Abstract
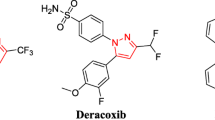
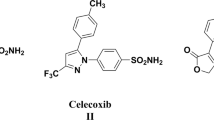
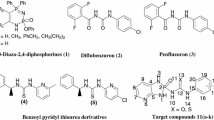
A new series of 4-(3-arylureido)phenyl-1,4-dihydropyridine urea derivatives were synthesized using simple three component condensation, reduction, and nucleophilic addition sequence in moderate to good yields. All the synthesized compounds 6a–j were evaluated for their anti-inflammatory [against the pro-inflammatory cytokines (tumor necrosis factor-alpha, TNF-α and interleukin-6, IL-6)] and anti-microbial activity (anti-bacterial and anti-fungal). Among all the compound screened, the compound 6b, 6f, and 6j were found to have promising anti-inflammatory activity, 74–83 % TNF-α and 91–96 % IL-6 inhibitory activity, respectively as compared to the standard dexamethasone (71 and 86 % inhibition) but at the MIC of 10 μM/ml. The compounds 6d–e and 6h exhibited relatively lower TNF-α and IL-6 inhibitory activity and found to be moderately potent anti-inflammatory agents. The compounds 6c–e, 6g, and 6i were found to be promising anti-bacterial and anti-fungal agents and remarkably some of the new compounds, viz. 6d and 6i were found be more potent than the standard ciprofloxacin or miconazole. It is to be noted that this is the first report on the anti-inflammatory activity evaluation of novel 1,4-dihydropyridine urea derivatives against the important molecular target, TNF-α, and IL-6.

Similar content being viewed by others
References
Desai B, Sureja D, Naliapara Y, Shah A, Saxena AK (2001) Synthesis and QSAR studies of 4-substituted phenyl-2,6-dimethyl-3,5-bis-N-(substituted phenyl)carbamoyl-1,4-dihydropyridines as potential antitubercular agents. Bioorg Med Chem 9:1993
Dominic SC, Raj MD (2009) Role of interleukin-6 in the anemia of chronic disease. Semin Arthritis Rheum 38:382
Eharkar PS, Desai B, Gaveria H, Varu B, Loriya R, Naliapara Y, Shah A, Kulkarni VM (2002) Three-dimensional quantitative structure–activity relationship of 1,4-dihydropyridines as antitubercular agents. J Med Chem 45:4858
Gaudio AC, Korolkovas A, Takahata Y (1994) Quantitative structure–activity relationships for 1,4-dihydropyridine calcium channel antagonists (nifedipine analogs): a quantum chemical/classical approach. J Pharm Sci 83:1110
Hwang C, Gatanaga M, Granger GA, Gatanaga T (1993) Mechanism of release of soluble forms of tumor necrosis factor/lymphotoxin receptors by phorbol myristate acetate-stimulated human THP-1 cells in vitro. J Immunol 151:5631–5638
Kirschbaum J (1968) Biological oxidations and energy conservation. J Chem Educ 45:28
Krishnamoorthy S, Honn KV (2006) Inflammation and disease progression. Cancer Metastasis Rev 25:481
Norcross BE, Klinedinst PE, Wertheimer FH (1962) The reduction of olefinic double bonds with dihydropyridines. J Am Chem Soc 84:797
Schellenberg KA, Weheimer FH (1965) A free-radical oxidation of a dihydropyridine. J Org Chem 30:1859
Shishoo CJ, Ravikumar T, Jain KS, Rathod IS, Gandhi TP, Satia MC (1999) Synthesis of novel 1,2-(un)substituted-3-amino-5-aryl-6-arylaminopyrazolo [3,4-d] pyrimidin-4 (5H)-ones and their biological activities. Indian J Chem 38:1075
Sirisha K, Bikshapathi D, Achaiah G, Reddy VM (2011) Synthesis, antibacterial and antimycobacterial activities of some new 4-aryl/heteroaryl-2,6-dimethyl-3,5-bis-N-(aryl)-carbamoyl-1,4-dihydropyridines. Eur J Med Chem 46:1564
Stout DM, Meyers AI (1982) Recent advances in the chemistry of dihydropyridines. Chem Rev 82:223
Surendra Kumar R, Idhayadhulla A, Jamal Abdul Nasser A, Selvin J (2011) Synthesis and antimicrobial activity of a new series of 1,4-dihydropyridine derivatives. J Serb Chem Soc 76:1
Swarnalatha G, Prasanthi G, Sirisha N, Madhusudhana Chetty C (2011) 1,4 Dihydropyridines: a multifunctional molecule—a review. Int J ChemTech Res 3:75
Tale RH, Rodge AH, Hatnapure GD, Keche AP (2011) The novel 3,4-dihydropyrimidin-2(1H)-one urea derivatives of N-aryl urea: synthesis, anti-inflammatory, antibacterial and antifungal activity evaluation. Bioorg Med Chem Lett 21:4648
Tanabe H, Tasaka S, Ohmori H, Gomi N, Sasaki Y, Machida T, Iino M, Kiue A, Naito S, Kuwano M (1998) Newly synthesized dihydropyridine derivatives as modulators of P-glycoprotein-mediated multidrug resistance. Bioorg Med Chem 6:2219
Tasaka S, Ohmori H, Gomi N, Iino M, Machida T, Kiue A, Naito S, Kuwano M (2001) Synthesis and structure–activity analysis of novel dihydropyridine derivatives to overcome multidrug resistance. Bioorg Med Chem Lett 11:275
Vijesha AM, Isloor AM, Peethambar SK, Shivananda KN, Arulmoli T, Isloor NA (2011) Hantzsch reaction: synthesis and characterization of some new 1,4-dihydropyridine derivatives as potent antimicrobial and antioxidant agents. Eur J Med Chem 46:5549
Acknowledgments
The authors would like to thank Mr. R. B. Ingle Department of Microbiology, Shri Shivaji College, Akola for biological screening studies of the newly synthesized compounds. We are very grateful to the editor and an anonymous reviewer for their invaluable comments and suggestions on our study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tale, R.H., Rodge, A.H., Hatnapure, G.D. et al. The synthesis, anti-inflammatory, and anti-microbial activity evaluation of new series of 4-(3-arylureido)phenyl-1,4-dihydropyridine urea derivatives. Med Chem Res 22, 1450–1455 (2013). https://doi.org/10.1007/s00044-012-0109-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0109-8