Abstract
The present study focuses on the standardization of the supercritical fluid extraction of the Bhut Jolokia (Capsicum chinense) which is abundantly grown in the north eastern region of India. The effect of process parameters pressure (75–225 bar), temperature (40–60 °C) and time (30–90 min) of oleoresin extraction process was studied. The standardized condition to obtain maximum extraction of Bhut Jolokia oleoresin was found to be 207 bar, 60 °C and 73 min. The oleoresin extract was analyzed for its antimicrobial, antioxidant and total phenolics content. Four strains of bacteria namely Escherichia coli (ATCC -11,229), Bacillus subtilis (ATCC- 11,774), Salmonella typhimurium (ATCC- 14,028) and Staphylococcus aureus (12,600) were used for the antimicrobial assay. It was observed that the highest inhibition was seen against E. coli, moderate inhibition was seen against S.aureus and S. typhi and partial/no zone of inhibition was observed against B. subtilis. The extract of Bhut Jolokia oleoresin showed radical scavenging activity of 58.6 ± 3.86% and total phenolics content of 4250 ± 2.26 mg GAE/100 g sample indicating Bhut Jolokia oleoresin as a good antioxidant and is also a good source of phenolic compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chilli is considered to be one of the popular commercial crops throughout the world and is a part of most of the recipies. It is known by different names globally, some of the prevalent names are red pepper, bell pepper, sweet pepper, pimento, cayenne and so forth. It belongs to the family of Solanaceae (Nightshade family), in India, it is cultivated in the southern part including Andhra Pradesh, Tamil Nadu and Karnataka and also in the northeast region [1].
Ghost pepper is a popular variety of chilli which is well known all over the world for its pungency. Ghost pepper (also known as Bhut Jolokia, King Chilly, Omrok) was earlier known as a hybrid variety of Capsicum chinense and Capsicum frutescens, but recently it has been reported as a distinct species, Capsicum assamicum [2]. Once known as the hottest chilli with Scoville heat units of 1,041,427 SHU, it is an indigenous fruit mainly grown in the northeastern region of India namely Assam, Manipur, Nagaland and Arunachal Pradesh [3]. The matured fruit of Bhut Jolokia is around 60–85 mm long and can be around 25–30 mm wide. Commercially it is used in dried as well as fresh forms. The major pungent principle in ghost pepper is capsaicin, reportedly found only in Capsicum fruit [4] followed by dihydrocapsaicin, norcapsaicin, nordihydrocapsaicin, homocapsaicin and homodihydrocapsaicin. Capsaicin is considered to be found in high amount in fruits of Capsicum chinense as compared to fruits of other chilli variety [5] and it is known to have potential therapeutic properties.
Oleoresin is generally a natural or synthetic blend of essential oil and resin. It can also be referred to a compound acquired by beneficiation of one or several functional units in the absolute extract by fractionation [6]. There are varieties of oleoresins generally categorized into three types as paprika oleoresin, red pepper oleoresin and Capsicum oleoresin. Paprika oleoresin can be used as a colouring agent generally in processed meats, dairy products, snacks, sauces and so forth. Red pepper oleoresin finds its usage in canned meats, various sausages etc. as it is a great source for colour as well as pungency. On being the most pungent chilli oleoresin, Capsicum oleoresin is mostly applied in plasters and pharmaceutical products as a superficial inflammation [6].
Spices are an important source of trade in many countries and are of great demands all over the world for applications in processed food industries, fragrant and cosmetics industries, spice oleoresins have found great application since they can be a great colouring agent as well as flavouring agents. Traditional extraction of these oleoresins involves various methods like adsorption, leaching, distillation of the spice oils followed by extraction using various solvents which are not desirable due to solvent residues in extracts. Using high temperature in these processes also leads to degrading of the thermolabile compounds thereby manufacturing products with low quality. In order to overcome these challenges, it is important to find the best separation technologies to provide yield in its purest form and also compounds with exceptional quality. One such novel technology considered to be a great extraction alternative was supercritical fluid extraction (SFE) process.
The solvents used in this process are known as supercritical fluids. They are substances at the pressure and temperature above their critical point. Above the critical point, the fluid is neither in their gaseous phase nor in the liquid phase. These properties allow the supercritical fluids for effective extraction with high mass transfer capacity [7]. Various solvents like water, ethane, propane so forth can be used as a supercritical fluid (SCF), but the most commercially used solvent is the supercritical CO2 since it is inert, harmless, inflammable, noncorrosive and widely available [8], and therefore it is also the most preferred supercritical solvent used in the food industry. The critical point of CO2 is 31.1 °C and 73.8 bar making it an appropriate solvent for extraction of a varying range of unstable and heat-labile compounds. With these advantages, the supercritical fluids are useful for extraction of valuable bioactive compounds (flavouring compound, pigment and other biomolecules). They can also be utilised for removing undesirable compounds like organic pollutants, toxins [9]. However, apart from these advantages, SFE also possesses certain disadvantages which should be taken care of including the density and thickness of the sample affects the SCF into the matrix, large number of variables may affect the extraction process. These issues can be fixed by designing an experimental design optimising the extraction conditions with minimum number of experiments.
In many applications SFE provides good yield or recovery which is comparable to conventional methods. The carbon dioxide is preferred solvent for bioactives, however to extract polar analytes addition of modifiers or co-solvents are used. Methanol is more commonly being reported for quantitative extraction. However, when it comes to extraction for food applications ethanol is preferred due to its GRAS status [10].
Looking into the demands of spice oleoresin in the global market and the need to overcome the various issues related to its extraction process, a systematic study was carried out in this paper involving standardization of the SFE of Bhut Jolokia to obtain the maximum extraction yield and capsaicin content and also analysing the influence of various process parameters (pressure, time and temperature) on the extraction process and yield of oleoresin. Further, the antioxidant and antimicrobial properties of the supercritical fluid extract of Bhut Jolokia oleoresin were also assesed.
2 Materials and methods
2.1 Materials
Ripe and fresh Bhut Jolokia was bought from the local market. The ripe chillies were washed to remove dirt and impurities. They were then sorted and dried in a tray drier at around 60 °C until the moisture content reduced near to constant. These dried pods were then grounded (mean particle size 0.5 mm) and kept in a cool, dry and dark place to avoid degradation of the colouring pigments in the sample. Characterization and extraction of this ground sample were carried out further and ethanol was used as an extraction solvent as it is commonly used for food applications and is generally recognised as safe (GRAS) for food applications.
2.2 Reagents
Standard of capsaicin (8-Methyl-N-vanillyl-trans-6-nonenamide) was from HiMedia, ethanol (for extraction) was from the Helix, HPLC grade methanol and acetonitrile from MEARCK, nutrient agar, nutrient broth, DTTP and Folin Ciocalteau reagent from HiMedia.
2.3 Experimental procedures
2.3.1 Apparatus
SFE was carried out using a Waters supercritical fluid extractor with 500 mL extracting capacity. The pressure, temperature and CO2 flow rate was controlled using Thar Instruments Process Suit software. For HPLC analysis, Waters gradient HPLC System was used. The system is equipped with HPLC pump 515, a UV/Vis—2489 detector, a refractive index detector- 2414 and an analytical C-18 column (4, 6 × 250 mm, 5 µm). Other equipment used were tray drier, laminar airflow, incubator shaker and UV spectrophotometer, atomic absorption spectrophotometer.
2.3.2 Proximate analysis of Bhut Jolokia
The chemical analysis and mineral composition of the Bhut Jolokia sample was performed following the standard analytical methods to check for its nutritional content. The moisture, ash, protein and fat content were evaluated by the standard methods [11]. The total carbohydrate content was evaluated by the difference method. The mineral content was analysed using atomic absorption spectrophotometer. All the analysis was carried out in triplicate and analytical grade reagents were used.
2.3.3 Extraction of Bhut Jolokia oleoresin
Extraction of the Bhut Jolokia oleoresin was carried out by SFE and conventional solvent extraction.
2.3.4 Soxhlet extraction
Soxhlet extraction of the Bhut Jolokia oleoresin was performed for a comparison study between the traditional method and the SFE method. The oleoresin extraction by Soxhlet extraction was carried out following the method suggested by Sarwa et al. [12] with slight modification. Around 5 g of the coarsely ground pods were extracted using Soxhlet apparatus. Acetone was used as a solvent and the extraction was performed for at least 6 h. The temperature was kept constant at around 65 °C.
2.3.5 Supercritical fluid extraction (SFE)
Chilli oleoresin was extracted using a supercritical extractor of laboratory scale. The extractor with a vessel capacity of 500 mL was used for the extraction. About 10 g of the sample was mixed with glass beads (1 mm in diameter) and placed inside the extraction vessel which was placed inside a column oven. The sample was packed tightly for uniform distribution of CO2 throughout the matrix. Pressurised CO2 was passed with a high-pressure pump and was made to pass through the extraction vessel with a constant flow rate of 5 mL/min. The required pressure level was maintained with a back-pressure regulator. The chiller was set at around − 5 °C to cool the pump heads and liquid CO2 to avoid cavitation issues. Ethanol (99.9%) was used as a co-solvent with a flow rate of 1 mL/min. It is used to elevate the solvent power of CO2 in the extraction of the oleoresin. Its flow rate was controlled by a co-solvent pump. The extraction vessel was heated and its temperature was controlled and monitored using the software. The supercritical CO2 was then passed through the heated micrometre valve to the extraction vessel leading to its expansion and thus extracting the compounds from the sample. The extract was further collected in a suitable vessel. It was then weighed and further evaluated using the following calculation:
where Y is yield, WE is weight of the extract and WS is weight of the sample. After each extraction, the solvent in the oleoresin extract was allowed to evaporate. Hot air oven at 60–65 °C was used for this purpose. Once the solvent evaporated the extract was collected in cryovials and stored at − 18 to − 20 °C until further analysis.
2.4 Analysis of Bhut Jolokia oleoresin
2.4.1 Estimation of capsaicin content
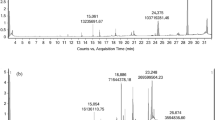
High performance liquid chromatography (HPLC) was used to quantify the capsaicin content of the oleoresin. Standard of capsaicin (8-Methyl-N-vanillyl-6-nonenamide) was used for calibration and the analytical method was followed as per the method described by Collins et al. [13]. A stock solution of 2000 ppm was prepared by dissolving the sample in methanol. The stock solution was diluted further to prepare standard solutions in methanol. They were further filtered using 0.45 µm syringe filter. Individual capsaicinoid peak detection was carried out using the gradient method.
A 20 µL of sample and the standard solution volume was injected for HPLC measurements. Methanol and water were used as mobile phase for the experiment to obtain individual capsaicinoid peaks at a good resolution. The calibration curve was obtained using the standard solution at various concentrations: 1000, 500, 100, 50 and 25 ppm. Operating conditions applied were: maintenance of ambient temperature, the flow rate was 1 mL/min and 20 min of run duration was considered. Gradient method was applied which involved running of the mobile phase consisting of 57% of solvent B (100% methanol) and 43% solvent A (10% methanol in water) for 10 min. This was followed by running of another 10 min of the mobile phase consisting of 68% solvent B and 32% solvent A. The eluents were scanned at 280 nm using a UV/Vis spectrophotometer.
2.4.2 Experimental design and data analysis
The software (Design Expert Version 12) was used for the statistical design of the experiment and data analysis. In this study, the design opted for experimentation was full factorial design. The extractions were carried out based on this design to standardise the SFE process for the chilli oleoresin. Table 1 shows the different process parameters as independent variables at the different levels selected for the study. The factors considered for this design were pressure (bar) (X1), temperature ( °C) (X2), and static time (min) (X3) and the response parameters were yield, capsaicin content and colour content of the Bhut Jolokia extract.
Analysis of variance (ANOVA) was performed to find the statistical significance of the variables, to check the fitting of the model terms. The model adequacy was examined by evaluating the model for the lack of fit test and R2. Response surface graphs were used to check the various effects of the variables on the responses. Second-order polynomial equation (Eq. (2)) was applied to locate the optimize parameters affecting the responses and validate the obtained design:
where k = 0, 1, 2, 3….Yk—Response, bk0—Constant, bki—Linear coefficient, bkii—Quadratic coefficient, bkij—Regression coefficient, Xi—Coded values for the independent variable.
The Design Expert 12 software was used for the numerical and graphical optimization of the process parameters by applying the constrains for the responses. Goals for each factor and responses were selected. The goals for the factors were set to experimental range whereas for the responses it was set to maximize the response value. The importance of each response was selected and they were further united into desirability function.
2.4.3 Anti-microbial activity of Bhut Jolokia oleoresin
The extract obtained using optimized parameters were used to perform antimicrobial assay since the screening of anti-bacterial assay of any promising plant extract should be carried out using either the pure substance or the crude extract [14, 15]. Diffusion techniques are commonly used for screening of antimicrobial or antifungal activity. Agar well diffusion technique was carried out as described in the paper published by Gurnani et al. [16] with a few modifications.
2.5 Test microorganisms
The extract was tested against two Gram positive strains and two Gram negative strains. The Gram positive strains selected were Staphylococcus aureus (ATCC—12,600) and Bacillus subtilis (ATCC -11,774) and the Gram negative strains were Salmonella typhimurium (ATCC-14028) and Escherichia coli (ATCC-11229). The bacterial strains were obtained from the Department of Molecular Biology and Biotechnology, Tezpur University, Assam. The nutrient broth, nutrient agar and all the plates and instruments to be used were sterilized by autoclaving at 121 °C for 15 min. The stock culture was made by inoculating the microbial strains in a freshly prepared nutrient broth. It was incubated at 37 °C for 24 h and was stored at − 20 °C until use.
2.6 Anti-bacterial assay
The anti-bacterial activity of the oleoresin obtained was carried out following the well diffusion method. The samples were diluted with 1% ethanol to a concentration of 20 mg/mL. Agar plates were prepared in a sterile condition by pouring 20 mL of nutrient agar in four different Petri plates each. After solidification of the plates, they were inoculated with the microbes using spread plate technique. The plates were further allowed to dry for 15 min before proceeding further. The plates were then punctured with 2 wells of 6 mm using sterile micro tips. 100 µL of the sample extracted at the optimized condition was used to fill the wells. 5 µg/mL of antibiotic (Ciprofloxacin) was taken as the positive control. The plates were incubated for 24 h at 37 ± 2 °C. The zone of inhibition was then later measured (in mm) to find the anti-microbial activity of the extracts. The sample was analyzed in triplicate.
2.7 Anti-oxidant activity
The anti-oxidant activity of the oleoresin sample was analyzed following the 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging method as described by Brand-Williams et al. [17]. The control was prepared by adding 4.73 mg of DPPH in 100 mL of methanol (HPLC grade). About 900 µL of the sample was then added with 4.5 mL of the control. It was finally incubated in room temperature for 45 min. Since DPPH is light sensitive, the solution was placed in the closed chamber without light. The absorbance was measured in a spectrophotometer at 517 nm [16, 18]. Methanol was taken as the blank. The assay was performed in triplicate and average values was reported. The % antioxidant activity was calculated using the following equation:
where Ac = Absorbance of control at 517 nm, As = Absorbance of the sample at 517 nm.
2.8 Total phenolics content
The total phenolics content in the extract was determined using the Folin-Ciocalteu assay as described by Tohma et al. [19] with some modifications. About 1 mL of the extract was prepared in ethanol. The sample was then added to a 25 mL volumetric flask having 9 mL of distilled water and 1 mL of Folin-Ciocalteu reagent. The mixture was allowed to stand for 5 min. 10 mL of 7% Na2CO3 was added to the mixture. After an incubation period of 90 min, the sample measured at 750 nm using a spectrophotometer. Similarly, the gallic acid standard was prepared and was used to create the calibration graph. The total phenolics content was evaluated using the Eq. (4) below and was further expressed as mg gallic acid equivalent (GAE)/100 g. The sample was analyzed in triplicates and the mean value of the absorbance was obtained.
where C—Total phenolic content in mg GAE/100 g, c—Concentration of gallic acid in mg/Ml, V—Volume of extract in mL, m—Mass of extract in g.
3 Results and discussion
3.1 Chemical composition of Bhut Jolokia
The proximate composition of the Bhut Jolokia powder was carried out following the standard analysis procedures. The composition was carbohydrate (60.22 ± 0.03), crude protein (12.05 ± 0.06), crude fat (7.39 ± 0.12), moisture (12.36 ± 0.73) and ash (7.98 ± 0.45), all the values were in percentage (%) (± SD). The analysis was carried out in triplicate and mean values were reported. However, Kuna et al. [20] reported much lower values of fat, fiber and protein content of King chilli. Variations might be due to changes in the agro-climatic conditions and also variability in the seasonal behaviour of the locations from which the samples were collected [5]. The oleoresin content of the Bhut Jolokia powder was estimated using acetone as a solvent. The average total yield obtained during soxhlet extraction was 6.23 g/100 g. The mineral composition of the Bhut Jolokia powder was also estimated using atomic absorption spectrophotometry. The major minerals found were potassium (31.38 ± 0.46), magnesium (1.53 ± 0.09), calcium (1.31 ± 0.33) and iron (0.07 ± 0.08). All mineral values were reported as mg/100 g (± SD).
3.2 Supercritical fluid extraction of Bhut Jolokia oleoresin
Standardisation of the extraction of the Bhut Jolokia oleoresin using supercritical CO2 was carried out by performing experiments as per the full factorial design with pressure, temperature and duration of the cycle as the three main variables. The responses obtained for the experiments are represented in the Table 1.
After the extraction process was over, the extract was allowed to evaporate in hot air oven (70 °C) leaving behind the oleoresin. It is a deep reddish-brown coloured, semi-viscous compound. The red colour gained is majorly due to the carotenoid content in Bhut Jolokia. The capsaicinoid content in the oleoresin finds numerous applications in food industry such as it is used as a flavouring agent, colouring agent, as well as a preservative to help in preservation of meat and other food products against the microbial activity.
3.3 Analysis of the oleoresin extract obtained using SFE
The capsaicin content which imparts hotness to the chilli was analyzed using HPLC analysis. The calibration graph of the standards with different concentration ranging from 0.025 to 1 mg/mL was obtained and used for quantification of the capsaicin content in the samples (Fig. 1). The linear regression analysis showed a very good relationship between the scalar response (area, A) and the independent variable (standard concentration, c). The coefficient of determination (R2) was found to be 0.986 and the regression equation obtained from the graph plot was:
3.4 Analysis of variance
Analysis of variance (ANOVA) was conducted on the experimental data and the significance of the independent parameters and its effect on the individual responses were estimated. It was observed that the quadratic model fitted well to the experimental data. The statistical significance for each response was calculated and represented in Table 2.
3.5 Effect of process parameters on yield and capsaicin content of Bhut Jolokia oleoresin
Supercritical CO2 extraction of Bhut Jolokia oleoresin was conducted following the full factorial experimental design. The effect of process variables on the selected response was analyzed using ANOVA test. The quadratic empirical equation was developed for selected responses to predict the response within the selected domain of the experimental range. It was observed that the independent variables pressure, temperature and duration affected the extraction process, which is discussed in the following section.
3.5.1 Effect on oleoresin yield
Analysis of variance (ANOVA) table for oleoresin yield showed that the linear effects of pressure and time were highly significant (p < 0.01). In terms of an interaction effect, the pressure–time interaction were highly significant (p < 0.01) and the rest of them were insignificant. The interaction effect between pressure (bar) and duration of the cycle (min) was plotted on a response surface as represented in Fig. 2. Oleoresin yield ranged from 1.02 to 12.75% for all the samples. The highest yield was obtained in the samples processed at 225 bar/60 °C/90 min, whereas the minimum yield was observed for the sample treated at 75 bar/40 °C/30 min. A good correlation was obtained as indicated by the R2 value of 0.922 for oleoresin yield. The lack of fit was found to be insignificant indicating that the model is validated and it would fit for optimization of extraction of oleoresin from Bhut Jolokia.
The interaction effect of pressure and duration of the cycle (time) on the oleoresin yield was plotted as shown in Fig. 2. Studying the response surface plot it was observed that oleoresin yield increased with rising pressure level from 75 to 225 bar, increase in temperature from 40 °C to 60 °C and increase in the duration of the cycle from 30 to 90 min. From the coefficients of the empirical equation shown in Table 2, it was observed that the linear effect pressure and time were positive on oleoresin yield indicating that with a higher level of pressure and time higher level of yield was obtained. Similar trend were reported by Putra et al. [21]; while extraction of wasted peanut skin by SC-CO2 and Perva-Uzunalić et al.[22]; while chilli pepper extraction with supercritical CO2. They observed that with an increase in pressure, yield percentage increased. This may be because an increase in pressure at fixed temperature leads to increase in density of the supercritical CO2 which imparts its effect on the solvating power of the supercritical fluid. The solvating power of supercritical fluid is considered as a function of its pressure [23]. The increased density amplifies the interaction between the molecules of the compound and carbon dioxide resulting in greater solubility of the compound.
However, as evident from the highest F value (169.38) it can be observed that the time had the most impact on the extraction of Bhut Jolokia oleoresin. With an increase in the extraction cycle, significant change in yield was observed. The oleoresin yield gradually increased from 1.02 to 12.75%.
A similar trend was obtained by Peusch et al.[23]. It was found that with an increase in time from 10 to 60 min there was an increase in the extraction yield. Yamini et al. [24] also reported while studying the effect of static-dynamic SFE isolation of fennel oil that with an increase in a dynamic time, extraction efficiency also increased. This effect may be because an increase in the extraction process time maximizes the contact of the analyte with the supercritical fluid thereby increasing its solubility in the fluid and hence increasing the extraction yield.
3.5.2 Effect on capsaicin content
The effect of process variables on the capsaicin content of the extract was analysed using ANOVA. It was observed that the effect of pressure level, temperatures and duration of cycle was significant (p < 0.01). The interaction effect of temperature and duration of the cycle only was significant (p < 0.01). The rest of the interaction effects were insignificant.
The capsaicin content ranged from 0.74 to 1.74% (Table 1) The highest value of capsaicin was obtained in the sample extracted at 75 bar/60 °C/30 min and the lowest value was obtained in the sample extracted at 225 bar/40 °C/90 min. The R2 value for the response capsaicin content was 0.90, indicating good correlation for the model. The lack of fit was found to be insignificant indicating that the model fit was adequate.
The interaction effect of the significant term, temperature and duration of the cycle (time) is represented in Fig. 3 below. Analysing the response surface plot, it was observed that with an increase in temperature and time lead to an increase in capsaicin content. It was observed that pressure was inversely related to colour value. As fluid pressure increased, the capsaicin content decreased gradually. Capsaicin content was observed to be maximum at 75 bar pressure, however, gradual increase in pressure to 225 bar displayed a gradual decrease of capsaicin content to minimum value.
A similar effect was also reported by de Aguiar et al. [25]. They extracted oleoresin from Jalapeno using SC-CO2. They noticed the highest capsaicin content was found to be at pressure 15 MPa. They stated that with an increase in pressure above 15 MPa some other compounds present in the malagueta pepper may be solubilized in the CO2, which might have resulted in competency for the solvent resulting in the reduction of capsaicin yield.
It was observed that the linear effect of pressure and time was negative on capsaicin content indicating that with a higher level of pressure and time lower level of capsaicin content was obtained. From the graph, it was noticeable that with an increase in duration cycle from 30 to 90 min the capsaicin content decreased gradually. Safaralie et al. [26] also reported a significant increase in essential oil with an increase in the dynamic time. However, the linear effect of temperature on capsaicin content was positive indicating that with higher temperature high level of capsaicin content can be obtained. The interaction effect of temperature and duration of cycle was found to be significant on the capsaicin content (p < 0.05). It was predicted by comparing the F value and its corresponding coefficient that temperature was the main factor affecting the capsaicin content.
4 Standardization of the SFE process
Based on the actual as well as the predicted values a point was optimized which provided a maximum desirability function and also selected the best combinations having higher desirability function. Optimization of the SFE process of Bhut Jolokia oleoresin was performed using Design Expert 12. The numerical and graphical optimization was done to represent the ideal operating conditions for Bhut Jolokia oleoresin extraction process. From the numerical optimization, the optimum process parameters were 207 bar/60 °C/73 min and the response obtained at the conditions were oleoresin yield 11.3% and capsaicin content 1.29% with desirability value of 0.64. The experimental range indicating the experimental conditions for the extraction was plotted in the form of overlay plot (Fig. 4), which indicate the experimental range for getting the optimum response values.
The predicted values obtained after optimization of the SFE process were compared with the actual values obtained after carrying out the SFE process with the optimized parameters of pressure, temperature and time. All the actual values were conducted in triplicate and the standard deviation of the actual responses were evaluated to find the relative deviation between the actual response and the predicted response. The relative deviation was found to be less than 5% of the predicted values.
5 Antimicrobial activity of Bhut Jolokia oleoresin
The Bhut Jolokia extract obtained using the optimized parameters was tested using the diffusion method to check for its antimicrobial property. The two most common diffusion technique used for the antimicrobial assay are: agar well diffusion method and disc diffusion method. Well diffusion method was preferred over disc diffusion method as the supercritical extract is difficult to dry on paper disc and capsaicin is not easily soluble thereby applying the disc diffusion assay would not have led to proper diffusion of the extract. The tested microorganisms used for this assay were 4 bacterial strains. 100 µL of the extract was used to carry out the test. The inhibitory effect was seen against S. aureus, E. coli and S. typhi. However, Gram positive B. subtilis showed partial/no inhibitory effect.
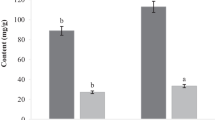
The inhibition zone was measured for each of the cultured plates and represented in Table 3. Effects on the microbial strains was observed as circular zones encircling the wells in which the antibiotic and the samples were loaded. It was seen that the effect of supercritical fluid extract of Bhut Jolokia was different for different microbial strains. Out of the four strains used in the experiment, E. coli showed the highest inhibitory effect with 10.2 mm zone of inhibition as compared to all other strains used. Cowan [14] also reported similar bactericidal effects of Capsicum species on E. coli. A moderate activity of the extract could be seen against S. typhi (5 mm) and S. aureus (8 mm). However, partial/no inhibitory effect was observed against B. subtilis. Similar lack of inhibition was reported for Capsicum species against B. subtilis [27].
The capsaicin content in the optimized extract of the Bhut Jolokia oleoresin could be the key component in the disintegration of the microbial membrane causing the inhibitory effect against the microbial variants. However, it may not be the only responsible compound for antimicrobial activity. Certain compounds having less/no polarity than capsaicin might be responsible for the inhibitory effect. The ethanol soluble fraction of terpenoids could also impart hindrance to microbial growth [14]. Cichewicz and Thorpe [27] suggested that variation in antibacterial activity of the various chilli extracts may be due to the presence of various concentrations of certain chemical compounds based on their variety or maturity. It was also stated that heating of the chilli extracts might also vary its chemical properties showing contradictory antimicrobial activity. Capsaicin had structural similarity with that of a natural and synthetic inhibitor of β-hydroxydecanoyl dehydratase enzyme responsible for growth in E. coli which could also be the mechanism behind various anti-microbial effect imparted by the chilli extract [28]. Further study towards the purified components of the supercritical fluid extract of the Bhut Jolokia might help in unveiling the causative mechanism of various chilli varieties showing bacterial inhibition.
6 Anti-oxidant activity and phenolics content of Bhut Jolokia oleoresin
The antioxidant activity of optimized extract of Bhut Jolokia oleoresin was analysed using DPPH radical scavenging activity. It was found that the optimized extract exhibited 58.6 ± 3.86% of DPPH radical scavenging activity at a concentration of 10 mg/mL. Similar results were observed in the literature by Sarpras et al. [18]. They performed a comparative analysis of antioxidant activity among 136 different Capsicum genotype of chillies and reported that the antioxidant activity in Capsicum chinense ranged from 40–83%, which supports the finding observed in the present study. The antioxidant activity of the Bhut Jolokia oleoresin extract is responsible mainly due to the capsaicin content of the oleoresin. It was found in a study carried out by Henderson et al. [29] that the mechanism behind action of capsaicin as a radical scavenging agent is mainly due to the presence of phenolic moiety containing groups of amides and imides. However, Kogure et al. [30] reported that the C7-benzyl carbon of the vanillin and 8-methyl-6-noneamide in capsaicin is the reason for oxidation inhibition by capsaicin and not the phenolic group.
The calibration graph was obtained to determine the total phenolics content of the optimized Bhut Jolokia extract using various concentration of gallic acid ranging from 0.00, i.e. 0.02 to 1 mg/mL. The concentration of the gallic acid (CGA) in the sample was determined using the regression equation represented below, where A is sample absorbance. The determination coefficient (R2) was found to be 0.993.
The total phenolics content for Bhut Jolokia oleoresin was found to be 4250 ± 2.26 mg GAE/100 g. This shows that Bhut Jolokia oleoresin is a good source of antioxidants, mostly phenolic content. Phenolic compounds are secondary metabolites which can donate hydrogen atoms leading to scavenging of free radicals.
7 Conclusions
From the present study of supercritical fluid extraction (SFE) of Bhut Jolokia oleoresin, it was observed that the supercritical fluid extraction of Bhut Jolokia yielded significant improvement in the extraction of oleoresin compared to the conventional extraction and was influenced by pressure, temperature and duration of the process. The experimental data obtained was used to develop an empirical model for the selected independent variables, which showed a good fit between independent variables and selected-response variables. Optimized parameters for maximum extraction of Bhut Jolokia oleoresin along with higher-level capsaicin content were found to be: pressure at 207 bar, temperature at 60 °C and time of extraction at 73 min. The maximum yield obtained following the optimized process parameters were: 11.81% of oleoresin and 1.30% of capsaicin content. It was also observed that the process retained the important bioactive compound like the capsaicin content of Bhut Jolokia thereby maintaining the quality of the extract. This indicates that the method is effective and can further be applied by various small-scale industries or start-ups to obtain maximum Bhut Jolokia oleoresin using SFE technique. The extract obtained by applying the optimized parameters showed anti-bacterial activity against Gram negative (E. coli, S. typhi) and Gram positive (S. aureus) bacteria. It also showed significant antioxidant property and has a good amount of phenolic compounds. Further kinetic study can be done to assess the effect of particle size and other co-solvents and characterisations of bioactive compounds using analytical methods.
References
Meetei NT, Singh AK, Singh BK, Mandal N (2016) Recent advances in naga king chilli (Capsicum chinense JACQ) research. Int J Agric, Environ Biotechnol 9(3):421–428. https://doi.org/10.5958/2230-732X.2016.00054.1
Purkayastha J, Alam SI, Gogoi HK, Singh L, Veer V (2012) Molecular characterization of’ Bhut Jolokia’the hottest chilli. J Biosci 37(4):757–768. https://doi.org/10.1007/s12038-012-9249-8
Dubey RK, Singh V, Upadhyay G, Pandey AK, Prakash D (2015) Assessment of phytochemical composition and antioxidant potential in some indigenous chilli genotypes from North East India. Food Chem 188:119–125. https://doi.org/10.1016/j.foodchem.2015.04.088
Hoffman PG, Lego MC, Galetto WG (1983) Separation and quantitation of red pepper major heat principles by reverse-phase high-pressure liquid chromatography. J Agric Food Chem 31(6):1326–1330. https://doi.org/10.1021/jf00120a044
Sanatombi K, Sharma GJ (2008) Capsaicin content and pungency of different Capsicum spp cultivars. Notulae Botanicae Horti Agrobotanici Cluj- Napoca 36(2):89–90. https://doi.org/10.15835/nbha362345
Prakash V, Eipeson WE (2003) 10 Post-harvest handling and processing of capsicums capsicum: the genus capsicum. CRC Press, Boca Raton, p 163
McHugh M, Krukonis V (2013) Supercritical fluid extraction: principles and practice. Elsevier, Amsterdam
Pourmortazavi SM, Saghafi Z, Ehsani A, Yousefi M (2018) Application of supercritical fluids in cholesterol extraction from foodstuffs: a review. J Food Sci Technol 55(8):2813–2823. https://doi.org/10.1007/s13197-018-3205-z
Pereira CG, Meireles MAA (2010) Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic perspectives. Food Bioprocess Technol 3(3):340–372. https://doi.org/10.1007/s11947-009-0263-2
Casas L, Mantell C, Rodríguez M, Torres A, Macías FA, De La Ossa EM (2007) Effect of the addition of cosolvent on the supercritical fluid extraction of bioactive compounds from Helianthus annuus L. J Supercrit Fluids 41(1):43–49. https://doi.org/10.1016/j.supflu.2006.09.001
AOAC(1990).Official methods of analyses of association of analytical chemist (15th ed.). Washington DC: AOAC
Sarwa KK, Mazumder B, Rudrapal M (2017) Effect of extraction methods and long term storage on capsaicinoids content of Bhut Jolokia fruits. Indian J Nat Prod Resour (IJNPR) Formerly Nat Prod Radiance (NPR) 8(1):69–77
Collins MD, Wasmund LM, Bosland PW (1995) Improved method for quantifying capsaicinoids in capsicum using high-performance liquid chromatography. Hort Science 30(1):137–139. https://doi.org/10.21273/HORTSCI.30.1.137
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12(4):564–582
Afolayan AJ, Meyer JJM (1997) The antimicrobial activity of 3, 5, 7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens. J Ethnopharmacol 57(3):177–181. https://doi.org/10.1016/s0378-8741(97)00065-2
Gurnani N, Gupta M, Mehta D, Mehta BK (2015) Chemical composition, total phenolic and flavonoid contents, and in vitro antimicrobial and antioxidant activities of crude extracts from red chilli seeds (Capsicum frutescens L.). J Taibah Univ Sci 10:462–470. https://doi.org/10.1016/j.jtusci.2015.06.011
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Sarpras M, Gaur R, Sharma V, Chhapekar SS, Das J, Kumar A, Yadava SK, Nitin M, Brahma V, Abraham SK, Ramchiary N (2016) Comparative analysis of fruit metabolites and pungency candidate genes expression between Bhut Jolokia and other capsicum species. PLoS ONE 11(12):e0167791. https://doi.org/10.1371/journal.pone.0167791
Tohma H, Gülçin İ, Bursal E, Gören AC, Alwasel SH, Köksal E (2017) Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc) determined by HPLC-MS/MS. J Food Measurement Charact 11(2):556–566. https://doi.org/10.1007/s11694-016-9423-z
Kuna A, Sahoo MR, Sowmya M, Mayengbam PD, Dasgupta M, Sreedhar M, Tholemfhuang S (2018) Nutrient and antioxidant properties of value added king chilli (capsicum chinense) products. Int J Curr Microbiol App Sci 7(6):1–8. https://doi.org/10.20546/ijcmas.2018.706.001
Putra NR, Rizkiyah DN, Zaini AS, Yunus MAC, Machmudah S, Idham ZB, Hazwan Ruslan MS (2018) Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide and soxhlet extraction. J Food Process Preserv 42(8):e13689. https://doi.org/10.1111/jfpp.13689
Perva-Uzunalić A, Škerget M, Weinreich B, Knez Ž (2004) Extraction of chilli pepper (var Byedige) with supercritical CO2: effect of pressure and temperature on capsaicinoid and colour extraction efficiency. Food Chemistry 87(1):51–58. https://doi.org/10.1016/j.foodchem.2003.10.016
Peusch M, Müller-Seitz E, Petz M, Müller A, Anklam E (1997) Extraction of capsaicinoids from chillies (Capsicum frutescens L) and paprika (Capsicum annuum L) using supercritical fluids and organic solvents. Zeitschrift für Lebensmitteluntersuchung und-Forschung A 204(5):351–355. https://doi.org/10.1007/s002170050089
Yamini Y, Sefidkon F, Pourmortazavi SM (2002) Comparison of essential oil composition of Iranian fennel (Foeniculum vulgare) obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Flavour Fragr J 17(5):345–348. https://doi.org/10.1002/ffj.1117
de Aguiar AC, Sales LP, Coutinho JP, Barbero GF, Godoy HT, Martínez J (2013) Supercritical carbon dioxide extraction of capsicum peppers: global yield and capsaicinoid content. J Supercrit Fluids 81:210–216. https://doi.org/10.1016/j.supflu.2013.05.008
Safaralie A, Fatemi S, Salimi A (2010) Experimental design on supercritical extraction of essential oil from valerian roots and study of optimal conditions. Food Bioprod Process 88(2–3):312–318. https://doi.org/10.1016/j.fbp.2009.02.002
Cichewicz RH, Thorpe PA (1996) The antimicrobial properties of chile peppers (capsicum species) and their uses in Mayan medicine. J Ethnopharmacol 52(2):61–70. https://doi.org/10.1016/0378-8741(96)01384-0
Molina-Torres J, Garcı́a-Chávez A, Ramı́rez-Chávez E (1999) Antimicrobial properties of alkamides present in flavouring plants traditionally used in mesoamerica: affinin and capsaicin. J Ethnopharmacol 64(3):241–248. https://doi.org/10.1016/S0378-8741(98)00134-2
Henderson DE, Slickman AM, Henderson SK (1999) Quantitative HPLC determination of the antioxidant activity of capsaicin on the formation of lipid hydroperoxides of linoleic acid: a comparative study against BHT and melatonin. J Agric Food Chem 47(7):2563–2570
Kogure K, Goto S, Nishimura M, Yasumoto M, Abe K, Ohiwa C, Sassa H, Kusumi T, Terada H (2002) Mechanism of potent antiperoxidative effect of capsaicin. Biochimica et Biophysica Acta (BBA)-General Subjects 1573(1):84–92. https://doi.org/10.1016/S0304-4165(02)00335-5
Acknowledgements
The authors acknowledge UGC, New Delhi for the financial support (F.30-437/2018(BSR)) under UGC-BSR Research Start-Up-Grant. The authors acknowledge Mr Prakash Kurmi, Sophisticated Analytical Instrumentation Centre (SAIC), Tezpur University for assistance in HPLC analysis; Department of Molecular Biology and Biotechnology, Tezpur University for the microbial strains.
Author information
Authors and Affiliations
Contributions
The first author DD has conducted the experiments, chamical and microbiological analysis and second author NRSH has contributed to experimental design, supercritical fluid extraction experiments and drafting the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deka, D., Swami Hulle, N.R. Supercritical fluid extraction of Bhut Jolokia oleoresin and its quality analysis. SN Appl. Sci. 3, 260 (2021). https://doi.org/10.1007/s42452-021-04218-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04218-y