Abstract
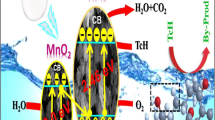
Crystalline hexagonal disk-shaped anatase TiO2 within manganese oxide (Mn2O3) nanorods has been fabricated to form manganese oxide-titanium dioxide (MnT) composites following sonochemical approach. The unique morphology of the synthesized MnT provides higher surface area to enhance the adsorption efficiency and promotes electron–hole separation. This appears particularly promising for efficient photocatalysis. The photocatalytic decolorization efficiency (PD) of sonosynthesized MnT (S-MnT) was tested via decomposition of an organic pollutant, malachite green oxalate under ultraviolet irradiation and compared to that of sol–gel synthesized MnT (H-MnT). The profound impact of hydrogen peroxide (H2O2) addition on the photoactivity was tested. S-MnT with addition of sonicated H2O2 showed the best photoactivity (PD 99.61% in 50 min) in comparison to sol–gel synthesized H-MnT (PD 92.14% in 60 min). The mechanism of degradation was found to depend on the formation of superoxide radicals (O −·2 ), hydroxyl radicals (·OH) and the defect concentration. The role of ultrasound in the overall degradation process was also discussed. Photoluminescence studies demonstrate that Mn2+ ions are in an octahedral and tetrahedral coordination in both the composites which is favourable for light emission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Non-silica mesoporous materials have inspired remarkable research interest as they are expected to serve as novel photocatalysts, semiconductors, electrode materials etc. [1]. Among these novel materials, titanium dioxide (TiO2) is one among the most investigated functional materials in recent years [2, 3]. Nevertheless, the main problem with TiO2 photocatalysis lies in the separation of photoinduced electron–hole pairs. To overcome this issue, different routes have been proposed which include combining the photocatalyst with another transition metal semiconductor [4], incorporation of non-metal ions [5] and by adding some sacrificial agent into the reaction system [6, 7]. Available methods for the formation of the binary composites include the sol–gel route [8], co-precipitation method [9], self-assembly [10] and a chelating agent-assisted precipitation method [11], although each suffers from conventional drawbacks. The widely used sol–gel route is characterized by poor dispersion and requirement of complexing agents to control the homogeneous crystallization of dopant inorganic precursors [12] while interfacial self-assembly methods [10] have various technical issues that remain unaddressed. In recent times, sonochemical synthesis of nanocomposites has gained momentum because of its cost-effectiveness and simplicity [13, 14]. Pressure and temperature changes during sonosynthesis result in the production of free radicals that in turn can reduce metal ions to metal or metal oxide nanoparticles [15, 16].
Aquatic ecosystems are continuously being contaminated by dye pollutants and have reached at an alarming point. These organic pollutants can be degraded to benign by-products by advanced oxidation processes which exploit the high reactivity of the hydroxyl radicals (·OH) as their driving force. The rate of generation of ·OH radical is, however, dependent on the type of crystal polymorphs. It is believed that a correlation exists between the rates of ·OH radical generation and the photocatalytic reactivity [17]. The photoinduced electrons and holes in the photocatalytic process react with H2O and O2 to give rise to several reactive oxygen species. Among the various reactive oxygen species, H2O2 is the most stable one. It is environment friendly and has high oxidative power [18, 19]. However, its behaviour on semiconducting particles is very complex. During photocatalytic reactions, it is not only formed but also gets decomposed. Kim et al. [20] have suggested that the difference in the reactivity of anatase and rutile TiO2 can be ascribed to their adsorption capability of the ·OH radicals. Many researchers have investigated the formation rate of ·OH radicals for various kinds of TiO2 powders along with the effect of small amounts of H2O2 addition [21,22,23,24] but none have discussed the method of addition of these agents.
In the present communication, two different Mn2O3–TiO2 (MnT) nanocomposites have been synthesized following conventional sol–gel route and an ultrasound assisted cost-effective technique. Two different morphologies, hexagonal nanodisks and nanorods were distinctly observed in the sonosynthesized composite in comparison to sol–gel synthesized composite which showed only hexagonal nanodisks. The focus of this work is to investigate the synergistic photocatalytic degradation of malachite green (MG) in aqueous conditions with addition of H2O2 under ultraviolet (UV) irradiation by the synthesized nanocomposites.
2 Experimental section
2.1 Materials required
Titanium (IV) n-butoxide, manganese acetate tetrahydrate was procured from Sigma Aldrich and sodium hydroxide (NaOH), ethanol, stearic acid, hydrogen peroxide (30% purified) from Merck and malachite green oxalate from PubChem.
2.2 Method of preparation
Ultrasonic irradiation (40 kHz, 480 W) served the base for synthesis of S-MnT nanocomposite. Titanium tetra butoxide (1.0 × 10−2 M) was added drop wise into 100 mL NaOH (1.0 × 10−3 M) in an ice bath. The mixture was then subjected to ultrasonic irradiation using a bath sonicator for a period of 3 h at a temperature of 80 °C. Fresh flow of water was introduced in the bath whenever required to maintain temperature. To this solution, 10 mL ethanolic solution of manganese acetate tetrahydrate (1.0 × 10−1 M) mixed with 0.5 M of melted (80 °C) stearic acid was added and the mixture was subjected to ultrasonic irradiation for 5 h. The final product (S-MnT) was washed with ethanol, filtered and dried at 120 °C for 24 h.
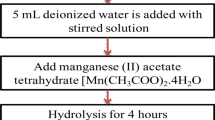
To properly understand the crucial influence of ultrasound governing the nanocomposite synthesis procedure, another MnT was prepared following the sol–gel route. Typically, titanium tetra butoxide (1.0 × 10−2 M) was added drop wise to 100 mL of (1.0 × 10−3 M) NaOH solution and stirred vigorously (700 rev/s) for a period of 4 h. Subsequently, 10 mL of ethanolic solution of manganese acetate tetrahydrate (1.0 × 10−1 M) mixed with 0.5 M of melted (80 °C) stearic acid was added. The contents were put in an autoclave, and heated at 130 °C for 24 h. The obtained product (H-MnT) was filtered, washed thrice with de-ionized water and dried at 200 °C for 5 h.
2.3 Characterization techniques
An X’Pert PRO PANalytical X-ray diffractometer was used to obtain the diffraction patterns of the synthesized materials. Elemental composition and morphology were ascertained with a Field emission Scanning Electron Micrograph (Carl Zeiss SUPRA 55VP Field emission SEM) coupled with an Energy dispersive spectrometer (EDS). Functional groups present in the materials were confirmed with an Infrared (IR) spectrometer (Perkin Elmer Spectrum one L120-000A). The emission and absorption spectra were recorded with a PTI QM 40 spectrometer and UV 3600 spectrometer respectively. A surface area analyser (Micromeritics Gemini VII-2390t) was used to measure the surface area. The samples were degassed at 100 °C and the total pore size distribution (PSD) was measured from the nitrogen desorption isotherm using the cylindrical pore model.
2.4 Degradation experiments
A UV–vis-NIR spectrophotometer was utilized to monitor the photocatalytic behaviour of MnT nanocomposites. The photocatalytic tests were carried out with an aqueous solution of malachite green oxalate (molecular formula C52H54N4O12, PubChem, CAS 2437-29-8) as the test contaminant in a reaction chamber enclosed within a wooden chamber fitted with UV lamps (Philips TUV 30 W T8). Arrangement of the reactor was such that UV light was directly incident on the samples. The photon flux has been calculated by a method discussed in M.D. Purkayastha et al. [2]. Prior to photocatalytic experiments, the aqueous dye solution was subjected to UV irradiation for 1 h to check whether photolysis occurs. Also, the adsorption behaviour of the photocatalysts for MG dye was tested without UV light irradiation. To achieve this, the photocatalysts were dispersed in the aqueous dye solution and stirred in dark for 1 h. The photocatalytic activities of both the samples were evaluated by measuring the decomposition of MG under UV light with time. Typically, 50 mL aqueous solution of MG dye (0.001 M) with 50 mg of the photocatalyst was stirred in the reactor. Prior to stirring, the system was dispersed by ultrasonic waves. After subjecting to UV irradiation, 5 mL aliquots of the reaction solutions were removed from the reactor every 10 min for analysis. These aliquots were centrifuged at 1000 rpm to exclude suspended particles, and the concentration of MG in the supernatant solutions was assessed by UV–Vis spectrometry at 618 nm, 315 nm and 418 nm absorption wavelengths for MG.
To analyse the influence of ultrasound on the behaviour of oxidizing species, photocatalytic degradation kinetic tests were repeated with optimal amounts of hydrogen peroxide (2 mL) added to S-MnT catalyst once directly and once after ultrasonication.
3 Results and discussion
3.1 S-MnT synthesis
Sonochemical methods of synthesis have been recently considered as a simple and efficient with the non-requirement of surfactants and complexing agents [25]. Addition of NaOH causes hydrolysis of titanium tetrabutoxide [Eq. (1)]. The hydrolytic species undergoes condensation and aggregation forming TiO2 under the effect of sonication at an elevated temperature of 80 °C [13, 26, 27].
Regarding the formation of Mn2O3, the precursor manganese acetate tetrahydrate is first dissolved in ethanol where it undergoes hydrolysis [9]. The use of stearic acid can modify the particle surface by introducing characteristic groups onto the surface of Mn2O3 to alter its physicochemical properties [28]. Also, the long carbon chains in stearic acid endow it with strong ability to disperse metal precursors [29]. The FTIR spectrum confirms the modification by showing a band corresponding to stearate. Similar predictions have been made by Kolodziejczak-Radzimska et al. [30]. Probably, changes in pressure and temperature due to ultrasonic irradiation caused Mn2+ ions to get oxidized to Mn2O3 that impeded the TiO2 particles to join forming rod like structures [31]. Similar observations have been made by Hu et al. [25] who synthesized connected rods of ZnO using a sonochemical process with an aqueous solution of Zn(NO3)2.6H2O at a temperature of 80 °C under ambient conditions.
3.2 Structural characterization
The diffraction pattern (Fig. 1a) of the pure TiO2 shows the presence of anatase peaks corresponding to the (1 0 1), (0 0 4), (2 0 0), (1 0 5), (211), (204), (116), (220) and (215) diffraction planes supported by JCPDS card no.78-2486. Figure 1b, c depicts the XRD patterns acquired from the two representative samples H-MnT and S-MnT. The diffraction peaks can be indexed to the mixtures of TiO2 anatase and bixbyite structure of Mn2O3 coinciding with JCPDS 24-0508. The S-MnT and H-MnT nano powders exhibited brown hue.
Sonication effect on size of the crystallites, and the phase was studied from the XRD pattern [32]. Both the composites favoured anatase phase of TiO2 in it. As shown in Fig. 1, the intensity of the (101) peak of TiO2 in the composites decreased remarkably in comparison to pure TiO2 anatase. However, the peaks of H-MnT appeared slightly sharper in comparison to S-MnT showing signs of improved crystalline structure. Probably, the step-wise hydrolysis of tetrabutyl titanate affected the process of crystallization in S-MnT. It could be concluded from this that ultrasound had some impact on the crystallinity. On calculating the crystallite size by Scherer formula [33] corresponding to the most intense (101) peak of TiO2 in the composites, it was observed that sizes (31.94 nm for H-MnT and 24.18 nm for S-MnT) are comparable. Overall, sonosynthesis has little or no influence on crystallite size and phase of the synthesized samples.
The energy dispersive X-ray (EDS) spectra of both H-MnT (Fig. 2a) and S-MnT (Fig. 2b) confirm the presence of manganese (5.6–6.6 keV), titanium (4.5–5 keV) and oxygen (0–1 keV) [34]. It can be observed that the atomic weight percentage of manganese in S-MnT is much higher which suggests better dispersion of manganese in the formation of S-MnT composite.
The typical FESEM images of the as-synthesized samples presented in Fig. 2 shows that the morphology changes drastically with change in synthesis techniques. The sample S-MnT prepared with ultrasound generated nanorods along with nanodisks (Fig. 2c, d), while only hexagonal nanodisks could be observed for H-MnT (Fig. 2e, f). It should be noted that these composite structures were synthesized maintaining all experimental conditions fixed except the use of ultrasound. Therefore, the evolution of diverse morphology is believed to be the result of ultrasound. The existence of diverse morphology in S-MnT could be because manganese impeded the hexagonal nanodisks to grow along preferred crystallographic direction forming nanorods under the influence of ultrasound. Thus, sonosynthesis can significantly influence the surface morphology of the nanoparticles which may have a profound effect on photocatalytic activity as well [35, 36].
The samples were dispersed in doubly distilled water in an ultrasonic bath and the absorbance spectrum was recorded (Fig. 3i). H-MnT showed absorption peaks at 238 nm and 377 nm corresponding to the UV region. These are characteristics peaks of TiO2 and found to coincide well with literature [37, 38]. Similar peaks were observed in the case of S-MnT. But the peak at 377 nm appeared red shifted by 5 nm. The Tauc Eq. (3) was utilized to calculate the optical band gap (Eg).
where α is the absorption coefficient and n signals the character of transition which assumes a value 1/2 for direct, allowed transition. The band gap of H-MnT was found to 2.44 eV (Fig. 3ii). The synergistic effect of ultrasound brings down the band gap energy to 2.14 eV for S-MnT (Fig. 3iii). Probably, ultrasound caused chemical bonding between Mn2O3 and TiO2 particles in the composite that led to band gap narrowing. The band gap energy values are lower than those reported by other researchers [39] which could be due to structural distortion resulting from oxygen deficiencies in the samples. Also, the S-MnT peaks showed higher intensity confirming the structural modification. Thus, sonochemistry is capable of tuning the band gaps and altering the light absorption properties of the samples as well.
IR spectra of S-MnT and H-MnT are illustrated in Fig. 4i. The peaks at 3400 cm−1 and 1627 cm−1 in both the samples can be assigned to the stretching vibrations of adsorbed water molecules and OH− in the lattice. H-MnT and S-MnT both show the characteristic peak of stearic acid at 1046 cm−1 used in their preparation which confirms that stearic acid is well incorporated in the structures [40]. The formation of bonds between metal and oxygen are confirmed from the peaks at 514 cm−1 and 730 cm−1 [29, 40].
Manganese, being a transition metal ion, has a wide emission wavelength range and its location in the TiO2 lattice actually decides its luminescence properties [41]. The photoluminescence (PL) properties of the samples under the UV excitation wavelength of 300 nm are presented in Fig. 4ii. Considering the Tanabe-Sugano diagrams [42] to demonstrate the PL mechanism, it can be assumed that due to UV excitation, electron transition occurs and the system is excited from the 4A2 ground state to the excited states of 2T1 and 4T2. These excited states are however not stable, and it is presumable that the system will relax and ultimately come down to the ground state 4A2 through a radiative transition. Previous studies have reported that a material can emit light corresponding to different wavelengths based on their arrangement within the lattice [43, 44]. H-MnT showed emissions at 469 nm and 551 nm while for S-MnT (Fig. 4ii) these peaks appeared slightly red shifted. This red shift can be attributed to the evolution of defects [45]. The peak at 551 nm corresponds to yellow-orange (and also red) emission and is associated with deeper defects while that at 469 nm corresponds to blue-green emission which can be associated with surface defects [46, 47]. Manganese is known to have variable oxidation states. Report [48] claims that the emission band of Mn4+ ion stretches from 600 to 710 nm and is due to the 2E → 4A2 transition. In the present case, a peak at 692 nm confirms the presence of Mn4+ ions in both the composites. Generally, the octahedral coordinated Mn2+ ions are known to yield an orange to red emission, whereas tetrahedral coordinated ones exhibit a green emission [49,50,51]. Thus, PL spectra confirm the presence of both octahedral coordinated Mn2+ ions and tetrahedral coordinated Mn2+ ions in the composites.
3.3 BET analysis results
The synthesized S-MnT sample (Fig. 5a) indicated type IV isotherm according to IUPAC classification and exhibited H-2 type hysteresis loop corresponding to mesoporous nature [52]. It also indicates the existence of narrow slit shaped pores [52]. At a relative pressure of 0.05, the first layer on the sample surface gets filled up which is indicated by the first inflection point A. The linear region (B) in the isotherm is attributed to the multilayer adsorption on the surface. BET method has been utilized to estimate the surface area using multi-point BET Eq. (4) indicated as follows: [52]
where Wm indicates the adsorbate weight covering the surface and W is the gas accumulated at P/Po. By plotting 1/[W (P/P0) − 1] versus P/Po, we obtained a linear curve (Fig. 5b) within 0.05 < P/P0 < 0.35 range. This proves that the isotherm data conforms well to the BET model.
The BJH method was then employed to determine the pore size distribution (Fig. 5c).
The surface area value for S-MnT is 18.14 m2/g. It is well known that mesopores and high surface area are beneficial for the adsorption and diffusion of the reactants [9]. As surface area increases, light harvesting capacity of the photocatalyst increases [53] which cause a significant increase in the interfacial charge transfer rate [54]. Also, the availability of active surface area differs depending on nanostructure dimensions [55]. Peter et al. [56] observed that reduction in dimensionality enhanced the available active surface area. S-MnT also shows a wider PSD which is suitable for energy applications and environmental remediation.
3.4 Analysis of photocatalytic properties
Malachite green (MG) is an organic pollutant present in aquatic ecosystems widely used in agriculture as a biocide [57]. The photocatalytic behaviour of the composites was analysed by monitoring their MG decomposition rates under UV radiation at neutral pH. The UV irradiation dose has been calculated as discussed in [2] and listed in Table 1. Dark experiments were carried out for 1 h and the adsorption ability of the composites was found to be less than 10% (Fig. 6i). Negative control experiments (without catalyst) were carried out and decomposition was found to be negligible in absence of photocatalyst. The MG solution along with the added photocatalyst was then subjected to UV irradiation and the progress of the reaction was followed by monitoring the intensity of MG absorption bands at 618 nm, 315 nm and 418 nm respectively. A change in absorbance intensity as function of time was observed (Figure S1). The kinetics of heterogeneous catalytic processes can be best described by Langmuir–Hinshelwood model that takes the sorption effect into consideration. Dye molecules tend to get adsorbed on the active sites of the photocatalyst before the reaction starts [58]. The expression for rate of the reaction (5) that changes with time is given by [59]
where A is the dye concentration at any time t, Ao is the initial dye concentration, kr is the reaction rate constant and K is the adsorption coefficient of the dye MG. Rate of photodegradation of organic compounds, in general, follow first order reaction kinetics. For pseudo first order reaction (KA ≪ 1)
where k1 is the pseudo first order reaction rate constant.To make the concentration–time relationship linear
On plotting − ln (At/Ao) as a function of time, the constant k1 can be easily determined from the slope of linear best fit. The decolourization efficiency was calculated using [60]
where Ao and At denote dye concentrations before and after photo-irradiation. Figure 6ii shows the time dependent percentage decolourization efficiency (PD) of MG solution (100 mg/L) under UV irradiation in contact with the composites, and also without the presence of the photocatalyst for MG 618 nm absorption peak. The PD values corresponding to 315 nm and 418 nm peaks of MG have been provided in Table S1 in Supplementary material. The composites were found to exhibit enhanced photoactivity in comparison to similar reported materials under UV radiation [61, 62]. It has already been discussed that H2O2 is a promising electron acceptor that tends to enhance the overall reaction rate [63]. However, addition of H2O2 in large amounts can have a deteriorating effect on it [64, 65]. Experiments were carried out with different amounts of H2O2 (0.5-5 mL) and 2 mL H2O2 was found to be the optimal dosage. To underline the sonication effect, this H2O2 was added in two ways-once directly and once after being sonicated. S-MnT with sonicated H2O2 showed the best photoactivity which confirmed that sonochemistry has a role in inhibition of electron–hole recombination. Probably, sonochemistry led to the uniform dispersion of H2O2 in the photocatalyst solution which accelerated the process of radical formation. The estimated values of k1, corresponding correlation coefficients (R2), PD efficiencies and error (chi-square χ2) values are listed in Table 1 (for pseudo first order). Figure 6iii provides the values of the pseudo first order rate constant obtained from the linear regression curves.
The stability is essential for its practical application. A cycle experiment of degrading MG in aqueous solutions over the composites was conducted. Results (Table 1) showed that S-MnT with sonicated H2O2 still held high photocatalytic activity after five cycles (Fig. 7).
The mechanism of photocatalysis is described as follows: On irradiating the reaction solution with UV light, photoexcited charge carriers get formed (Eq. (10)).
where hν = photons, h+ = holes, proton = H+, CB is conduction band, VB is valence band and e− = electrons.
Magesh et al. [66] calculated the conduction band potential (ECB) for TiO2 (Eg = 3.2 eV) in reference to normal hydrogen electrode (NHE) as − 0.29 eV while Li et al. [67] found that ECB for Mn2O3 (Eg = 3.2 eV) is − 1 eV. Clearly, ECB for Mn2O3 is more negative than that of TiO2. So, it is expected that at the junction of the MnT composite, there will be a transfer of excited electrons from Mn2O3 to the conduction band of TiO2.
The holes in the valence band will give rise to ·OH radicals by the oxidation of water as given by Eqs. (11) and (12).
Some excited electrons may get transferred to adsorbed oxygen molecules reducing molecular oxygen to superoxide radicals (O −·2 ) [23, 68,69,70,71].
Also, as ECB for TiO2 is more negative than that for O2/H2O2 (0.682 eV vs NHE), H2O2 may be produced in the process [72].
In case of added H2O2, Ti-peroxo species tend to be formed at the solution interface [73,74,75,76]. Ohno et al. [75] through FTIR and X-ray photoelectron spectroscopy (XPS) and Lousada et al. [77] through density functional theory studies have confirmed the formation of such peroxo species. These reactive oxygen species (·OH, O −.2 , H2O2 and peroxo species) ultimately lead to transformation of the chromophoric groups in the dye. MG is a dye containing nitrogen. Depending on the oxidation state of the nitrogen atom, MG may release NH4+, NO3− or even N2 [78]. However, the complete mineralization of the dye requires highly energetic free radicals which are preferentially generated under UV irradiation. In this context, Bae et al. [79] has documented that the TOC removal efficiencies of titania based catalysts are much higher under UV irradiation.
Also, the defect content in the composites could be responsible for the efficient photoactivity shown by them. In this regard, Zheng et al. [80] have explained that defects like interstitial oxygen generate shallow levels near the valence band while defects like oxygen vacancies act as electron acceptors. Thus, interfacial charge transfer prolongs the lifetime of the carriers by suppressing the recombination of electron–hole pairs. However, in the future, such parameters need to be studied on a theoretical platform.
A comparison of photocatalytic ability of synthesized MnT composites with other reported composites in degrading MG is illustrated in Table 2.
4 Conclusions
The present study outlined a reliable, cost-effective sonochemical method to synthesize binary nanocomposites of manganese and titanium oxides that can be extended for the preparation of other binary metal oxide composites. The composites were used to test the photocatalytic degradation of MG in the aqueous solution. The decolourization rate of S-MnT photocatalyst with ultrasound functionalized H2O2 was found to be higher than that of H-MnT, bare S-MnT and S-MnT with directly added H2O2. It could be proposed that unique nanorod–nanodisk morphology, defect concentration and sonochemistry played a vital role in reducing the chances of electron hole recombination thereby enhancing pollutant degradation rate. Luminescence studies also predict possible optoelectronic applications for transition metal oxide nanomaterials. The composites exhibited good stability predicting potential applications in waste water treatment, environment protection and even in energy preservation.
References
Soler-Illia GJAA, Sancchez C, Lebeau B, Patarin J (2002) Chemical strategies to design textured materials: from microporous and mesoporous oxides to nanonetworks and hierarchical structures. Chem Rev 102:4093–4138
Purkayastha MD, Middya S, Datta J, Ray PP, Biswas BD, Sarkar M, Darbha GK, Singh N, Majumder TP, Saha P, Das D (2019) The carrier transport properties and photodegradation ability of low temperature synthesized phase pure rutile titanium oxide nanostructured materials Mat. Chem Phys 226:362–370
Purkayastha MD, Sarkar M, Majumder TP (2019) Enhanced UV shielding properties of DAS based nanocomposites. Optik (Stuttg) 182:469–478
Purkayastha MD, Ray PP, Majumder TP, Sarkar M (2019) Spectroscopic characterization and photocatalytic degradation ability of ternary oxides of cobalt. Int J Innov Res Phys Accept Artic
Purkayastha MD, Singh N, Darbha GK, Majumder TP (2020) Strain influence on the structural properties of nitrogen and fluorine codoped TiO2. Optik (Stuttg) 206:164029
Harir M, Gaspar A, Kanawati B, Fekete A, Frommberger M, Martens D, Kettrup A, El Azzouzi M, Schmitt Kopplin P (2008) Photocatalytic reactions of imazamox at TiO2, H2O2 and TiO2/H2O2 in water interfaces: kinetic and photoproducts study. Appl Catal B Environ 84:524–532
Sivakumar S, Selvaraj A, Ramasam AK, Balasubramanian V (2013) Enhanced photocatalytic degradation of reactive dyes over FeTiO3/TiO2 heterojunction in the presence of H2O2. Water Air Soil Pollut 224:1529–1542
Song ZQ, Wang SB, Yang W, Mo L, Wang H, Yan H (2004) Synthesis of manganese titanate MnTiO3 powders by a sol-gel hydrothermal method. Mater Sci Eng, B 113:121–124
Xue M, Huang L, Wang JQ, Wang Y, Gao L, Zhu JH, Zou ZG (2008) The direct synthesis of mesoporous structured MnO2/TiO2 nanocomposite: a novel visible light active photocatalyst with large pore size. Nanotechnology 19:185604
Li J, Zeng HC (2006) Preparation of monodisperse Au/TiO2 nanocatalysts via self- assembly. Chem Mater 18:4270–4277
Kharkwal M, Uma S, Nagarajan R (2010) Use of a chelating agent for the synthesis of high surface area pyrophanite MnTiO3 powders Mater. Lett. 64:692–694
Sinha AK, Suzuki K (2005) Preparation and characterization of novel mesoporous ceria-titania. J Phys Chem B 109:1708–1714
Ghows N, Entezari MH (2010) Ultrasound with low intensity assisted the synthesis of nanocrystalline TiO2 without calcination. Ultrason Sonochem 17:878–883
Guo J, Zhu S, Chen Z, Li Y, Yu Z, Liu Q, Li J, Feng C, Zhang D (2011) Sonochemical synthesis of TiO2 nanoparticles on graphene for use as photocatalyst Ultrason. Sonochem 18:1082–1090
Arenas MC, Rodiguez-Nunez LF, Rangel D, Martinez-Alvarez O, Martinez-Alonso C, Castano VM (2013) Simple one step ultrasonic synthesis of anatase titania/polypyrrole nanocomposites Ultrason. Sonochem 20:777–784
Vinoth R, Karthik P, Devan K, Neppolian B, Muthupandian AK (2017) TiO2-NiO p–n junction nanocomposite with enhanced sonophotocatalytic activity under diffused sunlight Ultrason. Sonochem 35:655–663
Tryba B, Toyoda M, Morawski AW, Nonaka R, Inagaki M (2007) Photocatalytic activity and ·OH radical formation on TiO2 in the relation to crystallinity. Appl Catal B 71:163–168
Tachikawa T, Majima T (2010) Single molecule, single particle fluorescence imaging of TiO2 based photocatalytic reactions. Chem Soc Rev 39:4802–4819
Fujihira M, Satoh Y, Osa T (1981) Heterogeneous photocatalytic oxidation of aromatic compounds on titanium dioxide. Nature 298:206–208
Kim W, Tachikawa T, Moon G, Majima T, Choi W (2014) Molecular-level understanding of the photocatalytic activity difference between anatase and rutile nanoparticles. Angew Chem Int Ed 53:14036–14041
Hirakawa T, Yawata K, Nosaka Y (2007) Photocatalytic reactivity for O −·2 and ·OH radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl Catal A 325:105–111
Zhang J, Nosaka Y (2014) Mechanism of the OH radical generation in photocatalysis with TiO2 of different crystalline types. J Phys Chem C 118:10824–10832
Kakuma Y, Nosaka A, Nosaka Y (2015) Difference in TiO2 photocatalytic mechanism between rutile and anatase studied by the detection of active oxygen and surface species in water. Phys Chem Chem Phys 17:18691–18698
Nosaka Y, Nosaka A (2016) Chapter 7: Kinetic processes in the presence of photogenerated charge carriers. In: Schneider J, Bahnemann D et al (eds) Photocatalysis: fundamentals and perspectives. Royal Society of Chemistry, London, pp 165–184
Hu XL, Zhu YJ, Wang SW (2004) Sonochemical and microwave assisted synthesis of linked single crystalline ZnO rods. Mater Chem Phys 88:421–426
Prasad K, Pinjari DV, Pandit AB, Mhaske ST (2010) Phase transformation of nanostructured titanium dioxide from anatase to rutile via combined ultrasound assisted sol-gel method. Ultrason Sonochem 17:409–415
Ling J, Wang K, Wang Z, Huang H, Zhang G (2019) Enhanced piezoelectric-induced catalysis of SrTiO3 nanocrystal with well-defined facets under ultrasonic vibration. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2019.104819
Kockmann A, Hesselbach J, Zellmer S, Kwade A, Garnweitner G (2015) Facile surface tailoring of metal oxide nanoparticles via a two-step modification approach. RSC Adv 5:60993–60999
Enhessari M, Parviz A, Karamali E, Ozaee K (2012) Synthesis, characterisation and optical properties of MnTiO3 nanopowders. J Exp Nanosci 7:327–335
Kolodziejczak-Radzimska A, Jesionowski J (2014) Zinc oxide-from synthesis to application: a review. Materials (Basel) 7:2833–2881
Elser MJ, Berger T, Brandhunber D, Bernardi J, Diwald Knozinger E (2006) Particles coming together: electron centres in adjoined TiO2 nanocrystals. J Phys Chem 110:7605–7608
Monatzer M, Behzadnia A, Pakdel E, Rahimi MK, Moghadam MB (2011) Photoinduced silver on nano titanium dioxide as an enhanced antimicrobial agent for wool. J Photochem Photobiol, B 103:207–214
Patterson AL (1939) The Scherrer formula for x-ray particle size determination. Phys Rev 56:978–982
Das SK, Rou BK (2015) Magnetic and ferroelectric properties of Zn and Mn co-doped BaTiO3. Chin Phys B 24:067702
Smith AM, Nie S (2010) Semiconductor nanocrystals: structure, properties, and band gap engineering. Acc Chem Res 43:190–200
Zhang H, Zhu H, Sun R (2012) Fabrication of photocatalytic TiO2 nanoparticle film on PET fabric by hydrothermal method. Text Res J 82:747–754
Linsebigler AL, Lu G, Yates JT (1995) Photocatalysis on TiO2 surfaces: principles, mechanisms and selected results. Chem Rev 95:735–758
Binas V, Venieri D, Kotzias D, Kiriakidis G (2017) Modified TiO2 based photocatalysts for improved air and health quality. J Materiomics 3:3–16
Sharrouf M, Awad R, Roumie M, Marhaba S (2015) Structural, optical and room temperature magnetic study of Mn2O3 nanoparticles. Mater Sci Appl 6:850–859
Sharma YK, Kharkwal MK, Uma S, Nagarajan R (2009) Synthesis and characterisation of titanates of the formula MnTiO3 (M = Mn, Fe Co, Ni, Cd) by co-precipitation of mixed metal oxalates. Polyhedron 28:579–585
Gupta SK, Kadam RM, Gupta R, Sahu M, Natarajan V (2014) Evidence for stabilisation of manganese ion as Mn(II) and Mn(IV) in a-Zn2P2O7: Probed by EPR, luminescence and electrochemical studies. Mater Chem Phys 145:162–167
Tanabe Y, Sugano S (1954) On the absorption spectra of complex ions. J Phys Soc 9:753–766
Ye T, Dong Z, Zhao Y, Yu J, Wang F, Guo S, Zuo Y (2011) Controllable fabrication of perovskite SrZrO3 hollow cuboidal nanoshells Cryst. Eng Commun 13:3842–3847
Pathak N, Gupta SK, Ghosh PS, Arya A, Natarajan V, Kadam RM (2015) Probing local site environments and distribution of manganese in SrZrO3: PL and EPR spectroscopy complimented by DFT calculations. RSC Adv 5:17501–17513
Raul SZ, Marcelino BF, Umapada P (2011) Photoluminescence and Raman scattering in Ag-doped ZnO nanoparticles. J Appl Phys 109:014308
Souza AE, Santos GTA, Barra BC, Macedo WD Jr, Teixeira SR, Santos CM, Senos AMOR, Amaral L, Longo E (2012) Photoluminescence of SrTiO3: influence of particle size and morphology. Cryst Growth Des 12:5671–5679
da Silva LF, Avansi W, Moreira ML, Mesquita A, Maia LJQ, Andrés J, Longo E, Mastelaro VR (2012) Relationship between crystal shape, photoluminescence and local structure in SrTiO3 synthesized by microwave assisted hydrothermal method. J Nanomater 2012:890397
Wang B, Lin H, Xu J, Chen H, Wang YS (2014) CaMg2Al16O27: Mn4+ based red phosphor: a potential color converter for high-powered warm w-led. ACS Appl Mater Interfaces 6:22905–22913
Shang M, Geng D, Zhang Y, Li G, Yang D, Kang X, Lin J (2012) Luminescence and energy transfer properties of Ca8Gd2(PO4)6O2: A (A = Ce3+/Eu2+/Tb3+/Dy3+/Mn2+) phosphors. J Mater Chem 22:19094–19104
Geng D, Li G, Sheng M, Yang D, Zhang Y, Cheng Z, Lin J (2012) Colour tuning via energy transfer in Sr3In(PO4)3: Ce3+/Tb3+/Mn2+. J Mater Chem 22:14262–14271
Liu YF, Zhang X, Hao ZD, Wang XJ, Zhang JH (2011) Tunable full colour emitting Ca3Sc2Si3O12:Ce3+, Mn2+ phosphor via charge compensation and energy transfer. Chem Commun 47:10677–10679
Do DD (1998) Adsorption analysis: equilibrium and kinetics. Imperial College Press, London
Ahmed F et al (2014) Quantum-confinement induced enhancement in photocatalytic properties of iron oxide nanoparticles prepared by Ionic liquid. Ceram Int 40:15743–15751
Wei Z, Kowalska E, Verrett J, Colbeau-Justin C, Remita H, Ohtani B (2015) Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication hydrothermal reaction of titanates. Nanoscale 7:12392–12404
Flores NM, Pal U, Galeazzi R, Sandoval A (2014) Effect of morphology, surface area and defect content on the photocatalytic dye degradation performance of ZnO nanostructures. RSC Adv 4:41099–41110
Peter IJ, Praveen E, Vignesh G, Nithiananthi P (2017) Zinc nanostructures with different morphology for enhanced photocatalytic activity. Mater Res Express 4:124003
Raval NP, Shah PU, Shah NK (2017) Malachite green ‘a cationic dye’ and its removal from aqueous solution by adsorption. Appl Water Sci 7:3407–3445
Delagado P, Sans MT, Beltvan S (2007) Kinetic study for esterification of lactic acid with ethanol and hydrolysis of ethyl acetate using an ion exchange resin. Chem Eng J 120:111–118
Herrmann JM (2010) Photocatalysis fundamentals revisited to avoid mis-conceptions. Appl Catal B Environ 99:461–468
Menzinger M, Wolfgang R (1969) The meaning and use of the Arrhenius activation energy. Angew Chem 6:438–444
Tateoka A, Sekine Y, Tsuda T, Ohashi T (2005) Manganese oxide photodeposited onto titanium dioxide as a new environmental catalyst. Mater Sci Forum 480/481 117:117–122
Subrahmanyam C, Magureanu M, Laub D, Renken A, Kiwi-Minsker L (2007) Nonthermal plasma abatement of trichloroethylene enhanced by photocatalysis. J Phys Chem C 111:4315–4318
Wong CC, Chu W (2003) The hydrogen peroxide assisted photocatalytic degradation of alachlor in TiO2 suspensions. Environ Sci Technol 37:2310–2316
Ye H, Lu S (2013) Effect of hydrogen peroxide on the structure and photocatalytic activity of titania. Res Chem Intermed 41:139–149
Zang Y, Farnood R (2006) Effect of hydrogen peroxide on the photocatalytic degradation of tert-butyl ether. Top Catal 37:91–96
Magesh G, Viswanathan B, Viswanath RP, Varadarajan TK (2009) Photocatalytic behaviour of CeO2–TiO2 system for the degradation of methylene blue. Indian J Chem 48A:480–488
Li F, Wangyang P, Zada A, Humayun M, Wang B, Qu Y (2016) Synthesis of hierarchical Mn2O3 microspheres for photocatalytic hydrogen production Mater. Res Bull 84:99–104
Sahel K, Elsellami L, Mirali I, Dappozze F, Bouhent M, Guillard C (2016) Hydrogen peroxide and photocatalysis. Appl Catal B 188:106–112
Jiang T, Wang K, Guo T, Wu X, Zhang G (2020) Fabrication of Z-scheme MoO3/Bi2O4 heterojunction photocatalyst with enhanced photocatalytic performance under visible light irradiation. Chin J Catal 41:161–169
Sun X, Zhang J, Fu Z (2018) Polyoxometalate cluster sensitized with copper-viologen framework for efficient degradation of organic dye in ultraviolet, visible and near-infrared light. ACS Appl Mater Interfaces 10:35671–35675
Wang Z, Wang K, Li Y, Jiang L, Zhang G (2019) Novel BiSbO4/BiOBr nanoarchitecture with enhanced visible light driven photocatalytic performance: oxygen-induced pathway of activation and mechanism unveiling. Appl Surf Sci 498:143850
Mousavi M, Yangjeh AH, Abitorabi M (2016) Fabrication of novel magnetically separable nanocomposites using graphitic carbon nitride, silver phosphate and silver chloride and their applications in photocatalytic removal of different pollutants using visible light irradiation. J Colloid Interface Sci 480:218–231
Regazzoni AE, Mandelbaum P, Matsuyoshi M, Schiller S, Bilmes SA, Blesa MA (1998) Adsorption and photooxidation of salicylic acid on titanium dioxide: a surface complexation description. Langmuir 14:868–874
Li X, Chen C, Zhao J (2001) Mechanism of decomposition of H2O2 on TiO2 surfaces using visible light irradiation. Langmuir 17:4118–4122
Ohno T, Masaki Y, Hirayama S, Matsumura M (2001) TiO2 photocatalyzed epoxidation of 1-decene by H2O2 under visible light. J Catal 204:163–168
Pavel CC, Park S, Dreier A, Tesche B, Schmidt W (2006) Structural defects induced in ETS-10 by postsynthesis treatment with H2O2 solution. Chem Mater 18:3813–3820
Lousada CM, Johansson AJ, Brinck T, Jonsson M (2012) Mechanism of H2O2 decomposition on transition metal oxide surfaces. J Phys Chem C 116:9533–9543
Tanaka K, Padermpole K, Hisanaga T (2000) Photocatalytic degradation of commercial azo dyes. Water Res 34:327–333
Bae S, Kim S, Lee S, Choi W (2014) Dye decolourization test for the activity assessment of visible light photocatalysts: realities and limitations. Catal Today 224:21–28
Zheng YH, Chen CQ, Zhan YY, Lin XY, Zheng Q, Wei KM, Zhu JF, Zhu YJ (2007) Luminescence and photocatalytic activity of ZnO nanocrystals: correlation between structure and property. Inorg Chem 46:6675–6682
Hussain W, Malik H, Hussain RA, Hussain H, Greens IR, Marwat S, Bahadur A, Iqbal S, Farooq MU, Li H, Badshah A (2019) Synthesis of MnS from single and multi-source precursors for photocatalytic and battery applications. J Electron Mater 48:2278–2288
Biswas BD, Purkayastha MD, Majumder TP (2020) Effect of DAS on the optical and photocatalytic properties of metastable γ-MnS nanoparticles. Surf Interfaces 19:100469
Berberidou C, Poulios I, Xekoukoulotakis NP, Mantzavinos D (2007) Sonolytic, photocatalytic and sonophotocatalytic degradation of malachite green in aqueous solutions. Appl Catal B Environ 74:63–72
Ali H, Ahmad W, Haq T (2009) Decolourization and degradation of malachite green by Aspergillus flavus and Alternaria solani. Afr J Biotechnol 8:1574–1576
Khaparde AR, Acharya AS (2018) Efficient photocatalytic degradation of malachite green dye under visible irradiation by water soluble ZnS:Mn/ZnS core/shell nanoparticles. AIP Conf Proc 1953:030139
Baeissa ES (2016) Photocatalytic degradation of malachite green dye using Au/NaNbO3 nanoparticles. J Alloys Compd 672:564–570
Amar IA, Harara HM, Baqul QA, Qadir MAA, Altohami FA, Ahwidi MM, Abdalsamed IA, Saleh FA (2020) Photocatalytic degradation of malachite green dye under UV light irradiation using calcium-doped ceria nanoparticles. Asian J Nanosci Mater 3:1–14
Meena S, Vaya D, Das BK (2016) Photocatalytic degradation of malachite green dye by modified ZnO nanomaterial. Bull Mater Sci 39:1735–1743
Rathore R, Ameta R, Ameta SC (2014) Photocatalytic degradation of malachite green over nickel vanadate powder. Acta Chim Pharm Indica 4:213–220
Chen CC, Lua CS, Chung YC, Jan JL (2007) UV light induced photodegradation of malachite green on TiO2 nanoparticles. J Hazard Mater 141:520–528
Wang Q, Zhang C, Zhou L, Zhang D, Tong Z, Liu Y, Chen J (2018) Efficient photocatalytic degradation of malachite green in sea water by the hybrid of ZnO nanorods grown on 3D reduced graphene oxide (RGO)/Ni foam. Materials (Basel) 11:1004
Acknowledgements
Authors highly appreciate the DST-PURSE program of Kalyani University and the DST-FIST Project (SR/FST/PSI-175/2012). The authors are indebted to Dr Sayan Bhattacharyya, IISER Kolkata for surface area measurements. Sincere acknowledgement is due for the assistance under DST-FIST and UGC-SAP programs to the Dept. of Chemistry, Kalyani University. Gopala Krishna Darbha is thankful to the support from SERB, Ramanujan Fellowship Grant (SB/S2/RJN-006/2016) and SERB-ECR project (ECR/2017/000707), India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Purkayastha, M.D., Denrah, S., Singh, N. et al. Crystal structure dependent photocatalytic degradation of manganese and titanium oxides composites. SN Appl. Sci. 2, 1126 (2020). https://doi.org/10.1007/s42452-020-2933-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2933-7