Abstract
Superhydrophobic surfaces and coatings have gained significant attention for their potential in anti-corrosion applications, yet they present several challenges. These coatings typically lack the flexibility to function as freestanding films, limiting their ease of application and removal. Additionally, existing techniques often involve complex chemical processes and utilize materials that are costly and environmentally hazardous. In this study, waste polyethylene is valorized to produce flexible superhydrophobic films and coatings with anticorrosive properties. The process employs thermally induced phase separation using a bio-solvent and spin-casting. The resulting films demonstrate dual functionality as both anti-corrosion coatings and freestanding films. The maximum contact angle of anticorrosive film was found to be 148o. The maximum charge transfer resistances Rct of carbon steel and polymer coated film were 0.184 and 173 kΩ.cm2, respectively. The electrochemical impedance spectrometry (EIS) shows a corrosion inhibition efficiency of 99.39%, which confirms the superior anticorrosive properties of the coating. Importantly, the use of waste polyethylene offers a cost-effective and environmentally friendly solution to conventional superhydrophobic coatings, contributing to the circular economy and reducing plastic waste. Overall, this study presents an alternate strategy to develop anticorrosive superhydrophobic films, providing a template for utilizing waste polyethylene in corrosion protection applications.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Increasing corrosivity of iron-based alloys and steel necessitates effective anti-corrosion strategies. [1, 2]. Despite the inherent durability and strength of these alloys, their vulnerability to corrosion, particularly in marine environment, remains a significant impediment, resulting in substantial economic losses and casualties [3]. Recognizing the adverse impact of corrosion on metals and alloys, constituting approximately 3.4% of the global gross domestic product, there has been a continuous exploration of surface coating and modification techniques aimed at enhancing corrosion resistance [4]. Altering the surface to attain a superhydrophobic trait has become a progressively appealing method for protecting metals and alloys against oxidation [5]. In recent years, polymer-based superhydrophobic coatings and films have gained much attention due to their anti-wetting properties, as well as their availability and cost effectiveness [6, 7].
Two methods can enhance the hydrophobicity of a surface: one involves chemical modifications to reduce surface energy, while the other focuses on improving surface roughness by controlling topography [8, 9]. Natural examples, such as the lotus leaf, demonstrate this phenomenon that leads to CAs of 170° [8, 10, 11] Market analysis suggests a significant increase in worldwide interest for superhydrophobic coatings, projected to increase annually by 25.6% from 2019 to 2024 [10, 12].
Numerous research investigations have reported the application of superhydrophobic anti-corrosive coatings on steel and its alloys [13, 14]. These films, meshes, or coatings find extensive application in the realm of corrosion protection [15, 16] owing to their exceptional water-repellent characteristics. Superoleophilic and hydrophobic coating from perfluordecyltriethhoxysilane and silica particles was reported using spray method for steel surface [17]. Robust superhydrophobic coating from epoxy resin, polydimethylsiloxane, and modified SiO2 on various substrates were fabricated by suspension spraying approach for anti-corrosion applications [18]. A superhydrophobic coating was applied to a steel surface using a chemical etching method involving a blend of hydrochloric and nitric acid. Subsequently, treatment with lauric acid resulted in a water contact angle of 170o [19]. Anti-corrosive coating on steel was produced by employing polyurethane, SiO2 nanoparticles, and hexadecyltrimethoxysilane through a spin coating technique [20]. Finally, superamphiphobic stainless steel surfaces exhibiting excellent anticorrosion properties were synthesized. This process involved initial etching with hydrofluoric acid, followed by a cycle-etching approach consisting of repeated cycles of hydrofluoric acid etching and ultrasonic cleaning. The synthesis was completed with a final modification using fluoroalkylsilane [21].
In spite of being extensively employed in anti-corrosion applications, superhydrophobic surfaces pose various challenges that require careful consideration. Firstly, the application of superhydrophobic coatings involves chemical processes on metal surfaces, typically conducted in laboratory settings and requiring specialized manpower. Secondly, these coatings lack the flexibility to function as films that can be applied using adhesive tapes, limiting their potential for mechanical removal. While a few studies have explored freestanding anti-corrosive films, they often emphasize metal-organic compounds rather than readily available polymers [22, 23]. Moreover, the materials used in superhydrophobic coatings often incorporate low surface energy chemicals, such as fluorinated and/or silane compounds. Various fluoropolymers for the synthesis of superhydrophobic coatings are: polyvinylidene fluoride (PVDF) [24], poly(vinylidene-co-hexafluoropropylene) (PVDFHFP) [25], perfluoroalkyl alkyl pyrrole [26], poly[2-(perfluorooctyl)ethyl acrylate-ran-2-(dimethylamino) ethyl acrylate] [27], and mono-[2-(perfluorooctyl)ethyl] phosphate (FAP) [28]. Examples of silanes used in the synthesis of hydrophobic coatings include: (heptadecafluorotetrahydrodecyl)triethoxysilane (FAS-17) [4], and 1 H, 1 H, 2 H, 2 H-perfluorooctadecyltrichlorosilane (FDTS) [29]. Other low energy chemicals are alkyl ketene dimer (AKD) [30] and dodecane thiol [31]. These chemicals lead to increased material costs and environmental hazards. Additionally, the solvents employed in the coating process are predominantly fossil fuel-based, further contributing to environmental concerns. Therefore, replacing fossil-derived solvents with renewable and environmentally friendly alternatives becomes essential for dissolution needs [32]. Lastly, the chemicals utilized in these coatings are frequently virgin, contributing to heightened expenses for anti-corrosive applications.
The drawbacks associated with conventional superhydrophobic coatings highlight the need for more sustainable and cost-effective approaches, making the utilization of plastic waste a promising avenue for further investigation. Hence, this collective understanding prompts the exploration of alternative solutions. Specifically, the idea involves repurposing plastic waste, particularly polyethylene — which constitutes close to 40% of total plastic waste — for the development of anticorrosive superhydrophobic films and coatings.
Virgin polyethylene comes in various forms, and one notable variant is ultra-high molecular weight polyethylene (UHMWPE). Previous reports have discussed its utilization in combination with additives and fillers. UHMWPE was employed to synthesize anticorrosive superhydrophobic coatings in combination with Aluminum [33], diabase filler [34], maleic anhydride (MAH) [35], and Ti6Al4V alloy [36]. UHMWPE, a high-performance polymer renowned for its exceptional resistance to abrasion and impact, minimized impact and friction noise, favorable chemical resistance, and low coefficient of friction [37, 38]. To the best of the authors’ knowledge, waste polyethylene, specifically high-density polyethylene (HDPE), recognized as a commodity and inexpensive plastic which is readily found in plastic wastes [39, 40], has not been previously reported as the sole component for the development of anticorrosive superhydrophobic films and coatings.
In this work, we valorize waste HDPE into flexible superhydrophobic films and coatings with anticorrosive properties. By utilizing the bio-solvent cymene, a compound found naturally in plants and food items [41], we employed a dissolution process to prepare a polymer solution. This solution was spin-cast onto carbon steel, creating an extremely thin, rough surface. The surface was then annealed to control and strengthen the structure and close the pores. Subsequently, another layer of polymeric solution was spin-cast to produce anticorrosive superhydrophobic films and coatings with controlled thickness. These films can be applied to metal surfaces either chemically or mechanically through the use of adhesive tapes. Thus, these films demonstrate uniqueness by serving dual purposes as both anti-corrosion coatings and standalone or freestanding anti-corrosion films. Overall, this work provides a template for anticorrosive superhydrophobic films using waste polyethylene.
2 Materials and methods
p-Cymene (> 99%) was obtained from Lonwin Chemicals. HDPE was obtained in the form of discarded plastic bottles. Substrate supports were fabricated by cutting plain carbon steel plates into 5 cm2, then affixed onto a custom-made coater chuck from Ossila.
2.1 Preparation of single superhydrophobic coat (SHC) on carbon steel
3 g of waste HDPE was dissolved in 50 mL of p-Cymene, resulting in a concentration of 60 mg/mL, and heated at 130 °C for 20 min. A carbon steel, used as a substrate, was preheated to 120 °C. The solution was then laid onto the carbon steel and slowly spin-coated for 10 s at 400 rpm. The speed gradually increased to 1000 rpm for about 60 s, and finally to 3000 rpm for around 60 s. The explaination of using different speeds was discussed elsewhere [42]. A brief description is provided here: A clean substrate, preheated to 120 °C and measuring 5 cm2, was positioned onto the customized chuck of a spin coater. The chuck was specially designed to accommodate the substrate at high speeds, onto which the polymer solution was poured. To prevent solvent evaporation during spin coating, the lid was closed, and the spin coater was activated. The spin coating process consisted of three stages: an initial stage at a lower speed to promote polymer adhesion to the substrate, followed by a medium-speed stage to ensure uniform thickness, and a final high-speed stage to refine the film thickness and remove solvent molecules. Stage one involved spinning at 400 rpm for 5 s, stage two at 1000 rpm for 60 s, and stage three at 3000 rpm for 60 s. The resulting thin film, termed “single SHC on Carbon steel” was not subjected to further heating after spin coating. The average thickness of the coatings for the single SHC, heated SHC, and double SHC are approximately 80 µm, 34 µm, and 120 µm, respectively. Note that the corrosion results vary with the thickness of the coatings.
2.2 Preparation of heated-coat on carbon steel
Following the coating process, the carbon steel substrate was taken off the spin coater chuck and subjected to heating on a hot plate at 130 °C for 1–2 min until the opaque white film became clear, thereby producing the “heated-coat on Carbon steel.”
2.3 Preparation of double SHC on carbon steel
The hot carbon steel substrate with the heated-coat was placed back on the chuck. Another batch of heated polymer (waste HDPE solution) was poured over the heated-coat and spun at identical speeds as the single SHC preparation. This new layer was called “double SHC on Carbon steel.” After spin coating, the carbon steel substrate was taken off from the spin coater chuck. The resulting double hydrophobic coating can be manually peeled off using tweezers, if necessary, from the carbon steel substrate, and subsequently affixed to another surface using adhesive tape.
2.4 Anticorrosion study
The corrosion resistance investigation was carried out using a Potentiostat. Initially, the substrate (carbon steel) was roughened with 60-grit sandpaper and then treated with a superhydrophobic film derived from waste HDPE. Afterward, the sample underwent heating to 120 °C and received an additional coating layer according to the previous procedure. Both specimens were then submerged in a 3.5 wt% NaCl for various periods to assess their corrosion resistance characteristics.
3 Results and discussion
Figure 1 demonstrated the morphology of the single SHC on carbon steel (a-b), heated-coat on carbon steel (c-d), and double SHC on carbon steel (e-f). Figure 1 (a-b) demonstrates a phase-separated uniform thin film with nano fractures and high porosity. However, the internal structure of this coating is weak and fragile due to excessive pores and high porosity. Hence, it was heated, to strengthen the internal structure. The heating process led to the complete sealing of the porous structure, resulting in a decrease in the film thickness and an improvement in its strength (see Fig. 1c and d). This heating reduced the hydrophobicity of the coating, as discussed later, and thus another layer was coated as shown in Fig. 1 (e-f). It provides an extremely porous surface, which is required to produce an anti-corrosive film and coating. The average thickness of the superhydrophobic film or coating is 150 μm, consisting of a 30 μm base layer (heated-coat) and a 120 μm top layer (double-coat).
XRD analysis was conducted to explore the crystalline structure, with results depicted in Fig. 2. Both single and heated coats exhibited peaks at 22˚ and 24˚, characteristic of PE [43]. The Miller indices and green dotted lines show the peaks attributed to PE (orthorhombic) [44]. At first, the intensity of peaks in the single-coat was modest, yet it rose following heating, owing to improved crystallinity, a pattern mirrored in the double coat. This general augmentation in crystallinity was ascribed to the rearrangement of polymer chains and their denser alignment.
DSC was employed to assess thermal properties, complementing XRD results. Heating caused polymer molecules to absorb energy, soften, and allow chains to slide, eventually leading to melting. Figure 3 shows DSC spectra, indicating melting peaks at 129˚C for the double-coat and 128˚C for the single-coat. Although subtle, these results support XRD findings.
FTIR spectra analysis aimed to identify functional changes within the polymer chains. Peaks suggested that the polymer network retained HDPE’s original structure [45]. Distinctive characteristics observed in the spectra of the single SHC included peaks representing C-H asymmetric and symmetric bonds at 2915 cm− 1 and 2847 cm− 1, respectively, along with peaks indicating C-H bending at 1463 cm− 1 and C-H rocking at 730 cm− 1. Conversely, the heated-coat showed a peak at 1230 cm− 1, indicating C-O-C stretching and suggesting crosslinking through ether linkages upon annealing, enhancing intermolecular interactions and film robustness.
Furthermore, the single SHC exhibited lower transmittance (T%) compared to the heated-coat, attributed to its more amorphous nature causing increased scattering. Conversely, the heated-coat’s higher transmittance was attributed to a denser arrangement of polymer chains resulting from enhanced interpolymeric interactions. The double superhydrophobic coat shared characteristics with the heated-coat, reinforcing these observations (Fig. 4).
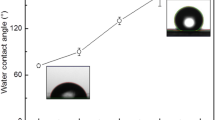
The degree of superhydrophobicity of the film was evaluated by measuring water contact angles (CAs) using distilled water. Initially, when a water droplet was placed on the carbon steel surface, it displayed slight hydrophilicity with a CA of 87° (Fig. 5a). Following the application of a single-coat, the contact angle increased to 145° (Fig. 5b). However, upon heating, the polymeric chains underwent reorganization and partial oxidation, resulting in a decrease in CA to 107° (Fig. 5c) [46]. Conversely, the double-coat film demonstrated remarkable superhydrophobicity, exhibiting a CA of 148° (Fig. 5d). This rise in the contact angle can be attributed to enhanced surface roughness and the presence of flaky microstructural aggregates characteristic of HDPE [47]. In the instance of the double-coat superhydrophobic surface, these flaky microstructural aggregates were formed through micro-stretching at the interface between the polymer and solvent-leaving regions. Such microstretches were generated by the expulsion of solvent molecules during spin coating rotations, followed by gradual condensation leading to polymer crystallization [11].
Anticorrosion studies— The electrochemical measurements were carried out to investigate the corrosion properties of the HDPE based superhydrophobic coatings on A36 carbon steel. For the comparison, electrochemical impedance testing of A36 carbon steel substrate, single layer HDPE based hydrophobic coatings, single layer heated HDPE based coatings and double layer HDPE based superhydrophobic coatings were tested over the immersion duration of 7 days in 3.5wt% NaCl solution. Experimental EIS data of all the samples were further modelled and fitted through Gamry EChem Analyst software to get the EIS parameters i.e. Rct, Rcoat, CPEdl, CPEcoat to propose corrosion resistance mechanism of the samples in the study. Symbols represents experimental data whereas solid lines in the plot indicate the fitted results. Figure 6 illustrates the schematic of the all the samples in this study along with their proposed equivalent electrical circuit (EEC) utilized in modelling experimental results. Modified Randle cell have been employed to model the corrosion behaviour of A36 steel, whereas cascaded two-time constant EEC have been adopted for numerous HDPE based coatings and respective values are presented in Table 1.
As shown in Fig. 6, electrical elements modelled for the present study contains Rs accounting for the solution resistance of 3.5wt% NaCl saline environment which is in series with the CPEdl and Rct accounting for the constant phase element developed as a result of double layer formed between the substrate and saline solution/coating whereas Rct represents charge transfer resistance at the interface of substrate and saline solution/coating. Moreover, CPEcoat and Rcoat represents the constant phase element and resistance due to HDPE based coatings. It’s notable that a constant phase element was used instead of a pure capacitive element to address deviations in experimental results from the ideal coating. This adjustment accounts for discrepancies encountered during the synthesis and testing of the samples, a common practice documented in literature.
Figure 7 reveals the Bode plot of A36 steel substrate revealing the impedance and phase angle responses to the wider spectrum of frequency ranging from mega hertz to milli hertz. A36 steel substrate seems to offer around 500Ω on Day 0 which decreases to 180 Ω as the duration of immersion increases to 24 h, this decrease in the impedance can be associated to the initiation of corrosion caused by the corrosion species from the salt solution. With further immersion duration upto Day 2, increase in the impedance value up to 250 Ω is observed which can be associated to the resistance offered by the passive oxide film formed as a result of corrosion initiated in Day 1 [48, 49]. Subsequent immersion duration of Day 5 and Day 7 further shows an increase in the impedance with the terminal value reaching up to 350 Ω and a shift in phase angle at lower frequency region from − 10 to -30 also reveals the formation of a film. This further indicates the development of passive oxide film on the A36 steel substrate. High frequency region in Fig. 7 (a) also reveal the solution resistance, which is around 10 Ω for the study, this is important as the solution resistance becomes un-noticeable for HDPE based coatings. Moreover, 7 (b) reveals the decrease of the bulge and its shift toward lower frequency region from the Day 0 to Day 1 and thereby remaining unchanged, this trend indicates the decrease in overall capacitive nature of corrosion and increase in resistive behaviour due to the development of passive oxide film as a result of successive immersion duration up to 7 days [50, 51].
Bode plot in Fig. 8 represents the impedance and phase curves of simple HDPE coating for the immersion of 7 days. It is evident from Fig. 8 (a) that the impedance on day 0 and day 1 are similar and superior which may be associated to the resistance of the coating in restricting corrosion species from reaching the steel substrate as Rcoat and Rct for day 0 lies in the range of 90kΩ and 23kΩ. As the immersion duration reaches upto 24 h, the coating resistance decreases to about 47kΩ which is complimented by increment in the charge transfer resistance of 76kΩ keeping the overall impedance nearly unchanged. But on day 2, the impedance reaches its lowest value indicating the initiation of corrosion as the corrosive species might have reached the steel substate through the pores and cavities of the HDPE coating surpassing the impedance offered by HD coat with Rcoat being 5.5kΩ and Rct being 1.7kΩ. with further immersion duration upto Day 5, the overall impedance is observed to be increase to which can be associated to the starting of formation of passive oxide film which tends to impede the corrosive species in reaching the steel substrate [52, 53]. When the immersion duration reaches upto 7 days, development of protective passive oxide film further improves the overall impedance with Rcoat value of 42kΩ and Rct 24kΩ. Figure 8 (b) reveals the phase angle curve indicating the behaviour of simple HD coating at respective immersion duration, phase angle curve of all the duration illustrates the straight line in mid to low frequency region at zero degree indicating the resistive components of the coating. However, for day 0, day 1 and day 7, capacitive dominating components of the coatings are prevalent in the higher frequency region. The transfer from capacitive to resistive and then back to capacitive behaviour can be attributed to the impedance offered by the HD coating, initiation of corrosion and then development of passive oxide film which further enhances the overall impedance of the coating system [54].
Figure 9 shows the bode plot of heated HDPE coating for respective immersion duration. Impedance on day 0 is comparatively higher which can be due to lack of corrosion species sweeping through the micropores and reaching substrate, thereby initiating corrosion. Overall impedance for rest of the testing duration seems to evident from Fig. 9 (a). However, closer observation indicates the trends followed by heated HD samples are quite similar to simple HD samples indicating the similar corrosion protection mechanism, i.e. development of passive layer of oxide which increases the impedance on day 5 before further decrease of corrosion resistance on day 7 which can be associated to the failure of passive film with further exposure to saline corrosive media. This decrease in impedance can be attributed to adverse effects of heat treatment of simple HDPE coating to obtain heat treated HDPE coat. Figure 9 (b) shows the phase angle curves revealing the transfer of capacitive behaviour to resistive behaviour as the duration of immersion increases from day 0 to day 7 in high frequency region, whereas the resistive behaviour predominates in the mid to low frequency region over the entire immersion duration.
Figure 10 reveals the bode plot for double layer HDPE coating for the immersion of 7 days. Impedance on day 0 is observed to be substantially enhanced as a result of two layers of coating heated and simple HDPE as seen in Fig. 10 (a), providing stiff protection and inhibiting the movement of corrosion species to the steel substrate with values of Rcoat reaching at 161 MΩ and Rct reaching above 1GΩ. However, as the immersion duration reaches 24 h, total impedance decreases to nearly 176kΩ indicating the contact of corrosion species with steel substrate thereby initiating corrosion. With further immersion duration, overall impedance decreases indicating the further corrosion taking place at the respective active site. However, increase in the impedance is observed at the seventh day of immersion that can be associated with the development of passive iron oxide film inhibiting the local movement of corrosion species and hence protecting the steel substrate. Phase angle curves of double layer HDPE samples are shown in Fig. 10 (b), capacitive behaviour of coat is observed upto mid frequency region before transiting toward resistive behaviour in lower frequency region for day 0. As the immersion duration reaches day 1, this transition from capacitive dominant behaviour takes place within the high frequency region and further immersion duration decreases the capacitive behaviour toward more resistive behaviour on day 2 and day 5. Finally, day 7 reveals the similar trend of increasing impedance indicating the similar corrosion protection mechanism of passive oxide film impeding the motion of corrosion species to reach the steel substrate. The capacitive loops of carbon steel and the double-coat superhydrophobic film are illustrated in Fig. 11. The Nyquist curve demonstrates a sequential enhancement in impedance, as indicated in the Bode plots. This enhancement is attributed to the blocking of the conductive path and the inhibition of electron flow to the carbon steel from corrosive media, resulting from the presence of the SHC layer.
4 Conclusion
This study highlights the effectiveness of superhydrophobic coatings, particularly those employing HDPE, in enhancing the corrosion resistance of carbon steel. Through comprehensive analysis of capacitive loops and impedance measurements, it has been demonstrated that the application of HDPE superhydrophobic coatings significantly impedes the corrosive action on the substrate. The charge transfer resistance values obtained for both single-coat and double-coat HDPE layers underscore their potential for mitigating corrosion. Nevertheless, it’s crucial to acknowledge that the effectiveness of the coatings decreases with time, highlighting the necessity for regular upkeep or the creation of more long-lasting coatings. In summary, the results of this research offer significant insights into the design and implementation of superhydrophobic coatings for corrosion prevention, potentially benefiting diverse industries such as maritime and infrastructure. Further research focusing on optimizing coating composition and exploring novel fabrication techniques will be crucial in advancing the field and addressing practical challenges in corrosion prevention.
References
Z. Sabet-Bokati, K. Sabet-Bokati, Z. Russell, K. Morshed-Behbahani, S. Ouanani, Anticorrosion shape memory-assisted self-healing coatings: a review. Prog Org. Coat. 188, 108193 (2024). https://doi.org/10.1016/j.porgcoat.2023.108193
L. Cheng, C. Liu, H. Zhao, L. Wang, Hierarchically self-reporting and self-healing photothermal responsive coatings towards smart corrosion protection. Chem. Eng. J. 467, 143463 (2023). https://doi.org/10.1016/j.cej.2023.143463
S. Xu, Q. Wang, N. Wang, X. Zheng, Fabrication of superhydrophobic green surfaces with good self-cleaning, chemical stability and anti-corrosion properties. J. Mater. Sci. 54, 13006–13016 (2019). https://doi.org/10.1007/s10853-019-03789-x
V. Chobaomsup, M. Metzner, Y. Boonyongmaneerat, Superhydrophobic surface modification for corrosion protection of metals and alloys. J. Coat. Technol. Res. 17, 583–595 (2020). https://doi.org/10.1007/s11998-020-00327-2
Z. GONG, L. J. WANG, X. WU, G. WANG, L.L.I.A.O. LÜ, Fabrication of Super Hydrophobic surfaces on copper by solution-immersion. Chin. J. Chem. Eng. 21, 920–926 (2013). https://doi.org/10.1016/S1004-9541(13)60569-8
J. Saleem, S. Safdar Hossain, Z.K.B. Moghal, G. McKay, Up-cycling waste polypropylene as superhydrophobic sheets. US Patent 11859065, 2024. https://www.freepatentsonline.com/11859065.html
J. Saleem, Z.K.B. Moghal, L. Sun, G. McKay, Valorization of mixed plastics waste for the synthesis of flexible superhydrophobic films. Adv. Compos. Hybrid. Mater. 7, 11 (2024). https://doi.org/10.1007/s42114-024-00829-2
H.Y. Erbil, A.L. Demirel, Y. Avcı, O. Mert, Transformation of a simple Plastic into a Superhydrophobic Surface, Science (80-.). 299 (2003) 1377–1380. https://doi.org/10.1126/science.1078365
J. Saleem, S. Safdar Hossain, Z.K.B. Moghal, G. McKay, Method to prepare superhydrophobic sheets from virgin and waste polypropylene. US Patent 11840609, 2023. https://www.freepatentsonline.com/11840609.html
X. Li, J. Wang, G. Yi, S.P. Teong, S.P. Chan, Y. Zhang, From waste plastic to artificial lotus leaf: Upcycling waste polypropylene to superhydrophobic spheres with hierarchical micro/nanostructure. Appl. Catal. B Environ. 342, 123378 (2024). https://doi.org/10.1016/j.apcatb.2023.123378
S.S. Latthe, C. Terashima, K. Nakata, A. Fujishima, Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of Lotus Leaf. Molecules. 19, 4256–4283 (2014). https://doi.org/10.3390/molecules19044256
S. Das, S. Kumar, S.K. Samal, S. Mohanty, S.K. Nayak, A review on Superhydrophobic Polymer Nanocoatings: recent development and applications. Ind. Eng. Chem. Res. 57, 2727–2745 (2018). https://doi.org/10.1021/acs.iecr.7b04887
Z. Yang, X. Liu, Y. Tian, Fabrication of super-hydrophobic nickel film on copper substrate with improved corrosion inhibition by electrodeposition process. Colloids Surf. Physicochem Eng. Asp. 560, 205–212 (2019). https://doi.org/10.1016/j.colsurfa.2018.10.024
Z. She, Q. Li, Z. Wang, L. Li, F. Chen, J. Zhou, Researching the fabrication of anticorrosion superhydrophobic surface on magnesium alloy and its mechanical stability and durability. Chem. Eng. J. 228, 415–424 (2013). https://doi.org/10.1016/j.cej.2013.05.017
Y. Liu, X. Yin, J. Zhang, S. Yu, Z. Han, L. Ren, A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta. 125, 395–403 (2014). https://doi.org/10.1016/j.electacta.2014.01.135
L.-K. Wu, X.-F. Zhang, J.-M. Hu, Corrosion protection of mild steel by one-step electrodeposition of superhydrophobic silica film. Corros. Sci. 85, 482–487 (2014). https://doi.org/10.1016/j.corsci.2014.04.026
N. Valipour Motlagh, F.C. Birjandi, J. Sargolzaei, N. Shahtahmassebi, Durable, superhydrophobic, superoleophobic and corrosion resistant coating on the stainless steel surface using a scalable method. Appl. Surf. Sci. 283, 636–647 (2013). https://doi.org/10.1016/j.apsusc.2013.06.160
D.-W. Li, H.-Y. Wang, Y. Liu, D.-S. Wei, Z.-X. Zhao, Large-scale fabrication of durable and robust super-hydrophobic spray coatings with excellent repairable and anti-corrosion performance. Chem. Eng. J. 367, 169–179 (2019). https://doi.org/10.1016/j.cej.2019.02.093
P. Varshney, S.S. Mohapatra, A. Kumar, Durable and Regenerable Superhydrophobic Coating on Steel Surface for Corrosion Protection. J. Bio- Tribo-Corrosion. 7, 76 (2021). https://doi.org/10.1007/s40735-021-00518-3
M.K. Meena, B.K. Tudu, A. Kumar, B. Bhushan, Development of polyurethane-based superhydrophobic coatings on steel surfaces. Philos. Trans. R Soc. Math. Phys. Eng. Sci. 378, 20190446 (2020). https://doi.org/10.1098/rsta.2019.0446
N. Wen, S. Peng, X. Yang, M. Long, W. Deng, G. Chen, J. Chen, W. Deng, A cycle-etching Approach toward the Fabrication of Superamphiphobic Stainless Steel Surfaces with excellent Anticorrosion properties. Adv. Eng. Mater. 19 (2017). https://doi.org/10.1002/adem.201600879
W.A. Zoubi, Y.G. Ko, Freestanding anticorrosion hybrid materials based on coordination interaction between metal-quinoline compounds and TiO2-MgO film. J. Colloid Interface Sci. 565, 86–95 (2020). https://doi.org/10.1016/j.jcis.2020.01.017
H. Jia, X. Yang, Q.-Q. Kong, L.-J. Xie, Q.-G. Guo, G. Song, L.-L. Liang, J.-P. Chen, Y. Li, C.-M. Chen, Free-standing, anti-corrosion, super flexible graphene oxide/silver nanowire thin films for ultra-wideband electromagnetic interference shielding. J. Mater. Chem. A 9, 1180–1191 (2021). https://doi.org/10.1039/D0TA09246K
A.B. Radwan, A.M.A. Mohamed, A.M. Abdullah, M.A. Al-Maadeed, Corrosion protection of electrospun PVDF–ZnO superhydrophobic coating, surf. Coat. Technol. 289, 136–143 (2016). https://doi.org/10.1016/j.surfcoat.2015.12.087
A. Bahgat Radwan, A.M. Abdullah, M.K. Hassan, The missing piece of the puzzle regarding the relation between the degree of superhydrophobicity and the corrosion resistance of superhydrophobic coatings. Electrochem. Commun. 91, 41–44 (2018). https://doi.org/10.1016/j.elecom.2018.05.008
M. Nicolas, Acids and alkali resistant sticky superhydrophobic surfaces by one-pot electropolymerization of perfluoroalkyl alkyl pyrrole. J. Colloid Interface Sci. 343, 608–614 (2010). https://doi.org/10.1016/j.jcis.2009.11.052
E. Yoshida, A. Nagakubo, Superhydrophobic surfaces of microspheres obtained by self-assembly of poly[2-(perfluorooctyl)ethyl acrylate-ran-2-(dimethylamino)ethyl acrylate] in supercritical carbon dioxide. Colloid Polym. Sci. 285, 1293–1297 (2007). https://doi.org/10.1007/s00396-007-1712-y
T. Fujii, Y. Aoki, H. Habazaki, Superhydrophobic hierarchical surfaces fabricated by anodizing of oblique angle deposited Al–Nb alloy columnar films. Appl. Surf. Sci. 257, 8282–8288 (2011). https://doi.org/10.1016/j.apsusc.2011.01.044
G. Wang, S. Liu, S. Wei, Y. Liu, J. Lian, Q. Jiang, Robust superhydrophobic surface on Al substrate with durability, corrosion resistance and ice-phobicity. Sci. Rep. 6, 20933 (2016). https://doi.org/10.1038/srep20933
L. Ejenstam, L. Ovaskainen, I. Rodriguez-Meizoso, L. Wågberg, J. Pan, A. Swerin, P.M. Claesson, The effect of superhydrophobic wetting state on corrosion protection – the AKD example. J. Colloid Interface Sci. 412, 56–64 (2013). https://doi.org/10.1016/j.jcis.2013.09.006
S. Niu, Y. Fang, R. Qiu, Z. Qiu, Y. Xiao, P. Wang, M. Chen, Superhydrophobic film based on Cu-dodecanethiol complex: Preparation and corrosion inhibition for Cu, Colloids surfaces a physicochem. Eng. Asp. 550, 65–73 (2018). https://doi.org/10.1016/j.colsurfa.2018.04.023
J. Saleem, Z.K.B. Moghal, R. Luque, G. McKay, Towards green membranes: repurposing waste polypropylene with a single plant-based solvent via tandem spin-casting and annealing. Chem. Eng. J. 148560 (2024). https://doi.org/10.1016/j.cej.2024.148560
X. Wang, H. Zhao, S. Wu, X. Suo, X. Wei, H. Li, Aluminum-polyethylene composite coatings with self-sealing induced anti-corrosion performances. J. Mater. Process. Technol. 282, 116642 (2020). https://doi.org/10.1016/j.jmatprotec.2020.116642
M. Skakov, M. Bayandinova, I. Ocheredko, B. Tuyakbayev, M. Nurizinova, A. Gradoboev, Influence of Diabase Filler on the structure and Tribological Properties of Coatings Based on Ultrahigh Molecular Weight Polyethylene, polymers (Basel). 15 (2023) 3465. https://doi.org/10.3390/polym15163465
K. Jeeva Jothi, A.U. Santhoskumar, S. Amanulla, K. Palanivelu, Thermally Sprayable Anti-corrosion Marine Coatings based on MAH-g-LDPE/UHMWPE Nanocomposites. J. Therm. Spray. Technol. 23, 1413–1424 (2014). https://doi.org/10.1007/s11666-014-0153-7
K. Anaya-Garza, A.M. Torres-Huerta, M.A. Domínguez-Crespo, J. Moreno-Palmerín, E. Ramírez-Meneses, Rodríguez-Salazar, corrosion resistance improvement of the Ti6Al4V/UHMWPE systems by the assembly of ODPA molecules by dip coating technique. Prog Org. Coat. 171, 107013 (2022). https://doi.org/10.1016/j.porgcoat.2022.107013
J. Saleem, P. Gao, J. Barford, G. McKay, Development and characterization of novel composite membranes for fuel cell applications. J. Mater. Chem. A 1, 14335 (2013). https://doi.org/10.1039/c3ta13855k
J. Saleem, A. Bazargan, J. Barford, G. McKay, Application of strong porous polymer sheets for Superior Oil spill recovery. Chem. Eng. Technol. 38, 482–488 (2015). https://doi.org/10.1002/ceat.201400068
J. Saleem, C. Ning, J. Barford, G. McKay, Combating oil spill problem using plastic waste. Waste Manag. 44 (2015). https://doi.org/10.1016/j.wasman.2015.06.003
J. Saleem, M. Adil Riaz, G. McKay, Oil sorbents from plastic wastes and polymers: a review. J. Hazard. Mater. 341, 424–437 (2018). https://doi.org/10.1016/j.jhazmat.2017.07.072
J.S.S. Quintans, P.P. Menezes, M.R.V. Santos, L.R. Bonjardim, J.R.G.S. Almeida, D.P. Gelain, A.A. de Araújo, Quintans-Júnior, improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in β-cyclodextrin. Phytomedicine. 20, 436–440 (2013). https://doi.org/10.1016/j.phymed.2012.12.009
J. Saleem, Z.K.B. Moghal, A.S. Luyt, R.A. Shakoor, G. McKay, Free-Standing Porous and Nonporous Polyethylene Thin films using spin coating: an alternate to the extrusion–stretching process. ACS Appl. Polym. Mater. 5, 2177–2184 (2023). https://doi.org/10.1021/acsapm.2c02183
J.-H. Lin, Y.-J. Pan, C.-F. Liu, C.-L. Huang, C.-T. Hsieh, C.-K. Chen, Z.-I. Lin, Lou, Preparation and Compatibility Evaluation of Polypropylene/High Density Polyethylene Polyblends. Mater. (Basel). 8, 8850–8859 (2015). https://doi.org/10.3390/ma8125496
A. Goto, S. Yamashita, M. Tagawa, Formation of Nanoscale Protrusions on Polymer Films after Atomic Oxygen Irradiation: Changes in Morphologies, Masses, and, F.T.-I.R. Spectra, Langmuir. 38 (2022) 3339–3349. https://doi.org/10.1021/acs.langmuir.1c02605
J. Saleem, Z.K.B. Moghal, G. McKay, Up-cycling plastic waste into swellable super-sorbents. J. Hazard. Mater. 453, 131356 (2023). https://doi.org/10.1016/j.jhazmat.2023.131356
J. Song, J. Liang, X. Liu, W.E. Krause, J.P. Hinestroza, O.J. Rojas, Development and characterization of thin polymer films relevant to fiber processing. Thin Solid Films. 517, 4348–4354 (2009). https://doi.org/10.1016/j.tsf.2009.03.015
J. Saleem, M.Z. Khalid Baig, U. Bin Shahid, R. Luque, G. McKay, Mixed plastics waste valorization to high-added value products via thermally induced phase separation and spin-casting. Green. Energy Environ. (2023). https://doi.org/10.1016/j.gee.2023.08.004
E. Sosa, R. Cabrera-Sierra, M. Oropeza, F. Hernández, N. Casillas, R. Tremont, C. Cabrera, I. González, Electrochemically grown passive films on carbon steel (SAE 1018) in alkaline sour medium. Electrochim. Acta. 48, 1665–1674 (2003). https://doi.org/10.1016/S0013-4686(03)00145-2
K. Sarkar, A. Mondal, A. Chakraborty, M. Sanbui, N. Rani, M. Dutta, Investigation of microstructure and corrosion behaviour of prior nickel deposited galvanised steels. Surf. Coat. Technol. 348, 64–72 (2018). https://doi.org/10.1016/j.surfcoat.2018.05.036
M. Feng, J. Qiu, X. Zhou, L. Wang, T. Li, J. Xie, M. Yang, Influence of flow on the corrosion behavior of pure iron in simulated geological repository conditions. Surf. Interfaces. 46, 103998 (2024). https://doi.org/10.1016/j.surfin.2024.103998
L. Wu, Z. Zhou, K. Zhang, X. Zhang, G. Wang, Electrochemical and passive film evaluation on the corrosion resistance variation of Fe-based amorphous coating affected by high temperature. J. Non Cryst. Solids. 597, 121892 (2022). https://doi.org/10.1016/j.jnoncrysol.2022.121892
A.A. Hermas, M.A. Salam, S.S. Al-Juaid, A.H. Qusti, M.Y. Abdelaal, Electrosynthesis and protection role of polyaniline–polvinylalcohol composite on stainless steel. Prog Org. Coat. 77, 403–411 (2014). https://doi.org/10.1016/j.porgcoat.2013.11.002
A.A. Hermas, XPS analysis of the passive film formed on austenitic stainless steel coated with conductive polymer. Corros. Sci. 50, 2498–2505 (2008). https://doi.org/10.1016/j.corsci.2008.06.019
A.A. Ganash, F.M. Al-Nowaiser, S.A. Al-Thabaiti, A.A. Hermas, Comparison study for passivation of stainless steel by coating with polyaniline from two different acids. Prog Org. Coat. 72, 480–485 (2011). https://doi.org/10.1016/j.porgcoat.2011.06.006
Funding
This publication was made possible by NPRP grant number NPRP12S-0325–190443 from the Qatar National Research Fund (a member of the Qatar Foundation). Open Access funding is provided by Qatar National Library.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saleem, J., Moghal, Z.K.B., Fayyaz, O. et al. Eco-friendly freestanding superhydrophobic thin films and coatings for corrosion protection. emergent mater. (2024). https://doi.org/10.1007/s42247-024-00794-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42247-024-00794-y