Abstract
This study aimed to screen the bioactive compounds from Prosopis juliflora leaf supercritical fluid extract and to assess its antimicrobial properties. Supercritical carbon dioxide and Soxhlet methods were used for extraction. The extract was subjected to Gas Chromatography-Mass Spectrometer (GC-MS) and Fourier Transform Infrared for the characterization of the phyto-components. When compared to soxhlet extraction, more components (35) were eluted by supercritical fluid extraction (SFE), according to GC-MS screening. Rhizoctonia bataticola, Alternaria alternata, and Colletotrichum gloeosporioides were all successfully inhibited by P. juliflora leaf SFE extract, which demonstrated strong antifungal properties with mycelium percent inhibition of 94.07%, 93.15%, and 92.43%, respectively, compared to extract from Soxhlet, which registered 55.31%, 75.63% and 45.13% mycelium inhibition respectively. Also, SFE P. juliflora extracts registered higher zone of inhibition 13.90 mm, 14.47 mm and 14.53 mm against all three test food-borne bacterial pathogens viz Escherichia coli, Salmonella enterica and Staphylococcus aureus respectively. Results obtained from GC-MS screening revealed that SFE is more efficient than soxhlet extraction in recovering the phyto-components. P. juliflora may provide antimicrobial agents, a novel natural inhibitory metabolite.
Similar content being viewed by others
Introduction
Chemical diversity has a variety of benefits for the crucial and difficult subject of discovering innovative medications. New drugs may be created from biomolecules or phytochemicals present in natural products like plant extracts. There are a variety of chronic and infectious diseases that can be cured using the numerous active components present in plants used in traditional medicine1. Thousands of phytochemicals are having antimicrobial, antioxidant, wound headlining, anticarcinogenic and antidiarrheal traits. Many studies reported plants as safe and broadly effective alternatives with fewer adverse effects1,2.
The shrub P. juliflora is known to contain different chemical compounds such as alkaloids, flavonoids, terpenoids, saponins, and phenolic compounds distributed in different parts of the plant body which makes the shrub of medicinal importance. P. juliflora (Sw.) DC, (Velvet Mesquite) contains 44 species, of which 40 are native to America, three to Asia, and one to Africa and belong to the family Fabaceae, subfamily Mimosoideae3. Medically active substances have been extracted from different parts of P. juliflora such as leaves, roots, stems, branches, bark as well as pollen. Crude extract from P. juliflora known to contain a varied class of secondary metabolites which has unique and combined therapeutic and antifungal traits4,5,6. The usage of this plentiful resource imparts a good option for yielding bioactive natural products that may serve as an important raw material for the pharmaceutical and chemical industries7.
The selection of the use of solvent and the extraction procedure in obtaining the antimicrobial metabolites from various plant parts play a major role8. There are various conventional extraction methods to obtain an extract from the plant. Among the techniques, soxhlet has been mostly used for a long time8. However, conventional extraction methods have the considerable drawback of solvent residue leftover in the extracts, time consumption and poor recovery. The new technique of Supercritical Fluid Extraction (SFE) has secured prime attention over the traditional techniques in the recovery of edible and essential oils in the field of natural products. Supercritical fluids extraction (SFE), or the extraction of components using solvents at high pressure, has attracted a lot of attention in recent years and has been used for a variety of applications, particularly in the food, pharmaceutical, and cosmetic industries9. Supercritical carbon dioxide (SC-CO2) is especially popular due to its inertness, non-toxicity, non-flammability, and low cost. SC-CO2 processes can be carried out at low temperature that results in preserving the original oil compositions and properties10. Supercritical fluid extraction (SFE), especially by supercritical carbon dioxide (SC-CO2) technique has been known as an alternative suitable tool for the extraction of essential oil and seed oil from various plants11. Pressurized fluids are used as solvents in the extremely selective SFE technique. When a fluid is driven to temperatures and pressures over its critical point, the liquid and gas phases become indistinguishable from one another, creating a supercritical fluid. At optimal conditions, SFE does not have any of the adverse effects of traditional organic solvents. Optimization of temperature and pressure has a major impact on fluid density, improved transport properties, higher extraction yield, and shorter extraction time in SFE. The most commonly used supercritical fluid (SF) is SC-CO2 because it has a moderate critical temperature (31.3 °C) and pressure (72.9 atm). Giving high-quality, solvent-free extracts at low temperatures, SC-CO2 extraction allows active compounds to be protected from thermal degradation, while providing more selective and efficient extraction by controlling the process temperature and pressure, so as to adjust CO2 density hence regulating the solvating power. The ability to control the extraction parameters is the major benefit of the supercritical fluid extraction process12,13,14,15.
After the extraction, it is very important to determine the components present in the extract. The application of chromatographic techniques is most suitable for the determination of various phytoconstituents (qualitatively and quantitatively) present in the extract. Gas chromatography-mass spectrometry (GC-MS) is a combined analytical technique used to determine and identify compounds present in any matrix sample. GC-MS with triple quadrupole is suitable equipment for an important role in the analysis of bioactive components and chemotaxonomic studies of medicinal plants containing biologically active components16.
There hasn't been any research published to date on the comparison of extraction techniques like SFE and Soxhlet through GC-MS analysis of the bioactive components of P. juliflora leaf extract. The Pesticide Residue and Food Quality Analysis Laboratory at the University of Agricultural Sciences, Raichur, Karnataka, India conducted research intending to characterize Phyto-components with antifungal traits present in he supercritical fluid and soxhlet P. juliflora leaves extract using GC-MS while keeping these facts in mind (Supplementry Table 1).
Results and discussion
Extraction yield and efficiency of P. juliflora leaf extract
The extraction yield and extraction efficiency of 14.10 g/100 g and 93.37% were recorded at SC-CO2 extraction whereas Soxhlet extraction registered 9.25 g/100 g and 61.25% respectively (Table 1). Based on the previous experiment result obtained, a pressure of 200 bar, and a temperature of 50 °C were considered as the optimum and best SC-CO2 extraction condition for obtaining the highest extraction efficiency from P. juliflora leaf powder. It is evident from the result that higher extraction yield and efficiency were found in SC-CO2 extraction compared to Soxhlet extraction. This might be since the increase in pressure increases the density of the CO2 thereby increasing the solvent strength and solubility of the oil in CO2. Naturally, a raise in pressure at a given temperature increases SC-CO2 density, hence enhancing its solubility. The diffusion coefficient is also lowered as a result of this action. This may be due to the comforting solute-solvent ejection brought on by the extremely compressed CO2 17,18,19,20,21.
Gas chromatography-mass spectrometry triple quadrupole (GCMS-MS) analysis
As expected, a wide variety of bioactive compounds could be found in Soxhlet and SFE extract. Twenty compounds were detected from the GCMS-MS analysis of soxhlet extract of P. juliflora leaves whereas thirty-five compounds were identified from the GCMS-MS analysis of a supercritical fluid extract of P. juliflora leaves. The chromatogram is depicted in Fig. 1, while the name of bioactive components with their retention time (RT), molecular formula, height, and area are presented in Tables 2 and 3. The major compounds identified in the P. juliflora soxhlet extract are Phenol, 3,5-bis(dimethyl ethyl), Benzene dicarboxylic acid, and Squalene. The major components detected in SFE extract of P. juliflora leaves are Pentanoic acid 5hydroxy 2,4, dibutyl phenyl ester, Phytol, Tetramethyl heptadecane, Neophytadiene, hexadecanal. Many other compounds were traced as low levels. All these major plant metabolites have a role as anti-inflammatory agents, anti-oxidants, and antimicrobial agents. More compounds are eluted in SFE extract compared to Soxhlet extract may be due to the special features of SFE. The unique characteristics of SFE, such as excellent extraction efficiency and selectivity, are caused by its liquid-like solubility and gas-like mass transport characteristics. Extraction of analytes present in low concentrations, cleaner extracts, and preservation of bioactive constituents could be achieved through SFE22. Even higher extraction yield can be achieved by providing close contact between the sample and extractant.
FTIR spectroscopy analysis of P. juliflora leaves extract
Phytochemical screening is an important step that leads to the isolation of new and novel compounds. The results of FTIR analysis of the P. juliflora leaf SFE extract revealed the presence of different functional groups in the extract (Fig. 2). Major peaks in the FTIR analysis showed the presence of alcohol, phenols, alkanes, aromatic, ether, carboxylic acid, aliphatic amines, primary and secondary amine. Similar preliminary phytochemical screening of the P. juliflora extract through FTIR also revealed that the plant contains Alkaloids, Flavonoids, Saponins, Tannins, Anthraquinone Glycoside, and Coumarins23,24.
Antimicrobial activity of P. juliflora leaf extract
The antimicrobial activity of Soxhlet and SC-CO2 P. juliflora leaf extract against the fungal plant pathogens viz., Rhizoctonia bataticola, Alternaria alternata and Colletotrichum gloeosporioides and food borne bacterial pathogens viz Escherichia coli, Salmonella enterica and Staphylococcus aureus is presented in Tables 4 and 5. SFE P. juliflora extract registered higher zone of inhibition 94.07%, 93.15% and 92.42% against all three test pathogens Rhizoctonia bataticola, Alternaria alternata and Colletotrichum gloeosporioides respectively whereas Soxhlet P. juliflora extract recorded 79.58%, 85.07% and 66.57% zone of inhibition respectively. SFE P. juliflora extract registered higher zone of inhibition 13.90 mm, 14.47 mm and 14.53 mm against all three test food-borne bacterial pathogens viz Escherichia coli, Salmonella enterica and Staphylococcus aureus respectively whereas Soxhlet P. juliflora extract recorded 5.10 mm, 5.20 mm and 6.10 mm zone of inhibition respectively. This result demonstrated that SFE extract has more antimicrobial potential compared to Soxhlet extract. Even though the extract was obtained from the same P. juliflora leaves, the method applied for extraction play a major role in the recovery of biomolecules from the raw material.
According to Saleh et al.5 the water-soluble leaf ethanolic extract of P. julifora displayed significant antibacterial activity against Staphylococcus sp. and E. coli. The results of the present investigation were in agreement with Raghavendra et al.25 that the activity of the aqueous extract of P. juliflora against Alternaria alternata showed 71.59% inhibition of mycelial growth. Deressaa and associates26 used methanol, acetone and aqueous extract of P. juliflora leaves against Colletotrichum gloeosporioides the results showed radial growth inhibition of 100 percent, 100 percent, 79.60 percent, respectively. Bazie et al.27 reported the activity of methanolic extract of P. juliflora against Colletotrichum musae, which showed a 30.70 mm zone of inhibition.
P. juliflora demonstrated significant antimicrobial activity and may be used to identify bioactive natural products that can serve as leads for developing new pharmaceuticals that address previously unmet needs28. The results indicate that leaves extracted from P. juliflora are a promising source of antimicrobial agents and may have therapeutic potential. A deeper study of this plant with its pure compounds may lead to the development of natural alternative antimicrobial compounds against plant and food borne pathogens. A similar study was carried out by Rizwana et al.29 who isolated two pentanoic acid compounds from Bluejack Oak and tested them for their antimicrobial potential and showed promising antifungal activity against Aspergillus niger and Aspergillus flavus.
Conclusion
Despite the fact that the extract was produced using the same P. juliflora leaves, the method of extraction's efficacy is extremely important. The GC-MS results revealed that the supercritical fluid extraction method is superior to the soxhlet extraction method for removing the bioactive components from P. juliflora leaves. Fungal pathogens such as Rhizoctonia bataticola, Alternaria alternata, and Colletotrichum gloeosporioides and bacterial food borne pathogens such as Escherichia coli, Salmonella enterica and Staphylococcus aureus are effectively suppressed by P. juliflora SFE extract because it includes antimicrobial substances including Phytol, Tetramethyl heptadecane, Neophytadiene, and Pentanoic acid 5hydroxy 2,4 dibutyl phenyl ester. P. juliflora could be a potential source for antimicrobial agents, a novel inhibitory metabolite.
Materials and methods
Raw materials
Fresh leaves of P. juliflora were collected around the campus of the University of Agricultural Sciences (UAS), Raichur, Karnataka State. Leaves were cut and dried in dehumidified air dryer (make: Bry Air Asia; model: FSD-600) at 45 °C and 15% relative humidity. The dried leaves were ground in a laboratory hammer mill to obtain a fine powder.
Chemicals
Chemicals used for the analysis included n-hexane (CAS 110-54-3), ethanol (CAS 64-17-5), Phenyl methyl siloxane (CAS CAS 68037-54-7) and Potassium bromide (CAS 7758-02-3). The solvents, chemicals, and reagents (analytical grade) used throughout the experiment were procured from M/s. Sigma Aldrich Chemicals, Germany and Merck, Germany.
Microbial culture
Authentic pure cultures of bacterial food borne pathogens were procured from American Type Culture Collection (ATCC), USA and Microbial Type Culture Collection (MTCC), Chandigarh, India, namely Escherichia coli (ATCC 0680P), Salmonella enterica (MTCC 98) and Staphylococcus aureus (MTCC 87). Pure fungal cultures of Rhizoctonia bataticola (ATCC 26020), Alternaria alternata (ATCC 66981), and Colletotrichum gloeosporioides (ATCC 20358) were collected from the Department of Plant Pathology, UAS, Raichur. Procured cultures were maintained in the appropriate media for further use.
Extraction of P. juliflora leaf extract
Supercritical fluid and Soxhlet extraction methods were employed for obtaining an extract from P. juliflora leaves.
Supercritical fluid extraction
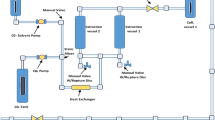
The supercritical carbon dioxide (SC-CO2) extraction system (Thar; SFE 500 system) was used for the extraction of P. juliflora leaf powder. Deionized water (5 °C) was used for cooling different zones in the SC-CO2 extraction system. Fifty grams of P. juliflora leaf powder were placed into the extractor vessel. The flow rates of supercritical CO2 and co-solvent (ethanol) were maintained at 20 and 2 g/min, respectively 30. The static extraction process was performed for 30 min. After attaining desired pressure (200 bar) and temperature (450C) dynamic extraction time (90 min) was started by opening the exit valve of the SC-CO2 extraction system. The static extraction time allowed the sample to soak in the CO2 and co-solvent to equilibrate the mixture at desired pressure and temperature. During the dynamic extraction time, CO2 carrying the crude extract flowed out of the extraction vessel and then into a collection vessel, where the CO2 was separated through the vent connected to the fume hood 31. The SFE instrument and scheme diagram of SC-CO2 is depicted in Fig. 3.
Soxhlet extraction
P. juliflora leaves extraction was carried out by the soxhlet extraction method using SOCS- PLUS apparatus (Make: Gerhardtz, model: SOX-416) with hexane as solvent. Accurately, 50 g of the P. juliflora leaf powder was taken into the thimble and placed in the sample compartment of the extractor. The sample compartment was attached to a 500 ml round bottom flask containing 300 ml hexane. The SOCS- PLUS apparatus was run at 85 °C for 90 min. Hexane in the extract was distilled out by using a rotary flash vacuum evaporator (Superfit, Rotavap; PBU-6D)32,33.
Extraction Yield The extraction yield was computed by using the following equation
where, M extract = Mass crude extract (g) M feed = Feed mass (g).
Extraction Efficiency The extraction efficiency was calculated as per the equation described. It is the ratio of the quantity of extract obtained during the process to the actual amount of extract present in 100 g of P. juliflora leaves
Preparation of extracts for GC-MS analysis
The extract obtained from both extraction methods were filtered through a 45 μm filter. The resulting solution was concentrated in vacuum to dryness to give a solvent free extract. The extract was stored in a refrigerator at 4 °C for further use.
GC-MS triple quadrupole analysis
GC-MS analysis was carried out in a combined 7890B gas chromatograph system Agilent make and mass spectrometer triple quadrupole fitted with an HP-5 MS fused silica column (5% phenyl methyl siloxane 30.0 m × 250 µm, film thickness 0.25 µm, interfaced with 7000D Agilent mass detector with (TQD) triple detector. Helium gas was used as carrier gas and was adjusted to column velocity flow of 1.0 ml/min.
Other GC oven conditions are 60 °C at initial temperature at 7 °C/min reaching to 270 °C with spitless, injection volume 1 µl and Mass condition ion source temperature, 280 °C with an injection temperature of 250 °C. The data were integrated using software and compiled with the compounds with the provided NIST Library software to identify unknown compounds and structures.
FTIR analysis of P. juliflora leaf powder extract
Supercritical fluid P. juliflora leaves extract was used for FTIR (Fourier Transform Infrared Spectroscopy) analysis with the Attenuated Total Reflectance (ATR) sampling method that introduces light on the sample to acquire structural and compositional information. About mg of the finely ground sample is then placed onto the face of a KBr plate. FTIR analysis was performed using the Shimadzu FTIR spectrometer 8000 series, between 4000 and 750 cm-1 which was used to detect the characteristic peaks and their functional groups. The peak values and the functional groups of P. juliflora leaf SFE extract were recorded.
Antifungal activity of P. juliflora leaf extract
The antifungal activity of Soxhlet and SFE P. juliflora leaf extract against the plant pathogens viz., Rhizoctonia bataticola, Alternaria alternata, and Colletotrichum gloeosporioides was carried out following poisoned food technique34. The potato dextrose agar (PDA) media was prepared and sterilized. Plant extract obtained from both extraction methods was exposed to a rotary flash vacuum evaporator for the complete removal of organic solvents used for the extraction. The extracts were resuspended in distilled water and sterilized by membrane filtration with pore 45 m (Whatman brand) and stored at 4 °C until use. A volume of 0.5 ml of plant extract (10 mg/ml) was aseptically poured into a Petri plate followed by the addition of 9.5 ml of melted PDA and was gently mixed. The inoculum disc of test fungus was aseptically inoculated upside down at the center of the Petri plate and incubated at 25 °C for 7 days.
The media plate with 0.5 ml of sterile distilled water in place of extract (without extract) was set as a negative control and the media plate supplemented with 0.1% hexaconazole was considered a positive control. The inhibition percentage of the fungal mycelia was measured on the 7th day of incubation.
where,
DC—Average diameter of the colony in the control plate.
DT—Average diameter of the colony in the treatment plate.
Antibacterial activity of P. juliflora leaf extract
Disc agar diffusion technique was employed for antibacterial bioassay. Petri plates were washed, rinsed with sterile distilled water, dried, wrapped in tin foil and kept in an autoclave at 100 °C for 15 min to sterilize. For testing antimicrobial activity against bacteria 20 ml of growth medium and 4 ml of bacterial inoculum were mixed and poured into separate sterilized Petri plates. Each mixture was thoroughly shaken to ensure uniform distribution of inoculum. The experiment was carried out in three replicates. Sterile paper discs measuring 6 mm in diameter, which absorbs about 0.1 ml of the extract (10 mg/ml) solution were employed for test in test samples. All test petri plates were kept at 5 °C for 40–50 min to allow the diffusion of the substances and then incubated at 35–37 °C for 18 h. The inhibition zones formed by the P. juliflora leaf extract were measured including the diameter of the paper disc.
For experimental research and field studies on plants
All procedures were conducted in accordance to the relevant institutional, national, and international guidelines and legislation.
Data availability
All data generated or analysed during this study are included in this published article.
References
Duraipandiyan, V., Ayyanar, M. & Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 6, 35–41. https://doi.org/10.1186/1472-6882-6-35 (2006).
Sasidharan, S., Chen, Y., Saravanan, D., Sundram, K. M. & Latha, L. Y. Extraction, isolation and characterization of bioactive compounds from plants extracts. Afr. J. Tradit. Complement. Altern. Med. 8(1), 1–10 (2011).
Prasad, M. N. V. and Tewari, J. C. P. juliflora (Sw) DC: Potential for bioremediation and bioeconomy. In Bioremediat. Bioeconomy, pp. 49–76 (2016).
Solanki, D. S. et al. Characterization of a novel seed protein of Prosopis cineraria showing antifungal activity. Int. J. Biol. Macromol. 116, 16–22. https://doi.org/10.1016/j.ijbiomac.2018.05.018 (2018).
Iman, S. & Abu-Dieyeh, M. H. Novel Prosopis julifora leaf ethanolic extract as natural antimicrobial agent against food spoiling microorganisms. Sci. Rep. 11, 7871. https://doi.org/10.1038/s41598-021-86509-3 (2021).
Lakshmibai, R. Phytochemical analysis and antioxidant activity of Prosopis Julifora thorn extract. Malaya J. Biosci. https://doi.org/10.13140/RG.2.2.35189.88806 (2018).
Prabha, D. S., Dahms, D. & Malliga, P. Pharmacological potentials of phenolic compounds from Prosopis spp.-a review. J. Coast. Life Med. 2(11), 918–924. https://doi.org/10.12980/JCLM.2.2014J27 (2014).
De Castro, M. L. & Garcıa-Ayuso, L. E. Soxhlet extraction of solid materials: an outdated technique with a promising innovative future. Anal. Chim. Acta 369(1–2), 1–10 (1998).
Sodeifian, G. & Ansari, K. Optimization of Ferulago Angulata oil extraction with supercritical carbon dioxide. J. Supercrit. Fluids 57(1), 38–43. https://doi.org/10.1016/j.supflu.2011.02.002 (2011).
Sodeifian, G., Sajadian, S. A. & Saadati Ardestani, N. Evaluation of the response surface and hybrid artificial neural network-genetic algorithm methodologies to determine extraction yield of Ferulago angulata through supercritical fluid. J. Taiwan Inst. Chem. Eng. 60, 165–173. https://doi.org/10.1016/j.jtice.2015.11.003 (2016).
Sodeifian, G. & Sajadian, S. A. Investigation of essential oil extraction and antioxidant activity of Echinophora platyloba DC. using supercritical carbon dioxide. J. Supercrit. Fluids 121, 52–62. https://doi.org/10.1016/j.supflu.2016.11.014 (2017).
Radzali, S. A., Markom, M. & Saleh, N. M. Co-solvent selection for Supercritical fluid extraction (SFE) of phenolic compounds from Labisia pumila. Molecules 25, 5859. https://doi.org/10.3390/molecules25245859 (2020).
Sodeifian, G., Sajadian, S. A. & Saadati Ardestani, N. Supercritical fluid extraction of omega-3 from Dracocephalum kotschyi seed oil: Process optimization and oil properties. J. Supercrit. Fluids 119, 139–149. https://doi.org/10.1016/j.supflu.2016.08.019 (2017).
Sodeifian, G., Sajadian, S. A. & Honarvar, B. Mathematical modelling for extraction of oil from Dracocephalum kotschyi seeds in supercritical carbon dioxide. Nat. Prod. Res. https://doi.org/10.1080/14786419.2017.1361954 (2017).
Sodeifian, G., Azizi, J. & Ghoreishi, S. M. Response surface optimization of Smyrnium cordifolium Boiss (SCB) oil extraction via supercritical carbon dioxide. J. Supercrit. Fluids 95, 1–7. https://doi.org/10.1016/j.supflu.2014.07.023 (2014).
Olivia, U. N., Goodness, U. C. & Ogugofor, M. O. Phytochemical profiling and GC-MS analysis of aqueous methanol fraction of Hibiscus asper leaves. Future J. Pharm. Sci. 7, 59. https://doi.org/10.1186/s43094-021-00208-4 (2021).
Sodeifian, G., Sajadian, S. A. & Saadati Ardestani, N. Optimization of essential oil extraction from Launaea acanthodes Boiss: Utilization of supercritical carbon dioxide and cosolvent. J. Supercrit. Fluids 116, 46–56. https://doi.org/10.1016/j.supflu.2016.05.015 (2016).
Sodeifian, G., Sajadian, S. A. & Saadati Ardestani, N. Extraction of Dracocephalum kotschyi Boiss using supercritical carbon dioxide: Experimental and optimization. J. Supercrit. Fluids 107, 137–144. https://doi.org/10.1016/j.supflu.2015.09.005 (2016).
Sodeifian, G., Ghorbandoost, S., Sajadian, S. A. & Saadati Ardestani, N. Extraction of oil from Pistacia khinjuk using supercritical carbon dioxide: Experimental and modeling. J. Supercrit. Fluids 110, 265–274. https://doi.org/10.1016/j.supflu.2015.12.004 (2016).
Sodeifian, G., Sajadian, S. A. & Saadati Ardestani, N. Experimental optimization and mathematical modeling of the supercritical fluid extraction of essential oil from Eryngium billardieri: Application of simulated annealing (SA) algorithm. J. Supercrit. Fluids 127, 146–157. https://doi.org/10.1016/j.supflu.2017.04.007 (2017).
Sodeifian, G., Ardestani, N. S., Sajadian, S. A. & Moghadamian, K. Properties of Portulaca oleracea seed oil via supercritical fluid extraction: Experimental and optimization. J. Supercrit. Fluids 135, 34–44. https://doi.org/10.1016/j.supflu.2017.12.026 (2018).
Autor, E. et al. Extraction of phenolic compounds from Populus salicaceae bark. Biomolecules 12, 539. https://doi.org/10.3390/biom12040539 (2022).
Badri, A. M. et al. Antioxidant activity and phytochemical screening of Prosopis julifora leaves extract. Adv. Med. Plant Res. https://doi.org/10.30918/AMPR.53.17.020 (2017).
Singh, S. Phytochemical analysis of different parts of P. juliflora. Int. J. Curr. Pharm. Res. 4(3), 59–61 (2012).
Raghavendra, M. P., Satish, S. & Raveesha, K. A. Alkaloids isolated from leaves of P. juliflora against Xanthomonaspathovars. Arch. Phytopathol. Plant Prot. 42(11), 1033–1041. https://doi.org/10.1080/03235400701621644 (2009).
Deressaa, T., Lemessab, F. & Wakjirab, M. Antifungal activity of some invasive alien plant leaf extracts against mango (Mangifera indica) anthracnose caused by Colletotrichum gloeosporioides. Int. J. Pest Manag. 61(2), 99–105 (2015).
Bazie, S., Amare, A. & Woldetsadik, K. Antifungal activity of some plant extracts against (Colletotrichum musae) the cause of postharvest banana anthracnose. J. Plant Pathol. Microbiol. 5(2), 2157–7471. https://doi.org/10.4172/2157-7471.1000226 (2014).
Davidson, P. M. Chemical preservatives and natural antimicrobial compounds. Food Microbiol. Fundam. Front. https://doi.org/10.1128/9781555818463.ch30 (2012).
Sarwar, R. et al. Isolation and characterization of two new antimicrobial acids from Quercus incana (Bluejack Oak). BioMed Res. Int. https://doi.org/10.1155/2018/3798105 (2018).
Pradhan, R. C. A. et al. Supercritical CO2 extraction of fatty oil from flaxseed and comparison with screw press expression and solvent extraction processes. J. Food Eng. 98(1), 393–397. https://doi.org/10.1016/j.jfoodeng.2009.11.021 (2010).
Palafox, J. O. et al. Extraction and characterization of oil from Moringa oleiferausing supercritical CO2 and traditional solvents. Am. J. Anal. Chem. 3(1), 946–949. https://doi.org/10.4236/ajac.2012.312A125 (2012).
Adhikari, P., Pandey, A., Agnihotri, V. & Pande, V. Selection of solvent and extraction method for determination of antimicrobial potential of Taxus wallichiana Zucc. Res. Pharm. https://doi.org/10.25081/rip.2018.v8.3487 (2018).
Malapit, C. A. Report on the extraction of moringa oil from moringa oil seeds. J. Lipid Sci. 1(1), 1–5 (2010).
Nene, Y. L. & Thapliyal, P. N. Fungicides in Plant Disease Control 507 (Oxford and IBH Publication Company, 1993).
Acknowledgements
The authors are thankful to the Pesticide Residue and Food Quality Analysis Laboratory (PRFQAL), University of Agricultural Sciences, Raichur for providing the necessary facilities in accomplishing the research work.
Funding
The experiment has been carried out under the Post Graduate Research garnt as it is a part of Masters degree research program.
Author information
Authors and Affiliations
Contributions
N.M.N.: Conducting experiment and Manuscript preparation; M.K.: Conducting experiment; M.M.: Head and Member of advisory team (Microbiology Part); M.B.: Professor and Head, Member of advisory team (Monitoring); U.N.: Member of advisory team (Extraction Part); V.K.: Analysis part; K.T.: Analysis part.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naik, N.M., Krishnaveni, M., Mahadevswamy, M. et al. Characterization of phyto-components with antimicrobial traits in supercritical carbon dioxide and soxhlet Prosopis juliflora leaves extract using GC-MS. Sci Rep 13, 4064 (2023). https://doi.org/10.1038/s41598-023-30390-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30390-9
- Springer Nature Limited