 µL of dNTP (2.5 mM) (Invitrogen, USA), 5 µL of 10 × buffer containing MgCl2 (Sigma-Aldrich, USA), 0.5 µL of (5 U/μL) Taq DNA polymerase (Invitrogen, USA), and 12.5 µL of MiliQ water, for a total volume of 25 µL. The amplification program used was as follows: 1 cycle at 80 °C for 1 min, followed by 94 °C for 3 min; 35 cycles of 94° for 1 min, annealing at 55 °C for 1 min and extension at 72 °C for 1 min; and a final extension of 72° for 7 min and subsequent maintenance at 4 °C. The reaction product was stored at – 20 °C until used for further analysis. The amplicons were visualized on a 1.5% agarose gel stained with ethidium bromide. The running time was 1 h, using a voltage of 80 V.
In addition to the positive-control samples, ultrapure water from Promega (Madison, WI, USA) was used as negative samples in PCR assays.
2.5 Sequencing of viral genomes found in positive samples and phylogenetic analysis
HAdV amplicons were purified using ReliaPrepTM DNA Clean-up and Concentration System kit (Promega, USA) and the amplicons were sequenced using the BigDye™ terminator cycle sequencing v3.1 kit (Applied Biosystems, USA) according to the manufacturer's instructions. The thermal cycle used was as follows: 96 °C for 1 min; 30 cycles of 96 °C for 10 s, 50 °C for 5 s, and 60 °C for 4 min; maintenance at 4 °C.
The sequencing reaction was purified with the BigDye® XTerminator™ Purification Kit (Applied Biosystems, USA) according to the manufacturer's recommendations and analyzed with ABI 3500 Genetic Analyzer equipment (Applied Biosystems, USA). The obtained sequences were identified by the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST) by comparison with available sequences.
2.6 Phylogenetic analysis
The nucleotide sequences were edited and aligned using the BioEdit Sequence Alignment Editor program v. 7.0.5.3, based on the pherogram. After editing, they were compared to the reference sequences obtained in BLAST. The reference sequences used were Adenovirus type 40 hexon gene (X51782.1, position 1819.0.2252) for genotype 40; Human adenovirus 41 isolated GyK253, complete genome (sequence KX868523.2, position 19407.0.19825) and Human mastadenovirus F strain HAdV41/Novosibirsk/NS12-N3542/2012/RUS hexon gene, partial cds (sequence MF589701.1, position 81.0.430) for genotype 41; and GgorAdV-B7 (sequence HQ292614.1, position 14788.0.15136) as an outgroup.
The phylogenetic tree was constructed using the MEGA 10.1.7 program based on neighbor-joining method; a bootstrap test with 1000 replicates was used. Genetic distances were calculated by the p-distance model. The tree was edited using iTOL: Interactive Tree Of Life v.5.
2.7 Rainfall index
The rainfall index for the period in which the samples were collected were downloaded from the Instituto Nacional de Meteorologia (INMET) database, and the correlation with HAdV genotyping was evaluated.
2.8 Statistical analyses
To verify the statistical association in the frequency distribution of viral types at the upstream and downstream collection points, and secondary vs tertiary treatment at downstream collection points, an exact Fischer tests were performed. For rainfall analysis and adenovirus frequency, the non-normal distribution of one of the variables was verified and spearman correlation was performed. In order to assess rainfall indexes in relation to the genotype, the non-normal distribution of the data was verified, and the Mann–Whitney test was carried out. GraphPad Prism 8.3.0 program was used for the analysis, and data were considered statistically significant at p < 0.05.
3 Results
A total of eight collections were analyzed, corresponding to the months of July, October, and November 2018 and June, July, August, October, and November 2019. Each collection consisted of 28 samples; 12 samples from the upstream site and 12 samples from the downstream site of each WWTP. Four samples were collected from WWTP 5 (two samples) and 13 (two samples), which have no downstream and upstream points; these samples were all considered as downstream. Of the 224 samples, HAdV was detected in 27.2% (61/224) collected samples (Table 1).
Downstream samples displayed a significant higher prevalence of HAdVs (38.3%, 49/128), as compared to prevalence observed for the upstream samples (12.5%, 12/96) (Fig. 1) (p < 0.0001).
With the exception of the tertiary-level WWTP-9, HAdVs were detected in all remaining water bodies impacted by WWTPs. Despite HAdVs have been detected regardless of the sewage treatment achieved, the HAdV positivity was different (p = 0.0153) in downstream samples collected from receiving water bodies impacted by secondary-level WWTPs (55–22/40) in comparison with those impacted by tertiary-level WWTPs (30–22/72) (Table 2).
Of the 61 HAdV-positive samples, 60 were sequenced. After phylogenetic analysis it was possible to observe the presence of genotypes 40 in 20% (12/60) and 41 in 80% (48/60) of the samples (Fig. 2; Table 2). Also, the presence of genotype 40 or 41 was independent of the type of treatment (secondary or tertiary) of each WWTP and the point of collection, upstream or downstream (Table 2). It is important to note that samples may have mixtures of HAdV genotypes. Considering this observation, our results may show the predominant occurrence of one HAdV genotype per sample collected. PCR reactions tend to enrich and detect nucleic acid molecules that are overrepresented, as the first amplification cycles of a specific target lead to concurrent inhibition of the amplification of other targets. Pherogram analyses in sequencing assays, which showed bases with well-defined peaks along the sequences and with a low level of noise, confirm the existence of predominant genotypes for each sample (data not shown).
Both genotypes have tropism for the gastrointestinal tract and are consequently associated with the development of gastroenteritis. Phylogenetic analysis also made it possible to observe a possible association of genotypes with precipitation. Despite the overall predominance of genotype 41, it was verified a trend in increasing the frequency of genotype 40 associated with higher pluviosity. Indeed, pluviosity values associated with genotype 40 were frequently higher (Mann–Whitney mean rank = 42.25) than those associated with genotype 41 (Mann–Whitney mean rank = 27.56) (p = 0.005) (Fig. 3).
Considering the possible association between the prevalence of genotype 40 in periods of rain and genotype 41 in periods of drought, an analysis was carried out to verify the statistical difference between the variables. Indeed, the distribution of precipitation was different between the two genotypes (Spearman correlation; rs = -− 0.098; p > 0.05) (Fig. 3).
4 Discussion
In Brazil, contamination of water bodies through the release of untreated sewage represents a public health problem that directly affects the most vulnerable and low-income populations [12]. In addition, the treatments currently applied by WWTPs are not able to completely remove or inactivate pathogens [22]. For the first time, all 14 WWTPs from Brazilian Federal District had their receiving water bodies surveyed for the presence of HAdV.
HAdV was detected in 27.2% (61/224) of the samples obtained from 13 out of 14 WWTPs. Despite the HAdV leak from both secondary- and tertiary-level WWTPs, the secondary-level plants responded for a higher frequency of water body contamination. The presence of HAdVs in some water samples was expected, given the stability of their genome in comparison with RNA viruses and their higher frequency and abundance in waters compared to other enteric viruses [23]. In Brazil, similar low prevalence was observed in waterstream samples in Porto Alegre (21.4%) [19]. Worldwide, low HAdVs prevalence was also observed in samples from a river water in Italy (21.6%) and wastewater samples in Taiwan (27.3%) and Morocco (45.5%) [24,25,26]. Oppositely, high prevalence of HAdVs were found in Brazil. Studies demonstrated high prevalence in treated effluents samples in São Paulo (100%) [15], in environmental samples in Florianópolis (64%) [27] and in the Sinos River watershed region, southern region of Brazil (75%) [28]. Important studies demonstrated high prevalence of HAdVs around the world, such as studies from Italy (60%), South Africa (62.5 and 64%), Tunisia (64%), Norway (92%), Greece (76.9 to 92.3%), Poland (92.1%) and United Kingdom (100%) [10, 29,30,31,32,33,34,35]. The wide range of results from the different studies is related to the different water matrices, types of treatment, sampling point and detection methodologies. Anyway, the presence of HAdVS in diverse water samples in diverse regions of the world strengthens the need of environmental virology monitoring.
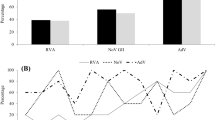
Despite the low-cost of use of fecal indicator bacteria (FIB) to monitor water quality, there are still some limitations. There are sources other than human feces that give rise to FIB, and the presence of FIB does not necessarily correlate with the presence of human enteric viruses. Outbreaks of viral-related illnesses among FIB-free waters or water with quality criteria based on FIB demonstrate that risks to health may occur when FIB alone are used as a water control quality index [36]. Virus particles should thus be considered as complementary measures to bacterial indicators, once the potential role of water in transmission of viruses and the outcomes should not be excluded. In addition, recent studies demonstrated the presence of several types of enteric viruses in water matrices, such as Rotaviruses A (RVA), Noroviruses (NoVs), Hepatoviruses (HVA) and Adenoviruses (HAdVs) [10, 37,38,39,40,41,42,43]. Some authors note that HAdVs can be used as virological indicators of human contamination and, consequently, a marker for water quality. Furthermore, characteristics such as persistence and stability make HAdV a possible indicator of water quality. In addition, HAdVs are also widely distributed in a variety of water matrices, including lakes and rivers, sewage systems, treated waters, and swimming pools [17, 44].
On the other hand, the use of viruses as indicators of contamination faces some barriers, including the difficulty in recovering these particles and standardizing a detection method. Variability in the HAdV positivity rate found in different studies can be attributed to factors such as concentration and molecular methods used, season, and geographic location [1]. In this study, we successfully used an adsorption-elution method with negatively charged membranes for viral concentration [18]. Conventional PCR was used for molecular detection. Other studies used adsorption-elution methods with negatively charged membranes and demonstrated good recovery of viral particles [45,46,47]. In another study, this method was shown to be less susceptible to the action of organic compounds, which are potential inhibitors of molecular techniques [48]. Other advantages include low cost and easy execution of the technique [19]. The use of quantitative real-time PCR, a methodology with greater sensitivity than the conventional PCR, which may also explain the higher rates of detection in other studies. Finally, the concentration methods used in these studies (ultrafiltration and filtration systems, respectively) are different from those used here. For instance, the study performed in Porto Alegre [19] used the same concentration and detection methods as our study and, similar results were found.
It is widely known that adenovirus is more resistant to sewage treatment compared to other enteric viruses [10, 49], and the treatment level accomplished is a key factor that affects the positivity rate of HAdVs in effluents. Here, we observed a significant lower rate of HAdV positivity in downstream samples after tertiary treatment compared to secondary one. In the Brazilian context, another study had already demonstrated decreasing in the prevalence and concentration of HAdVs after tertiary treatment [15]. In fact, the higher resistance of HAdVs to secondary treatments was also documented, showing that conventional and standard wastewater treatment techniques are inadequate and ineffective for eliminating enteric adenoviruses [10]. In addition, although several types of treatment process effectively reduce the adenovirus load, the virus is still detected in the final effluent (even after tertiary treatment) [50]. In our study, despite a lower HAdVs positivity was observed after tertiary treatment, the viability and concentration of remaining HAdVs were not assessed. It is particularly important, once the tertiary treatment and disinfection process may impact the viability of HAdVs particles and its genomes [15].
All HAdV strains were sequenced and a phylogenetic analysis was performed to identify the genotypes of HAdV. Only one sequence could not be identified due to the difficulty of obtaining the electropherogram with good quality. Phylogenetic analysis revealed two genotypes. All samples belonged to species F, with 20% of sequencing as genotype 40 (12/60) and 80% as genotype 41 (48/60). Species F is primarily associated with gastroenteric disorders. Initial epidemiological studies on the prevalence of HAdV-40 and HAdV-41 reported that the occurrence rates of these genotypes were approximately equal [51]. However, the pattern of occurrence of these genotypes has changed in recent years, with a reduction in HAdV-40 and an increase of HAdV-41. In addition, several epidemiological studies report a predominance of genotype 41 in cases of pediatric gastroenteritis. The increase in the prevalence of HAdV may be associated with antigenic drift [52,53,54]. In water matrices, the genotype 41 is also the most prevalent genotype in several regions worldwide such as Michigan, Poland, Norway, South Africa, Tunisia, Taiwan, Singapore, Germany, Italy [10, 29, 31, 32, 55,56,57,58]. These data strengthen the results obtained in this study that also point the genotype 41 as the most prevalent in the capital of Brazil.
Through genotyping, it was uncovered a possible relationship between the presence of a specific genotype and rainfall, confirmed by the evaluation of precipitation levels in each sampling. We observed that genotype 40 had its detection frequency in months of higher rainfall. No other studies comparing the rainfall index and HAdV genotype emergence have been published. Studies assessing the prevalence of HAdVs in wastewater and seasonality have shown conflicting results. This may be explained by the climatic and environmental conditions and linked to the variation and the specificity of the climate in each region [10, 30, 32, 58, 59]. Here, the sampling method did not allow the assessment of seasonality, and any conclusion would be mere speculation. Thus, further studies should be conducted to investigate this correlation.
5 Conclusion
For the first time, the prevalence and genetic diversity of HAdV in treated effluents in the Brazilian Federal District was demonstrated. As expected, the largest number of positive samples was found downstream of the collection point. The presence of genotypes 40 and 41 was observed in WWTPs evaluated, with the dominance of enteric adenoviruses genotype 41. Finally, the genotype 40 was associated with the rainy season and genotype 41 with the drought period. This study had an exploratory character and was the first carried out with the objective of evaluating viral contamination in the WWTPs in the Brazilian Federal District. We highlight the necessity of continuous viral contamination monitoring of WWTPs and the establishment of effective disinfection treatment of wastewater to prevent the waterborne disease outbreaks.
Availability of data and material
Data transparency.
Code availability
Software application or custom code.
References
Iaconelli M, Muscillo M, Della Libera S, Fratini M, Meucci L, De Ceglia M, Giacosa D, La Rosa G (2016) One-year surveillance of human enteric viruses in raw and treated wastewaters, downstream river waters, and drinking waters. Food Environ Virol 9:79–88. https://doi.org/10.1007/s12560-016-9263-3
Sriwanna P, Chieochansin T, Vuthitanachot C, Vuthitanachot V, Theamboonlers A, Poovorawan Y (2013) Molecular characterization of human adenovirus infection in Thailand, 2009–2012. Virol J 10:1. https://doi.org/10.1186/1743-422X-10-193
Toth K, Lee SR, Ying B, Spencer JF, Tollefson AE, Sagartz JE, Kong IK, Wang Z, Wold WSM (2015) STAT2 Knockout Syrian Hamsters support enhanced replication and pathogenicity of human adenovirus, revealing an important role of type i interferon response in viral control. PLoS Pathog 11:1–22. https://doi.org/10.1371/journal.ppat.1005084
Lenaerts L, De Clercq E, Naesens L (2008) Clinical features and treatment of adenovirus infections. Rev Med Virol 18:357–374. https://doi.org/10.1002/rmv
HAdV Working Group (2019) Available online: http://hadvwg.gmu.edu/. Accessed 11 Sept 2019
Kosulin K (2019) Intestinal HAdV infection: tissue specificity, persistence, and implications for antiviral therapy. Viruses 11:15–21
Primo D, Pacheco GT, Timenetsky M, Luchs A (2018) Surveillance and molecular characterization of human adenovirus in patients with acute gastroenteritis in the era of rotavirus vaccine, Brazil, 2012–2017. J Clin Virol 109:35–40. https://doi.org/10.1016/j.jcv.2018.10.010
Elmahdy EM, Ahmed NI, Shaheen MNF, Mohamed ECB, Loutfy SA (2019) Molecular detection of human adenovirus in urban wastewater in Egypt and among children suffering from acute gastroenteritis. J Water Health 17:287–294. https://doi.org/10.2166/wh.2019.303
Rodríguez-Lázaro D, Cook N, Ruggeri FM, Sellwood J, Nasser A, Nascimento MSJ, D’Agostino M, Santos R, Saiz JC, Rzezutka A et al (2012) Virus hazards from food, water and other contaminated environments. FEMS Microbiol Rev 36:786–814. https://doi.org/10.1111/j.1574-6976.2011.00306.x
Ibrahim C, Hassen A, Pothier P, Mejri S, Hammami S (2018) Molecular detection and genotypic characterization of enteric adenoviruses in a hospital wastewater. Environ Sci Pollut Res 25:10977–10987. https://doi.org/10.1007/s11356-018-1399-2
Dias J, Pinto RN, Vieira CB, de Abreu CA (2018) Detection and quantification of human adenovirus (HAdV), JC polyomavirus (JCPyV) and hepatitis A virus (HAV) in recreational waters of Niterói, Rio de Janeiro, Brazil. Mar Pollut Bull 133:240–245. https://doi.org/10.1016/j.marpolbul.2018.05.031
Prado T, Miagostovich MP (2014) Virologia ambiental e saneamento no Brasil: uma revisão narrativa. Cad Saude Publica 30:1367–1378. https://doi.org/10.1590/0102-311X00109213
Fong TT, Lipp E (2005) Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiol Mol Biol Rev 69:357–371. https://doi.org/10.1128/MMBR.69.2.357
La Rosa G, Pourshaban M, Iaconelli M, Muscillo M (2010) Quantitative real-time PCR of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy. Ann Ist Super Sanità 46:266–273. https://doi.org/10.4415/ANN_10_03_07
Prado T, de Castro BA, Barbosa MRF, Garcia SC, de Jesus Melo AM, Sato MIZ (2019) Performance of wastewater reclamation systems in enteric virus removal. Sci Total Environ 678:33–42. https://doi.org/10.1016/j.scitotenv.2019.04.435
Girardi V, Demoliner M, Gularte JS, Spilki FR (2019) ‘Don’t put your head under water’: enteric viruses in Brazilian recreational waters. New Microbes New Infect 29:100519. https://doi.org/10.1016/j.nmni.2019.100519
Rames E, Roiko A, Stratton H, Macdonald J (2016) Technical aspects of using human adenovirus as a viral water quality indicator. Water Res 96:308–326. https://doi.org/10.1016/j.watres.2016.03.042
Katayama H, Shimasaki A, Ohgaki S (2002) Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Appl Environ Microbiol 68:1033–1039. https://doi.org/10.1128/AEM.68.3.1033-1039.2002
Vecchia et al (2012) First description of Adenovirus, Enterovirus, Rotavirus and Torque teno virus in water samples collected from the Arroio Dilúvio, Porto Alegre, Brazil. Braz J Biol 72:323–329
Hata A, Katayama H, Kitajima M, Visvanathan C, Nol C, Furumai H (2011) Validation of internal controls for extraction and amplification of nucleic acids from enteric viruses in water samples. Appl. Environ. Microbiol. 77:4336–4343. https://doi.org/10.1128/AEM.00077-11
Khamrin P, Okame M, Thongprachum A, Nantachit N, Nishimura S, Okitsu S, Maneekarn N, Ushijima H (2011) A single-tube multiplex PCR for rapid detection in feces of 10 viruses causing diarrhea. J Virol Methods 173:390–393. https://doi.org/10.1016/j.jviromet.2011.02.012
Okoh AI, Sibanda T, Gusha SS (2010) Inadequately treated wastewater as a source of human enteric viruses in the environment. Int J Environ Res Public Health 7:2620–2637. https://doi.org/10.3390/ijerph7062620
Verani M, Federigi I, Donzelli G, Cioni L, Carducci A (2019) Science of the total environment human adenoviruses as waterborne index pathogens and their use for quantitative microbial risk assessment. Sci Total Environ 651:1469–1475. https://doi.org/10.1016/j.scitotenv.2018.09.295
La Rosa G, Sanseverino I, Della Libera S, Iaconelli M, Ferrero VEV, Barra Caracciolo A, Lettieri T (2017) The impact of anthropogenic pressure on the virological quality of water from the Tiber River, Italy. Lett Appl Microbiol 65:298–305. https://doi.org/10.1111/lam.12774
Lim MCY, Wang YF, Huang SW, Yang JY, Wang JR (2015) High Incidence of mammalian orthoreovirus identified by environmental surveillance in Taiwan. PLoS ONE 10:1–14. https://doi.org/10.1371/journal.pone.0142745
Amdiouni H, Faouzi A, Fariat N, Hassar M, Soukri A, Nourlil J (2012) Detection and molecular identification of human adenoviruses and enteroviruses in wastewater from Morocco. Lett Appl Microbiol 54:359–366. https://doi.org/10.1111/j.1472-765X.2012.03220.x
Rigotto C, Victoria M, Moresco V, Kolesnikovas CK, Corrêa A, Souza DSM, Miagostovich MP, Simões CMO, Barardi CRM (2010) Assessment of adenovirus, hepatitis A virus and rotavirus presence in environmental samples in Florianopolis, South Brazil. J Appl Microbiol 109:1979–1987. https://doi.org/10.1111/j.1365-2672.2010.04827.x
Rodrigues MT, Henzel A, Staggemeier R, de Quevedo DM, Rigotto C, Heinzelmann L, do Nascimento CA, Spilki FR (2015) Human adenovirus spread, rainfalls, and the occurrence of gastroenteritis cases in a Brazilian basin. Environ Monit Assess. https://doi.org/10.1007/s10661-015-4917-4
Iaconelli M, Valdazo-González B, Equestre M, Ciccaglione AR, Marcantonio C, Della Libera S, La Rosa G (2017) Molecular characterization of human adenoviruses in urban wastewaters using next generation and Sanger sequencing. Water Res 121:240–247. https://doi.org/10.1016/j.watres.2017.05.039
Adefisoye MA, Nwodo UU, Green E, Okoh AI (2016) Quantitative PCR detection and characterisation of human adenovirus, rotavirus and Hepatitis A virus in discharged effluents of two wastewater treatment facilities in the Eastern Cape, South Africa. Food Environ Virol 8:262–274. https://doi.org/10.1007/s12560-016-9246-4
Osuolale O, Okoh A (2015) Incidence of human adenoviruses and Hepatitis A virus in the final effluent of selected wastewater treatment plants in Eastern Cape Province, South Africa. Virol J 12:1–8. https://doi.org/10.1186/s12985-015-0327-z
Wieczorek M, Krzysztoszek A, Witek A (2015) Species-specific identification of human adenoviruses in sewage. Polish J Microbiol 64:23–28. https://doi.org/10.33073/pjm-2015-003
Farkas K, Marshall M, Cooper D, McDonald JE, Malham SK, Peters DE, Maloney JD, Jones DL (2018) Seasonal and diurnal surveillance of treated and untreated wastewater for human enteric viruses. Environ Sci Pollut Res 25:33391–33401. https://doi.org/10.1007/s11356-018-3261-y
Fumian TM, Vieira CB, Leite JPG, Miagostovich MP (2013) Assessment of burden of virus agents in an urban sewage treatment plant in Rio de Janeiro, Brazil. J Water Health 11:110–119. https://doi.org/10.2166/wh.2012.123
Grøndahl-Rosado RC, Yarovitsyna E, Trettenes E, Myrmel M, Robertson LJ (2014) A one year study on the concentrations of norovirus and enteric adenoviruses in wastewater and a surface drinking water source in Norway. Food Environ Virol 6:232–245. https://doi.org/10.1007/s12560-014-9161-5
Sun S, Shi Y, Tong HI, Kang W, Wang Z, Allmann E, Lu Y (2016) Effective concentration, recovery, and detection of infectious adenoviruses from environmental waters. J Virol Methods 229:78–85. https://doi.org/10.1016/j.jviromet.2016.01.002
Ibrahim C, Cherif N, Hammami S, Pothier P, Hassen A (2015) Quantification and molecular characterization of Norovirus after two wastewater treatment procedures. Water Air Soil Pollut 226:1–13. https://doi.org/10.1007/s11270-015-2402-x
Ibrahim C, Cherif N, Hammami S, Pothier P, Hassen A (2016) Quantification and genotyping of Rotavirus A within two wastewater treatment processes. Clean: Soil, Air, Water 44:393–401. https://doi.org/10.1002/clen.201400588
Ibrahim C, Hammami S, Pothier P, Khelifi N, Hassen A (2020) The performance of biological and tertiary wastewater treatment procedures for rotaviruses A removal. Environ Sci Pollut Res 27:5718–5729. https://doi.org/10.1007/s11356-019-05487-2
Ibrahim C, Hammami S, Khelifi N, Pothier P, Hassen A (2020) The effectiveness of activated sludge procedure and UV-C254 in Norovirus inactivation in a Tunisian industrial wastewater treatment plant. Food Environ Virol 12:250–259. https://doi.org/10.1007/s12560-020-09434-0
Ibrahim C, Hamdi R, Hammami S, Pothier P, Khelifi N, Hassen A (2020) Inactivation of Hepatovirus A in wastewater by 254 nm ultraviolet-C irradiation. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-11601-6
Cadamuro RD, Viancelli A, Michelon W, Fonseca TG, Mass AP, Krohn DMA, Peter NRW, Fongaro G (2021) Enteric viruses in lentic and lotic freshwater habitats from Brazil’s Midwest and South regions in the Guarani Aquifer area. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-13029-y
Shi D, Ma H, Miao J, Liu W, Yang D, Qiu Z, Shen Z, Yin J, Yang Z, Wang H et al (2021) Levels of human Rotaviruses and Noroviruses GII in urban rivers running through the city mirror their infection prevalence in populations. Sci Total Environ 754:142–203. https://doi.org/10.1016/j.scitotenv.2020.142203
Silva HD, García-Zapata MTA, Anunciação CE (2011) Why the use of adenoviruses as water quality virologic marker? Food Environ Virol 3:138–140. https://doi.org/10.1007/s12560-011-9069-2
Hsu BM, Chen CH, Kung CM, Wan MT, Shen SM (2007) Evaluation of enterovirus recovery in surface water by different adsorption and elution procedures. Chemosphere 66:964–969. https://doi.org/10.1016/j.chemosphere.2006.06.054
Spilki FR, da Luz RB, Fabres RB, Soliman MC, Kluge M, Fleck JD, Rodrigues MT, Comerlato J, Cenci A, Cerva C et al (2013) Detection of human adenovirus, rotavirus and enterovirus in water samples collected on dairy farms from Tenente Portela, Northwest Of Rio Grande do Sul, Brazil. Brazilian J Microbiol 44:953–957. https://doi.org/10.1590/S1517-83822013000300046
Katayama H, Haramoto E, Oguma K, Yamashita H, Tajima A, Nakajima H, Ohgaki S (2008) One-year monthly quantitative survey of noroviruses, enteroviruses, and adenoviruses in wastewater collected from six plants in Japan. Water Res 42:1441–1448. https://doi.org/10.1016/j.watres.2007.10.029
Hata A, Katayama H, Kojima K, Sano S, Kasuga I, Kitajima M, Furumai H (2014) Effects of rainfall events on the occurrence and detection efficiency of viruses in river water impacted by combined sewer overflows. Sci Total Environ 468–469:757–763. https://doi.org/10.1016/j.scitotenv.2013.08.093
Sidhu JPS, Sena K, Hodgers L, Palmer A, Toze S (2018) Comparative enteric viruses and coliphage removal during wastewater treatment processes in a sub-tropical environment. Sci Total Environ 616–617:669–677. https://doi.org/10.1016/j.scitotenv.2017.10.265
Liu P, Herzegh O, Fernandez M, Hooper S, Shu W, Sobolik J, Porter R, Spivey N, Moe C (2013) Assessment of human adenovirus removal by qPCR in an advanced water reclamation plant in Georgia, USA. J Appl Microbiol 115:310–318. https://doi.org/10.1111/jam.12237
Arashkia A, Bahrami F, Farsi M, Nejati B, Jalilvand S, Nateghian A, Rahbarimanesh A, Shoja Z (2019) Molecular analysis of human adenoviruses in hospitalized children < 5 years old with acute gastroenteritis in Tehran. Iran J Med Virol 91:1930–1936. https://doi.org/10.1002/jmv.25539
Verma H, Chitambar SD, Varanasi G (2009) Identification and characterization of enteric adenoviruses in infants and children hospitalized for acute gastroenteritis Harsha. J Med Virol 81:60. https://doi.org/10.1002/jmv
Li L, Phan TG, Nguyen TA, Kim KS, Seo JK, Shimizu H, Suzuki E, Okitsu S, Ushijima H (2005) Molecular epidemiology of adenovirus infection among pediatric population with diarrhea in asia. Microbiol Immunol 49:121–128. https://doi.org/10.1111/j.1348-0421.2005.tb03711.x
Dey RS, Ghosh S, Chawla-Sarkar M, Panchalingam S, Nataro JP, Sur D, Manna B, Ramamurthy T (2011) Circulation of a novel pattern of infections by enteric adenovirus serotype 41 among children below 5 years of age in Kolkata, India. J Clin Microbiol 49:500–505. https://doi.org/10.1128/JCM.01834-10
Fong TT, Phanikumar MS, Xagoraraki I, Rose JB (2010) Quantitative detection of human adenoviruses in wastewater and combined sewer overflows influencing a Michigan river. App Environ Microbiol 76:715–723. https://doi.org/10.1128/AEM.01316-09
Myrmel M, Lange H, Rimstad E (2015) A 1-year quantitative survey of Noro-, Adeno-, Human Boca-, and Hepatitis E Viruses in raw and secondarily treated sewage from two plants in Norway. Food Environ Virol 7:213–223. https://doi.org/10.1007/s12560-015-9200-x
Aw TG, Gin KYH (2010) Environmental surveillance and molecular characterization of human enteric viruses in tropical urban wastewaters. J Appl Microbiol 109:716–730. https://doi.org/10.1111/j.1365-2672.2010.04701.x
Kuo HW, Chen LZ, Shih MH (2015) High prevalence of type 41 and high sequence diversity of partial hexon gene of human adenoviruses in municipal raw sewage and activated sludge. J Appl Microbiol 119:1181–1195. https://doi.org/10.1111/jam.12907
Lipp EK, Kurz R, Vincent R, Rodriguez-Palacios C, Farrah SR, Rose JB (2001) The effects of seasonal variability and weather on microbial fecal pollution and enteric pathogens in a subtropical estuary. Estuaries 24:266–276. https://doi.org/10.2307/1352950
Funding
This work was supported for Fundação de Apoio à Pesquisa do Distrito Federal (FAP / DF); (Grant number 193.000.713 / 2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quintão, T.S.C., Silva, F.G., Pereira, A.L. et al. Detection and molecular characterization of enteric adenovirus in treated wastewater in the Brazilian Federal District. SN Appl. Sci. 3, 691 (2021). https://doi.org/10.1007/s42452-021-04678-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04678-2