Abstract
A one-step procedure has been developed for the synthesis of new Hantzsch 1,4-dihydropyridines (1,4-DHPs) with high yields from four-component reaction of 4-hydroxybenzaldehyde, acetylacetone, various primary amine and barbituric acid in EtOH in the presence of 3-methyl-1-sulfonic acid imidazolium chloride {[Msim]Cl} as an acidic ionic liquid. In this work {[Msim]Cl} acts as a more efficient and green catalyst in the multicomponent reaction, leading to a simple procedure of synthesis, short reaction times, less pollution, high yields of the products, low cost of chemicals and uses less toxic solvents.
Similar content being viewed by others
1 Introduction
1,4-Dihydropyridines (1,4-DHPs) are one of N-heterocyclic, biologically active compounds and naturally occurring molecules [1, 2]. 1,4-DHPs are also considered as key starting materials to synthesize various classes of biologically and pharmacologically active compounds [3, 4]. They are used as calcium channel blockers [5], anti-inflammatory [6, 7], antiviral [8], antitumor [9], anticancer [10], analgesic activities [11]. In additions, 1,4-DHP derivatives are employed as heptatoprotective and antidiabetic agents for the treatment of cardiovascular diseases such as hypertension [12,13,14].
Some methods for the synthesis of 1,4-DHP derivatives have been reported in the literature. Nevertheless, these methods generally call for harsh reaction conditions, high temperature, using of expensive reagents, create wastes, long reaction time, needing strongly acidic condition, using multi-steps, need a complex synthetic route and no agreement with the green chemistry strategies [15], Therefore, facile and highly efficient synthetic methods to 1,4-DHP derivatives are highly desirable [16].
One method to address this challenge includes employing multicomponent reactions (MCRs). MCRs strategy offer a significant importance over linear-strategy synthesis due to their flexible, convergent, atomic efficient nature and provide a maximum structural complexity with a minimum number of synthetic steps [17]. Reportedly, in MCRs a wide range of catalysts has been explored in the synthesis of 1,4-DHP derivatives including l-proline [18, 19], aspartic acid [20], p-toluenesulfonic acid monohydrate [21,22,23], TiO2 nano wires [24], phosphootungstic acid (H3PW12O40) [25], silica-coated nano-Fe3O4 [26], cellulose sulfuric acid [27], chitosan supported copper(II)sulfate (CSCS) [28], porcine pancreatic lipase [29], Magnetic dextrin nanobiomaterial [30], SO3H‐functionalized nano‐MGO‐D‐NH2 [31], guanidinylated chitosan magnetic nano catalyst [32], and pyrimidine-2,4-diamine (PDA)-functionalized silica-coated magnetic nanocatalyst (Fe3O4/SiO2-PDA) [33]. Therefore, the discovery of new and efficient catalysts in combination with an operationally simple work up and eco-friendly conditions is highly desirable [15].
Ionic liquids have fascinated a considerable attention in the past few decades due to they have unique properties including an inexpensive, easy to prepare, environmental friendly, non-toxic catalyst for various organic synthesis, high thermal, non-hazardous, non-volatility, a simple and rapid to handle, economically viable, non-flammability, recyclability, provide a high selectivity, and can be used to dissolve a wide range of materials [34,35,36,37]. In addition, ionic liquids are used as catalyst and media in multicomponent reactions such as triethylamine-bonded sulfonic acid {[Et3N–SO3H]Cl} [38], [Bmim]PF6 [39], and sulfamic acid (H2NSO3H) [40].
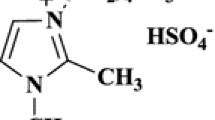
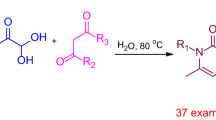
As a part of our interest with green chemistry, herein we aim to expand the application of ionic liquids in multicomponent reactions. Therefore, 3-methyl-1-sulfonic acid imidazolium chloride {[Msim]Cl}3 was synthesized as Bronsted acidic ionic liquid (Scheme 1) and employed as an efficient catalyst for the synthesis of new 1,4-DHP derivatives 8a–8i (Scheme 2).
2 Results and discussion
The optimized conditions of a model reaction were carried out from the reaction of 4-hydroxybenzaldehyde 4 (2.0 mmol), acetylacetone 5 (2.0 mmol), 4-aminomorpholine 6a (2.0 mmol) and barbituric acid 7 (2.0 mmol) in EtOH (20 mL) in the presence of {[Msim]Cl} 3 as a catalyst at room temperature. In these optimized conditions, EtOH was found to be an excellent solvent for this reaction (Table 1, entry 5).
To evaluate the efficiency of {[Msim]Cl} 3 in comparison with the reported catalysts, the model reaction was screened in some of these catalysts including l-proline, DABCO, copper, DAHP and ZnCl2. Interestingly, as shown in Table 2, an efficient activity of catalysis {[Msim]Cl} 3 was observed and the desired product was collected in higher yield with a shorter reaction time.

The effect of catalytic concentration was investigated on the model reaction in EtOH. Initially, at the catalytic concentration of {[Msim]Cl} 3 (5 mol%), the reaction was carried out under the same reaction time and afforded product 8a 76% in yield (Table 2, entry 7). By increasing the amount of catalysis {[Msim]Cl} 3 from 5 to 10 mol%, the highest yield of the corresponding product 8a was obtained (Table 2, entry 8). Increasing the catalyst loading to 15 mol%, no a significant yield was obtained at this concentration (Table 2, entry 9). As a result, 10 mol% of catalyst {[Msim]Cl} 3 was chosen in EtOH as the most appropriate conditions for the one-pot synthesis of novel 1,4-DHP derivatives 8a–8i at room temperature. To evaluate the significance of {[Msim]Cl} 3 as catalyst, the model reaction was tested in the absence of {[Msim]Cl} 3 and found that no yield of corresponding product 8a was obtained after 24 h (Table 2, entry 1).
The 1H and 13C NMR, FT-IR and Mass spectra data of all synthesized compounds are consistent with the expected structures. The 1H NMR spectra of 1,4-DHP derivatives 8a–8i show a broad singlet at the lowest field for NH groups, two singles at 1.82–2.14 ppm for two CH3 groups and a one singlet at 4.95–5.24 ppm for fused pyridine proton (CH group) at the expected region. In the 13C NMR spectra of all synthesized compounds, the appearance of signals at the lowest field is due to carbon resonance of the C=O group. The other chemical shifts of 13C NMR spectra were displayed in the expected regions. Also, the appearance of absorption band at 3437–3109 cm−1 in the FT-IR spectra of synthetic compounds (8a–8i), the characteristic of the NH groups is a good evidence in support of the expected compounds. The all synthesized 1,4-DHP derivatives 8a–8i are illustrated in Table 3.
The plausible mechanism of the reaction in the presence of catalyst {[Msim]Cl} 3 is shown in Scheme 3. Initially, a nucleophilic addition of barbituric acid A to activated 4-hydroxybenzaldehyde B in the presence of catalyst {[Msim]Cl} 3 led to intermediate C which becomes involved in the addition reaction with acetylacetone D and a subsequent addition of primary amine to adduct E led to intermediate F by via losing water molecule. Ultimately, a further intramolecular cyclization of intermediate F led to desired compounds.
3 Experimental
All solvents and chemicals were purchased from Sigma-Aldrich. All reactions were carried out under an atmosphere of air. Melting points were recorded using a Gallenkamp melting point apparatus in capillary tubes. 1H and 13C NMR spectra were recorded on a Bruker AV-400 (1H: 400 MHz) and AV-400 (13C: 100.5 Hz) spectrometer (Sheffield, UK) at room temperature in deuterated dimethyl sulfoxide and chloroform as solvent (1H NMR: DMSO-d6: δ 2.50 ppm; 13C NMR: DMSO-d6: δ 39.52 ppm, 1H NMR: CDCl3: δ 7.26 ppm; 13C NMR: δ 77.16 ppm). For 1H NMR, the completed protons of decoupling J are evaluated by Hz unite. Fourier Transform infrared (FT-IR) spectra were recorded on a Perkin Elmer Paragon 100 FT-IR spectrophotometer (Sheffield, UK), the recording absorbance were taken between 4000–750 cm−1. High-resolution mass spectrum (HRMS) was performed by using a Micro Mass LCT operating in Electrospray mode (ES) (Sheffield, UK). All synthesized compounds were purified by flash chromatography with silica gel 60 Å (230–400 mesh). Analytical properties of TLC were performed by using plates precoated with silica gel 60 UV 255 (Merck). UV light and an alkaline aqueous solution of potassium permanganate (KMnO4) were utilized to visualize all synthesized compounds.
Synthesis of ionic liquid {[Msim]Cl} ( 3 )
To a solution of 1-methylimidazole (1.64 g, 20 mmol) dissolved in dry DCM (100 mL) in a round-bottomed flask (200 mL), chlorosulfonic acid (2.42 g, 20.8 mmol) was added. The reaction mixture was stirred for 30 min, halted for 10 min and the DCM was decanted. The resulting mixture was washed with (3 × 100 mL) DCM and dried under vacuum to afford 1.5 g (90%) {[Msim]Cl} 3 as a viscous colorless oil; 1H NMR (400 MHz, DMSO-d6, δ, ppm): 3.87 (s, 3H, CH3), 7.67 (d, J = 2.36 Hz, 1H, CH), 7.70 (d, J = 2.5 Hz, 1H, CH), 9.06 (s, 1H, CH), 14.22 (s, 1H, SO3H). 13C NMR (100 MHz, DMSO-d6, δ, ppm): 35.6 (CH3), 120.8 (CH), 123.5 (CH), 137.5 (CH).
General method for synthesis of 1,4-DHP derivatives 8a ( – 8i )
In a round-bottom flask (50 mL), 4-hydroxybenzaldehyde 4 (0.24 g, 2 mmol), acetylacetone 5 (0.2 g, 2 mmol), various primary amine 6a–6i (2 mmol) and barbituric acid 7 (0.24 g, 2 mmol) in EtOH (20 mL) were mixed and stirred at room temperature. Ionic liquid {[Msim]Cl} 3 (0.04 g, 10 mol%) was added and the mixture was stirred at room temperature for an appropriate time 2 h. After the completion of the reaction (indicated by TLC, petroleum ether: ethyl acetate: 6:4), the resulting mixture was diluted with water (20 mL) and extracted with ethyl acetate (3 × 20 mL). The combined organic layers were dried over anhydrous MgSO4, filtered and concentrated. The cured products were purified by flash chromatography to afford the titled barbituric acid derivatives.
6-Acetyl-5-(4-hydroxyphenyl)-7-methyl-8-morpholino-5,8-dihydropyrido [2,3-d pyrimidine-2,4(1H, 3H)-dione ( 8a )
Follow general method, using 4-hydroxybenzaldehyde 4 (0.24 g, 2.0 mmol), acetylacetone 5 (0.2 g, 2.0 mmol), 4-aminomorpholine 6a (0.2 g, 2.0 mmol) and barbituric acid 7 (0.24 g, 2.0 mmol) for 2 h. The crude compound was purified by flash chromatography (petroleum ether: ethyl acetate: 6: 4, Rf = 0.3) to give 0.18 g (90%) 8a as a yellow solid; m.p 203–204 °C; 1H NMR (400 MHz, CDCl3, δ, ppm): 12.31 (s, 1H, NH), 7.85 (d, J = 8.8 Hz, 2H, Ar–H), 7.00 (d, J = 8.9 Hz, 2H, Ar–H), 5.13 (s, 1H, CH), 3.83–3.79 (m, 4H, 2CH2), 3.12–3.01 (m, 4H, 2CH2), 2.06 (s, 3H, CH3), 1.90 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, δ, ppm): 17.2 (CH3), 25.0 (CH3), 28.3 (CH), 49.2 (CH2), 58.8 (CH2), 100 (C, Ar), 114.6 (C, Ar), 124.6 (CH, Ar), 130.9 (CH, Ar), 149.3 (C=O), 160.7 (C=O), 195.2 (C=O). FT-IR (KBr, cm−1): 3437 (N–H), 3037 (C–H, Ar), 2965 (C–H), 2856 (C–H), 1609 (C=O), 1568 (C=C, Ar). HR-MS (ESI+): m/z Cald for C20H22N4O5 ([M + H+]) 398.1600, found 398.1594.
6-Acetyl-8-(cyclohexylmethyl)-5-(4-hydroxyphenyl)-7-methyl-5,8-dihydro pyrido [2,3-d] pyrimidine-2,4(1H,3H)-dione ( 8b )
Follow general method, using 4-hydroxybenzaldehyde 4 (0.24 g, 2.0 mmol), acetylacetone 5 (0.2 g, 2.0 mmol), cyclohexanemethylamine 6b (0.22 g, 2.0 mmol) and barbituric acid 7 (0.24 g, 2.0 mmol) for 2 h. The crude compound was purified by flash chromatography (petroleum ether: ethyl acetate: 6: 4, Rf = 0.4) to give 0.16 g (85%) 8b as a white solid; m.p 197–198 °C; 1H NMR (400 MHz, CDCl3, δ, ppm): 11.02 (s, 1H, NH), 7.77 (d, J = 8.9 Hz, 2H, Ar–H), 7.01 (d, J = 8.5 Hz, 2H, Ar–H), 4.99 (s, 1H, CH), 3.11 (d, J = 6.5 Hz, 2H, CH2), 2.04 (s, 3H, CH3), 1.95 (s, 3H, CH3), 1.81–1.69 (m, 4H, CH2), 1.27–1.16 (m, 4H, CH2), 1.03–0.95 (m, 2H, CH2). 13C NMR (100 MHz, CDCl3, δ, ppm): 21.0 (CH3), 27.2 (CH2), 27.3 (CH3), 29.2 (CH2), 30.8 (CH2), 40.2 (CH), 49.4 (CH2), 95.6 (C), 117.9 (C, Ar), 126.8 (CH, Ar), 131.4 (CH, Ar), 163.7 (C=O), 193.3 (C=O). FT-IR (KBr, cm−1): 3231 (N–H), 3010 (C–H, Ar), 2928 (C–H), 2856 (C–H), 1683 (C=O), 1546 (C=C, Ar). HR-MS (ESI+): m/z Cald for C23H27N3O4 ([M + H+]) 410.1456, found 410.1452.
6-Acetyl-8-butyl-5-(4-hydroxyphenyl)-7-methyl-5,8-dihydro pyrido [2,3-d] pyrimidine-2,4(1H,3H)-dione ( 8c )
Follow general method, using 4-hydroxybenzaldehyde 4 (0.24 g, 2.0 mmol), acetylacetone 5 (0.2 g, 2.0 mmol), butylamine 6c (0.14 g, 2.0 mmol) and barbituric acid 7 (0.24 g, 2.0 mmol) for 2 h. The crude compound was purified by flash chromatography (petroleum ether: ethyl acetate: 6: 4, Rf = 0.5) to give 0.13 g (93%) 8c as a white solid; m.p 180–181 °C; 1H NMR (400 MHz, CDCl3, δ, ppm): 10.92 (s, 1H, NH), 7.80 (d, J = 3.7 Hz, 2H, Ar–H); 7.02 (d, J = 8.6 Hz, 2H, Ar–H), 5.02 (s, 1H, CH), 3.29 (m, 2H, CH2), 2.06 (s, 3H, CH3), 1.91 (s, 3H, CH3), 1.61–1.56 (m, 2H, CH2), 1.45–14.3 (m, 2H, CH2), 0.94 (t, J = 7.3 Hz, 3H, CH3). 13C NMR (100 MHz, CDCl3, δ, ppm): 13.7 (CH3), 17.2 (CH3), 19.9 (CH3), 28.1 (CH2), 31.9 (CH), 42.9 (CH2), 58.8 (C), 95.3 (C, Ar), 116.2 (CH, Ar), 128.8 (CH, Ar), 132.4 (C), 163.7 (C, Ar), 164.8 (C=O), 191.1 (C=O), 194.4 (C=O). FT-IR (KBr, cm−1): 3242 (N–H), 2862 (C–H, Ar), 2931 (C–H), 2875 (C–H), 1679 (C=O), 1592 (C=C, Ar). HR-MS (ESI+): m/z Cald for C20H23N3O4 ([M + H+]) 370.1350, found 370.1345.
6-Acetyl-8-cyclopropyl-5-(4-hydroxyphenyl)-7-methyl-5,8-dihydropyrido [2,3-d] pyrimidine-2,4(1H, 3H)-dione ( 8d )
Follow general method, using 4-hydroxybenzaldehyde 4 (0.24 g, 2.0 mmol), acetylacetone 5 (0.2 g, 2.0 mmol), cyclopropylamine 6d (0.1 g, 2.0 mmol) and barbituric acid 7 (0.24 g, 2.0 mmol) for 2 h. The crude compound was purified by flash chromatography (petroleum ether: ethyl acetate: 6: 4, Rf = 0.5) to give 0.1 g (90%) 8d as a yellow solid; m.p 177–178 °C; 1H NMR (400 MHz, CDCl3, δ, ppm): 10.71 (s, 1H, NH), 7.81 (d, J = 8.7 Hz, 2H, Ar–H), 7.08 (d, J = 4.3 Hz, 2H, Ar–H), 5.06 (s, 1H, CH), 2.67–2.65 (m, 1H, CH), 2.13 (s, 3H, CH3), 2.05 (s, 3H, CH3), 0.85 (dd, J = 6.7, 2.2 Hz, 2H, CH2), 0.67 (dd, J = 5.3, 1.6 Hz, 2H, CH2). 13C NMR (100 MHz, CDCl3, δ, ppm): 11.2 (CH3), 15.1 (CH2), 20.3 (CH3), 25.9 (CH2), 30.6 (CH), 40.4 (CH), 93.6 (CH, Ar), 112.8 (CH, Ar), 122.3 (C), 131.2 (C, Ar), 163.7 (C=O), 194.3 (C=O). FT-IR (KBr, cm−1): 3090 (N–H), 3008 (C–H, Ar), 2921 (C–H), 2813 (C–H), 1683 (C=O), 1604 (C=C, Ar). HR-MS (ESI+): m/z Cald for C19H19N3O4 ([M + H+]) 354.1789, found 354.1785.
8-(4-(1H-Pyrrol-1-yl)phenyl)-6-acetyl-5-(4-hydrox yphenyl)-7-methyl-5,8-dihy dropyrido [2,3-d] pyrimidine-2,4 (1H,3H)-dione ( 8e )
Follow general method, using 4-hydroxybenzaldehyde 4 (0.24 g, 2.0 mmol), acetylacetone 5 (0.2 g, 2.0 mmol), 4-pyrrol-1-yl-aniline 6a (0.3 g, 2.0 mmol) and barbituric acid 7 (0.24 g, 2.0 mmol) for 2 h. The crude compound was purified by flash chromatography (petroleum ether: ethyl acetate: 6: 4, Rf = 0.3) to give 0.16 g (80%) 8e as a yellow solid; m.p 256–257 °C; 1H NMR (400 MHz, CDCl3, δ, ppm): 12.50 (s, 1H, NH), 7.38 (d, J = 8.7 Hz, 1H, Ar–H), 7.19 (t, J = 8.4 Hz, 2H, Ar–H), 7.08 (t, J = 8.4 Hz, 2H, Ar–H), 6.99 (t, J = 2.5 Hz, 1H, CH-pyrrole), 6.74 (dd, J = 6.5, 2.4 Hz, 1H, CH-pyrrole), 6.38 (t, J = 2.4 Hz, 1H, CH-pyrrole), 5.24 (s, 1H, CH), 2.14 (s, 3H, CH3), 2.03 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, δ, ppm): 20.5 (CH3), 30.3 (CH3), 41.5 (CH), 97.9 (CH, pyrrole), 109.4 (CH, pyrrole), 110.3 (C, Ar), 115.2 (C, Ar), 118.4 (CH, Ar), 122.5 (CH, Ar), 123.6 (CH, Ar), 125.9 (C, Ar), 149.5 (C=O), 160.8 (C=O), 197.6 (C=O). FT-IR (KBr, cm−1): 3242 (N–H), 3066 (C–H, Ar), 2928 (C–H), 2866 (C–H), 1601 (C=O), 1570 (C=C, Ar). HR-MS (ESI+): m/z Cald for C26H22N4O4 ([M + H+]) 455.3222, found 455.3219.
6-Acetyl-5-(4-hydroxyphenyl)-7-methyl-8-(4-(2-methylthiazol-4-yl)phenyl)-5,8-dihydro pyrido[2,3-d] pyrimidine-2,4 (1H,3H)-dione ( 8f )
Follow general method, using 4-hydroxybenzaldehyde 4 (0.24 g, 2.0 mmol), acetylacetone 5 (0.2 g, 2.0 mmol), 4-(2-methyl-1,3-thiazol-4-yl) aniline 6f (0.38 g, 2.0 mmol) and barbituric acid 7 (0.24 g, 2.0 mmol) for 2 h. The crude compound was purified by flash chromatography (petroleum ether: ethyl acetate: 6: 4, Rf = 0.2) to give 0.16 g (82%) 8f as an orange solid; m.p 267–268 °C; 1H NMR (400 MHz, CDCl3, δ, ppm): 12.55 (s, 1H, NH), 7.86 (d, J = 8.5 Hz, 2H, Ar–H), 7.69 (d, J = 8.5 Hz, 2H, Ar–H), 7.29 (d, J = 4.9 Hz, 2H, Ar–H), 7.16 (d, J = 8.4 Hz, 2H, Ar–H), 6.73 (s, 1H, CH-thiazole), 5.22 (s, 1H, CH), 2.78 (s, 3H, CH3), 2.12 (s, 3H, CH3), 2.05 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, δ, ppm): 20.3 (CH3), 21.2 (CH3), 31.8 (CH3), 39.4 (CH), 100.2 (C, thiazole), 112.6 (C, thiazole), 114.2 (CH, Ar), 116.6 (CH, Ar), 125.3 (CH, Ar), 126.6 (C, Ar), 130.5 (C, Ar), 142.1 (C=O), 169.8 (C=O), 196.3 (C=O). FT-IR (KBr, cm−1): 3225 (N–H), 3063 (C–H, Ar), 2921 (C–H), 2870 (C–H), 1606 (C=O), 1575 (C=C, Ar). HR-MS (ESI+): m/z Cald for C26H22N4O4S ([M + H+]) 487.4573, found 487.4577.
6-Acetyl-5-(4-hydroxyphenyl)-7-methyl-8-((5-methylfuran-2-yl)methyl)-5,8-dihy dro pyrido[2,3-d]pyrimidine-2,4 (1H,3H)-dione ( 8g )
Follow general method, using 4-hydroxybenzaldehyde 4 (0.24 g, 2.0 mmol), acetylacetone 5 (0.2 g, 2.0 mmol), (5-methylfuran-2-yl) methanamine 6g (0.22 g, 2.0 mmol) and barbituric acid 7 (0.24 g, 2.0 mmol) for 2 h. The crude compound was purified by flash chromatography (petroleum ether: ethyl acetate: 6:4, Rf = 0.3) to give 0.17 g (85%) 8g as a yellow solid; m.p 200–201 °C; 1H NMR (400 MHz, CDCl3, δ, ppm): 11.01 (s, 1H, NH), 7.79 (d, J = 8.7 Hz, 2H, Ar–H), 6.99 (d, J = 8.6 Hz, 2H, Ar–H), 6.12 (d, J = 3.1 Hz, 1H, furan), 5.91 (dd, J = 4.2, 2.2 Hz, 1H, furan), 5.08 (s, 1H, CH), 4.40 (s, 2H, CH2), 2.08 (s, 3H, CH3), 2.02 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, δ, ppm): 12.0 (CH3), 16.2 (CH3), 28.6 (CH2), 40.8 (CH), 100.3 (CH, furan), 109.2 (CH, furan), 111.8 (CH, Ar), 117.9 (CH, Ar), 130.3 (C, Ar), 161.8 (C=O), 194.8 (C=O). FT-IR (KBr, cm−1): 3109 (N–H), 3003 (C–H, Ar), 2921 (C–H), 2815 (C–H), 1683 (C=O), 1604 (C=C, Ar). HR-MS (ESI+): m/z Cald for C22H21N3O5 ([M + H+]) 408.8550, found 408.8545.
6-Acetyl-5-(4-hydroxyphenyl)-7-methyl-8-phenethyl-5,8-dihydropyrido [2,3-d] pyrimidine-2,4 (1H, 3H)-dione ( 8h )
Follow general method, using 4-hydroxybenzaldehyde 4 (0.24 g, 2.0 mmol), acetylacetone 5 (0.2 g, 2.0 mmol), 2-phenylethylamine 6h (0.24 g, 2.0 mmol) and barbituric acid 7 (0.24 g, 2.0 mmol) for 2 h. The crude compound was purified by flash chromatography (petroleum ether: ethyl acetate: 6: 4, Rf = 0.5) to give 0.18 g (90%) 8h a white solid; m.p 197–198 °C; 1H NMR (400 MHz, CDCl3, δ, ppm): 10.92 (s, 1H, NH), 7.34–7.21 (m, 9H, Ar–H), 4.95 (s, 1H, CH), 3.49 (t, J = 10.3 Hz, 2H, CH2), 2.88 (t, J = 7.3 Hz, 2H, CH2), 2.01 (s, 3H, CH3), 1.82 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, δ, ppm): 13.9 (CH2), 16.7 (CH3), 25.9 (CH2), 37.3 (CH2), 45.2 (CH), 95.8 (CH, Ar), 112.6 (CH, Ar), 124.6 (CH, Ar), 130.0 (C, Ar), 132.3 (C, Ar), 150.3 (C=O), 162.5 (C=O), 196.4 (C=O). FT-IR (KBr, cm−1): 3230 (N–H), 3063 (C–H, Ar), 2926 (C–H), 2870 (C–H), 1606 (C=O), 1568 (C=C, Ar). HR-MS (ESI+): m/z Cald for C24H23N3O4 ([M + H+]) 418.2674, found 418.2673.
6-Acetyl-8-(3,4-dimethoxybenzyl)-5-(4-hydroxyphenyl)-7-methyl-5,8-dihydropyrido[2,3-d] pyrimidine-2,4(1H,3H)-dione ( 8i )
Follow general method, using 4-hydroxybenzaldehyde 4 (0.24 g, 2.0 mmol), acetylacetone 5 (0.2 g, 2.0 mmol), 2,4-dimethoxybenzylamine 6i (0.32 g, 2.0 mmol) and barbituric acid 7 (0.24 g, 2.0 mmol) for 2 h. The crude compound was purified by flash chromatography (petroleum ether: ethyl acetate: 6: 4, Rf = 0.2) to give 0.18 g (93%) 8i as a yellow solid; m.p 223–224 °C; 1H NMR (400 MHz, CDCl3, δ, ppm): 11.14 (s, 1H, NH), 7.74 (d, J = 8.6 Hz, 1H, Ar–H), 7.09 (d, J = 8.4 Hz, 1H, Ar–H), 6.96 (d, J = 8.7 Hz, 2H, Ar–H), 6.44 (d, J = 7.2 Hz, 2H, Ar–H), 5.02 (s, 1H, CH), 4.39 (s, 2H, CH2), 3.81 (s, 3H, OCH3), 3.80 (s, 3H, OCH3), 2.04 (s, 3H, CH3), 2.02 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, δ, ppm): 21.1 (CH3), 28.3 (CH3), 30.1 (CH2), 40.3 (CH), 42.8 (OCH3), 55.2 (OCH3), 100.5 (CH, Ar), 106.2 (C, Ar), 112.9 (CH, Ar), 114.6 (CH, Ar), 118.6 (C, Ar), 125.4 (CH, Ar), 130.2 (C, Ar), 159.7 (C=O), 162.2 (C=O), 196.2 (C=O). FT-IR (KBr, cm−1): 3088 (N–H), 3006 (C–H, Ar), 2965 (C–H), 2834 (C–H), 1683 (C=O), 1548 (C=C, Ar). HR-MS (ESI+): m/z Cald for C25H25N3O6 ([M + H+]) 464.1789, found 464.1785.
4 Conclusion
In summary, a convenient and green method was introduced for the synthesis of new 1,4-DHP derivatives by the one-pot, four-component reaction in EtOH in the presence of 3-methyl-1-sulfonic acid imidazolium chloride {[Msim]Cl}as catalyst at room temperature. Interesting properties of {[Msim]Cl} include non-toxic nature, easy to remove and offer potential advantages over conventional catalyst. This modified strategy gives increased performance for the synthesis of new 1,4-DHP derivatives. The delightful properties of this protocol include the exploitation of environmentally benign catalyst, easy work up and excellent yields of desired products. Further studies on evaluating the biological activities of these derivatives are in progress.
References
Stout DM, Meyers A (1982) Recent advances in the chemistry of dihydropyridines. Chem Rev 82(2):223–243. https://doi.org/10.1021/cr00048a004
Maleki A, Firouzi-Haji R, Hajizadeh Z (2018) Magnetic guanidinylated chitosan nanobiocomposite: a green catalyst for the synthesis of 1,4-dihydropyridines. Int J Biol Macromol 116:320–326. https://doi.org/10.1016/j.ijbiomac.2018.05.035
Maleki A, Eskandarpour V, Rahimi J, Hamidi N (2019) Cellulose matrix embedded copper decorated magnetic bionanocomposite as a green catalyst in the synthesis of dihydropyridines and polyhydroquinolines. Carbohydr Polym 208:251–260. https://doi.org/10.1016/j.carbpol.2018.12.069
Mannhold R, Jablonka B, Voigt W, Schoenafinger K, Schraven E (1992) Calcium-and calmodulin-antagonism of elnadipine derivatives: comparative SAR. Euro J Med Chem 27(3):229–235. https://doi.org/10.1016/0223-5234(92)90006-M
Triggle DJ (2007) Calcium channel antagonists: clinical uses—past, present and future. Biochem Pharmacol 74(1):1–9. https://doi.org/10.1016/j.bcp.2007.01.016
Briukhanov V, Zverev I, Elkin V (1994) The effect of calcium antagonists on the development of inflammatory edema in rats. Eksp Klin Farmakol 57(2):47–49
Tale RH, Rodge AH, Hatnapure GD, Keche AP, Patil KM, Pawar RP (2013) The synthesis, anti-inflammatory, and anti-microbial activity evaluation of new series of 4-(3-arylureido)phenyl-1,4-dihydropyridine urea derivatives. Med Chem Res 22(3):1450–1455. https://doi.org/10.1007/s00044-012-0109-8
Hamy F, Brondani V, Flörsheimer A, Stark W, Blommers MJ, Klimkait T (1998) A new class of HIV-1 Tat antagonist acting through Tat− TAR inhibition. Biochemistry 37(15):5086–5095. https://doi.org/10.1021/bi972947s
Mohamed MF, Darweesh AF, Elwahy AH, Abdelhamid IA (2016) Synthesis, characterization and antitumor activity of novel tetrapodal 1,4-dihydropyridines: p53 induction, cell cycle arrest and low damage effect on normal cells induced by genotoxic factor H2O2. RSC Adv 6(47):40900–40910. https://doi.org/10.1039/C6RA04974E
Sirisha K, Achaiah G, Reddy VM (2010) Facile synthesis and antibacterial, antitubercular, and anticancer activities of novel 1,4-dihydropyridines. Arch Pharm 343(6):342–352. https://doi.org/10.1002/ardp.200900243
Gullapalli S, Ramarao P (2002) L-type Ca2+ channel modulation by dihydropyridines potentiates κ-opioid receptor agonist induced acute analgesia and inhibits development of tolerance in rats. Neuropharmacology 42(4):467–475. https://doi.org/10.1016/S0028-3908(01)00200-3
Bossert F, Meyer H, Wehinger E (1981) 4-Aryldihydropyridines, a new class of highly active calcium antagonists. Angew Chem Int Ed Engl 20(9):762–769. https://doi.org/10.1002/anie.198107621
Haller H (2008) Effective management of hypertension with dihydropyridine calcium channel blocker-based combination therapy in patients at high cardiovascular risk. Int J Clin Pract 62(5):781–790. https://doi.org/10.1111/j.1742-1241.2008.01713.x
Ruiz E, Rodríguez H, Coro J, Niebla V, Rodríguez A, Martínez-Alvarez R, de Armas HN, Suárez M, Martín N (2012) Efficient sonochemical synthesis of alkyl 4-aryl-6-chloro-5-formyl-2-methyl-1,4-dihydropyridine-3-carboxylate derivatives. Ultrason Sonochem 19(2):221–226. https://doi.org/10.1016/j.ultsonch.2011.07.003
Maleki B, Atharifar H, Reiser O, Sabbaghzadeh R (2019) Glutathione-coated magnetic nanoparticles for one-pot synthesis of 1,4-dihydropyridine derivatives. Polycycl Aromat Compd 52(3):1–14. https://doi.org/10.1080/10406638.2019.1614639
Tayebee R, Fattahi Abdizadeh M, Erfaninia N, Amiri A, Baghayeri M, Kakhki RM, Maleki B, Esmaili E (2019) Phosphotungstic acid grafted zeolite imidazolate framework as an effective heterogeneous nanocatalyst for the one-pot solvent-free synthesis of 3,4-dihydropyrimidinones. Appl Organomet Chem 33(8):4959. https://doi.org/10.1002/aoc.4959
Orru RV, de Greef M (2003) Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis 2003:1471–1499. https://doi.org/10.1055/s-2003-40507
Bhattacharjee D, Sutradhar D, Chandra AK, Myrboh B (2017) L-proline as an efficient asymmetric induction catalyst in the synthesis of chromeno [2, 3-d] pyrimidine-triones, xanthenes in water. Tetrahedron 73(25):3497–3504. https://doi.org/10.1016/j.tet.2017.05.025
Albadi J, Shirini F, Ghabezi B, Seiadatnasab T (2017) Melamine trisulfonic acid catalyzed regioselective nitration of aromatic compounds with sodium nitrate under solvent-free conditions. Arab J Chem 10:S509–S513. https://doi.org/10.1016/j.arabjc.2012.10.011
Ahad A, Farooqui M (2017) Organocatalyzed domino reactions: diversity oriented synthesis of pyran-annulated scaffolds using in situ-developed benzylidenemalononitriles. Res Chem Intermed 43(4):2445–2455. https://doi.org/10.1007/s11164-016-2772-8
Cherkupally SR, Mekala R (2008) P-TSA catalyzed facile and efficient synthesis of polyhydroquinoline derivatives through Hantzsch multi-component condensation. Chem Pharm Bull 56(7):1002–1004. https://doi.org/10.1248/cpb.56.1002
Rahmati A, Khalesi Z (2012) A one-pot, three-component synthesis of spiro [indoline-isoxazolo [4′, 3′: 5, 6] pyrido [2, 3-d] pyrimidine] triones in water. Tetrahedron 68(40):8472–8479. https://doi.org/10.1016/j.tet.2012.07.073
Xie YB, Ye SP, Chen WF, Hu YL, Li DJ, Wang L (2017) Brønsted-acid-catalyzed multicomponent one-pot reaction: efficient synthesis of polysubstituted 1,2-dihydropyridines. Asian J Org Chem 6(6):746–750. https://doi.org/10.1002/ajoc.201700127
Dastkhoon S, Tavakoli Z, Khodabakhshi S, Baghernejad M, Abbasabadi MK (2015) Nanocatalytic one-pot, four-component synthesis of some new triheterocyclic compounds consisting of pyrazole, pyran, and pyrimidinone rings. New J Chem 39(9):7268–7271. https://doi.org/10.1039/C5NJ01046B
Khalafi-Nezhad A, Divar M, Panahi F (2013) Nucleosides as reagents in multicomponent reactions: one-pot synthesis of heterocyclic nucleoside analogues incorporating pyrimidine-fused rings. Tetrahedron Lett 54(3):220–222. https://doi.org/10.1016/j.tetlet.2012.11.003
Ghashang M, Jabbarzare S, Tavakoli H, Banisadeghi H, Hosein Sokhanvar A, Lotfi M, Chami A (2015) Growth of the nano-islands of barium aluminum oxide nano-spheres on the surface of Al2O3–MgO composite: preparation and evaluation of their catalytic activity. Curr Nanosci 11(1):95–100
Safari J, Banitaba SH, Khalili SD (2011) Cellulose sulfuric acid catalyzed multicomponent reaction for efficient synthesis of 1,4-dihydropyridines via unsymmetrical Hantzsch reaction in aqueous media. J Mol Catal A Chem 335(1–2):46–50. https://doi.org/10.1016/j.molcata.2010.11.012
Dekamin MG, Kazemi E, Karimi Z, Mohammadalipoor M, Naimi-Jamal MR (2016) Chitosan: an efficient biomacromolecule support for synergic catalyzing of Hantzsch esters by CuSO4. Int J Biol Macromol 93:767–774. https://doi.org/10.1016/j.ijbiomac.2016.09.012
Jiang L, Ye W, Su W (2019) One-pot multicomponent synthesis of highly functionalized 1,4-dihydropyridines using porcine pancreatic lipase. Chem Res Chin Univ 35(2):235–238. https://doi.org/10.1007/s40242-019-8277-4
Maleki A, Hassanzadeh-Afruzi F, Varzi Z, Esmaeili MS (2020) Magnetic dextrin nanobiomaterial: An organic-inorganic hybrid catalyst for the synthesis of biologically active polyhydroquinoline derivatives by asymmetric Hantzsch reaction. Mater Sci Eng 109:110502. https://doi.org/10.1016/j.msec.2019.110502
Alinezhad H, Tarahomi M, Maleki B, Amiri A (2019) SO3H-functionalized nano-MGO-D-NH2: synthesis, characterization and application for one-pot synthesis of pyrano [2, 3-d] pyrimidinone and tetrahydrobenzo [b] pyran derivatives in aqueous media. Appl Organomet Chem 33(3):4661. https://doi.org/10.1002/aoc.4661
Maleki A, Firouzi-Haji R, Hajizadeh Z (2018) Magnetic guanidinylated chitosan nanobiocomposite: a green catalyst for the synthesis of 1,4-dihydropyridines. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2018.05.035
Taheri-Ledari R, Rahimi J, Maleki A (2019) Synergistic catalytic effect between ultrasound waves and pyrimidine-2,4-diamine-functionalized magnetic nanoparticles: applied for synthesis of 1,4-dihydropyridine pharmaceutical derivatives. Ultrason Sonochem 59:104737. https://doi.org/10.1016/j.ultsonch.2019.104737
Maleki B, Akbarzadeh E, Babaee S (2015) New basic ionic liquid from ethan-1,2-diyl bis (hydrogen sulfate) and DBU (1,8-diazobicyclo [5.4. 0] undec-7-ene) as an efficient catalyst for one-pot synthesis of xanthene derivatives. Dyes Pigm 123:222–234. https://doi.org/10.1016/j.dyepig.2015.08.009
Olivier-Bourbigou H, Magna L, Morvan D (2010) Ionic liquids and catalysis: recent progress from knowledge to applications. Appl Catal A 373(1–2):1–56. https://doi.org/10.1016/j.apcata.2009.10.008
Salvi P, Mandhare A, Sartape A, Pawar D, Han SH, Kolekar S (2011) Brønsted acidic ionic liquids promoted cyclocondensation reaction: synthesis of 1,8-dioxo-octahydroxanthene. C R Chim 14(10):883–886. https://doi.org/10.1016/j.crci.2011.04.008
Tayebee R, Jomei M, Maleki B, Razi MK, Veisi H, Bakherad M (2015) A new natural based ionic liquid 3-sulfonic acid 1-imidazolopyridinium hydrogen sulfate as an efficient catalyst for the preparation of 2H-indazolo[2,1-b]phthalazine-1,6,11(13H-triones). J Mol Liq 206:119–128. https://doi.org/10.1016/j.molliq.2015.02.021
Zare A, Khanivar R, Merajoddin M, Kazem-Rostami M, Ahmad-Zadeh MM, Moosavi-Zare AR, Hasaninejad A (2012) Triethylamine-bonded sulfonic acid [Et3N-SO3H] Cl as an efficient and homogeneous catalyst for the synthesis of 12-aryl-8, 9, 10, 12-tetrahydrobenzo [a] xanthen-11-ones. Iran J Catal 2(3):107–114
Shirvan SA, Ghahremanzadeh R, Moghaddam MM, Bazgir A, Zarnani AH, Akhondi MM (2012) A novel method for the synthesis of spiro [indoline-pyrazolo [4′, 3′: 5, 6] pyrido [2, 3-d] pyrimidine] triones by alum as a reusable catalyst. J Heterocycl Chem 49(4):951–954. https://doi.org/10.1002/jhet.898
Kamal A, Babu KS, Vardhan MV, Hussaini SA, Mahesh R, Shaik SP, Alarifi A (2015) Sulfamic acid promoted one-pot three-component synthesis and cytotoxic evaluation of spirooxindoles. Bioorgan Med Chem Lett 25(10):2199–2202. https://doi.org/10.1016/j.bmcl.2015.03.054
Acknowledgements
Authors gratefully thank to Sheffield University, UK for 1H, 13C NMR, FT-IR and HRMS spectra. This work was financially supported by the Ministry of Higher Education and Scientific Research (Iraq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jassem, A.M., Almashal, F.A.K., Mohammed, M.Q. et al. A catalytic and green method for one-pot synthesis of new Hantzsch 1,4-dihydropyridines. SN Appl. Sci. 2, 359 (2020). https://doi.org/10.1007/s42452-020-2165-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2165-x