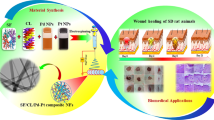
Abstract
Bone, skin and soft tissue chronic wounds emanating from burns or bacterial infections which persist due to prolonged tissue inflammation contribute to a delay in wound healing. Electrospun biomimetic scaffolds produced from biodegradable polymers have proven to be a better alternative due to their large surface area to volume ratio and ability to release the drug directly to the wound surface allowing fast and sustained absorbance over the affected wound area. In this study, poly lactic acid (PLA) (20% w/v) and collagen-based (PLA/C) fibrous scaffolds (electrospun at a voltage of 22 kV, flow rate of 0.1 mL/min) containing varying concentrations of silver sulphadiazine (1% w/w, 0.75% w/w) (Ag+S) and Aspalathus linearis (AL) fermented extract (0.025%, 0.1% and 0.5% w/w), were designed and fabricated to increase antimicrobial penetration and cellular biocompatibility. The elastic modulus of samples revealed that incorporating 1% Ag+S and A. linearis extract to PLA solution culminates in a fiber with the superlative stiffness of 2.1.1 GPa. The antimicrobial effect of the scaffolds was evaluated against S. aureus, P. aeruginosa, MRSA and E. coli. PLA/C–Ag+S/AL scaffolds and showed antibacterial activity against both gram +ve and gram −ve bacteria. They were nontoxic to the cells and provided favorable substrates for the neonatal epidermal keratinocytes cells to undergo cell attachment and proliferation. PLA/C–Ag+S/AL scaffolds have a great potential for use in chronic wounds as well as in tissue and bioengineering applications.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Chronic wounds occur as a result of continuous stimulation of the immune system leading to prolonged tissue inflammation, hence contributing to a delay in wound healing [1, 2]. Chronic wounds have been treated with topical antiseptic agents [3], anti-microbial therapy, systemic antibiotics [2, 4], surgical debridement [5], and granulocyte-colony stimulating factors. However, these treatments do have their limitation such as diagnostic uncertainty, reoccurrence of the wound and inability to deliver the drug directly to its site of action and sometimes poor absorption [6]. Some of these treatment options expose patients to serious risks. Although advances have been made in multidisciplinary wound care and have resulted in improved clinical outcomes [7], the economic and social impact of non-healing chronic wounds continue to grow [1, 8]. As a result of increasing growth in populations prone to dysfunctional wound healing, there is therefore an urgent and unmet need for novel strategies or techniques which can both prevent and treat these complications resulting from chronic wounds. Tissue engineering therefore offers the promising potential to create functional skin. The synergistic and combined efforts of biomedical engineers, molecular and cell biologists, material and pharmaceutical scientists, have yielded promising therapies essentially for non-healing wounds otherwise called chronic wounds [9].
Silver based products exhibit broad-spectrum antibacterial activity against a wide range of micro-organisms at concentrations as low as 0.1% w/w [10,11,12] and have been seen to be effective against immunogenic bacteria when incorporated into a composite [13]. Heo et al. developed a multilayered co-electrospun scaffold containing silver sulfadiazine as a prophylactic against osteomyelitis which was seen to have great potential in management of bone infections [14].
Currently, antioxidants are being introduced into the treatment of chronic wounds. Antioxidant vitamins, plant products such as carotenoids and polyphenolic flavonoids are potent antioxidant and wound healing substances in nature [15]. These free radical scavengers, flavonoids inhibit lipid peroxidation and promote vascular relaxation to prevent or delay the occurrence pathological changes associated with oxidative stress. Rooibos is endemic only to South Africa and it is one of the 278 species of the Aspalathus genus. Aspalathus linearis is rich in polyphenols which have antioxidant properties [16]. Lee and Bae evaluated the anti-inflammatory effects of aspalathin and nothofagin from Rooibos (A. linearis) in vitro and in vivo suggesting potential wound healing activity [17]. Pringle et al. [18] showed that the pro-inflammatory nature of fermented rooibos may have therapeutic value for wounds characterized with a delayed initial inflammatory phase, as seen in chronic wounds.
Wound dressings materials were originally produced from natural materials such as plant fibers and animal fats to cover the wound area [19]. This has greatly evolved in the modern day wherein artificial dressing materials can be produced by various advanced technologies to create multifunctional wound dressings [19, 20]. The two essential requirements of suitable modern wound dressings include rapid hemostasis and good antibacterial property. Electro-spun wound dressings have advantages over the present wound dressing because of their large surface area to volume ratio which allows for cell proliferation and attachment [21].
Chronic wounds usually exhibit an elevated level of matrix metalloproteinases (MMPs) [22], fibroblasts in chronic wound are unable to secrete tissue inhibitors of MMPs thus preventing extracellular matrix (ECM) and granulation tissue formation. Collagen based wound dressings are uniquely suited to address the issue of elevated levels of MMPs by acting as a ‘sacrificial substrate’ in the wound [22]. This study therefore seeks to fabricate nano-fibrous scaffolds from biodegradable polymers [23], incorporate a rooibois extract which has been postulated to have an inherent antibacterial and wound healing activity [18]. In this study polylactic acid (PLA) and collagen-based (PLA/C) nano-fibrous scaffolds containing varying concentrations of silver sulphadiazine (Ag+S) and A. linearis (AL) fermented extract (PLA/C–Ag+S/AL), were designed and fabricated to increase antimicrobial penetration and cellular biocompatibility.
2 Methods
2.1 Materials
Poly Lactic acid (PLA 250,000 g/mol is ~ 20 wt%) (Merck KGaA, Darmstadt, Germany), Collagen (Fish Collagen type 1 Shanghai Macklin Biochemical Co. Ltd, China), Silver sulfadiazine (Xi’an Shunyi Biochemical Technology Co., Ltd, China.) 1,1,1,3,3,3 hexafluoro-2-propanolol (HFIP) (Shanghai Macklin Biochemical Co. Ltd, Pudong China), Phosphate buffered saline (Quality biological, USA), Trypsin (Amresco Inc., Ohio, USA), Dulbesco Modified Eagle Medium (Merck KGaA, Darmstadt, Germany), Fetal Bovine serum (Merck KGaA, Darmstadt, Germany), Disodium hydrogen phosphate, potassium dihydrogen phosphate (Merck KGaA, Darmstadt, Germany), Dialysis membranes (white gridded 47 mm, Merck KGaA, Darmstadt, Germany), Water used in all the tests was Milli-Q water (Millipore, USA). Bacterial strains Pseudomonas aeruginosa 1546 (P. aeruginosa), Staphylococcus aureus 1706 (S. aureus) and Methicillin-resistant Staphylococcus aureus 1615 (MRSA) and Escherichia coli 1808 were obtained from wound swabs of patients with diabetic foot ulcers. All other chemical reagents and solvents were of analytical grade and were used for this research without further purification.
Plant material: Fermented rooibos A. linearis was procured from Rooibos Ltd (Clanwilliam, South Africa). Identification of the plant was carried out at Plant Bioactives Group, Post-Harvest and Agro-Processing Technologies, Agricultural Research Council (Infruitec-Nietvoorbij), Private Bag X5026, Stellenbosch 7599, South Africa and this research was carried out in accordance with local legislation and permission from the Agricultural Research Council of South Africa. The plant material (100 g) was extracted in a 1:10 plant material-to-water ratio for 30 min with hot water (93 °C) according to Muller et al. [24] with yield of 18.5%. The extracts were filtered through Whatman No. 4 filter paper, cooled and freeze-dried. Retention samples of fermented rooibos extract was coded ALI_L0091_1028B1_1604. A voucher specimen of this material has been deposited in a publicly available Agricultural Research Council of South Africa.
2.2 Electrospun PLA/C–Ag+S/AL scaffolds design and fabrication
Four different solutions of 20% w/v PLA polymer were dissolved separately in HFIP in four different containers respectively, at 25 °C. Collagen (4% w/v) was dissolved in the same solvent and each PLA solution was added to each collagen solution. Two concentrations of silver sulfadiazine powder were added directly to each PLA solution at concentrations of 0.75, and 1% w/w. The mixture was stirred vigorously at 80 °C for about 4 h until a clear and transparent solution was obtained, then 25 mg, 50 mg and 100 mg of powdered of AL was added to EF1, EF2 and EF3 and kept stirred until the new solution became very clear and homogeneous (Table 1). The final solution obtained was then allowed to cool at room temperature. The fourth solution contained only the unreinforced PLA which had the dissolved PLA only without the addition of collagen, AL and silver sulfadiazine for comparison purposes.
The morphology of the fibrous scaffolds is usually influenced by various factors ranging from viscosity, flow rate, concentration, conductivity as well as the surface tension of the polymer solution which is electrospun. 20% w/v polymer solutions of PLA were prepared. Both PLA and collagen were all dissolved using absolute dichloromethane respectively. All mixtures were stirred vigorously for about 4 h until a clear solution was obtained upon dissolution of all the components. Electro-spinning of the prepared solution as shown in Table 1 was carried out using 20 mL plastic syringes equipped with 21 G gauge needles. The loaded syringe was then placed in a syringe pump and operated at a flow rate of 0.1 mL/min. The solution was electrospun at a voltage of 25 kV onto a static collector wrapped with aluminum foil paper. The distance between the tip of the gauge needle to the collector was set at 22 cm.
The fabricated nanofibrous scaffolds were cross-linked using 20% v/v glutaraldehyde vapor for 5 h and 0.05% N-(3-dimethylaminopropyl)-N′-ethyl carbodiimide hydrochloride in order to enhance their mechanical properties using the method of Maleki et al. [25]. The scaffolds were compressed on a compression machine (Model MP-15; Technosearch Instruments, Mumbai, India) under a pressure of 10 kg, at room temperature to improve their mechanical properties, uniformity and to minimize the void contents between nanofibers. The samples were then washed with 0.1 M glycine-water, three times, periodically at intervals of 30 min to remove the excess of glutaraldehyde.
2.3 Morphological characterization
2.3.1 Scanning electron microscopy
In order to determine the surface morphology of the fabricated scaffolds, scanning electron microscopy (SEM) (Phenom ProX, by Phenom world Eindhoven, Netherlands) was used at an accelerating voltage of 15 kV. The scaffolds were cut into small pieces of 5 mm × 5 mm. The samples were sputter coated with gold for 20 s. The 5 mm by 5 mm cut samples were then mounted over the studs using a carbon tape and analyzed at 15 kV as stated above to perform the experiment. All the samples were analyzed in triplicate.
2.3.2 Determination of the porosity of the electrospun scaffolds
The porosity of the scaffolds was determined using the ‘liquid displacement method’ [26] with ethanol used as the displacement liquid. The weight of the scaffold used was noted as ‘w’. The scaffold was then immersed in 5 mL of ethanol and volume of ethanol used was noted as V1. It was allowed to stand for 5 min. After 5 min, the volume of ethanol present in the ethanol-impregnated scaffold was noted as V2. The remaining volume of ethanol after removal of the ethanol-impregnated scaffold was noted as V3. The volume was measured using a calibrated cylindrical vial (Smith Scientific glassware Edenbridge, Kent UK). The experiment was performed in triplicate and the average porosity of the scaffolds calculated using Eq. 1.
2.4 Mechanical characterization
Tensile strength was measured using a Universal Testing Machine (Instron-series 3369, USA), fitted with a 50 kN load cell. The cross-linked scaffolds were cut into sections of dimensions 50 mm x 10 mm and tested in ambient temperature of 20 °C and humidity of 60%. The rectangular sections were cut out and then tapered using a template to control the failure location. The resulting scaffolds were then mounted between two clamps and stretched at a rate of 50 mm/min with an applied load range of about 50 N and gauge length of 50 mm. The tensile strength was recorded in triplicate at room temperature and average value was calculated.
2.5 Chemical characterization
2.5.1 Differential scanning calorimetry
Analysis of any possible polymer interaction and the thermal transitions was recorded using Differential Scanning calorimetry (DSC: Mettler Toledo, USA). A fixed amount of the sample scaffold (5 mg of the scaffolds) was used during the analysis. The weighed scaffolds were taken into aluminum pans and were then hermetically sealed. An empty pan was used as reference. The scans were recorded from 37 to 450 °C, at heating rate of 10 °C/min, under nitrogen.
2.5.2 Attenuated total reflectance Fourier transform infrared spectroscopy
The spectra were recorded in the range of wave numbers between 500 and 4000 cm−1 to characterize the absorption bands of the nano fibers EF1, EF2, EF3 and EF4. Samples of nanofibrous scaffolds were dehydrated by vacuum drying (45 °C) and later placed over the diamond crystal for the FTIR analysis. Twenty scans were recorded for each spectrum. Smoothing was done where necessary to reduce the noise, without loss of any peak. The absorptions peaks/bands were studied to know if there was any chemical interaction occurring as a result of the co-formulation.
2.5.3 Measurement of Ag+ release
A modification of the method by Heo et al. [14] was utilized. The amount of the Ag+ ion released periodically from the electrospun scaffolds was detected using MS (mass Spectrometry). The electrospun scaffolds, loaded with varying concentrations (0.75 and 1 g) of silver Sulfadiazine (Ag+S), were cut into a circular shape with a diameter of 10 mm. Each prepared specimen scaffold was then put in a glass vial with 10 mL of pH 7.4 PBS (Phosphate buffered saline). These specimens were then placed in an incubator and agitated at 120 rpm at a temperature of 37 °C ± 0.5 for 5 days. 1 mL of the supernatant from each of the samples was extracted and diluted with 9 mL using a mixture of nitric acid and hydrochloric acid, optimally in a molar ratio of 1:3 for mass spectrometry analysis (ICP-MS Agilent 7900 series CA, USA).
2.6 Biological characterization
2.6.1 Antibacterial activity assessment
Typed Clinical isolates of Pseudomonas aeruginosa 1546 (P. aeruginosa), Staphylococcus aureus 1706 (S. aureus) and Methicillin-resistant Staphylococcus aureus 1615 (MRSA) and Escherichia coli 1808 obtained from wound swabs of patients managing chronic wounds were utilized for this study. In vitro antibacterial activity of the PLA/C–Ag+S/AL electrospun nano-fibrous scaffold against each bacterial strain was determined via disc diffusion test [27, 28] using 150-mm-diameter Mueller–Hinton (Carolina Biological Supply Co. Burlington, NC) agar plates. Utilizing turbidity consistent with a McFarland standard of 0.5, the agar plates were inoculated via organism spread in saline suspension. PLA/C–Ag+S/AL electrospun scaffolds were cut into 10 mm diameter circles and placed on the inoculated test organisms and then observed for any zone of inhibition around the scaffolds. An antibiotic disc of Gentamicin was placed at the center of the plates to serve as a control. The plates were incubated at 35 °C for 24 h. Clear inhibition zones around the PLA/C–Ag+S/AL electrospun nano-fibrous discs indicate that the scaffolds possess antimicrobial activity. Each assay was performed in triplicate.
2.6.2 Cytotoxicity assessment
An MTT assay was used to test for cytotoxicity property of the PLA/C–Ag+S/AL electrospun scaffolds. Cells cultured on wells without a scaffold were used as the control. After obtaining ethical clearance (Ethical Approval No. CMUL/HREC/10/18/451) and verbal parental consent, the foreskin of a 21-day old baby was obtained from the parents. The fore skin was collected after circumcision in a private hospital (it should be noted that the fore skins are usually discarded as medical waste after circumcision) and was kept in bottles containing PBS (Quality biological, USA) which contains 0.5 µg/mL amphotericin B, 100 IU/mL of gentamicin and 100 µg/mL of Streptomycin. Keratinocytes were prepared in the culture room. Utilizing double enzymatic digestion, isolation of keratinocytes was carried out [28, 29]. The cell pellets was placed in a flask and then divided into 2 groups, the first with no feeder and the second group into a type 1 collagen scaffold (Shanghai Macklin Biochemical Co. Ltd, China). The number of cells seeded on the sampled scaffolds in the 24-well plates were determined by optimizing the number of cells on the 24-Well Plate. The keratinocytes distribution and confluence were viewed using a Microscope (Omax, China) and the suitable number of cells for 5 days of culture was then determined. The cells were then prepared for MTT assay as per method by Lee et al. [30].
2.6.3 Antioxidant activity of the of the electrospun scaffolds using DPPH assay
Free radical scavenging activity of the electrospun scaffold was assessed to determine the antioxidant activity of the A. linearis incorporated into the scaffolds. The scaffolds were assayed spectrophotometrically using a modification of a method described by Ilomuanya et al. [31]. Using ascorbic acid as the standard and ethanol as a control, radical scavenging activity of the scaffolds against 1,1 diphenyl1-1-picryl-1-hydrazyl (DPPH) radical via UV–Vis absorbance at 517 nm was determined. Here, ascorbic acid was used as a standard. A commercial antioxidant (Citrix® Topix Pharmaceuticals, Inc. NY, USA) was also evaluated and compared with the fabricated scaffolds.
2.7 Statistical analysis
Statistical analysis was done using SPSS, Inc. version 11 (Chicago, IL). All the values were expressed as mean ± standard deviations, and differences with p values (*p < 0.05) were considered statistically significant.
3 Results
3.1 Morphological characterization
SEM images of the electrospun scaffolds as shown in Fig. 1a showed the unreinforced nanofiber EF4 had the highest average fiber diameter of 22.7 µm. Reinforced PLA functionalized with the ECM component collagen, silver sulphadiazine and A. linearis extract (C–Ag+S/AL) comprising EF1, EF2 and EF3 had smaller average fiber diameter 15.5, 15.4 and 12.8 µm respectively (Fig. 1b). The fiber diameters of the scaffolds are greater than the native ECM, however the pliability of the scaffolds would still enable infiltrating cells to undergo cellular attachment and facilitate wound healing. The fibers have smaller diameters than the conventional wound dressing PolyMem silver dressing®. Polylactide solution has good electrical conductivity compared to its composites. In the absence of Ag+S (EF3 and EF4), reduced conductivity is expected when compared to EF1 and EF2 which contain silver ions that increase the conductivity in solution. This conductivity engenders the stretching of the solution and produces PLA fibers of smaller diameter [32]. Low conductivity reduced the charge in the electrospinning jet with bead formation in EF 1, EF2 and EF3. Beads and junctions observed in the composites could be attributed to agglomeration and random dispersion of hydrophilic constituents.
3.2 Mechanical characterization
The mechanical behavior of the scaffolds is strongly correlated to the material composition during electrospinning. Tensile testing was performed to assess the mechanical properties of each type of electrospun nanofiber scaffolds. The ductility of the unreinforced PLA nanofiber sample EF4 was 20.5% (Fig. 2a), which was lower than the ductility of EF1 to EF3. Addition of 0.75 g Ag+S and 0.05% w/w A. linearis impaired the elastic properties of PLA nanofibers. The addition of 1 g Ag+S collagen and 0.025% w/w AL (EF 1) improved PLA’s ductility to a maximum magnitude of 39.8%, hence showing that reinforcements had improved interfacial adhesion at the matrix interface. This restricts easy glide of the filler particles over the matrix giving rise to higher ductility [33].
a Ductility, b ultimate tensile strength, c elastic modulus of electrospun PLA nanofibers containing different concentrations of Ag+S and A. linearis extract EF1, EF2 EF3 and EF4 respectively (p < 0.05; * represents statistical significant difference in varying parameters of EF4 from EF1, EF2 and EF3)
3.3 Chemical characterization
The DSC results of samples displayed in Fig. 3e–h showed endothermic peaks. The chemical structure of the fabricated PLA/C–Ag+S/AL scaffolds were measured by FTIR. The characteristics spectrum of sample EF 4 PLA unreinforced (Fig. 3d) showed peaks corresponding to C=O stretching at 1748 cm−1, C–O (ester) stretching at 1125 cm−1 and C–O–C group at 1080 cm−1. EF1 nanofiber peaks were found at 1751 cm−1, 1125 cm−1 and 1080 cm−1 (Fig. 3a) while EF2 peaks were found at 1752 cm−1, 1129 cm−1 and 1084 cm−1 (Fig. 3b) and EF3 nanofiber peaks were found at 1748 cm−1, 1181 cm−1 and 1080 cm−1 (Fig. 3c) respectively. Distinct peaks of polyphenols where observed in this spectra. The distinct peak at 3224 cm is characteristic of the stretching vibration of phenolic –OH groups. Also peak at 2922 cm is attributed to stretching vibration of CH2 and CH3. The scaffolds absorption bands were noted to be very similar without the presence of new peaks. This therefore showed that there were no chemical changes in the nanofibers as a result of co-formulation or the addition of components such as collagen, AgSD and A. linearis to the PLA polymer. These results also further prove that the components were well incorporated into the nanofiber which did not impede the release of Ag+S from the scaffolds. The total quantitative values released Ag+S obtained from the nanofiber containing 0.75 and 1% Ag+S were 58 and 141 parts per billion (ppb) respectively (Fig. 4).
Total amount of silver ions (ppb) released from PLA/C–Ag+S/AL electrospun nanofibrous scaffolds containing varying concentrations of Ag+ over a period of 5 days from the scaffolds (EF1 and EF2) loaded with varying concentration of silver (*p < 0.05) using ICP-MS. PLA/C-/AL and PLA representing EF3 and EF4 were utilized as control (ND not detected)
3.4 Biological characterization
3.4.1 Cell viability/proliferation
Cell viability/proliferation behavior on EF1, to EF4 was assessed using MTT assay (Fig. 5). Keratinocyte cells cultured on the plate without any scaffold were used as control.
The measurement of keratinocytes cell proliferation after day 1 and day 7 from EF1 [PLA/C–Ag+S (1%)/AL (25 mg)]; EF2 [PLA/C–Ag+S (0.75%)/AL (50 mg)]; EF3 [PLA/C-/AL (100 mg)]; EF4 [PLA] electrospun nanofibrous scaffolds (*p < 0.05) (β represents statistical significant difference compared to cell proliferation after 24 h)
These results indicated that the scaffolds were nontoxic to the cells. EF3 showed similar percentage of cell proliferation with control, all the scaffolds showed statistically significant difference compared to percentage cell proliferation after 24 h. EFI and EF2 had lower cell proliferation when compared to other scaffolds EF3 and EF4 that did not contain Ag+S, however percentage cell infiltration was significantly higher than control. The optimized concentration of Ag+S in EF2 adequately supported cell growth and hence was deemed nontoxic to the cells.
3.4.2 Antibacterial activity
Figure 6 shows the antibacterial activity of the electrospun scaffolds against both gram +ve and gram −ve bacteria. Silver agents are known to have broad spectrum activity against both gram negative and positive bacteria and are useful for controlling bacterial growth. The antimicrobial activity of EF1 and 2 scaffolds loaded with 1 and 0.75% Ag+S respectively; EF3 containing no Ag+S, and EF4 were evaluated against Staphylococcus aureus, Pseudomnas aeruginosa, Methicillin resistant Staphylococcus aureus and Escherichia coli. These organisms were chosen based on their clinical importance and their roles in chronic wounds [36]. EF4 did not show any zone of inhibition and thus elicited no activity against the microorganisms utilized. EF1 and EF2 showed comparable zones of inhibition to PolyMem silver dressing® and gentamicin control disc.
Antibacterial activity of EF1 [PLA/C–Ag+S (1%)/AL (25 mg)]; EF2 [PLA/C–Ag+S (0.75%)/AL (50 mg)]; EF3 [PLA/C-/AL (100 mg)]; EF4 [PLA] electrospun nanofibrous scaffolds and control discs and wound dressings showing zones of inhibition. (p < 0.05; # represents statistically significant difference compared to PolyMem silver dressing® control) n = 7
3.4.3 Antioxidant activity
The wound-healing property and antioxidant activity co-exist in many plant species from a variety of families [18] hence the incorporation of A. linearis extract in the scaffold fabrication. There was an increase in DPPH free radical scavenging with increasing scaffold concentration of A. linearis extract. EF3 scaffolds showed the highest DPPH radical scavenging activity with EF4 showing no antioxidant activity at all as a result of the absence of the A. linearis extract (Fig. 7). EF2 and EF3 exhibited antioxidant activity (64.73 and 68.9%, respectively), at 15 mg/mL, in comparison with commercially available silver dressing, PolyMem silver dressing®. It was a better antioxidant scaffold and would be useful in the wound healing cascade. The DPPH scavenging activity of the scaffolds also showed that the electrospinning activity of the polymer would not adversely affect the antioxidant activity of the scaffold when utilized as a wound dressing material.
4 Discussion
Electrospun scaffolds utilized in bioengineering have been fabricated with various polymers to ensure their biomimetic composition which facilitates cell adhesion and optimal recovery from cellular injury. Electro spinning utilizing only polymers and pharmaco-therapeutic agents have been seen to achieve positive results, however functionalization of these polymers via addition of components present in the extracellular matrix and plant antioxidant materials to enhance the wound healing properties of this polymers will increase the intrinsic value of the biomaterial. To this end we have developed PLA/C–Ag+S/AL electrospun scaffolds containing collagen (an ECM component), A. linearis extract (an antioxidant) and Silver sulfadiazine (an antibacterial) for management of wounds. The ultimate tensile strength (UTS) of electrospun nanofibres, expresses the maximum strength that can be withstood prior to fracture under tension. The good strength exhibited by composites could be attributed to improved crystallinity, their good dispersion and adhesion to the PLA fibre (Fig. 2b). The poor strength exhibited by unreinforced PLA could be attributed to poor interfacial bonding caused by the presence of non-cellulose and impurity [33]. The Elastic modulus of scaffolds revealed that incorporating 0.75 g Ag+S and 0.05% w/w A. linearis (EF2) to PLA solution culminated in a nanofiber with the superlative stiffness of 2.11 GPa, highest compared to all fabricated nanofibers (Fig. 2c). This stiffness allows ease of utilization as a wound dressing application.
The addition of collagen, Ag+S and A. linearis extract to PLA solution prior to electrospinning elevated the hydrophilic nature of PLA which in turn improved its biocompatibility features. The thermogram behaviour of EF 1 and 3 were similar but the intensity of peaks were different (wider in EF 1). The first thermal event showed peaks at 111 °C, 89 °C and 112 °C (Fig. 3e–h): this could be attributed to dehydration (loss of water). Polylactide is a highly hydrophobic polymer in solid state [35] and in order to improve its biocompatibility, its structure needs to be modified to enhance its hydrophilic nature. Their associated enthalpies were calculated to give 81.8 J/g, 25.8 J/g and 165.3 J/g for EF 1, 2 and 3 (Fig. 3e–g), indicating obvious differences in composites’ macromolecules in relation to their water holding capacity and strength of water–polymer interaction. The hydrophobic nature of PLA could be responsible for the absence of water loss region in the thermo gram of PLA electrospun nano fibre (Fig. 3h). Keratinocytes grew and proliferated excellently on EF1, 2 and 3 scaffolds. All the scaffolds provided favorable substrates for the keratinocytes. The fabricated scaffolds play a role like that of extracellular matrix simulated by providing a high surface area to volume ratio critical for cell attachment and proliferation. Biochemical interaction between cells and collagen resulted from the binding of collagen I to receptors on the cell membrane, mediated by fibronectin, an ECM glycoprotein [30]. The enhanced interaction between the keratinocytes and the scaffolds thus would invariably facilitate wound healing via fibronectin mediation especially in situations where the healing process had been impaired. The electrospinning process was not affected by the incorporation of the Ag+S into the scaffolds. Since Ag+S was well permeated into the electrospun scaffolds, it had no impact on the electrical charge during electrospinning. The release of silver ions from the scaffolds is an important parameter which ensures elicitation of antibacterial activity. The amount of silver released is proportional to its antimicrobial efficacy hence nanofiber with 1% Ag+S had a larger zone of inhibition than others during the antimicrobial study.
The presence of silver ions however led to a decrease in percentage of cell proliferation, which was not statistically significant (Fig. 5). Stereo regularities have a great impact on the physical and chemical properties of PLA such as its mechanical and thermal properties [34].
To evaluate if the loaded scaffolds had the expected antibacterial activity, gentamicin impregnated antibiotic disc was used as control and placed at the center of the agar plates. No zones of inhibition were observed around the PLA only nanofiber. This confirmed that PLA had no antibacterial activity. Zones of inhibition were however seen around the antibiotic disc and around EF1 and EF2 due to the incorporation of silver sulfadiazine, this activity was comparable with the control PolyMem silver dressing®. EF3 showed activity as a result of the incorporation of A. linearis but not comparable with that of the control disc and PolyMem silver dressing®. EF 1 nanofibrous scaffolds had the higher zone of inhibition compared to EF 2 and this was further confirmed from the mass spectrometry Ag+ ion release studies where the amount of silver ion released form sample was far above that released from EF 2 and this also matched previous research by Heo et al. [14]. EF1 scaffolds showed larger inhibition against the microorganisms than EF 2. From previous reports, Ag+ has well known activity against bacteria [14], though the mechanisms of action at which Ag+ works is unclear but it is postulated that it works by damage to the intracellular protein of the organism, hence causing killing the bacteria [10]. It has also been postulated that Ag+ penetrates the bacterial cell body and denatures its biological components (DNA) leading to cell death and prevents the growth of bacteria [10, 14]. Previous research has also confirmed that silver sulfadiazine has been clinically useful in the biomedical field and has higher activity against methicillin resistant S. aureus. Hence, larger zones of inhibition were observed around the scaffolds on the MRSA than others and this is very similar to a report by Heo et al. [14]. Overall, it can be inferred that EF1 and 2 have good antibacterial activity against all the organisms. A. linearis (Rooibos tea) has been reported to have an antibacterial activity and this was evident from the present study as zones of inhibition were noticed around EF 3 which contained no Ag+S. However, the zone of inhibition noted was lower than that of EF 1 and EF 2 which had larger zones of inhibition. In the present study, however it can be confirmed that addition of AgSD to A. linearis will help improve its antibacterial activity as evident from the antimicrobial study carried out making these scaffolds a good potential for application in wound healing.
5 Conclusion
PLA/C–Ag+S/AL electrospun scaffolds containing collagen (an ECM component) and A. linearis extract which was incorporated into a PLA scaffold containing Ag+S a known antibacterial has been developed for wound healing applications. The antimicrobial activity of the scaffolds loaded were evaluated against S. aureus, P. aeruginosa, MRSA and E. coli. PLA/C–Ag+S/AL scaffolds showed antibacterial activity against both gram +ve and gram −ve bacteria, they were nontoxic to the cells, and provided favorable substrates for the neonatal epidermal keratinocytes cells to undergo cell attachment and proliferation. PLA/C–Ag+/AL scaffolds have a great potential for use in chronic wounds as well as in tissue and bio-engineering applications.
Abbreviations
- PLA:
-
Poly lactic acid
- PLA/C:
-
Poly lactic acid/collagen-based
- Ag+S:
-
Silver sulphadiazine
- AL:
-
Aspalathus linearis fermented extract
- PLA/C–Ag+S/AL:
-
Electrospun nano-fibrous scaffolds containing collagen, silver sulfadiazine and A. linearis fermented extract
- MRSA:
-
Methicillin-resistant staphylococcus aureus
- SEM:
-
Scanning electron microscopy
- DSC:
-
Differential scanning calorimetry
- PBS:
-
Phosphate buffered saline
References
Frykberg RG, Banks J (2015) Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 4(9):560–582. https://doi.org/10.1089/wound.2015.0635
Dhall S, Do DC, Garcia M, Kim J, Mirebrahim SH, Lyubovitsky J, Lonardi S, Nothnagel EA, Schiller N, Martins-Green M (2014) Generating and reversing chronic wounds in diabetic mice by manipulating wound redox parameters. J Diabetes Res 2014:562625. https://doi.org/10.1155/2014/562625
Shankaran V, Brooks M, Mostow E (2013) Advanced therapies for chronic wounds: NPWT, engineered skin, growth factors, extracellular matrices. Dermatol Ther 26:215–221. https://doi.org/10.1111/dth.12050
Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K (2006) Clinical approach to wounds: debridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg 117:72S–109S. https://doi.org/10.1097/01.prs.0000225470.42514.8f
Falanga V, Brem H, Ennis WJ, Wolcott R, Gould LJ, Ayello EA (2008) Maintenance debridement in the treatment of difficult-to-heal chronic wounds. Recommendations of an expert panel. Ostomy Wound Manag. https://doi.org/10.1001/archderm.134.3.293
Brett D (2008) A review of collagen and collagen-based wound dressings. Wounds 20(12):13–29
Phillips CJ, Humphreys I, Fletcher J, Harding K, Chamberlain G, Macey S (2015) Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int Wound J 13(6):1193–1197. https://doi.org/10.1111/iwj.12443
Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, Car J (2017) The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev 6(1):15. https://doi.org/10.1186/s13643-016-0400-8
Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL (2007) Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 27:3115–3124. https://doi.org/10.1016/j.biomaterials.2006.01.022
Laura C, Milena S, Giovanna B, Cristina BM, Giuseppina S, Giampiero B (2013) Characterization of silver sulfadiazine-loaded solid lipid nanoparticles by thermal analysis. J Therm Anal Calorim 111:2149–2155. https://doi.org/10.1007/s10973-012-2709-4
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83. https://doi.org/10.1016/j.biotechadv.2008.09.002
Ito K, Saito A, Fujie T, Nishiwaki K, Miyazaki H, Kinoshita M, Saitoh D, Ohtsubo S, Takeoka S (2015) Sustainable antimicrobial effect of silver sulfadiazine-loaded nanosheets on infection in a mouse model of partial-thickness burn injury. Acta Biomater 24:87–95. https://doi.org/10.1016/j.actbio.2015.05.035
Heo DN, Yang DH, Lee JB, Bae MS, Kim JH, Moon SH, Chun HJ, Kim CH, Lim HN, Kwon IK (2013) Burn-wound healing effect of gelatin/polyurethane nanofiber scaffold. J Biomed Nanotechnol 9:511–515. https://doi.org/10.1166/jbn.2013.1509
Heo M, Lee SJ, Heo DN, Lee D, Lim H, Moon J, Kwon K (2018) Multilayered co-electrospun scaffold containing silver sulfadiazine as a prophylactic against osteomyelitis: characterization and biological in vitro evaluations. Appl Surf Sci 432(B):308–316. https://doi.org/10.1016/j.apsusc.2017.04.147
Dias DA, Urban S, Roessner U (2012) A historical overview of natural products in drug discovery. Metabolites 2:303–336. https://doi.org/10.3390/metabo2020303
Hendricks R, Pool EJ (2010) The in vitro effects of rooibos and black tea on immune pathways. J Immunoassay Immunochem 31:169–180. https://doi.org/10.1080/15321811003617537
Lee W, Bae SP (2015) Anti-inflammatory effects of aspalathin and nothofagin from rooibos (Aspalathus linearis) in vitro and in vivo. Inflammation. https://doi.org/10.1007/s10753-015-0125-1
Pringle NA, Koekemoer TC, Holzer A, Young C, Venables L, van de Venter M (2018) Potential therapeutic benefits of green and fermented rooibos (Aspalathus linearis) in dermal wound healing planta medica. Planta Med 84:645–652. https://doi.org/10.1055/a-0578-8827
Shah JB (2011) The history of wound care. J Am Coll Certif Wound Spec 3(3):65–66. https://doi.org/10.1016/j.jcws.2012.04.002
Benskin LL (2012) PolyMem® Wic® Silver® rope: a multifunctional dressing for decreasing pain, swelling, and inflammation. Adv Wound Care 1(1):44–47. https://doi.org/10.1089/wound.2011.0285
Sadri M, Arab-Sorkhi S, Vatani H, Bagheri-Pebdeni A (2015) New wound dressing polymeric nanofiber containing green tea extract prepared by electrospinning method. Fibers Polym 16:1742. https://doi.org/10.1007/s12221-015-5297-7
Thomas Hess C (2011) Checklist for factors affecting wound healing. Adv Skin Wound Care 24(4):192. https://doi.org/10.1097/01.ASW.0000396300.04173.ec
Ulery BD, Nair LS, Laurencin CT (2011) Biomedical applications of biodegradable polymers. J Polym Sci Part B Polym Phys 49(12):832–864. https://doi.org/10.1002/polb.22259
Muller CJF, Malherbe CJ, Chellan N, Yagasaki K, Miura Y, Joubert E (2018) Potential of rooibos, its major C-glucosyl flavonoids, and Z-2-(β-d-glucopyranosyloxy)-3-phenylpropenoic acid in prevention of metabolic syndrome. Crit Rev Food Sci Nutr 58:227–246. https://doi.org/10.1080/10408398.2016.1157568
Maleki M, Latifi M, Amani-Tehran M, Mathur S (2013) Electrospun core–shell nanofibers for drug encapsulation and sustained release. Polym Eng Sci 53(8):1770–1779. https://doi.org/10.1002/pen.23426
Lee Y, Jeong J, Youn I, Lee WH (1997) Modified liquid displacement method for determination of pore size distribution in porous membranes. J Membr Sci 130(1–2):149–156. https://doi.org/10.1016/S0376-7388(97)00017-3
Valaperta R, Tejada MR, Frigerio M, Moroni A, Ciulla E, Cioffi SP, Costa E (2010) Staphylococcus aureus nosocomial infections: the role of a rapid and low-cost characterization for the establishment of a surveillance system. New Microbiol 33:223–232
Weinstein MP (2018) M100 performance standards for antimicrobial susceptibility testing, 29th edn. 127-133 ISBN: 978-1-68440-032-4
Chaudhari AA, Vig K, Baganizi DR, Sahu R, Dixit S, Dennis V, Singh SR, Pillai SR (2016) Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review. Int J Mol Sci 17(12):1974. https://doi.org/10.3390/ijms17121974
Lee JS, Lee SU, Che CY, Lee JE (2015) Comparison of cytotoxicity and wound healing effect of carboxymethylcellulose and hyaluronic acid on human corneal epithelial cells. Int J Ophthalmol 8(2):215–221. https://doi.org/10.3980/j.issn.2222-3959.2015.02.01
Ilomuanya MO, Akhimien T, Aghaizu C, Adeyinka O, Ajayi T (2018) Polyherbal antioxidant topical preparation comprising ethanol extract of Tetracarpidium conophorum and Ocimum gratissimum: formulation and evaluation. Dhaka Univ J Pharm Sci 17(2):213–219. https://doi.org/10.3329/dujps.v17i2.39178
Zong X, Kim K, Fang D, Ran S, Hsiao BS, Chu B (2002) Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 43:4403. https://doi.org/10.1016/S0032-3861(02)00275-6
Adeosun SO, Taiwo O, Akpan EI, Gbenebor OP, Gbagba S, Olaleye S (2016) Mechanical characteristics of groundnut shell particle reinforced polylactide nano fibre. Rev Mater 21(2):482–491. https://doi.org/10.1590/S1517-707620160002.0045
Huda MS, Drzal LT, Mohanty AK, Misra M (2008) Effect of fiber surface-treatments on the properties of laminated biocomposites from poly(lactic acid) (PLA) and kenaf fiber. Compos Sci Technol 68:424–432. https://doi.org/10.1016/j.compscitech.2007.06.022
Hendrick E, Frey M (2014) Increasing surface hydrophilicity in poly(lactic acid) electrospun fibers by addition of Pla-b-Peg Co-polymers. J Eng Fibers Fabr 6(2):153–164. https://doi.org/10.1177/155892501400900219
Kostenko V, Lyczak J, Turner K, Martinuzzi RJ (2010) Impact of silver-containing wound dressings on bacterial biofilm viability and susceptibility to antibiotics during prolonged treatment. Antimicrob Agents Chemother 54(12):5120–5131. https://doi.org/10.1128/AAC.00825-10
Acknowledgements
The content is solely the responsibility of the authors.
Author information
Authors and Affiliations
Contributions
MOI conceived the study. MOI, BOS and AS helped design and coordinate the study, MOI, ACA, SOA, CLE and WW carried out the experimental studies and drafted the manuscript. AS, EJ and DB provided information on the plant extract utilized in the study. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Availability of data and materials
Data is contained in the manuscript.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval was obtained from the Health Research and Ethics committee of the College of Medicine University of Lagos. CMUL/HREC No. CMUL/HREC/10/18/451. Verbal parental consent for the foreskin of a 21-day old baby was obtained verbally from the parents. The fore skin was collected after circumcision in a private hospital (it should be noted that the fore skins are usually discarded as medical waste after circumcision).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ilomuanya, M.O., Adebona, A.C., Wang, W. et al. Development and characterization of collagen-based electrospun scaffolds containing silver sulphadiazine and Aspalathus linearis extract for potential wound healing applications. SN Appl. Sci. 2, 881 (2020). https://doi.org/10.1007/s42452-020-2701-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2701-8