Abstract
Spinal epidural hematomas (SEH) are a rare hemorrhagic event occurring after trauma, epidural anesthesia, or operative inventions. However, in 40–50% of cases, they occur spontaneously. Spontaneous spinal epidural hematomas (SSEH) are rare in occurrence with an estimated incidence of 1 case per million annually. Pregnancy is an independent risk factor. Sudden neck or back pain, often in combination with a rapid onset of neurological symptoms, is the most common presentation of SEH (1). A 36-year-old Caucasian female with rheumatoid arthritis (RA) presented to the emergency department approximately 48 h after an uncomplicated vaginal delivery. She sought medical attention due to constant headaches and neck pain that started during active labor. An MRI of the spine revealed an extensive SEH spreading from C1 to L5. The patient was without neurological symptoms or deficits and was successfully treated conservatively without any sequelae. Even though the definitive cause of this case of SEH will remain unknown, several possible synergistic mechanisms have been identified. These include female gender, full-term pregnancy, physical activity with increased intraabdominal pressure (i.e., Valsalva maneuver), systemic administration of platelet aggregation inhibitor (PAI), and iatrogenic manipulation such as spinal epidural anesthesia. Even though autoimmune and inflammatory disorders have been described in the literature to be rare sources of hemorrhage in the spinal canal, it is unclear whether the patient’s RA should be regarded as an individual risk factor.
Key Facts
-
Spinal epidural hematomas are a rare hemorrhagic event characterized by an accumulation of blood between the vertebrae and the dura of the spinal canal, and can be a clinical challenge to diagnose due to the insidious presentation, with symptoms ranging from asymptomatic to non-specific head or neck pain, or neurological deficits.
-
The etiology of spontaneous spinal epidural hematomas remains largely unknown, although there are many predisposing factors, including vascular malformation, anticoagulation therapy, trauma, iatrogenic manipulations, and hypertension.
-
The differential diagnosis to spinal epidural hematomas are several, e.g., meningitis, migraine, subarachnoid or intracranial hemorrhage, pituitary apoplexy, venous sinus thrombosis, and thrombosis, and radiological examination is key to diagnose and map the distribution of the hemorrhage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal epidural hematomas (SEH) are a rare hemorrhagic event characterized by an accumulation of blood in the loose areolar tissue between the vertebrae and the dura of the spinal canal. Though often asymptomatic, devastating neurological consequences can occur if there is compression of the spinal cord. SEH most often occurs after trauma, epidural anesthesia, or operative inventions [1]. There are several well-documented predisposing factors for developing SEH, such as anticoagulant therapy, inherited coagulation disorders (i.e., hemophilia), vascular malformations (i.e., arteriovenous malformation), arthritis, and iatrogenic causes such as spinal or epidural puncture [2]. Spontaneous spinal epidural hematomas (SSEH) are rare in occurrence with an estimated incidence of 1 case per million annually, with the highest incidence in the age range 50- to 80-year-old subjects. Pregnancy is an independent risk factor [1]. The pathophysiology of SSEH is not conclusive and some data suggest that the source of bleeding is of venous origin [3,4,5,6], whereas other data suggests an arterial source [7]. Autoimmune or inflammatory disorders have also been described in the literature to be rare sources of hemorrhage in the spinal canal [1, 8, 9].
Non-specific neck or back pain is considered to be the earliest sign of SEH, though painless presentations of SEH have been described [3]. In a systematic review by Soltani et al. in 2019, all 16 patients with SEH included in the review presented with symptoms of back or neck pain [6]. The hemorrhage in the extradural space may lead to spinal cord or nerve root compression that may potentially cause neurological deficits or even death. Hence, prompt diagnosis is key; however, because of its rarity, and sometimes modest symptoms, it may present a clinical challenge.
Pregnancy results in an increased blood volume and increased pressure on the vascular walls which may lead to rupturing of vessels and subsequent SSEH, especially if vascular pathology is present. Because pregnancy is considered a hypercoagulable state (with hypercoagulability persisting through the first 2 weeks post-delivery), in the absence of predisposing factors, the incidence of SSEH is extremely low in this patient population [6]. Pre-eclampsia has been reported in a few cases of SSEH; however, it has not been identified as an individual risk factor [7]. To our knowledge, no cases of SEH have been reported to debut during labor, although there are reports on cases of SEH days up to weeks following delivery.
We herein report on a rare occurrence of extensive SEH, with a distribution extending to the majority of vertebrae, in a postpartum patient with rheumatoid arthritis (RA). This case report aims to disclose and discuss the possible causes of SEH, as well as the diagnostics.
Case Report
A 36-year-old Caucasian female, gravida 2 and para 2, with a BMI of 29 and healthy apart from rheumatoid arthritis (RA) presented to the emergency department approximately 48 h after vaginal delivery of her second child. She sought medical attention due to constant headache and neck pain that started during active labor. For headache relief, non-opioid-based analgesics had been given, according to local protocols, without any effect.
Nine years earlier, the patient had been diagnosed with RA, which before to pregnancy was treated with methotrexate and etanercept. This drug regime was interrupted when pregnancy was planned and instead a daily dose of prednisolone and NSAID was administered until gestational week 20. Thereafter, a single dose of prednisolone was administered daily. During pregnancy, the patient had an uncomplicated course except for mild arthralgia and neck stiffness. The patient had no medical record of vascular or hematological pathology. Blood count including platelets was normal. Autoimmune blood analyses of beta-2-glycoprotein and cardiolipin antibodies were all tested negative. Seven years earlier, the patient had given birth to a healthy full-term baby.
There were no complications during pregnancy and no signs of preeclampsia. The baby had a normal intrauterine growth curve. Due to the patient’s RA, she was checked more frequently at the maternal healthcare center during the pregnancy. At gestational week 39 + 4, labor started spontaneously. Early in the phase of active labor, the patient received spinal epidural anesthesia (EDA) of sufentanil, which provides a complete analgesia in early labor without a sympathectomy or motor blockade. The patient also received nitrous oxide gas during labor. The EDA catheter was successfully placed at the first attempt at the L2-L3 level using the loss of resistance technique and rounded bevel cutting edge and sharp needle (Tuohy Epidural Needle 18 G). The loss of resistance was felt at an approximate depth of 5 cm and the catheter was placed 7 cm into the epidural space. The EDA procedure was without complications: no accidental dural puncture or signs of bleeding. Routine cardiotocography (CTG) was recorded during labor. Maternal intrapartum fever was noted whereupon a dose of antibiotics was given intravenously. In the final stages of labor, decelerations on the CTG were observed and an infusion of oxytocin was given to accelerate the delivery. Soon thereafter, the child was delivered with the aid of vacuum extraction as a consequence of the CTG changes and maternal fatigue. The baby had an APGAR score of 3 at 1 min, which improved to 4 at 5 min, and 8 at 10 min upon delivery. Shortly after partum, the patient complained of neck pain, radiating towards the head. The patient recalls the onset of pain while carrying out the Valsalva maneuver during active labor and described it as being similar to that of her RA-related neck pain. The fact that the neck pain mimicked the patient’s regular neck complaints, in combination with efficient pain relief from standard dosages of paracetamol and ibuprofen, probably contributed to that the patient’s neck sensation was not considered alarming at the maternity ward. The patient was discharged from the hospital approximately 24 h after giving birth, as she fulfilled the regional criteria for early admission to home (discharge < 48 h after delivery with a follow-up within 72 h), consistent with the national guidelines for postpartum care (Supplementary table 1 and 2).
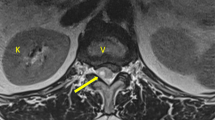
The morning after, the patient returned to the hospital due to a worsening headache. The headache was still radiating from the neck, involving the whole head with no correlation to body position. The patient had taken full dosages of paracetamol and ibuprofen, but as this point without any pain relief. Pain intensity was rated 9 out of 10 on a verbal numerical rating scale (NRS). The patient’s vital parameters were within the normal range (see Supplementary material). All blood samples were within a normal range including normal activated partial thromboplastin time, prothrombin time, and platelet count, and there was no sign of meningitis or any other source of infection, and the neurological examination did not reveal anything deficits: no paralysis, paresis, paresthesia, or signs of increased intracranial pressure. The pupillary light reflex was normal. Hence, there were no clinical signs of severe intracranial bleeding and the patient’s problem of constant and severe neck pain and headache was at that time unexplained. Opioids were given in addition to paracetamol and ibuprofen and a CT of the cervical spine was performed, showing no fractures. A decrease in the joint space of the left facet joint at the C2-C3 level, as well as some osteophytes in the posterior aspect at the same level, was noted (Fig. 1). Minor signs of disc degeneration, previously known to the patient, were also shown. These changes had not progressed during pregnancy. There were no signs of infectious origin or increased intracranial pressure (ICP), but a spinal pathology had not been ruled out. An MRI of the neck and subsequently of the full spine was therefore performed the following day for further diagnostics, and revealed an extensive epidural hematoma extending from C1 of the cervical spine to the lumbar regions of L5 (Fig. 2). The hematoma in the thoracic region shows compression of the epidural space, suggesting conditions that might affect the flow of the cerebrospinal fluid (CSF) (Fig. 2).
Sagittal MRI STIR sequence of cervical, thoracic, and lumbar spine showing an extensive and widespread epidural hematoma reaching from occiput to L5, with a width of approximately 4–5 mm at the thoracic region and 3–4 mm at the lumbar region. The liquor space is compressed to approximately 1 mm in width. No intra-medullar signal changes. A subcutaneous edema is also seen at the level of Th12-L3, likely a consequence of pressure on the soft tissue as a consequence of the patent being bedridden. MRI, magnetic resonance imaging; L, lumbar vertebrae; Th, thoracic vertebrae; STIR, short-TI inversion recovery
Due to the MRI findings, the patient was admitted to the neuro-intensive care unit for neurological monitoring and continuous morphine infusion for pain relief. The SEH was conservatively treated as the patient remained stable without progressive symptoms or development of neurological deficits. The patient remained with moderate to mild headache relieved by standard pain relief and short-acting morphine tablets for approximately 2 weeks prior to dismissal from the hospital, after which the symptoms fully regressed. No sequelae or other medical complications developed and the baby showed normal development.
Discussion
We herein describe an unusual case of SEH spanning over 24 vertebras in a 36-year-old woman with RA that developed severe neck pain and headache while performing a Valsalva maneuver during vaginal delivery. The average size of an SSEH extends across 3.9 levels [6], making this case rare. According to the literature, a key clinical feature in many SSEH cases is well-localized back pain, often following minimal physical activity [10]. It might be speculated that the patient had a spontaneous rupture of an epidural vessel during labor resulting in SSEH. The course was prolonged and difficult, which would likely have substantially increased the risk of SEH from straining. However, we believe that the most plausible explanation for this exceptional SEH case is of multifactorial origin. Another key feature following localized pain is a sudden onset of neurological deficits [5]. Interestingly, the patient in our case study did not develop any neurologic dysfunction, which is very rare. In a systematic review of 16 cases of SSEH in pregnancy, only one case did not progress with neurological symptoms [11]. According to the patient’s MRI scan, the CSF space was significantly reduced due to the hematoma, particularly in the thoracic region of the vertebrae (Fig. 2).
The etiology of SSEH remains largely unknown, although there are many predisposing factors, including vascular malformation, anticoagulation therapy, trauma, iatrogenic manipulations, and hypertension [12,13,14,15,16,17]. The increased blood volume and increased pressure exerted on the vascular walls during pregnancy has also been reported as a risk for spontaneous rupture of vessels and subsequent SSEH. The risk is further increased if the patient has pre-eclampsia or known vascular pathology [4]. Still, pregnancy and postpartum status are both a state of hypercoagulation and hence reducing the risk of hemorrhage during and after delivery [7].
The patient in this case suffered from RA, an autoimmune disease assumed to predispose to cardiac and vascular diseases [18]. We have, however, not found any published data showing RA to be a predisposing factor for SEH. Although one could speculate whether a systemic inflammatory disease would potentially affect the collagenous structures such as the dura mater and vascular walls increasing the risk of SEH.
The patient received spinal epidural anesthesia during the early stages of labor. The most common complication of epidural anesthesia is a post-dural puncture headache (PDPH). It most frequently occurs in cases in which the dura mater is accidentally punctured during the procedure [19], which can be revealed by the extravasation of blood. No such complication has been reported in this case and there was no positional aspect to the patient’s headache, a defining feature of PDPH. Spinal-epidural anesthesia, however, has been documented to be an iatrogenic cause of intracranial hypotension resulting in a venous dilatation, which is considered to cause headache via meningeal traction and subdural effusions as well as possible hematomas occurring via the rupture of bridging veins [20]. On the MRI scans, there is a small amount of liquid surrounding the C1 vertebra, implying that a minor dural tear or rift might have occurred, e.g., due to spinal epidural anesthesia or during a Valsalva maneuver during vaginal child delivery. The cervical spine is a common location for dural rifts and vertebral osteophytes have been described as a possible cause of dural rifts [21]. In this particular case, there are no osteophytes visible at the level of where the minor liquid collection is found (Fig. 3). However, the reduced amount of CSF surrounding the cerebellum and medulla (Fig. 4) suggests a mild form of sagging brain, which is often associated with low CSF pressure headaches. Additional brain scans would have been required to address this issue in further detail. It cannot completely be ruled out that the fluid collection at C1 is caused by CSF leakage from a dural tear; however, it is highly unlikely that this small amount of fluid would cause a significant drop in ICP. Intracranial hypotension usually develops over weeks or months, but it is a known cause of widespread epidural or subdural hematoma [22]. In our case, there is no sign of a subdural hematoma at the clivus or cervical cranial junction. As there are no additional scans of the brain, no further analysis of intracranial hemorrhage can be made. Additional differential diagnoses to SSEH such as subarachnoid or intracranial hemorrhage, pituitary apoplexy, and sinus venous thrombosis could also be considered in this and similar cases, as these diagnoses may present with analog symptoms.
The physically demanding exercise of child labor and a Valsalva maneuver could lead to increased intra-abdominal pressure that in turn could cause spontaneous rupturing of the epidural vessels. It is possible that the sudden onset of pain that the patient experienced during the end-stage of delivery corresponds to the extravasation of blood in the epidural space. Jacob et al. describe a middle-aged woman with spinal vasculitis resulting in an acute epidural spinal hemorrhage which first presented with a sudden non-traumatic onset of stabbing back pain followed by headache [23], although it has also been argued in the literature that patients presenting with SEH postpartum usually do so days or weeks after delivery, suggesting that the pregnancy-induced dilation of epidural vessels, rather than the mechanical straining of labor, is the predominant cause of SSEH in pregnancy [1].
A traumatic mechanism such as a minor bone fracture induced by mild trauma, for example, by neck distortion during active labor, could explain the sudden onset of neck pain. Due to the patient’s RA, thrusting the chin against her chest was associated with discomfort. There were, however, no direct signs of fracture or indirect signs such as bone edema in the vertebrae (Fig. 2). Even though micro-fracturing of the vertebrae cannot be fully ruled out, it is unlikely to cause a massive epidural hemorrhage and a fracture-induced vascular puncture is considered low in this case.
In addition to the patient’s RA, there had been a continuous long-term administration of modest amounts of glucocorticosteroids such as prednisolone (7.5–10 g per os daily, see supplementary material). Even though glucocorticoids are known to affect vascular tone and resistance, as well as atherogenesis, there is to our knowledge, however, no correlation between glucocorticoid administration and the incidence of SSEH [23].
The patient has no history of coagulopathy or bleeding catastrophes, and laboratory hematological findings were normal. Furthermore, postpartum status implies hypercoagulable homeostasis, lowering the risk for hemorrhages. Once the patient’s baby was born, however, the patient was administered full-dose ibuprofen in combination with paracetamol the following 48 h in an attempt to dampen the neck and head pain. Long-time aspirin usage as PAI therapy has been reported to be a risk factor for developing an SEH of the spine [24, 25], and according to local protocols, PAIs are withdrawn at least 12 h prior to neuraxial interventions (e.g., epidural anesthesia). This is in order to minimize the risk for SEH. It seems plausible that the therapeutic dose of NSAID ingested postpartum during 48 h would potentially have contributed to the slow extravasation of blood into the epidural space and therefore enabled the formation of an extensive SEH.
In addition to the anti-platelet effect of ibuprofen, a venous origin of the hematoma would also contribute to a slow formation and limited mass effect of the hematoma, enabling the spinal cord to adapt to the pressure changes [5]. The pressure of the epidural venous system is lower than the intrathecal pressure at the same level [10]. Interestingly, Miyagi et al. [26] describe rapid deteriorating neurological deficits after the initial onset of dorsalgia in patients with SEH, which they suspected to have an arterial origin due to the quick formation of the hematoma and spinal cord compression. Hence, the time-lapse of the clinical presentation of an SEH, in combination with predisposing factors, may provide an insight into the origin of the extravasated blood since the source of the bleeding (arterial vs venous) may influence the rate of hematoma formation. One could argue that an elongated SEH would imply a more favorable prognosis since an extensive distribution of the epidural blood mass has less impact on the spinal cord and intrathecal pressure. However, a spread hematoma might denote a greater blood volume within the epidural space, causing more compression on the spinal cord.
The assumption that the SEH in this case was of venous origin is further supported by the post-pregnant status of the patient. During pregnancy, epidural venous pressure is increased due to an elevation of the intraabdominal pressure caused by the hypertrophic uterus, and a direct compression of the vena cava [27]. Those two physiological conditions divert a portion of the venous return from the lower extremities and pelvic region into the vertebral venous system, resulting in a higher blood volume in the extradural venous plexus predisposing to dilation and rupture of the epidural vessels [6]. The epidural veins lack valves, implying that elevated venous pressure in the epidural space during pregnancy may increase the risk for SSEH, especially during straining, coughing, or strenuous efforts such as a Valsalva maneuver [4, 7].
Furthermore, there were no radiological signs of an arteriovenous fistula or malformation. Female gender and pregnancy are predisposing for spontaneous dissection of the coronary arteries [28], vertebral arteries [29], and splenic arteries [27]. Estrogen and progesterone excess during the third trimester of pregnancy has been proposed to cause degenerative structural changes in arterial and venous vessels [27]. It remains unclear if the same degenerative changes occur in other smaller vessels, such as those of the epidural venous plexus.
Conclusions
The incidence of SSEH is very low in the general population, the diagnosis is a clinical challenge, and the etiology of SSEH is partly unknown. In this case study, we describe an unusual case of SEH in a female RA patient that developed neck and head pain during labor. The MRI scan revealed an SEH extending over 24 vertebrae. Despite this extensive hematoma, no neurological symptoms developed and the patient was successfully treated conservatively without any sequelae. Even though the definitive cause of this case of SEH will remain unknown, several possible synergistic mechanisms have been identified. These include female gender, full-term pregnancy, physical activity with increased intraabdominal pressure (i.e., a Valsalva maneuver), systemic administration of PAI, and iatrogenic manipulation such as spinal-epidural anesthesia. Even though autoimmune and inflammatory disorders have been described in the literature to be rare sources of hemorrhage in the spinal canal, it is unclear whether the patient’s RA should be regarded as an individual risk factor.
Data Availability
All journal information from which this case report is based is stored in accordance with national regulations in the local journal system NCS Cross used in Västerbotten County, Umeå, Sweden.
Code Availability
Not applicable.
References
Kakitsubata Y, Theodorou SJ, Theodorou DJ, Miyata Y, Ito Y, Yuki Y, et al. Spontaneous spinal subarachnoid hemorrhage associated with subdural hematoma at different spinal levels. Emerg Radiol. 2010;17(1):69–72.
Sage DJ. Epidurals, spinals and bleeding disorders in pregnancy: a review. Anaesth Intensive Care. 1990;18(3):319–26.
Senelick RC, Norwood CW, Cohen GH. “Painless” spinal epidural hematoma during anticoagulant therapy. Neurology. 1976;26(3):213–25.
Carroll SG, Malhotra R, Eustace D, Sharr M, Morcos S. Spontaneous spinal extradural hematoma during pregnancy. J Matern Fetal Med. 1997;6(4):218–9.
Liu Z, Jiao Q, Xu J, Wang X, Li S, You C. Spontaneous spinal epidural hematoma: analysis of 23 cases. Surg Neurol. 2008;69(3):253–60 (discussion 60).
Soltani S, Nogaro MC, Jacqueline Kieser SC, Wyatt MC, Kieser DC. Spontaneous spinal epidural hematomas in pregnancy: a systematic review. World Neurosurg. 2019;128:254–8.
Yonekawa Y, Mehdorn HM, Nishikawa M. Spontaneous spinal epidural hematoma during pregnancy. Surg Neurol. 1975;3(6):327–8.
Klingler JH, Glasker S, Shah MJ, Van Velthoven V. Rupture of a spinal artery aneurysm attributable to exacerbated Sjogren syndrome: case report. Neurosurgery. 2009;64(5):E1010-1 (discussion E1).
Massand MG, Wallace RC, Gonzalez LF, Zabramski JM, Spetzler RF. Subarachnoid hemorrhage due to isolated spinal artery aneurysm in four patients. AJNR Am J Neuroradiol. 2005;26(9):2415–9.
Beatty RM, Winston KR. Spontaneous cervical epidural hematoma. A consideration of etiology J Neurosurg. 1984;61(1):143–8.
Soltani S, Nogaro MC, Rougelot C, Newell N, Lim K, Kieser DC. Spontaneous spinal epidural haematomas in children. Eur Spine J. 2019;28(10):2229–36.
Aksay E, Kiyan S, Yuruktumen A, Kitis O. A rare diagnosis in emergency department spontaneous spinal epidural hematoma. Am J Emerg Med. 2008;26(7):835 e3–5.
Stetkarova I, Jelinkova L, Janik V, Peisker T. Spontaneous spinal epidural hematoma after abrupt sneezing with prompt recovery of severe paraparesis. Am J Emerg Med. 2014;32(12):1555 e3–5.
Silber SH. Complete nonsurgical resolution of a spontaneous spinal epidural hematoma. Am J Emerg Med. 1996;14(4):391–3.
Miller JB, Khalsa G, Vohra T. Spontaneous spinal epidural hematoma presenting as flank pain and constipation. Am J Emerg Med. 2010;28(4):536 e3–5.
Sivakumaran R, King A, Bodi I, Chandler CL, Walsh DC. Spontaneous epidural spinal haematoma in children caused by vascular malformations. Eur Spine J. 2016;25(2):614–8.
Hsieh CT, Chang CF, Lin EY, Tsai TH, Chiang YH, Ju DT. Spontaneous spinal epidural hematomas of cervical spine: report of 4 cases and literature review. Am J Emerg Med. 2006;24(6):736–40.
Blasi C. The autoimmune origin of atherosclerosis. Atherosclerosis. 2008;201(1):17–32.
Jabbari A, Alijanpour E, Mir M, Bani Hashem N, Rabiea SM, Rupani MA. Post spinal puncture headache, an old problem and new concepts: review of articles about predisposing factors. Caspian J Intern Med. 2013;4(1):595–602.
Lin JP, Zhang SD, He FF, Liu MJ, Ma XX. The status of diagnosis and treatment to intracranial hypotension, including SIH. J Headache Pain. 2017;18(1):4.
Williams EC, Buchbinder BR, Ahmed S, Alston TA, Rathmell JP, Wang J. Spontaneous intracranial hypotension: presentation, diagnosis, and treatment. Anesthesiology. 2014;121(6):1327–33.
Pabaney AH, Mirza FA, Syed NA, Ahsan H. Spontaneous dural tear leading to intracranial hypotension and tonsillar herniation in Marfan syndrome: a case report. BMC Neurol. 2010;10:54.
Jacob JT, Tanaka S, Wood CP, Wijdicks EF, Lanzino G. Acute epidural spinal hemorrhage from vasculitis: resolution with immunosuppression. Neurocrit Care. 2012;16(2):311–5.
Baba Y, Tsuboi Y, Fujino Y, Takahashi M, Yamada T. [Extended spontaneous spinal epidural anterior hematoma over multiple spinal segments. A case report]. Rinsho Shinkeigaku. 2000;40(6):566–70.
Weber J, Hoch A, Kilisek L, Spring A. Spontaneous intraspinal epidural hematoma secondary to use of platelet aggregation inhibitors. Dtsch Med Wochenschr. 2001;126(31–32):876–8.
Miyagi Y, Miyazono M, Kamikaseda K. Spinal epidural vascular malformation presenting in association with a spontaneously resolved acute epidural hematoma. Case report J Neurosurg. 1998;88(5):909–11.
Nolte JE, Rutherford RB, Nawaz S, Rosenberger A, Speers WC, Krupski WC. Arterial dissections associated with pregnancy. J Vasc Surg. 1995;21(3):515–20.
Bucciarelli E, Fratini D, Gilardi G, Affronti G. Spontaneous dissecting aneurysm of coronary artery in a pregnant woman at term. Pathol Res Pract. 1998;194(2):137–9.
Mass SB, Cardonick E, Haas S, Gopalani S, Leuzzi RA. Bilateral vertebral artery dissection causing a cerebrovascular accident in pregnancy. A case report J Reprod Med. 1999;44(10):887–90.
Acknowledgements
This manuscript had not been written if it had not been thanks to our senior medical colleagues at The Umeå University Hospital, Umeå, Sweden. They have shared our interest in the case and contributed with their clinical experiences and provided valuable feedback. We would therefore like to express our gratitude to the following: Simona Negri, MD, Senior specialist in obstetrics; Lukas Bobinski, MD, Ph.D., Senior specialist in spine surgery; Professor Lars-Owe Koskinen, MD, Ph.D., Senior specialist in neurosurgery; Thomas Lindqvist, MD, Senior specialist in neuro-radiology; Kerstin Adamsson, MD, Senior specialist in emergency medicine; Siv Törnell, MD, Senior specialist in anesthesia; Stefan Engstrand, MD, Senior specialist in rheumatology.
Funding
Open access funding provided by Umea University. The case report was supported by a research grant from Region Västerbotten (RV-3451 60 003) to ATB.
Author information
Authors and Affiliations
Contributions
ATB is the main author of this manuscript having the main role in the assembly of information and in the writing of the manuscript. JÅ has contributed by writing sections of the manuscript, providing and organizing radiological data, and images, as well as in the proofreading process. ATB has as a receiving doctor at the emergency department had a major role in the initial medical care of the patient. JÅ has been involved in the patient as consulted primary on call in the spine surgery unit, Umeå University hospital.
Corresponding author
Ethics declarations
Ethics Approval
No ethical permit was required to write this case report.
Consent to Participate
Informed consent to participate in the case report was obtained from the individual participant included in the study.
Consent for Publication
Informed consent for publication was obtained from the individual participant included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bäck, A.T., Åkerstedt, J. Extensive Spinal Epidural Hematoma as the Cause of Postpartum Headache and Neck Pain After Epidural Anesthesia: a Case-Based Report. SN Compr. Clin. Med. 4, 168 (2022). https://doi.org/10.1007/s42399-022-01238-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01238-6