Abstract
Materials in general and copper in particular, have a wide variety of industrial applications. However, they can easily corrode in aggressive environments. The anticorrosive action of copper by the essential oil of Allium sativum in a 0.5M H2SO4 solutions has been studied using electrochemical techniques: electrochemical impedance spectroscopy and potentiodynamic polarization. The polarization curves indicate that the studied oil acts as a cathodic inhibitor retarding the cathodic reaction. An appropriate electrical equivalent circuit model was used to calculate the electrochemical impedance parameters. The results obtained showed that the inhibition efficiency depends on the inhibitor concentration and the temperature. This efficiency reached 97% at a concentration of 1.0 g/L. Some thermodynamic parameters for activation process and metal dissolution such as activation energy (Ea) activation entropy (ΔSa) and the activation enthalpy (ΔHa) were also calculated and discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to its excellent thermal conductivity, electrical conductivity, ductility, and good workability, copper is widely used in various fields, such as machinery manufacturing, power transmission, cooling and heating systems, military industry, and so on [1,2,3,4]. In order to obtain a shiny final copper surface, which is required in some applications, or to remove scale deposits that reduce the heating efficiency of some copper-based equipment, a sulphuric acid solution is frequently used to remove any undesirable products formed at the surface (oxidation films, incrustations, scale etc.) [3,4,5]. Therefore, corrosion of copper can be caused during this chemical treatment after the scale or oxides have been removed, which implies the dissolution of the metal and the consumption of the acid used. This also implies important economic losses for the industrialists [6, 7]. Corrosion phenomenon can be reduced by the use of inhibitors.
According to the literature, several studies have been carried out in this field for hundreds of years [8,9,10]. In 1860 Great Britain announced the discovery of the world's first corrosion inhibitor. Since this discovery, a lot of work has been done to find and develop different types of organic and inorganic inhibitors [11]. Previous studies have found that the presence of heteroatom's such as nitrogen, sulphur and phosphorus in the molecular structure of these inhibitors improves their inhibition effectiveness. Therefore, they can establish a coordination bonds between the free doublets of these heteroatom's and the empty d-orbital's of transition metals, which indicate the formation of an effective barrier film that isolates the metal substrate from the corrosive environment [12, 13]. However, some of these inhibitors have disadvantages: high cost, toxic to humans, risk of pollution to the environment [14]. In this context, corrosionists have always sought alternatives by focusing on biodegradable, economical, renewable plant products that do not present any risk to human health and the environment [15, 16].
According to Ajeigbe et al. [17] and Barakat et al. [18], plant extracts have been used for decades as effective corrosion inhibitors, their property due to their richness in complex organic compounds containing heteroatom’s, aromatic rings and multiple bonds (double and triple bonds) have also been identified as adsorption centers that interact with active sites on the surface of a metal. For this reason, multiple essential oils from different plants have also been studied to prevent metal corrosion. Among these plants, Rosmarinus officinals L [19], Nigella Sativa [20], Artemisia herba-alba's [21], Juniperusphoenicea [22] and Cinnamon [3]. All these oils have shown good inhibitory properties.
Garlic or Allium sativum, which belongs to the Liliaceaes family has been widely used since antiquity due to its therapeutic properties in the field of pharmacology [23, 24].
It has been evaluated as effective corrosion inhibitors in various media by several investigators. Table 1 shows some of the work of A. sativum used as corrosion inhibitors of different materials in different environments.
The essential oil of A. sativum is characterized by a high amount of organo-sulphur compounds such as trisulfide, methyl2-propenyl; trisulfide, di-2-propenyl and diallyl disulfide [30]. The last compound has been tested as a corrosion inhibitor of copper corrosion in 0.5M sulfuric acid medium by Tan et al. [4]. The existence of these products in our essential oil means that it may be an excellent inhibitor candidate for reducing copper corrosion in acidic media. Therefore, the aim of this work is to study the inhibition of copper corrosion in 0.5M sulphuric acid media in the absence and presence of A. sativum essential oil using electrochemical techniques such as potentiodynamic study, electrochemical impedance spectroscopy (EIS) and surface analysis by SEM/EDX.
2 Materials and methods
2.1 Extraction of the essential oil of A. sativum
The essential oil of garlic was extracted by hydrodistillation in a simple distillation unit, from fresh garlic bulbs of A. sativum (Meknes region, Morocco), prepared and distilled according to the following method: 500 g of garlic cloves were mixed with 500 ml of distilled water in a domestic blender for 1 min, and then placed in a 1L flask. The distillation was carried out for 4 h. The distillate (hydrolat + essential oil) was collected in a flask and separated with a separating funnel using diethyl ether as solvent. The organic phase, containing the essential oil, obtained was passed through a rotavapor to get rid of the solvent. The product obtained is kept in a dark brown glass container and stored in a refrigerator at 2 to 5 °C.
2.2 Preparation of the material
The metal samples used in this study are copper samples with the following chemical composition (in % by weight): 0.019 P, < 0.001 Fe, < 0.001 As, < 0.001 Mn, < 0.002 Sb, < 0.001 Al, 0.009 Sn, 0.003 Ni, 0.015 Pb, < 0.005 Ag, < 0.001 Bi, < 0.001 S, < 0.005 C and the rest is Cu. The copper samples were abraded with different grades of SiC abrasive papers(from 180 to 2000), followed by cleaned carefully according to ASTM G-81, and finally drying at room temperature. The aggressive solution were prepared by diluting analytical grade H2SO4 (98 wt.%) with distilled water. The concentration of the A. sativum oil used in the present study ranged from 0.5 to 2.0 g/L, and the blank solution was also prepared for comparison.
2.3 GC–MS analysis
The chromatographic analysis of the studied essential oil was carried out at the University Centre for Analysis, Expertise, Technology Transfer and Incubator CUA2TI of IBN TOFAIL University in Kenitra, using a 456-GC type gas chromatograph coupled to an EVOQ TQ type mass spectrometer. The fragmentation is performed by electronic impact at 70 eV. The column used is a Rxi-5Sil MS type capillary column (30 m × 0.25 mm ID × 0.25 µm df). The temperature of the column is initially programmed at 40 °C for 8 min at a rate of 2 °C/min up to 200 °C or 20 min remaining. The carrier gas is helium with a flow rate of 1.50 ml/min. The injection temperature is maintained at 250 °C and the injection volume is 1 µl. The apparatus is connected to a computer system managing a NIST MS Search2.0 mass spectrum library and driven by Ms Data review software allowing to follow the evolution of the chromatographic analyses. The identification of the constituents has been made on the basis of the comparison of their retention times with those of the standard compounds in the computerized database (NIST 2014).
2.4 Electrochemical measurements
The electrochemical measurements were carried out using a potentiostat/galvanostat/PGZ100 controlled by Volta Master 4 analysis software. The electrochemical experiments were carried out in the assembly of a three-electrode electrochemical cell which the size is 50 ml, using a copper plate as working electrode (with an exposed surface area of 1 cm2), a platinum rod as counter electrode and saturated calomel electrode (SCE) as reference electrode.
EIS measurement was performed in the frequency range of 100 kHz to 10 MHz at an open circuit potential (OCP) with 10 points per decade. The applied AC signal amplitude is 10 mV ms. All experiments were performed after 30 min immersion of the copper in a 0.5M H2SO4 solutions in the absence and presence of different concentrations of the studied oil.
The potentiodynamic polarization curves were obtained by sweeping the working electrode potential from − 1200 to 600 mV with a sweep rate of 1 mV/s. All electrochemical experiments were carried out at a temperature of 298 K and were repeated three times and presented values were obtained by averaging.
2.5 Analysis of surface morphology
In order to confirm the formation of a natural film on the cooper surface exposed to the 0.5M H2SO4 solutions for 6 h in the absence and presence of the A. sativum oil was carried out by analysis using Scanning Electron Microscope (Quantra 450) coupled with EDX analyses. These analyses were carried out at the MASCIR Foundation—Rabat.
3 Results and discussion
3.1 Analysis by GC–MS
GC–MS analysis identified 16 compounds representing 99.815% of the total constituents listed in Table 2.
Allium sativum essential oil from the region of Meknes is composed essentially of organosulphurates and hydrocarbons, comparing these results with the literature [31,32,33], it was noticed that there is a great variation in the level of the elements identified, as well as in their percentages of existence in the oil studied, this variation may be due to the origin of the plant [34] as it may be due to the operating conditions of extraction [35].
Satyal [36] was able to identify 33 organosulfur compounds for Spanish garlic oil, the majority of which are Diallyltrisulfide (33.4%), Allyl methyl trisulfide (19.2%), and Diallyl disulfide with a percentage of (20.8%), whereas Egyptian garlic oil had diallyl disulfide (25.2%), allyl methyl trisulfide (23.8%), and diallyltrisulfide (21.1%) as major constituents [33]. The major constituents in this study are: Diallyl disulfide (26.623%), Trisulfide, methyl2-propenyl (16.459%) and Trisulfide, di-2-propenyl (34.104%) shown in Table 3.
3.2 Polarization curve
The polarization curves of copper in the 0.5M H2SO4 solutions in the absence and presence of the essential oil of A. sativum at 298 K are shown in Fig. 1:
In the literature the behavior of copper in an aerated sulphuric acid solution has been studied by several researchers [37,38,39,40], the cathodic reaction was the summation of Eqs. (1) and (2):
Hydrogen reduction (1):
Reduction of oxygen (2):
The cathodic reduction of oxygen can be expressed either by a direct transfer of \(4e^{ - }\) as described by Eq. (2) or by \(2e^{ - }\) in two consecutive steps involving reduction with hydrogen peroxide (H2O2) first [41, 42]:
followed by a further reduction:
The anodic dissolution of copper takes place in two continuous stages.
It has been concluded that the reaction rate of the first stage (reaction 5) is much faster than that of the second stage (reaction 6); therefore, the second stage is considered to be a key stage in the corrosion process [43].
The anodic and cathodic polarization curves shown in Fig. 1 show that the corrosion potential has been shifted to more negative values by increasing the concentration of the inhibitor.
On the other hand, the cathodic current density has been remarkably reduced compared to the anodic current density, implying that the rate of cathodic corrosion has been reduced. Also, it can be observed that the cathodic curves are not parallel to the white curve, which states that the inhibitor causes the modification of the cathodic corrosion mechanism of copper in an aerated 0.5M H2SO4 solutions by influencing the cathodic oxygen reduction reaction more than the anodic copper dissolution reaction [14].
The electrochemical parameters obtained from the extrapolation of the Tafel lines are listed in Table 4. These parameters included the corrosion potential (Ecorr), the cathodic Tafel slope(βc) and the corrosion current density (icorr). In addition, the corrosion inhibition efficiency (ηpp) was obtained using the following formula [43]:
where icorr° and icorr are the corrosion current densities values in absence and presence of inhibitor, respectively.
The parameters presented in Table 4 show that all corrosion potentials are transferred to more negative potentials with a degree of offset of − 150 mV exceeding a value of − 85 mV. This observation reveals that the studied compound is cathodic in nature [4, 13, 44].
Moreover, the icorr values decrease towards lower current densities than the uninhibited solution with increasing concentration, which means the increase of the inhibition efficiency up to a value of 97.6% at a concentration 1 g/L. This phenomenon proves the formation of a barrier film on the surface of copper against corrosive attack [45].
The polarization resistance (Rp) values of copper in 0.5M H2SO4 increases from 397 Ω for the blank to 15182Ω for 1.0 g/L concentration of A. sativum (Table 4).
3.3 Electrochemical impedance spectroscopy
-
1
In order to obtain information on the protection mechanism (charge transfer, diffusion, and adsorption) of copper by A. sativum essential oil in 0.5M H2SO4 solutions, electrochemical impedance measurements has been performed. Many works [46, 47], have shown that the electrochemical impedance measurements are likely to reveal the elementary steps involved in the global corrosion and/or protection processes. Cruz et al. [48] and Bentiss et al. [49] have studied the inhibition mechanism of some inhibitors using electrochemical impedance measurements, seems to prove that this technique is particularly well adapted to the determination of the mode of action of the inhibitors, to the evaluation of the dielectric characteristics of the formed film and makes it possible to follow their evolution according to many parameters.
The Nyquist diagrams of copper immersed in the 0.5M H2SO4 solutions obtained experimentally without and with the addition of different concentrations of A. sativum essential oil studied are recorded in Fig. 2. The observed experiments tested of triplicate measurements are highly reproducible giving standard deviations.
The impedance diagrams presented in the Nyquist plane are characterized by the presence of two badly separated capacitive loops, we can confirmed this result by the bode diagram, a two time-constants in bode plot appears in the inhibited sample as shown in Fig. 2b. The semicircle at the high frequency part is related to the resistance and capacitance of adsorbed inhibitor films present on the copper surface, the second time constant appearing at low frequencies corresponds to the charge transfer phenomenon at the copper/H2SO4 interface [3].
From the bode presentation, concerning the bode presentation of blank solution (Fig. 2a) we can observed the presence of one time-constant attributed to charge transfer process.
We also observe that the impedance display of copper in the solutions containing the inhibitor has considerably changed shape and size compared to the blank one. While the essential oil of A. sativum leads to changes in impedance behavior [3], the increase in the size (namely Rp = diameter)of these loops, with the concentration of inhibitor is justified, indicating an increase in the protective power by the formation of an inhibitor film that blocks the formation of the corrosion product [50]. This result reflects the influence of A. sativum on the process at the copper/acidic medium interface [51].
The Nyquist impedance spectra of copper in 0.5M H2SO4 were modelled by the equivalent circuit shown in Fig. 3a, and All impedance spectra obtained from the copper electrode exposed for 30 min in H2SO4 solutions containing A. sativum were analyzed by the equivalent circuit shown in Fig. 3b. The following are the components of the suggested equivalent circuit: Rs represents the solution resistance, Rct the charge transfer resistance, Rf the film resistance, and Qf and Qdl the constant phase elements [51, 52].
As can be seen in Fig. 3, the capacitor has been replaced by a constant phase element, indicating the presence of a dissimilar frequency response. The EPC impedance is defined as follows [53]:
where Q is a constant in Ω/cm2 sn, ω is the angular frequency in rad/s, n is the exponent of CPE with − 1 < n < 1. CPE can represent an inductance (n = − 1), a Warburg impedance (n = 0.5), a pure capacitance (n = 1), or a resistance (n = 0). The smaller value of n, the higher the surface roughness [54].
The electrochemical parameters obtained from the impedance diagrams and the inhibiting efficiency (E%) are grouped in Table 4. The values of the polarization resistance Rp of all systems are calculated using the following equation:
Inhibition efficiency (ηimp%) is calculated by the following equation [45]:
where RP° and RP are the charge transfer resistance in absence and presence of the inhibitor, respectively.
From the data in Table 5, it can be seen that, when the concentrations of A. sativum increase to a value of 1 g/L, the values of Rct increase, while the values of Qdl decrease, this is due to the formation of a protective film on the metal surface by the adsorption of Allium sativum, which slows down the corrosion process of copper in H2SO4 by the action of the inhibitor [50]. The decrease in Qdl value may be due to a decrease in the local dielectric constant and/or an increase in the thickness of the double layer [50]. As a consequence, it can be said that the inhibitor molecules act by adsorption at the solution-metal interface [51].
Similarly, when the concentration of the inhibitor increases to a value of 1 g/L, it can be seen that the Rf values increase, while the Qf values decrease to the optimal concentration (1 g/L of A. sativum essential oil). These evolutions indicate a thickening of the film formed and a decrease in permeability through it [55]. Consequently, the decrease in Qf values shows that the adsorption layer formed by Allium sativum on the metal surface is stable and thick [56]. In the presence of 1.0 g/L of Allium sativum we notice that the diameter of Rp has obviously increased from 350 Ω cm2 to 14,302 Ω cm2, which implies a better protection of the copper by our oil in an acidic environment.
Comparing the performance of our oil to other green inhibitors such as cinnamon essential oil and lotus zizyphus extract which have been tested for the same metal in an acidic medium we notice that the maximum efficiency that can be achieved with cinnamon essential oil is 89.62% [3] as well as obtaining a maximum value of 90.57% for the extract of lotus zizyphus [50], while with the oil of A. sativum the effectiveness has reached 97.5% Table 5, so we can see that our inhibitor admits a better inhibitory effect for copper in an acidic medium compared to these two green inhibitors.
3.4 Temperature effect
In order to evaluate the influence of temperature on the inhibition efficiency of the investigated essential oil, the polarization curves of copper were performed before and after addition of A. sativumin 0.5M H2SO4 solutions at a concentration of 1.0 g/L in the temperature range from 298 to 328 K after 30 min of immersion time.
Figure 4 shows the polarization curves of copper in the 0.5M H2SO4 solutions in the absence (a) and presence (b) of the inhibitor. The electrochemical parameters are summarized in Table 5. It can be seen from Table 5 that the cathodic and anodic current densities increase with increasing temperature. Thus, this compound still show superior inhibition performance to prevent copper from corrosion by forming a firm adsorption film onto copper substrate.
On the other hand, it can be seen that the curves in Fig. 4a are more or less parallel to each other, only for the anode region, whereas the cathode region admits a change in the shape of the cathode curve from T = 298 K to T = 308 K, which implies that temperature does not affect the anode reaction but only the cathode reaction and the speed of the latter.
Although in the presence of the inhibitor Fig. 4b, it can be seen that the curves are more or less parallel for the two cathodic and anodic regions, it can be said then that in the presence of the inhibitor the temperature does not affect the mechanism but only the corrosion rate.
Compared with that in blank solution, the icorr value decreases remarkably with the addition of 1.0 g/L d’E.O. at each temperature, implying that this essential oil considerably inhibit the corrosion reaction of MS.
Also by comparing the curves of Fig. 4 we can see a decrease in the current density icorr compared to the bank solution at different temperatures as well as a decrease in the inhibition efficiency with temperature which can be due to the weakening of the adsorbed film on the substrate surface, which suggests a physical adsorption of our inhibitor on the metal surface [57].
The effect of temperature on the inhibited acid-metal action is very complex, many changes may occur on the metal surface, such as rapid desorption of the inhibitor as well as the inhibitor itself, may undergo decomposition, rearrangement or both at the same time [3, 50].
Further, Table 6 indicates that corrosion efficiency decreases with increasing temperature, this decrease in efficiency is attributed to the increased dissolution of copper in acidic media, and/or partial desorption of the inhibitor from the metal surface [58,59,60]. These results indicate physisorption of the inhibitor on the metal surface of copper [50, 58].
3.5 Parameters for activation of the corrosion process
The calculation of thermodynamic parameters such as activation energy, activation enthalpy and activation entropy allow the quantification of the corrosion inhibition efficiency in order to determine and interpret the adsorption type adopted by an inhibitor.
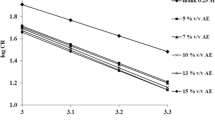
In order to estimate these parameters shown in Table 7, plots of the Arrhenius curves, the logarithm of the corrosion rate (ln(icorr)) vs (1000/T) are used (Fig. 5a). The activation energy of the corrosion process Ea can be calculated using the following equation [61, 62]:
where R is the perfect gas constant, A is the pre-exponential Arrhenius constant and T is the absolute temperature.
The activation entropy (ΔSa) and the activation enthalpy (ΔHa) were obtained on plotting (Ln (icorr/T)) vs (1000/T) (Fig. 5b), where (− ΔHa/R) is the slope and [Ln (R/Nh) + (ΔSa/R)] the intersection of the straight line with the y-axis according to the alternative formula of the Arrhenius equation [61,62,63]:
where h is the Planck's constant, N is the Avogadro number, T is the absolute temperature, ΔSa is the activation entropy and ΔHa is the activation enthalpy. The obtained results are grouped in Table 7.
The Arrhenius curves obtained in the presence of 1 g/L of A. sativum essential oil presented in Fig. 5 are compared to those of copper in a 0.5M H2SO4 solutions in the absence of inhibitor.
According to the literature, the variation of kinetic parameters adopts a specific type of adsorption. The presence of the studied inhibitor increased the energy barrier (Ea) of the inhibiting solution above that of the blank and hence the inhibition process, which promotes electrostatic interactions with the metal surface and the adsorption of the protective film is of physical nature [3, 63,64,65]. In contrast to an equivalent chemical adsorption of a decrease in Ea in the presence of an inhibitor, which is accompanied by an increase in the effectiveness of the inhibition with temperature [66, 67].
The results shown in Table 6 indicate an increase in Ea values in the presence of inhibitor compared to the blank solution, which suggests a physisorption at the metal surface, while the studied inhibitor blocks the cathodic sites by constructing a barrier that mainly inhibits the corrosion process [62]. Furthermore, the positive values of ΔHa indicate the endothermic character of the copper dissolution process and inform that the dissolution of copper is slow in the presence of inhibitor [61, 63]. Also, the negative values of the entropy (ΔSa) indicate a decrease in the degree of randomness which occurred when the reactants are transformed to activated complexes [61, 63].
3.6 Surface analysis
In order to confirm the results obtained by the electrochemical measurements. Qualitative microscopic SEM analyses coupled with quantitative EDX analyses were performed. Figure 6 shows the surface of recently abraded copper immersed in the 0.5M H2SO4 solutions without inhibitor, it can be found that the homogeneous corrosion of the entire copper surface is very dense and orderly in the blank solution. However, the corrosion degree of the Cu surface is very serious. However, the addition of the studied essential oil causes a decrease in the corrosion degree on the copper surface and a non-uniform protective film is formed on the surface of the copper (Fig. 7).
The EDX analysis (Fig. 6) shows the presence of oxygen, which is a corrosive element for copper and therefore justifies the corrosion of copper. These findings are confirmed by the SEM analysis. Figure 7 shows also the appearance of a sulphur peak which is the initial element of the chemical composition of A. sativum essential oil. These observations proves the formation of a protective barrier film created when the tested essential oil interact with the copper surface in acid solution.
3.7 The mechanism of inhibition
Generally, organic molecules presented in our essential oil are adsorbed on the metal surface and prevent further dissolution of metal by blocking either the cathodic or anodic reaction or both. Organic inhibitors, capable of forming insoluble complexes, or chelates, with metallic ions present on the surface of metal [68]. The inhibition efficiency of our corrosion inhibitor (major constituents)against the corrosion of copper in 0.5M H2SO4 can be explained on the basis of the number of adsorption sites, their charge density, molecular size, mode of interaction with the metal surface and the ability to form a metallic complex. The π electrons and free electrons on the S atoms form bonds with the metal surface (copper); see Fig. 8.
According to the potential–pH diagram [69], the protective Cu2O and CuO oxide films can be easily dissolved at low pH values. As shown in Fig. 8, the dissolution of copper in acidic medium proceeded according to the following steps:
where Cu(I)ads is an adsorbed species at the copper surface and does not diffuse into the bulk solution. The cathode reduction of oxygen can be expressed as follow:
According to the literatures [70] two mechanisms have been proposed to explain the high inhibiting efficiency recorded for the inhibitors designated as INH. One mechanism attributes the phenomenon to the formation of an adsorbed layer of INH i.e.,
where Cu: INH(ads) refers to INH adsorbed on the copper surface. The other mechanism postulates a protective Cu(I)IN film on the surface, which inhibits the anodic dissolution reaction, i.e.,
The Cu(I)IN complex is favored at more anodic potentials in less acidic media; however, the adsorbed species is formed in more acidic media under cathodic potentials.
4 Conclusion
In the present study, the inhibition effect of A. sativum essential oil against the corrosion of copper in 0.5 H2SO4 was evaluated via electrochemical and surface studies. From the above results and discussion, we can conclude that:
-
The analysis of the oil by GPC-MS showed that the oily fraction of garlic contains highly reactive sulphur molecules of which three are the main compounds (diallyldisulphide, trisulphide, methyl-2-propenyl and trisulphide, di-2-propenyl).
-
The essential oil of A. sativum is endowed with a good inhibitory efficiency of up to 97.6% for a concentration of 1 g/L.
-
EIS results show that the Nyquist diagrams obtained show two capacitive loops, one at high frequency which can be associated with the adsorption of a film on the surface of the copper, and the other at low frequency which can be attributed to the phenomenon of charge transfer at the copper/H2SO4 interface.
-
The study of the temperature effect shows that our inhibitor has good inhibitory efficacy values in an ascending temperature range, which shows a good temperature resistance.
-
The thermodynamic parameters confirm the physisorption of the studied inhibitor on the copper surface in 0.5M sulphuric acid media.
-
SEM analysis coupled with EDX confirms the presence of a protective layer on the substrate surface.
References
Qiang Y, Zhang S, Xu S, Li W (2016) Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution. J Colloid Interface Sci 472:52–59. https://doi.org/10.1016/j.jcis.2016.03.023
Hegazy MA, Nazeer AA, Shalabi K (2015) Electrochemical studies on the inhibition behavior of copper corrosion in pickling acid using quaternary ammonium salts. J Mol Liq 209:419–427. https://doi.org/10.1016/j.molliq.2015.05.043
Dahmani K, Galai M, Cherkaoui M, El hasnaoui A, El Hessni A (2017) Cinnamon essential oil as a novel eco-friendly corrosion inhibitor of copper in 0.5M sulfuric acid medium. JMES 8(5):1676–1689
Tan B, Zhang S, Qiang Y, Guo L, Feng L, Liao C, Chen S (2018) A combined experimental and theoretical study of the inhibition effect of three disulfide-based flavouring agents for copper corrosion in 0.5M sulfuric acid. J Colloid Interface Sci 526:268–280. https://doi.org/10.1016/j.jcis.2018.04.092
Qiang Y, Zhan”g S, Guo L, Zheng X, Xiang B, Chen S, (2017) Experimental and theoretical studies of four allyl imidazolium-based ionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros Sci 119:68–78. https://doi.org/10.1016/j.corsci.2017.02.021
Quartarone G, Battilana M, Bonaldo L, Tortato T (2008) Investigation of the inhibition effect of indole-3-carboxylic acid on the copper corrosion in 0.5M H2SO4. Corros Sci 50(12):3467–3474. https://doi.org/10.1016/j.corsci.2008.09.032
Li H, Zhang S, Tan B et al (2018) Investigation of Losartan Potassium as an eco-friendly corrosion inhibitor for copper in 0.5M H2SO4. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.112789
Antonijevic MM, Petrovic MB (2008) Copper corrosion inhibitors. A review. Int J Electrochem Sci 3:1–28
Khaled KF (2008) Adsorption and inhibitive properties of a new synthesized guanidine derivative on corrosion of copper in 0.5M H2SO4. Appl Surf Sci 255(5):1811–1818. https://doi.org/10.1016/j.apsusc.2008.06.030
Mihajlović MP, Antonijević MM (2015) Copper corrosion inhibitors. Period 2008–2014. A review. Int J Electrochem Sci 10:1027–1053
Zhang J, Zhang L, Tao G (2018) A novel and high-efficiency inhibitor of 5-(4-methoxyphenyl)-3h-1,2-dithiole-3-thione for copper corrosion inhibition in sulfuric acid at different temperatures. J Mol Liq. https://doi.org/10.1016/j.molliq.2018.09.095
Xu Y, Zhang S, Li W, Guo L, Xu S, Feng L, Madkour LH (2018) Experimental and theoretical investigations of some pyrazolo-pyrimidine derivatives as corrosion inhibitors on copper in sulfuric acid solution. Appl Surf Sci 459:612–620. https://doi.org/10.1016/j.apsusc.2018.08.037
Tan B, Zhang S, Qiang Y, Li W, Liu H, Xu C, Chen S (2019) Insight into the corrosion inhibition of copper in sulfuric acid via two environmentally friendly food spices: combining experimental and theoretical methods. J Mol Liq. https://doi.org/10.1016/j.molliq.2019.110891
Raja PB, Sethuraman MG (2008) Natural products as corrosion inhibitor for metals in corrosive media—a review. Mater Lett 62(1):113–116. https://doi.org/10.1016/j.matlet.2007.04.079
Fateh A, Aliofkhazraei M, Rezvanian AR (2017) Review of corrosive environments for copper and its corrosion inhibitors. Arab J Chem 13:481–544. https://doi.org/10.1016/j.arabjc.2017.05.021
Mo S, Luo H-Q, Li N-B (2016) Plant extracts as “green” corrosion inhibitors for steel in sulphuric acid. Chem Pap. https://doi.org/10.1515/chempap-2016-0055
Ajeigbe SO, Basar N, Hassan MA, Aziz M (2017) Optimization of corrosion inhibition of essential oils of Alpinia galanga on mild steel using response surface methodology. J Eng Appl Sci 12(9):2763–2771
Barakat Y, Hassan A, Paraka MA (1998) Corrosion Inhibition of mild steel in aqueous solution containing H2S by some naturally occurring substances. Materialwiss Werkstoffech 9(7):365
Tiskar M, Galai M, EbnTouhami M, Satrani B, Ghanmi M, El Ammari Y, Chaouch A (2016) Inhibition of carbon steel Corrosion in Hydrochloric Solution with natural inhibitor case: Rosmarinus officinalis L. Int J Acad Stud 2(11):478–492
Benabida M, Galai M, Cherkaoui M, Dagdag O (2016) Potentials of nigella sativa oil as inhibition towards the corrosion of mild steel in neutral media. Anal Bioanal Electrochem 8(8):962–976
Tiskar M, Galai M, El Ammari Y, EbnTouhami M, Satrani B, Ghanmi M, Ettahir A, Chaouch A (2017) Chemical composition and inhibitory effect of Artemisia herba-alba’s essential oil on carbon steel corrosion in hydrochloric acid (1M). Int J Acad Stud 3(1):01–13
Tiskar M, Galai M, Elhadiri H, EbnTouhami M, Sfaira M, Satrani B, Ghanmi M, Chaouch A, Touir R (2016) Juniperus phoenicea essential oil as green corrosion inhibitor for mild steel in molar hydrochloric acid. J Matér Tech 104:609. https://doi.org/10.1051/mattech/2017003
Pilettia R, Zanettib M, Jungb G, Muneron de Mello JM, Dalcantonc F, Soaresa C, GracherRiellaa H, Antônio Fiori M (2019) Microencapsulation of garlic oil by β-cyclodextrin as a thermal protection method for antibacterial action. Mater Sci Eng C 94:139–149
Cardelle-Cobas A, Soria AC, Corzo-Martínez A, Villamiel M (2010) A comprehensive survey of garlic functionality. In: Pacurar M, Krejci G (eds) Book of garlic consumption and health publisher. Nova Science Publishers Inc., Hauppauge, pp 1–60. https://doi.org/10.13140/2.1.4992.6728
Al Mhyawi SR (2014) Corrosion inhibition of aluminum in 0.5M HCl by garlic aqueous extract oriental. J Chem 30(2):541–552. https://doi.org/10.13005/ojc/300218
Barreto LS, Tokumoto MS, Guedes IC, Melo HG, Amado FDR, Capelossi VR (2017) Evaluation of the anticorrosion performance of peel garlic extract as corrosion inhibitor for ASTM 1020 carbon steel in acidic solution. Matéria (Rio de Janeiro). https://doi.org/10.1590/s1517-707620170003.0186
Yang B, Chen W, Mu DH (2014) Study of garlic extract as a green corrosion inhibitor for mild steel in acidic media. Adv Mater Res 960–961:118–125. https://doi.org/10.4028/www.scientific.net/amr.960-961.118
Devikala S, Kamaraj P, Arthanareeswari M, Patel MB (2019) Green corrosion inhibition of mild steel by aqueous Allium sativum extract in 3.5% NaCl. Mater Today Proc 14:580–589. https://doi.org/10.1016/j.matpr.2019.04.182
Afia L, Benali O, Salghi R, EbensoEno E, Jodeh S, Zougagh M, Hammouti B (2014) Steel corrosion inhibition by acid garlic essential oil as a green corrosion inhibitor and sorption behavior. Int J Electrochem Sci 9(2014):8392–8406
Satyal P, Craft JD, Dosoky NS, Setzer WN (2017) The chemical compositions of the volatile oils of garlic (Allium sativum) and wild garlic (Allium vineale). J Foods 6:63. https://doi.org/10.3390/foods6080063
Pyun MS, Shin S (2006) Antifungal effects of the volatile oils from Allium plants against Trichophyton species and synergism of the oils with ketoconazole. Phytomedicine 13:394–400
Douiri L, Boughdad A, Assobhei O, Moumni M (2013) Chemical composition and biological activity of Allium sativum essential oils against Callosobruchus maculatus. IOSR J Environ Sci Toxicol Food Technol 3:30–36
Romeilah RM, Fayed SA, Mahmoud GI (2010) Chemical compositions, antiviral and antioxidant activities of seven essential oils. J Appl Sci Res 6:50–56
Daouda Toure (2015) Etudes Chimique Et Biologique Des Huiles Essentielles De Quatre Plantes Aromatiques Medicinales De Côte D’ivoire. Chimie organique. Université Felix HouphoeutBoigny, Côte d’Ivoire, 2015. Français. ffNNT: 29/2015ff. fftel-01222964f
Boukhatem MN, Ferhat A, Kameli A (2019) Méthodes d’extraction et de distillation des huiles essentielles : revue de littérature. Rev Agrobiol 9(2):1653–1659
Satyal P, Craft J, Dosoky N, Setzer W (2017) The chemical compositions of the volatile oils of garlic (Allium sativum) and wild garlic (Allium vineale). Foods (Basel, Switzerland). https://doi.org/10.3390/foods6080063
Khaled KF (2008) Adsorption and inhibitive properties of a new synthesized guanidine derivative on corrosion of copper in 0.5M H2SO4. Appl Surf Sci 255(5):1811–1818. https://doi.org/10.1016/j.apsusc.2008.06.030
Quartarone G, Moretti G, Bellomi T, Capobianco G, Zingales A (1998) Using indole to inhibit copper corrosion in aerated 0.5M sulfuric acid. Corrosion 54(8):606–618. https://doi.org/10.5006/1.3287636
Wang DKY, Coller BAW, Macfarlane DR (1993) A kinetic model for the dissolution mechanism of copper in acidic sulfate solutions. Electrochim Acta 38:2121
Smyrl WH (1981) Comprehensive treatise of electrochemistry, vol 4. Plenum Press, New York, p 116
Kaesche H (1985) Mettalic corrosion. NACE, Houston, p 124
Jinturkarp YC, Han KN (1998) Dissolution and corrosion inhibition of copper, zinc and their alloys. Corrosion 54(2):106–114
Tan B, Zhang S, Qiang Y, Feng L, Liao C, Xu Y, Chen S (2017) Investigation of the inhibition effect of Montelukast Sodium on the copper corrosion in 0.5 mol/L H2SO4. J Mol Liq 248:902–910. https://doi.org/10.1016/j.molliq.2017.10.111
El Faydy M, Galai M, EbnTouhami M, Obot IB, Lakhrissi B, Zarrouk A (2017) Anticorrosion potential of some 5-amino-8-hydroxyquinolines derivatives on carbon steel in hydrochloric acid solution: gravimetric, electrochemical, surface morphological, UV–visible, DFT and Monte Carlo simulations. J Mol Liq 248:1014–1027. https://doi.org/10.1016/j.molliq.2017.10.125
Qiang Y, Zhang S, Wang L (2019) Understanding the adsorption and anticorrosive mechanism of DNA inhibitor for copper in sulfuric acid. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2019.06.190
Ouakki M, Rbaa M, Galai M, Lakhrissi B, Rifi EH, Cherkaoui M (2018) Experimental and quantum chemical investigation of imidazole derivatives as corrosion inhibitors on mild steel in 1.0 M hydrochloric acid. J Bio Tribo Corros 4:35
Kadiri L, Galai M, Ouakki M, Essaadaoui Y, Ouass A, Cherkaoui M, Rifi EL, Lebkiri A (2018) J Anal Bioanal Electrochem 10(2):249–268
Cruz J, Martínez R, Genesca J, Ochoa E (2004) Experimental and theoretical study of 1-(2-ethylamino)-2-methylimidazoline as an inhibitor of carbon steel corrosion in acid media. J Electroanal Chem 566:111
Bentiss F, Traisnel M, Vezin H, Lagrenée M (2003) Linear resistance model of the inhibition mechanism of steel in HCl by triazole and oxadiazole derivatives: structure–activity correlations. Corros Sci 45:371
Jmiai A, El Ibrahimi B, Tara A, El Issami S, Jbara O, Bazzi L (2018) Alginate biopolymer as green corrosion inhibitor for copper in 1 M hydrochloric acid: experimental and theoretical approaches. J Mol Struct 1157:408–417. https://doi.org/10.1016/j.molstruc.2017.12.060
Rehim SSA, Hassan HH, Amin MA (2002) Corrosion and corrosion inhibition of Al and some alloys in sulphate solutions containing halide ions investigated by an impedance technique. Appl Surf Sci 189:279
M’hanni N, Galai M, Anik T, EbnTouhami M, Rifi EH, Asfari Z, Touir R (2017) Influence of additives selected calix [4] arenes on electroless copper plating using hypophosphite as reducing agent. Surf Coat Technol 310:8–16
Galai M, Ouassir J, EbnTouhami M, Nassali H, Benqlilou H, Belhaj T, Berrami K, Mansouri I, Oauki B (2017) α-Brass and (α + β) brass degradation processes in azrou soil medium used in plumbing devices. J Bio Tribo Corros 3:30
Zheng X, Zhang S, Li W, Yin L, He J, Wua J (2014) Investigation of 1-butyl-3-methyl-1H-benzimidazolium iodide as inhibitor for mild steel in sulfuric acid solution. Corros Sci 80:383
Rahmouni K, Keddam M, Srhiri A, Takenouti H (2005) Corrosion of copper in 3% NaCl solution polluted by sulphide ions. Corros Sci 47:3249–3266
Kardas G (2005) The inhibition effect of 2-thiobarbituric acid on the corrosion performance of mild steel in HCl solutions. J Mater Sci 41:337
Essaadaoui Y, Galai M, Ouakki M, Kadiri L, Ouass A, Cherkaoui M, Rifi E, Lebkiri A (2019) Study of the anticorrosive action of eucalyptus camaldulensis extract in case of mild steel in 1.0 M HCl. J Chem Technol Metall 54(2):431–442
Deyab MA (2015) Egyptian licorice extract as a green corrosion inhibitor for copper in hydrochloric acid solution. J Ind Eng Chem 22:384–389. https://doi.org/10.1016/j.jiec.2014.07.036
Deyab MA (2013) Effect of halides ions on H2 production during aluminum corrosion in formic acid and using some inorganic inhibitors to control hydrogen evolution. J Power Sources 242(2013):86
Deyab MA (2007) Effect of cationic surfactant and inorganic anions on the electrochemical behavior of carbon steel in formation water. Corros Sci 49(2007):2315
Ouakki M, Galai M, Cherkaoui M, Rifi E, Hatim Z (2018) Inorganic compound (Apatite doped by Mg and Na) as a corrosion inhibitor for mild steel in phosphoric acidic medium. Anal Bioanal Electrochem 10(7):943–960
Rbaa M, Galai M, El Kacimi Y, Ouakki M, Touir R, Lakhrissi B, EbnTouhami M (2017) Adsorption properties and inhibition of carbon steel corrosion in a hydrochloric solution by 2-(4,5-diphenyl-4,5-dihydro-1h-imidazol-2-yl)-5 methoxyphenol, Portugaliae. Electrochim Acta 35(6):323–338
Dkhireche N, Galai M, El Kacimi Y, Rbaa M, Ouakki M, Lakhrissi B, EbnTouhami M (2018) New quinoline derivatives as sulfuric acid inhibitor’s for mild steel. Anal Bioanal Electrochem 10(1):111–135
Hong S, Chen W, Lao HQ, Li NB (2012) Inhibition effect of 4-amino-antipyrine on the corrosion of copper in 3 wt.% NaCl solution. Corros Sci 57:270
Nabah R, Benhiba F, Ramli Y, Ouakki M, Cherkaoui M, Oudda H, Touir R, Warad I, Zarrouk A (2018) Corrosion inhibition study of 5, 5-diphenylimidazolidine-2, 4-dione for mild steel corrosion in 1 M HCl solution: experimental, theoretical computational and Monte Carlo simulations studies. Anal Bioanal Electrochem 10(10):1375–1398
Oguzie EE (2008) Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corros Sci 50:2993
Oguzie EE, Onuoha GN, Onuchukwu AL (2005) Inhibitory mechanism of mild steel corrosion in 2 M sulphuric acid solution by methylene blue dye. Mater Chem Phys 89:305
Mistry BM, Patyl NS, Sahoo S, Junhari S (2012) Experimental and quantum chemical studies on corrosion inhibition performance of quinoline derivatives for MS in 1N HCl. Bull Mater Sci 35:459–469
Guo L, Dong W, Zhang S (2014) Theoretical challenges in understanding the inhibition mechanism of copper corrosion in acid media in the presence of three triazole derivatives. RSC Adv 4:41956
Khaled KF, Amin MA (2009) Dry and wet lab studies for some benzotriazole derivatives as possible corrosion inhibitors for copper in 1.0 M HNO3. Corros Sci 51:2098–2106
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mzioud, K., Habsaoui, A., Ouakki, M. et al. Inhibition of copper corrosion by the essential oil of Allium sativum in 0.5M H2SO4 solutions. SN Appl. Sci. 2, 1611 (2020). https://doi.org/10.1007/s42452-020-03393-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03393-8