Abstract
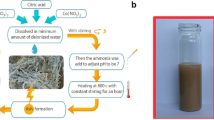
In this study, the modified Hummer method was used to prepare graphene oxide (GO) and magnetic graphene oxide (α-Fe2O3/GO). The as-obtained Go and α-Fe2O3/GO were characterized using Fourier Transform Infrared, Raman spectroscopy, X-ray diffraction, and Brunauer Emmett Teller. The adsorptive capacity of these materials towards cadmium (Cd2+) and lead (Pb2+) has been studied. An adsorption process with regeneration was carried out, such as equilibrium time, effect of the initial concentration of Cd2+ and Pb2+, effect of the amount of the GO, and α-Fe2O3/GO and pH effect. The adsorption of an aqueous solution of Cd2+ and Pb2+ with an initial concentration of 10–3 M of the two heavy metals onto 0.1 g of the prepared materials reached an equilibrium time in 2 h with an adsorption rate of more than 90% for both metals. The fine morphology of the adsorbents facilitated the rapid diffusion of the metals studied in the pores, which increased the kinetic. The kinetics can be described by the pseudo-second-order model with R2 = 0.993 and R2 = 0.997 for Cd2+ and Pb2+, respectively. The thermodynamic study reveals that the adsorption process is spontaneous, exothermic, and random as temperature increases. The adsorption mechanism included physical adsorption, ion exchange and possibly surface complexation. According to the results obtained and the ease of obtaining α-Fe2O3/GO, we can say that it is a promising adsorbent for Cd2+ and Pb2+. The magnetic nanoparticle α-Fe2O3/GO can be recovered using a magnet. As a result, it is a reusable and recyclable adsorbent.











Similar content being viewed by others
References
Rabia A, Yaseen M, Mukhtar A, Klemeš JJ, Saqib S, Ullah S, Al-Sehemi AG et al (2020) Lead and cadmium removal from wastewater using eco-friendly biochar adsorbent derived from rice husk, wheat straw, and corncob. Clean Eng Technol 1:100006. https://doi.org/10.1016/j.clet.2020.100006
Bhatluri KK, Manna MS, Ghoshal AK, Saha P (2017) Separation of cadmium and lead from wastewater using supported liquid membrane integrated with in-situ electrodeposition. Electrochim Acta 229:1–7. https://doi.org/10.1016/j.electacta.2017.01.090
Cheng S, Zhao S, Guo H, Xing B, Liu Y, Zhang C, Ma M (2022) High-efficiency removal of lead/cadmium from wastewater by MgO modified biochar derived from crofton weed. Bioresour Technol 343:126081. https://doi.org/10.1016/j.biortech.2021.126081
Cui N, Laiye Qu, Gang Wu (2022) Heavy metal accumulation characteristics and physiological response of Sabina chinensis and Platycladus orientalis to atmospheric pollution. J Environ Sci 112:192–201. https://doi.org/10.1016/j.jes.2021.05.013
Ding C, Xie A, Yan Z, Li X, Zhang H, Tang N, Wang X (2021) Treatment of water-based ink wastewater by a novel magnetic flocculant of boron-containing polysilicic acid ferric and zinc sulfate. J Water Process Eng 40:101899. https://doi.org/10.1016/j.jwpe.2020.101899
Chyad TF, Al-Hamadani RFC, Hammood ZA, Ali GA (2021) Removal of Zinc(II) ions from industrial wastewater by adsorption on to activated carbon produced from pine cone. Mater Today Proc. https://doi.org/10.1016/j.matpr.2021.07.016
Huy DH, Seelen E, Liem-Nguyen V (2020) Removal mechanisms of cadmium and lead ions in contaminated water by stainless steel slag obtained from scrap metal recycling. J Water Process Eng 36:101369. https://doi.org/10.1016/j.jwpe.2020.101369
Li T, Yang T, Yu Z, Xu G, Han Q, Luo G, Du J, Guan Y, Guo C (2021) Trivalent chromium removal from tannery wastewater with low cost bare magnetic Fe3O4 nanoparticles. Chem Eng Process Process Intensif 169:108611. https://doi.org/10.1016/j.cep.2021.108611
Li X-D, Xin L, Rong W-T, Liu X-Y, Deng W-A, Qin Y-C, Li X-L (2021) Effect of heavy metals pollution on the composition and diversity of the intestinal microbial community of a pygmy grasshopper (Eucriotettix oculatus). Ecotoxicol Environ Saf 223:112582. https://doi.org/10.1016/j.ecoenv.2021.112582
Liu T, Lawluvy Y, Shi Y, Ighalo JO, He Y, Zhang Y, Yap P-S (2021) Adsorption of cadmium and lead from aqueous solution using modified biochar: a review. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.106502
Ma M, Du Y, Bao S, Li J, Wei H, Lv Y, Song X, Zhang T, Du D (2020) Removal of cadmium and lead from aqueous solutions by thermal activated electrolytic manganese residues. Sci Total Environ 748:141490. https://doi.org/10.1016/j.scitotenv.2020.141490
Marichamy MK, Kumaraguru A, Jonna N (2021) Particle size distribution modeling and kinetic study for coagulation treatment of tannery industry wastewater at response surface optimized condition. J Clean Prod 297:126657. https://doi.org/10.1016/j.jclepro.2021.126657
Masoumi H, Ghaemi A, Gilani HG (2021) Elimination of lead from multi-component lead–nickel–cadmium solution using hyper-cross-linked polystyrene: experimental & RSM modeling. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.106579
Nair K, Manu SB, Azhoni A (2021) Sustainable treatment of paint industry wastewater: current techniques and challenges. J Environ Manag 296:113105. https://doi.org/10.1016/j.jenvman.2021.113105
Radelyuk I, Tussupova K, Klemeš JJ, Persson KM (2021) Oil refinery and water pollution in the context of sustainable development: developing and developed countries. J Clean Prod 302:126987. https://doi.org/10.1016/j.jclepro.2021.126987
Rezania S, Mojiri A, Park J, Nawrot N, Wojciechowska E, Marraiki N, Zaghloul NSS (2022) Removal of lead ions from wastewater using lanthanum sulfide nanoparticle decorated over magnetic graphene oxide. Environ Res 204:111959. https://doi.org/10.1016/j.envres.2021.111959
Rha S, Jo HY (2021) Data describing characteristics of waste foundry dust (WFD), sorbent obtained before and after batch sorption tests using As(III) and Cr(VI) aqueous solutions. Data Brief 35:106921. https://doi.org/10.1016/j.dib.2021.106921
Sharma MD, Krupadam RJ (2021) Adsorption-desorption dynamics of synthetic and naturally weathered microfibers with toxic heavy metals and their ecological risk in an estuarine ecosystem. Environ Res. https://doi.org/10.1016/j.envres.2021.112198
Stando G, Hannula P-M, Kumanek B, Lundström M, Janas D (2021) Copper recovery from industrial wastewater—synergistic electrodeposition onto nanocarbon materials. Water Resour Ind 26:100156. https://doi.org/10.1016/j.wri.2021.100156
Tao X, Hu X, Wen Z, Ming Y, Li J, Liu Y, Chen R (2022) Highly efficient Cr(VI) removal from industrial electroplating wastewater over Bi2S3 nanostructures prepared by dual sulfur-precursors: insights on the promotion effect of sulfate ions. J Hazard Mater 424:127423. https://doi.org/10.1016/j.jhazmat.2021.127423
Tran TK, Kim N, Le QC, Nguyen MT, Leu HJ, Thi KNV (2021) Electrochemical preparation and characterization of polyaniline enhanced electrodes: an application for the removal of cadmium metals in industrial wastewater. Mater Chem Phys 261:124221. https://doi.org/10.1016/j.matchemphys.2021.124221
Uugwanga MN, Kgabi NA (2021) Heavy metal pollution index of surface and groundwater from around an abandoned mine site, Klein Aub. Phys Chem Earth Parts A/B/C. https://doi.org/10.1016/j.pce.2021.103067
Vogel N, Murawski A, Schmied-Tobies MIH, Rucic E, Doyle U, Kämpfe A, Höra C et al (2021) Lead, cadmium, mercury, and chromium in urine and blood of children and adolescents in Germany—human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Int J Hyg Environ Health 237:113822. https://doi.org/10.1016/j.ijheh.2021.113822
Wang C, Li T, Yu G, Deng S (2021) Removal of low concentrations of nickel ions in electroplating wastewater using capacitive deionization technology. Chemosphere 284:131341. https://doi.org/10.1016/j.chemosphere.2021.131341
Zamora-Ledezma C, Negrete-Bolagay D, Figueroa F, Zamora-Ledezma E, Ni M, Alexis F, Guerrero VH (2021) Heavy metal water pollution: a fresh look about hazards, novel and conventional remediation methods. Environ Technol Innov 22:101504. https://doi.org/10.1016/j.eti.2021.101504
Zhou H, Zhao X, Kumar K, Kunetz T, Zhang Y, Gross M, Wen Z (2021) Removing high concentration of nickel(II) ions from synthetic wastewater by an indigenous microalgae consortium with a revolving Algal Biofilm (RAB) system. Algal Res 59:102464. https://doi.org/10.1016/j.algal.2021.102464
Berbar Y, Hammache ZE, Bensaadi S, Soukeur R, Amara M, Van der Bruggen B (2019) Effect of functionalized silica nanoparticles on sulfonated polyethersulfone ion exchange membrane for removal of lead and cadmium ions from aqueous solutions. J Water Process Eng 32:100953. https://doi.org/10.1016/j.jwpe.2019.100953
D Trishitman, A Cassano, A Basile, NK Rastogi (2020) 9—reverse osmosis for industrial wastewater treatment. In: Basile A, Cassano A, Rastogi NK (eds) Current trends and future developments on (bio-) membranes. Elsevier, pp 207–28. https://doi.org/10.1016/B978-0-12-816777-9.00009-5
Atanassova M (2021) Solvent extraction chemistry in ionic liquids: an overview of f-ions. J Mol Liq 343:117530. https://doi.org/10.1016/j.molliq.2021.117530
Xiong C, Li Y, Wang G, Fang L, Zhou S, Yao C, Chen Q et al (2015) Selective removal of Hg(II) with polyacrylonitrile-2-amino-1,3,4-thiadiazole chelating resin: batch and column study. Chem Eng J 259:257–265. https://doi.org/10.1016/j.cej.2014.07.114
Sun Y, Lan J, Du Y, Li Z, Liao X, Du D, Ye H, Zhang TC, Chen S (2020) Efficient removal of heavy metals by synergistic actions of microorganisms and waste molasses. Bioresour Technol 302:122797. https://doi.org/10.1016/j.biortech.2020.122797
Kessler E (1978) The role of parenteral hyperalimentation in the management of the burnt patient. Proc Mine Med Off Assoc S A 58(426):1–9
Ferrucci A, Vocciante M (2021) Improved management of water resources in process industry by accounting for fluctuations of water content in feed streams and products. J Water Process Eng 39:101870. https://doi.org/10.1016/j.jwpe.2020.101870
Yang S-S, Chen Y-d, Zhang Ye, Zhou H-M, Ji X-Y, He L, Xing D-F, Ren N-Q, Ho S-H, Wei-Min Wu (2019) A novel clean production approach to utilize crop waste residues as co-diet for mealworm (Tenebrio molitor) biomass production with biochar as byproduct for heavy metal removal. Environ Pollut 252:1142–1153. https://doi.org/10.1016/j.envpol.2019.06.028
Kadari M, Makhlouf M (2021) Graphene oxide (GO): synthesis, characterization and application to the retention of mercuric ions in aqueous solutions. Mater Today Proc. https://doi.org/10.1016/j.matpr.2021.09.426
Khan et al., Graphene oxide/PVC composite papers functionalized with p-phenylenediamine as high-performance sorbent for the removal of heavy metal ions
Chaabane L, Beyou E, Baouab MHV (2021) Preparation of a novel zwitterionic graphene oxide-based adsorbent to remove of heavy metal ions from water: modeling and comparative studies. Adv Powder Technol 32(7):2502–2516. https://doi.org/10.1016/j.apt.2021.05.011
Jalali K, Pajootan E, Bahrami H (2019) Elimination of hazardous methylene blue from contaminated solutions by electrochemically magnetized graphene oxide as a recyclable adsorbent. Adv Powder Technol 30(10):2352–2362. https://doi.org/10.1016/j.apt.2019.07.018
Mehta D, Mazumdar S, Singh SK (2015) Magnetic adsorbents for the treatment of water/wastewater—a review. J Water Process Eng 7:244–265. https://doi.org/10.1016/j.jwpe.2015.07.001
Xie Y, Qian D, Dongliang Wu, Ma X (2011) Magnetic halloysite nanotubes/iron oxide composites for the adsorption of dyes. Chem Eng J 168(2):959–963. https://doi.org/10.1016/j.cej.2011.02.031
Mak S-Y, Chen D-H (2004) Fast adsorption of methylene blue on polyacrylic acid-bound iron oxide magnetic nanoparticles. Dyes Pigm 61(1):93–98. https://doi.org/10.1016/j.dyepig.2003.10.008
Rashidi NH, Ibrahim WAW, Kamboh MA, Sanagi MM (2017) New magnetic graphene-based inorganic–organic sol-gel hybrid nanocomposite for simultaneous analysis of polar and non-polar organophosphorus pesticides from water samples using solid-phase extraction. Chemosphere 166:21–30. https://doi.org/10.1016/j.chemosphere.2016.09.054
Cushing BL, Kolesnichenko VL, O’Connor CJ (2004) Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem Rev 104(9):3893–3946. https://doi.org/10.1021/cr030027b
Gradzielski M, Langevin D, Farago B (1996) Experimental investigation of the structure of nonionic microemulsions and their relation to the bending elasticity of the amphiphilic film. Phys Rev E 53(4):3900–3919. https://doi.org/10.1103/PhysRevE.53.3900
Gunko YK, Cristmann U, Kessler VG (2002) Synthesis and structure of the first FeII heterometallic alkoxide [(THF)NaFe(OtBu)3]2—a possible precursor for new materials. Eur J Inorg Chem 2002(5):1029–1031. https://doi.org/10.1002/1099-0682(200205)2002:5%3c1029::AID-EJIC1029%3e3.0.CO;2-X
Xu J, Yang H, Wuyou Fu, Kai Du, Sui Y, Chen J, Zeng Yi, Li M, Zou G (2007) Preparation and magnetic properties of magnetite nanoparticles by sol-gel method. J Magn Magn Mater 309(2):307–311. https://doi.org/10.1016/j.jmmm.2006.07.037
Sivashankar R, Sathya AB, Vasantharaj K, Sivasubramanian V (2014) Magnetic composite an environmental super adsorbent for dye sequestration—a review. Environ Nanotechnol Monit Manag 1–2:36–49. https://doi.org/10.1016/j.enmm.2014.06.001
Pourzamani H, Parastar S, Hashemi M (2017) The elimination of xylene from aqueous solutions using single wall carbon nanotube and magnetic nanoparticle hybrid adsorbent. Process Saf Environ Prot 109:688–696. https://doi.org/10.1016/j.psep.2017.05.010
Kyzas GZ, Deliyanni EA, Lazaridis NK (2014) Magnetic modification of microporous carbon for dye adsorption. J Colloid Interface Sci 430:166–173. https://doi.org/10.1016/j.jcis.2014.05.049
Shao G, Yonggen Lu, Fangfang Wu, Yang C, Zeng F, Qilin Wu (2012) Graphene oxide: the mechanisms of oxidation and exfoliation. J Mater Sci 47(10):4400–4409. https://doi.org/10.1007/s10853-012-6294-5
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Yue Wu, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45(7):1558–1565. https://doi.org/10.1016/j.carbon.2007.02.034
He Y, Yi C, Zhang X, Zhao W, Yu D (2021) Magnetic graphene oxide: Synthesis approaches, physicochemical characteristics, and biomedical applications. TrAC Trends Anal Chem 136:116191. https://doi.org/10.1016/j.trac.2021.116191
Luo Li, Yang Y, Haipu Li Ru, Ding QW, Yang Z (2018) Size characterization of silver nanoparticles after separation from silver ions in environmental water using magnetic reduced graphene oxide. Sci Total Environ 612:1215–1222. https://doi.org/10.1016/j.scitotenv.2017.09.024
Chua CK, Pumera M (2014) Chemical reduction of graphene oxide: a synthetic chemistry viewpoint. Chem Soc Rev 43(1):291–312
Kadari M, Kaid M, Ali MB, Villemin D (2017) Selective study of elimination of Cd(II) and Pb(II) from aqueous solution by novel hybrid material. J Chin Adv Mater Soc 5(3):149–157. https://doi.org/10.1080/22243682.2017.1329027
Zenki M, Minamisawa K, Yokoyama T (2005) Clean Analytical methodology for the determination of lead with arsenazo III by cyclic flow-injection analysis. Talanta 68(2):281–286. https://doi.org/10.1016/j.talanta.2005.07.059
Rohwer H, Collier N, Hosten E (1995) Spectrophotometric study of arsenazo III and its interactions with lanthanides. Anal Chim Acta 314(3):219–223. https://doi.org/10.1016/0003-2670(95)00279-9
Faniyi IO, Fasakin O, Olofinjana B, Adekunle AS, Oluwasusi TV, Eleruja MA, Ajayi EOB (2019) The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches. SN Appl Sci 1(10):1181. https://doi.org/10.1007/s42452-019-1188-7
Xu C, Shi X, Ji A, Shi L, Zhou C, Cui Y (2015) Fabrication and characteristics of reduced graphene oxide produced with different green reductants. Édité par Bing Xu. PLoS One 10(12):e0144842. https://doi.org/10.1371/journal.pone.0144842
Abasali karaj abad Z, Nemati A, Khachatourian AM, Golmohammad M (2020) Synthesis and characterization of RGO/Fe2O3 nanocomposite as an efficient supercapacitor electrode material. J Mater Sci Mater Electron 31(17):14998–15005. https://doi.org/10.1007/s10854-020-04062-7
Sanchez JS, Pendashteh A, Palma J, Anderson M, Marcilla R (2017) Anchored Fe3O4 nanoparticles on RGO nanosheets as high-power negative electrodes for aqueous batteries. ChemElectroChem 4(6):1295–1305. https://doi.org/10.1002/celc.201700048
Wang Y, Alsmeyer DC, McCreery RL (1990) Raman spectroscopy of carbon materials: structural basis of observed spectra. Chem Mater 2(5):557–563. https://doi.org/10.1021/cm00011a018
Kudin KN, Ozbas B, Schniepp HC, Prud’homme RK, Aksay IA, Car R (2008) Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett 8(1):36–41. https://doi.org/10.1021/nl071822y
Stobinski L, Lesiak B, Malolepszy A, Mazurkiewicz M, Mierzwa B, Zemek J, Jiricek P, Bieloshapka I (2014) Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J Electron Spectrosc Relat Phenom 195:145–154. https://doi.org/10.1016/j.elspec.2014.07.003
Biswal S, Bhaskaram DS, Govindaraj G (2020) α-Fe2O3/reduced graphene oxide nanocomposite: interfacial effect on the magnetic property. J Supercond Novel Magn 33(6):1629–1632. https://doi.org/10.1007/s10948-019-05211-8
Biswal S, Bhaskaram DS, Govindaraj G (2018) Graphene oxide: structure and temperature dependent magnetic characterization. Mater Res Express 5(8):086104. https://doi.org/10.1088/2053-1591/aad1cf
Bhowmik RN, Saravanan A (2010) Surface magnetism, morin transition, and magnetic dynamics in antiferromagnetic α-Fe2O3 (Hematite) nanograins. J Appl Phys 107(5):053916. https://doi.org/10.1063/1.3327433
Mohan VB, Jayaraman K, Bhattacharyya D (2020) Brunauer–Emmett–Teller (BET) specific surface area analysis of different graphene materials: a comparison to their structural regularity and electrical properties. Solid State Commun 320:114004. https://doi.org/10.1016/j.ssc.2020.114004
Mercier G, Klechikov A, Hedenström M, Johnels D, Baburin IA, Seifert G, Mysyk R, Talyzin AV (2015) Porous graphene oxide/diboronic acid materials: structure and hydrogen sorption. J Phys Chem C 119(49):27179–27191. https://doi.org/10.1021/acs.jpcc.5b06402
Kuśmierek K, Świątkowski A (2015) The influence of different agitation techniques on the adsorption kinetics of 4-chlorophenol on granular activated carbon. React Kinet Mech Catal 116(1):261–271. https://doi.org/10.1007/s11144-015-0889-1
Priddy SA, Hanley TR (2003) Effect of agitation on removal of acetic acid from pretreated hydrolysate by activated carbon. Appl Biochem Biotechnol 106(1–3):353–364. https://doi.org/10.1385/ABAB:106:1-3:353
Khalid A, Ihsanullah MZ (2018) A comparative study on the adsorption of eriochrome black T dye from aqueous solution on graphene and acid-modified graphene. Arab J Sci Eng 43(5):2167–2179. https://doi.org/10.1007/s13369-017-2543-x
Ouyang D, Zhuo Y, Liang Hu, Zeng Q, Yuehua Hu, He Z (2019) Research on the adsorption behavior of heavy metal ions by porous material prepared with silicate tailings. Minerals 9(5):291. https://doi.org/10.3390/min9050291
Cozmuta LM, Cozmuta AM, Peter A, Nicula C, Nsimba EB, Tutu H (2012) The influence of ph on the adsorption of lead by Na-clinoptilolite: kinetic and equilibrium studies. Water SA 38:269–278
Z Tang, H Wu, Q Wen, L Hu (2019) Effect of adsorbent dosage to adsorbate concentration ratio on the adsorption of Cd(II) on coal gangue. In: Zhan L, Chen Y, Bouazza A (eds) Proceedings of the 8th international congress on environmental geotechnics volume 1. Environmental science and engineering. Springer, Singapore, pp 428–35. https://doi.org/10.1007/978-981-13-2221-1_45
Al-Shehri HS, Almudaifer E, Alorabi AQ, Alanazi HS, Alkorbi AS, Alharthi FA (2021) Effective adsorption of crystal violet from aqueous solutions with effective adsorbent: equilibrium, mechanism studies and modeling analysis. Environ Pollut Bioavailab 33(1):214–226. https://doi.org/10.1080/26395940.2021.1960199
Biswas S, Bal M, Behera S, Sen T, Meikap B (2019) Process optimization study of Zn2+ adsorption on biochar-alginate composite adsorbent by response surface methodology (RSM). Water 11(2):325. https://doi.org/10.3390/w11020325
Gao J, Liu Yu, Li X, Yang M, Wang J, Chen Y (2020) A Promising and cost-effective biochar adsorbent derived from Jujube Pit for the removal of Pb(II) from aqueous solution. Sci Rep 10(1):7473. https://doi.org/10.1038/s41598-020-64191-1
Gebreslassie YT (2020) Equilibrium, kinetics, and thermodynamic studies of malachite green adsorption onto Fig (Ficus Cartia) leaves. J Anal Methods Chem 2020:1–11. https://doi.org/10.1155/2020/7384675
Gorzin F, Abadi MBR (2018) Adsorption of Cr(VI) from aqueous solution by adsorbent prepared from paper mill sludge: kinetics and thermodynamics studies. Adsorpt Sci Technol 36(1–2):149–169. https://doi.org/10.1177/0263617416686976
Ayawei N, Ebelegi AN, Wankasi D (2017) Modelling and interpretation of adsorption isotherms. J Chem 2017:1–11. https://doi.org/10.1155/2017/3039817
Belhachemi M, Addoun F (2011) Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl Water Sci 1(3–4):111–117. https://doi.org/10.1007/s13201-011-0014-1
Adeola AO, de Lange J, Forbes PBC (2021) Adsorption of antiretroviral drugs, efavirenz and nevirapine from aqueous solution by graphene wool: kinetic, equilibrium, thermodynamic and computational studies. Appl Surf Sci Adv 6:100157. https://doi.org/10.1016/j.apsadv.2021.100157
Anthony ET, Ojemaye MO, Okoh AI, Okoh OO (2020) Synthesis of CeO2 as promising adsorbent for the management of free-DNA harboring antibiotic resistance genes from tap-water. Chem Eng J 401:125562. https://doi.org/10.1016/j.cej.2020.125562
Iriarte-Velasco U, Álvarez-Uriarte JI, González-Marcos MP, González-Velasco JR (2014) Microcolumn adsorption studies of acid/basic dyes related to the physicochemical properties of the adsorbent. Coloration Technol 130(1):62–72. https://doi.org/10.1111/cote.12058
Xu Z, Cai J-G, Pan B-C (2013) Mathematically modeling fixed-bed adsorption in aqueous systems. J Zhejiang Univ Sci A 14(3):155–176. https://doi.org/10.1631/jzus.A1300029
William K, Serkan Emik G, Öngen A, Özcan HK, Aydın S (2019) Modelling of adsorption kinetic processes—errors, theory and application. In: Edebali S (ed) Advanced sorption process applications. IntechOpen. https://doi.org/10.5772/intechopen.80495
El-Nemr MA, Yılmaz M, Ragab S, El Nemr A (2022) Biochar-SO prepared from pea peels by dehydration with sulfuric acid improves the adsorption of Cr6+ from water. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02378-4
Avelar D, Viana F, Pires BC, Nascimento TA, Mano V, Borges KB (2017) Polyaniline-deposited cellulose fiber composite prepared via in situ polymerization: enhancing adsorption properties for removal of meloxicam from aqueous media. RSC Adv 7(21):12639–12649. https://doi.org/10.1039/C6RA27019K
Hokmabadi F, Zadmard R, Akbarzadeh A, Tafakori V, Jalali MR, Ahmadian G (2022) Synthesis of a New Chitosan-p-Tert-Butylcalix[4]arene polymer as adsorbent for toxic mercury ion. R Soc Open Sci 9(5):211223. https://doi.org/10.1098/rsos.211223
Korake SR, Jadhao PD (2021) Investigation of taguchi optimization, equilibrium isotherms, and kinetic modeling for cadmium adsorption onto deposited silt. Heliyon 7(1):e05755. https://doi.org/10.1016/j.heliyon.2020.e05755
Morrison SR (1990) Adsorption and desorption. In: Morrison SR (ed) The chemical physics of surfaces. Springer US, Boston, pp 251–295
Singh R, Bhateria R (2020) Experimental and modeling process optimization of lead adsorption on magnetite nanoparticles via isothermal, kinetics, and thermodynamic studies. ACS Omega 5(19):10826–10837. https://doi.org/10.1021/acsomega.0c00450
Bermúdez-Salguero C, Gracia-Fadrique J (2011) Analysis of Gibbs adsorption equation and thermodynamic relation between Gibbs standard energies of adsorption and micellization through a surface equation of state. J Colloid Interface Sci 355(2):518–519. https://doi.org/10.1016/j.jcis.2010.12.040
Horsfall JM, Spiff AI (2005) Effects of temperature on the sorption of Pb2+ and Cd2+ from aqueous solution by Caladium bicolor (Wild Cocoyam) biomass. Electron J Biotechnol 8(2):162–169. https://doi.org/10.2225/vol8-issue2-fulltext-4
Petrovic B, Gorbounov M, Soltani SM (2022) Impact of surface functional groups and their introduction methods on the mechanisms of CO2 adsorption on porous carbonaceous adsorbents. Carbon Capture Sci Technol 3:100045. https://doi.org/10.1016/j.ccst.2022.100045
Han R, Zou W, Li H, Li Y, Shi J (2006) Copper(II) and Lead(II) removal from aqueous solution in fixed-bed columns by manganese oxide coated zeolite. J Hazard Mater 137(2):934–942. https://doi.org/10.1016/j.jhazmat.2006.03.016
Miralles N, Valderrama C, Casas I, Martínez M, Florido A (2010) Cadmium and lead removal from aqueous solution by grape stalk wastes: modeling of a fixed-bed column. J Chem Eng Data 55(9):3548–3554. https://doi.org/10.1021/je100200w
Lung I, Stan M, Opris O, Soran M-L, Senila M, Stefan M (2018) Removal of Lead(II), Cadmium(II), and Arsenic(III) from aqueous solution using magnetite nanoparticles prepared by green synthesis with Box–Behnken design. Anal Lett 51(16):2519–2531. https://doi.org/10.1080/00032719.2018.1446974
Amarasinghe BM, Williams RA (2007) Tea waste as a low cost adsorbent for the removal of Cu and Pb from wastewater. Chem Eng J 132(1–3):299–309. https://doi.org/10.1016/j.cej.2007.01.016
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kadari, M., Makhlouf, M., Khaoua, O.O. et al. The Removal Efficiency of Cadmium (Cd2+) and Lead (Pb2+) from Aqueous Solution by Graphene Oxide (GO) and Magnetic Graphene Oxide (α-Fe2O3/GO). Chemistry Africa 6, 1515–1528 (2023). https://doi.org/10.1007/s42250-023-00586-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00586-7