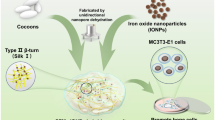
Abstract
Bacterial infection is a major problem following bone implant surgery. Moreover, poly-l-lactic acid/carbon nanotube/hydroxyapatite (PLLA/CNT/HAP) bone scaffolds possess enhanced mechanical properties and show good bioactivity regarding bone defect regeneration. In this study, we synthesized silver (Ag)-doped CNT/HAP (CNT/Ag-HAP) nanohybrids via the partial replacing of calcium ions (Ca2+) in the HAP lattice with silver ions (Ag+) using an ion doping technique under hydrothermal conditions. Specifically, the doping process was induced using the special lattice structure of HAP and the abundant surface oxygenic functional groups of CNT, and involved the partial replacement of Ca2+ in the HAP lattice by doped Ag+ as well as the in situ synthesis of Ag-HAP nanoparticles on CNT in a hydrothermal environment. The resulting CNT/Ag-HAP nanohybrids were then introduced into a PLLA matrix via laser-based powder bed fusion (PBF-LB) to fabricate PLLA/CNT/Ag-HAP scaffolds that showed sustained antibacterial activity. We then found that Ag+, which possesses broad-spectrum antibacterial activity, endowed PLLA/CNT/Ag-HAP scaffolds with this activity, with an antibacterial effectiveness of 92.65%. This antibacterial effect is due to the powerful effect of Ag+ against bacterial structure and genetic material, as well as the physical destruction of bacterial structures due to the sharp edge structure of CNT. In addition, the scaffold possessed enhanced mechanical properties, showing tensile and compressive strengths of 8.49 MPa and 19.72 MPa, respectively. Finally, the scaffold also exhibited good bioactivity and cytocompatibility, including the ability to form apatite layers and to promote the adhesion and proliferation of human osteoblast-like cells (MG63 cells).
Graphic abstract










Similar content being viewed by others
References
Dang HP, Shabab T, Shafiee A et al (2019) 3D printed dual macro-, microscale porous network as a tissue engineering scaffold with drug delivering function. Biofabrication 11(3):035014. https://doi.org/10.1088/1758-5090/ab14ff
Kalsi S, Singh J, Sehgal SS et al (2021) Biomaterials for tissue engineered bone scaffolds: a review. Mater Today Proc 81(2):888–893. https://doi.org/10.1016/j.matpr.2021.04.273
Feng P, Zhao RY, Tang WM et al (2023) Structural and functional adaptive artificial bone: materials, fabrications, and properties. Adv Funct Mater 33(23):2214726. https://doi.org/10.1002/adfm.202214726
Shao HF, Nian ZH, Jing ZL et al (2022) Additive manufacturing of hydroxyapatite bioceramic scaffolds with projection based 3D printing. Chin J Mech Eng Addit Manuf Front 1(2):100021. https://doi.org/10.1016/j.cjmeam.2022.100021
Esmi A, Jahani Y, Yousefi AA et al (2019) PMMA-CNT-HAP nanocomposites optimized for 3D-printing applications. Mater Res Express 6(8):085405. https://doi.org/10.1088/2053-1591/ab2157
Zhang L, Yang GJ, Johnson BN et al (2019) Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater 84:16–33. https://doi.org/10.1016/j.actbio.2018.11.039
Qi F, Wang Z, Yang L et al (2023) A collaborative CeO2@metal-organic framework nanosystem to endow scaffolds with photodynamic antibacterial effect. Mater Today Chem 27:101336. https://doi.org/10.1016/j.mtchem.2022.101336
Qian GW, Zhang LM, Shuai Y et al (2023) 3D-printed CuFe2O4-MXene/PLLA antibacterial tracheal scaffold against implantation-associated infection. Appl Surf Sci 614:156108. https://doi.org/10.1016/j.apsusc.2022.156108
Yang CS, Zhou L, Geng XD et al (2022) New dual-function in situ bone repair scaffolds promote osteogenesis and reduce infection. J Biol Eng 16(1):23. https://doi.org/10.1186/s13036-022-00302-y
Wu YZ, Liao Q, Wu L et al (2021) ZnL2-BPs integrated bone scaffold under sequential photothermal mediation: a win–win strategy delivering antibacterial therapy and fostering osteogenesis thereafter. ACS Nano 15(11):17854–17869. https://doi.org/10.1021/acsnano.1c06062.s001
Zan J, Shuai Y, Zhang J et al (2023) Hyaluronic acid encapsulated silver metal organic framework for the construction of a slow-controlled bifunctional nanostructure: antibacterial and anti-inflammatory in intrauterine adhesion repair. Int J Biol Macromol 230:123361. https://doi.org/10.1016/j.ijbiomac.2023.123361
Agnihotri R, Gaur S, Albin S (2020) Nanometals in dentistry: applications and toxicological implications—a systematic review. Biol Trace Elem Res 197(1):70–88. https://doi.org/10.1007/s12011-019-01986-y
Feng P, Shen SP, Shuai Y et al (2023) PLLA grafting draws GO from PGA phase to the interface in PLLA/PGA bone scaffold owing enhanced interfacial bonding. Sustain Mater Technol 35:e00566. https://doi.org/10.1016/j.susmat.2023.e00566
Riaz M, Zia R, Ijaz A et al (2018) Synthesis of monophasic Ag doped hydroxyapatite and evaluation of antibacterial activity. Mater Sci Eng C 90:308–313. https://doi.org/10.1016/j.msec.2018.04.076
Uskoković V (2020) Ion-doped hydroxyapatite: an impasse or the road to follow? Ceram Int 46(8):11443–11465. https://doi.org/10.1016/j.ceramint.2020.02.001
Mansour SF, El-Dek SI, Dorozhkin SV et al (2017) Physico-mechanical properties of Mg and Ag doped hydroxyapatite/chitosan biocomposites. New J Chem 41(22):13773–13783. https://doi.org/10.1039/c7nj01777d
Sobczak-Kupiec A, Pluta K, Drabczyk A et al (2018) Synthesis and characterization of ceramic-polymer composites containing bioactive synthetic hydroxyapatite for biomedical applications. Ceram Int 44(12):13630–13638. https://doi.org/10.1016/j.ceramint.2018.04.199
Li XJ, Yuan Y, Liu LY et al (2020) 3D printing of hydroxyapatite/tricalcium phosphate scaffold with hierarchical porous structure for bone regeneration. Bio-Des Manuf 3(1):15–29. https://doi.org/10.1007/s42242-019-00056-5
Mohiti-Asli M, Pourdeyhimi B, Loboa EG (2014) Novel, silver-ion-releasing nanofibrous scaffolds exhibit excellent antibacterial efficacy without the use of silver nanoparticles. Acta Biomater 10(5):2096–2104. https://doi.org/10.1016/j.colsurfb.2019.01.064
Yang YW, Cheng Y, Deng F et al (2021) A bifunctional bone scaffold combines osteogenesis and antibacterial activity via in situ grown hydroxyapatite and silver nanoparticles. Bio-Des Manuf 4(3):452–468. https://doi.org/10.1007/s42242-021-00130-x
Karunakaran G, Cho EB, Kumar GS et al (2019) Ascorbic acid-assisted microwave synthesis of mesoporous Ag-doped hydroxyapatite nanorods from biowaste seashells for implant applications. ACS Appl Bio Mater 2(5):2280–2293. https://doi.org/10.1021/acsabm.9b00239
Karthieka RR, Prakash T (2023) Influence of applied bias on direct conversion X-ray sensing capability of nanocrystalline Ca9Ag(PO4)6(OH)2. Mater Sci Semicond Process 162:107517. https://doi.org/10.1016/j.mssp.2023.107517
Jin S, Li JD, Wang J et al (2018) Electrospun silver ion-loaded calcium phosphate/chitosan antibacterial composite fibrous membranes for guided bone regeneration. Int J Nanomed 13:4591–4605. https://doi.org/10.2147/IJN.S167793
Dong Y, Chen AN, Yang T et al (2023) Microstructure evolution and mechanical properties of Al2O3 foams via laser powder bed fusion from Al particles. Adv Powder Mater 2(4):100135. https://doi.org/10.1016/j.apmate.2023.100135
Tian XY, Wu LL, Gu DD et al (2022) Roadmap for additive manufacturing: toward intellectualization and industrialization. Chin J Mech Eng Addit Manuf Front 1(1):100014. https://doi.org/10.1016/j.cjmeam.2022.100014
Niu PD, Li RD, Fan ZQ et al (2023) Inhibiting cracking and improving strength for additive manufactured AlxCoCrFeNi high entropy alloy via changing crystal structure from BCC-to-FCC. Addit Manuf 71:103584. https://doi.org/10.1016/j.addma.2023.103584
Chen AN, Su J, Li YJ et al (2023) 3D/4D printed bio-piezoelectric smart scaffolds for next-generation bone tissue engineering. Int J Extreme Manuf 5:032007. https://doi.org/10.1088/2631-7990/acd88f
Gao CD, Yao X, Deng YW et al (2023) Laser-beam powder bed fusion followed by annealing with stress: a promising route for magnetostrictive improvement of polycrystalline Fe81Ga19 alloys. Addit Manuf 68:103516. https://doi.org/10.1016/j.addma.2023.103516
Ji HR, Zhao MC, Xie B et al (2021) Corrosion and antibacterial performance of novel selective-laser-melted (SLMed) Ti-xCu biomedical alloys. J Alloy Comp 864:158415. https://doi.org/10.1016/j.jallcom.2020.158415
Hassan AA, Radwan HA, Abdelaal SA et al (2021) Polycaprolactone based electrospun matrices loaded with Ag/hydroxyapatite as wound dressings: morphology, cell adhesion, and antibacterial activity. Int J Pharmaceut 593:120143. https://doi.org/10.1016/j.ijpharm.2020.120143
Esfahani H, Salahi E, Tayebifard A et al (2014) Influence of zinc incorporation on microstructure of hydroxyapatite to characterize the effect of pH and calcination temperatures. J Asian Ceram Soc 2(3):248–252. https://doi.org/10.1016/j.jascer.2014.05.001
Nikolova V, Kircheva N, Dobrev S et al (2023) Lanthanides as calcium mimetic species in calcium-signaling/buffering proteins: the effect of lanthanide type on the Ca2+/Ln3+ competition. Int J Mol Sci 24(7):6297. https://doi.org/10.3390/ijms24076297
Saini RK, Bagri LP, Bajpai A (2019) Nano-silver hydroxyapatite based antibacterial 3D scaffolds of gelatin/alginate/poly(vinyl alcohol) for bone tissue engineering applications. Colloids Surf B 177:211–218. https://doi.org/10.1016/j.colsurfb.2019.01.064
Nagyne-Kovacs T, Meszaros B, Molnar M et al (2020) Hydrothermal synthesis of Sr-doped hydroxyapatite and its antibacterial activity. Period Polytech Chem Eng 64(1):54–60. https://doi.org/10.3311/PPch.14062
Jacobs A, Gaulier M, Duval A et al (2019) Silver doping mechanism in bioceramics—from Ag+:doped HAp to Ag°/BCP nanocomposite. Crystals 9(7):326. https://doi.org/10.3390/cryst9070326
Wang JR, Gong X, Hai J et al (2018) Synthesis of silver–hydroxyapatite composite with improved antibacterial properties. Vacuum 152:132–137. https://doi.org/10.1016/j.vacuum.2018.03.015
Ciobanu CS, Iconaru SL, Pasuk I et al (2013) Structural properties of silver doped hydroxyapatite and their biocompatibility. Mater Sci Eng C 33(3):1395–1402. https://doi.org/10.1016/j.msec.2012.12.042
Maleki-Ghaleh H, Siadati MH, Fallah A et al (2021) Effect of zinc-doped hydroxyapatite/graphene nanocomposite on the physicochemical properties and osteogenesis differentiation of 3D-printed polycaprolactone scaffolds for bone tissue engineering. Chem Eng J 426:131321. https://doi.org/10.1016/j.cej.2021.131321
Li HP, Song XQ, Li BE et al (2017) Carbon nanotube-reinforced mesoporous hydroxyapatite composites with excellent mechanical and biological properties for bone replacement material application. Mater Sci Eng C 77:1078–1087. https://doi.org/10.1016/j.msec.2017.04.048
Khan AS, Hussain AN, Sidra L et al (2017) Fabrication and in vivo evaluation of hydroxyapatite/carbon nanotube electrospun fibers for biomedical/dental application. Mater Sci Eng C 80:387–396. https://doi.org/10.1016/j.msec.2017.05.109
Im YO, Lee SH, Kim T et al (2017) Utilization of carboxylic functional groups generated during purification of carbon nanotube fiber for its strength improvement. Appl Surf Sci 392:342–349. https://doi.org/10.1016/j.apsusc.2016.09.060d
Afroze JD, Abden MJ, Alam MS et al (2016) Development of functionalized carbon nanotube reinforced hydroxyapatite magnetic nanocomposites. Mater Lett 169:24–27. https://doi.org/10.1016/j.matlet.2016.01.060
Guo WT, Wang XC, Yang CY et al (2022) Microfluidic 3D printing polyhydroxyalkanoates-based bionic skin for wound healing. Mater Future 1(1):015401. https://doi.org/10.1088/2752-5724/ac446b
Feng JW, Fu JZ, Yao XH et al (2022) Triply periodic minimal surface (TPMS) porous structures: from multi-scale design, precise additive manufacturing to multidisciplinary applications. Int J Extreme Manuf 4(2):022001. https://doi.org/10.1088/2631-7990/ac5be6
Li QT, Xu S, Feng Q et al (2021) 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact Mater 6(10):3396–3410. https://doi.org/10.1016/j.bioactmat.2021.03.013
Han W, Kong LB, Xu M (2022) Advances in selective laser sintering of polymers. Int J Extreme Manuf 4(4):042002. https://doi.org/10.1088/2631-7990/ac9096
Chen XM, Zhang LY, Zheng M et al (2015) Quantitative nanomechanical characterization of the van der Waals interfaces between carbon nanotubes and epoxy. Carbon 82:214–228. https://doi.org/10.1016/j.carbon.2014.10.065
Zhao Y, Qiu YH, Fang ZX et al (2022) Preparation and characterization of Sr-substituted hydroxyapatite/reduced graphene oxide 3D scaffold as drug carrier for alendronate sodium delivery. Ceram Int 48(24):36601–36608. https://doi.org/10.1016/j.ceramint.2022.08.219
Gupta N, Gupta SM, Sharma SK (2019) Carbon nanotubes: synthesis, properties and engineering applications. Carbon Lett 29(5):419–447. https://doi.org/10.1007/s42823-019-00068-2
Sezer HK, Eren OM (2019) FDM 3D printing of MWCNT re-inforced ABS nano-composite parts with enhanced mechanical and electrical properties. J Manuf Process 37:339–347. https://doi.org/10.1016/j.jmapro.2018.12.004
Li PB, Tan WT, Gao MM et al (2021) Strengthening of the magnesium matrix composites hybrid reinforced by chemically oxidized carbon nanotubes and in situ Mg2Sip. J Alloys Compd 858:157673. https://doi.org/10.1016/j.jallcom.2020.157673
Jaswal R, Shrestha S, Shrestha BK et al (2020) Nanographene enfolded AuNPs sophisticatedly synchronized polycaprolactone based electrospun nanofibre scaffold for peripheral nerve regeneration. Mater Sci Eng C 116:111213. https://doi.org/10.1016/j.msec.2020.111213
Sukhorukova IV, Sheveyko AN, Shvindina NV et al (2017) Approaches for controlled Ag+ ion release: influence of surface topography, roughness, and bactericide content. ACS Appl Mater Interfaces 9(4):4259–4271. https://doi.org/10.1021/acsami.6b15096
Shi J, Wang J, Liang LB et al (2021) Carbothermal synthesis of biochar-supported metallic silver for enhanced photocatalytic removal of methylene blue and antimicrobial efficacy. J Hazard Mater 401:123382. https://doi.org/10.1016/j.jhazmat.2020.123382
Qin ZJ, Zheng YK, Wang YH et al (2021) Versatile roles of silver in Ag-based nanoalloys for antibacterial applications. Coord Chem Rev 449:214218. https://doi.org/10.1016/j.ccr.2021.214218
Webster RD (2023) Electrochemistry combined with electron paramagnetic resonance (EPR) spectroscopy for studying catalytic and energy storage processes. Curr Opin Electrochem 40:101308. https://doi.org/10.1016/j.coelec.2023.101308
Shukla AK, Alam J, Ansari MA et al (2019) Selective ion removal and antibacterial activity of silver-doped multi-walled carbon nanotube/polyphenylsulfone nanocomposite membranes. Mater Chem Phys 233:102–112. https://doi.org/10.1016/j.matchemphys.2019.05.054
Qian GW, Wang JZ, Yang LM et al (2023) A pH-responsive CaO2@ZIF-67 system endows a scaffold with chemodynamic therapy properties. J Mater Sci 58(3):1214–1228. https://doi.org/10.1007/s10853-022-08103-w
Jayaramudu T, Varaprasad K, Reddy KK et al (2020) Chitosan-pluronic based Cu nanocomposite hydrogels for prototype antimicrobial applications. Int J Biol Macromol 143:825–832. https://doi.org/10.1016/j.ijbiomac.2019.09.143
Kung ML, Tai MH, Lin PY et al (2017) Silver decorated copper oxide (Ag@CuO) nanocomposite enhances ROS-mediated bacterial architecture collapse. Colloids Surf B 155(1):399–407. https://doi.org/10.1016/j.colsurfb.2017.04.041
Xin Q, Shah H, Nawaz A et al (2019) Antibacterial carbon-based nanomaterials. Adv Mater 31(45):1804838. https://doi.org/10.1002/adma.201804838
Oladapo BI, Zahedi SA, Adeoye AOM (2019) 3D printing of bone scaffolds with hybrid biomaterials. Compos Part B Eng 158:428–436. https://doi.org/10.1016/j.compositesb.2018.09.065
Zadpoor AA (2014) Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater Sci Eng C 35(1):134–143. https://doi.org/10.1016/j.msec.2013.10.026
Diogo GS, Marques CF, Freitas-Ribeiro S et al (2022) Mineralized collagen as a bioactive ink to support encapsulation of human adipose stem cells: a step towards the future of bone regeneration. Biomater Adv 133:112600. https://doi.org/10.1016/j.msec.2021.112600
Qi FW, Liao RB, Wu P et al (2023) An electrical microenvironment constructed based on electromagnetic induction stimulates neural differentiation. Mater Chem Front 7(8):1671–1683. https://doi.org/10.1039/d2qm01193j
Lee SH, Lee KG, Lee J et al (2023) Three-dimensional kagome structures in a PCL/HA-based hydrogel scaffold to lead slow BMP-2 release for effective bone regeneration. Bio-Des Manuf 6(1):12–25. https://doi.org/10.1007/s42242-022-00219-x
Acknowledgements
This work was supported by the following funds: (1) the National Natural Science Foundation of China (Nos. 52275393 and 51935014); (2) Hunan Provincial Natural Science Foundation of China (Nos. 2021JJ20061, 2020JJ3047, and 2019JJ50588); (3) Jiangxi Provincial Natural Science Foundation of China (No. 20224ACB204013); (4) the Project of State Key Laboratory of High Performance Complex Manufacturing; (5) Technology Innovation Platform Project of Shenzhen Institute of Information Technology 2020 (No. PT2020E002); (6) Guangdong Province Precision Manufacturing and Intelligent Production Education Integration Innovation Platform (No. 2022CJPT019); and (7) Independent Exploration and Innovation Project of Central South University (No. 1053320220553). The authors would like to thank eceshi (www.eceshi.com) for the ICP-AES/MS test and Shiyanjia Lab (www.shiyanjia.com) for the EPR analysis.
Author information
Authors and Affiliations
Contributions
CJS and XXS were involved in conceptualization, investigation, writing—original draft; KW and YLG were involved in visualization and resources; KW and FY helped in writing—review & editing; and PF contributed to supervision.
Corresponding author
Ethics declarations
Conflict of interest
CJS is an associate editor for Bio-Design and Manufacturing and was not involved in the editorial review or the decision to publish this article. The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shuai, C., Shi, X., Wang, K. et al. Ag-doped CNT/HAP nanohybrids in a PLLA bone scaffold show significant antibacterial activity. Bio-des. Manuf. 7, 105–120 (2024). https://doi.org/10.1007/s42242-023-00264-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-023-00264-0