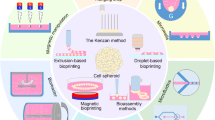
Abstract
Three-dimensional (3D) bioprinting is a computer-assisted technology which precisely controls spatial position of biomaterials, growth factors and living cells, offering unprecedented possibility to bridge the gap between structurally mimic tissue constructs and functional tissues or organoids. We briefly focus on diverse bioinks used in the recent progresses of biofabrication and 3D bioprinting of various tissue architectures including blood vessel, bone, cartilage, skin, heart, liver and nerve systems. This paper provides readers a guideline with the conjunction between bioinks and the targeted tissue or organ types in structuration and final functionalization of these tissue analogues. The challenges and perspectives in 3D bioprinting field are also illustrated.







Similar content being viewed by others
References
Ozbolat IT, Yu Y (2013) Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Bio-Med Eng 60(3):691–699
Lee JM, Yeong WY (2016) Design and printing strategies in 3D bioprinting of cell-hydrogels: a review. Adv Healthc Mater 5(22):2856–2865
Mir TA, Nakamura M (2017) 3D-Bioprinting: towards the era of manufacturing human organs as spare parts for healthcare and medicine. Tissue Eng Part B Rev 23(3):245–256
Gauvin R, Chen YC, Jin WL, Soman P, Zorlutuna P, Nichol JW, Bae H, Chen S, Khademhosseini A (2012) Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 33(15):3824–3834
Zhang YS, Yue K, Aleman J, Mollazadehmoghaddam K, Bakht SM, Yang J, Jia W, Dell’Erba V, Assawes P, Shin SR (2017) 3D bioprinting for tissue and organ fabrication. Ann Biomed Eng 45(1):148–163
Kengla C, Atala A, Sang JL (2015) Chapter 15-bioprinting of organoids. In: Haley M (ed) Essentials of 3D biofabrication and translation. Elsevier Inc, USA
Chang R, Nam Y, Sun W (2008) Direct cell writing of 3D microorgan for in vitro pharmacokinetic model. Tissue Eng Part C-Methods 14(2):157–166
Dababneh AB, Ozbolat IT (2014) Bioprinting technology: a current state-of-the-art review. J Manuf Sci Eng 136(6):061016
Ozbolat IT, Hospodiuk M (2016) Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76(37):321–343
Gudapati H, Dey M, Ozbolat I (2016) A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials 102:20–42
Schiele NR, Corr DT, Huang Y, Raof NA, Xie Y, Chrisey DB (2010) Laser-based direct-write techniques for cell printing. Biofabrication 2(3):032001
Hospodiuk M, Dey M, Sosnoski D, Ozbolat IT (2017) The bioink: a comprehensive review on bioprintable materials. Biotechnol Adv 35(2):217–239
Yao X, Peng R, Ding JD (2013) Cell-material interactions revealed via material techniques of surface patterning. Adv Mater 25(37):5257–5286
Jungst T, Smolan W, Schacht K, Scheibel T, Groll J (2016) Strategies and molecular design criteria for 3D printable hydrogels. Chem Rev 116(3):1496–1539
Yu L, Ding JD (2008) Injectable hydrogels as unique biomedical materials. Chem Soc Rev 37(8):1473–1481
Ahmed EM (2015) Hydrogel: preparation, characterization, and applications: a review. J Adv Res 6(2):105–121
Marchant RE (2011) Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices 8(5):607–626
Alakpa E, Jayawarna V, Lampel A, Burgess K, West C, Bakker SJ, Roy S, Javid N, Fleming S, Lamprou D (2016) Tunable supramolecular hydrogels for selection of lineage-guiding metabolites in stem cell cultures. Chem 1(2):298–319
Discher DE, Janmey P, Wang YL (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310(5751):1139–1143
Wells RG (2008) The role of matrix stiffness in regulating cell behavior. Hepatology 47(4):1394–1400
Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Müller R, Hubbell JA (2003) Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol 21(5):513–518
Richardson TP, Peters MC, Ennett AB, Mooney DJ (2001) Polymeric system for dual growth factor delivery. Nat Biotechnol 19(11):1029–1034
Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJ, Groll J, Hutmacher DW (2013) 25th Anniversary article: engineering hydrogels for biofabrication. Adv Mater 25(36):5011–5028
Murphy SV, Aleksander S, Anthony A (2013) Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res, Part A 101(1):272–284
Carrow JK, Kerativitayanan P, Jaiswal MK, Lokhande G, Gaharwar AK (2015) Chapter 13-polymers for bioprinting. In: Haley M (ed) Essentials of 3D biofabrication and translation. Elsevier Inc, USA
Burdick JA, Chung C, Jia X (2005) Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules 6(1):386–391
Wu LB, Ding JD (2004) In vitro degradation of three-dimensional porous poly(D, L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 25(27):5821–5830
Khalil S, Sun W (2009) Bioprinting endothelial cells with alginate for 3D tissue constructs. Trans ASME J Biomech Eng 131(11):111002
Malheiro A, Wieringa P, Mota C, Baker M, Moroni L (2016) Patterning vasculature: the role of biofabrication to achieve an integrated multicellular ecosystem. ACS Biomater Sci Eng 2(10):1694–1709
Melchiorri AJ, Fisher JP (2015) Chapter 20-bioprinting of blood vessels. In: Haley M (ed) Essentials of 3D biofabrication and translation. Elsevier Inc, USA
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773–785
Muschler GF, Nakamoto C, Griffith LG (2004) Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg 86–A:1541–1558
Norotte C, Marga FS, Niklason LE, Forgacs G (2009) Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30(30):5910
Lu HJ, Feng ZQ, Gu ZZ, Liu CJ (2009) Growth of outgrowth endothelial cells on aligned PLLA nanofibrous scaffolds. J Mater Sci-Mater Med 20(9):1937–1944
Xu C, Chai W, Huang Y, Markwald RR (2012) Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol Bioeng 109(12):3152–3160
Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS (2012) Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 11(9):768–774
Bertassoni LE, Cecconi M, Manoharan V, Nikkhah M, Hjortnaes J, Cristino AL, Barabaschi G, Demarchi D, Dokmeci MR, Yang Y, Khademhosseini A (2014) Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 14(13):2202–2211
Fan R, Piou M, Darling E, Cormier D, Sun J, Wan J (2016) Bio-printing Cell-laden Matrigel-agarose Constructs. J Biomater Appl 31(5):684–692
Bertassoni LE, Cardoso JC, Manoharan V, Cristino AL, Bhise NS, Araujo WA, Zorlutuna P, Vrana NE, Ghaemmaghami AM, Dokmeci MR, Khademhosseini A (2014) Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 6(2):024105
Skardal A, Zhang J, McCoard L, Xu X, Oottamasathien S, Prestwich GD (2010) Photocrosslinkable Hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng Part A 16(8):2675–2685
Salgado AJ, Coutinho OP, Reis RL (2004) Bone Tissue engineering: state of the art and future trends. Macromol Biosci 4(8):743–765
Knothe MLT (2003) Whither flows the fluid in bone? An osteocyte’s perspective. J Biomech 36(10):1409–1424
Hing KA (1825) Bone repair in the twenty-first century: biology, chemistry or engineering? Philos Trans 2004(362):2821–2850
Larsen M, Mishra R, Miller M, Dean D (2015) Chapter 17-bioprinting of bone. In: Haley M (ed) Essentials of 3D biofabrication and translation. Elsevier Inc, USA
Sun W, Puzas JE, Sheu TJ, Liu X, Fauchet PM (2007) Nano- to microscale porous silicon as a cell interface for bone-tissue engineering. Adv Mater 19(7):921–924
Fedorovich NE, De Wijn JR, Verbout AJ, Alblas J, Dhert WJ (2008) Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng Part A 14(1):127–133
Gruene M, Deiwick A, Koch L, Schlie S, Unger C, Hofmann N, Bernemann I, Glasmacher B, Chichkov B (2011) Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng Part C Methods 17(1):79–87
Byambaa B, Annabi N, Yue K, Trujillo-de Santiago G, Alvarez MM, Jia W, Kazemzadeh-Narbat M, Shin SR, Tamayol A, Khademhosseini A (2017) Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv Healthc Mater 6(16):1700015
Keriquel V, Oliveira H, Remy M, Ziane S, Delmond S, Rousseau B, Rey S, Catros S, Amedee J, Guillemot F, Fricain JC (2017) In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci Rep 7(1):1778
Fedorovich NE, Wijnberg HM, Dhert WJ, Alblas J (2011) Distinct tissue formation by heterogeneous printing of osteo- and endothelial progenitor cells. Tissue Eng Part A 17(15–16):2113–2121
Carlier A, Skvortsov GA, Hafezi F, Ferraris E, Patterson J, Koc B, Van Oosterwyck H (2016) Computational model-informed design and bioprinting of cell-patterned constructs for bone tissue engineering. Biofabrication 8(2):025009
Hung BP, Naved BA, Nyberg EL, Dias M, Holmes CA, Elisseeff JH, Dorafshar AH, Grayson WL (2016) Three-dimensional printing of bone extracellular matrix for craniofacial regeneration. ACS Biomater Sci Eng 2(10):1806–1816
Naumann A, Dennis JE, Awadallah A, Carrino DA, Mansour JM, Kastenbauer E, Caplan AI (2002) Immunochemical and mechanical characterization of cartilage subtypes in rabbit. J Histochem Cytochem Off Jo Histochem Soc 50(8):1049–1058
Umlauf D, Frank S, Pap T, Bertrand J (2010) Cartilage biology, pathology, and repair. Cell Mol Life Sci 67(24):4197–4211
Liu M, Zeng X, Ma C, Yi H, Ali Z, Mou X, Li S, Deng Y, He N (2017) Injectable hydrogels for cartilage and bone tissue engineering. Bone Res 5:17014
Cui X, Breitenkamp K, Finn MG, Lotz M, D’Lima DD (2012) Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 18(11–12):1304–1312
Zhang YS, Yue K, Aleman J, Mollazadeh-Moghaddam K, Bakht SM, Yang J, Jia W, Dell’Erba V, Assawes P, Shin SR, Dokmeci MR, Oklu R, Khademhosseini A (2016) 3D bioprinting for tissue and organ fabrication. Ann Biomed Eng 45(1):148–163
Lai K, Xu T (2015) Chapter 18-bioprinting of cartilage: recent progress on bioprinting of cartilage. In: Haley M (ed) Essentials of 3D biofabrication and translation. Elsevier Inc, USA
Schuurman W, Levett PA, Pot MW, van Weeren PR, Dhert WJ, Hutmacher DW, Melchels FP, Klein TJ, Malda J (2013) Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol Biosci 13(5):551–561
Park JY, Choi JC, Shim JH, Lee JS, Park H, Kim SW, Doh J, Cho DW (2014) A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 6(3):035004
Daly AC, Critchley SE, Rencsok EM, Kelly DJ (2016) A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 8(4):045002
Shi W, Sun M, Hu X, Ren B, Cheng J, Li C, Duan X, Fu X, Zhang J, Chen H, Ao Y (2017) Structurally and functionally optimized silk-fibroin-gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv Mater 29(29):170189
Kundu J, Shim JH, Jang J, Kim SW, Cho DW (2015) An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med 9(11):1286–1297
Pescosolido L, Schuurman W, Malda J, Matricardi P, Alhaique F, Coviello T, van Weeren PR, Dhert WJ, Hennink WE, Vermonden T (2011) Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules 12(5):1831–1838
Apelgren P, Amoroso M, Lindahl A, Brantsing C, Rotter N, Gatenholm P, Kolby L (2017) Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLoS ONE 12(12):e0189428
Stichler S, Bock T, Paxton N, Bertlein S, Levato R, Schill V, Smolan W, Malda J, Tessmar J, Blunk T, Groll J (2017) Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(epsilon-caprolactone) for MSC chondrogenesis. Biofabrication 9(4):044108
Rhee S, Puetzer JL, Mason BN, Reinhartking CA, Bonassar LJ (2016) 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater Sci Eng 2(10):1800–1805
Vijayavenkataraman S, Lu WF, Fuh JY (2016) 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication 8(3):032001
Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M (2003) Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res 42(1):1–36
Koch L, Michael S, Reimers K, Vogt PM, Chichkov B (2015) Chapter 13-bioprinting for skin. In: Geraghty F (ed) 3D bioprinting and nanotechnology in tissue engineering and regenerative medicine. Elsevier Inc, USA
Michael S, Sorg H, Peck CT, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K (2013) Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS ONE 8(3):e57741
Lineen E, Namias N (2008) Biologic dressing in burns. J Craniofac Surg 19(4):923–928
Lee W, Debasitis JC, Lee VK, Lee JH, Fischer K, Edminster K, Park JK, Yoo SS (2009) Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 30(8):1587–1595
Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo SS, Dai G, Karande P (2014) Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng Part C Methods 20(6):473–484
Cubo N, Garcia M, Del Canizo JF, Velasco D, Jorcano JL (2016) 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication 9(1):015006
Pourchet LJ, Thepot A, Albouy M, Courtial EJ, Boher A, Blum LJ, Marquette CA (2017) Human skin 3D bioprinting using scaffold-free approach. Adv Healthc Mater 6(4):1601101
Farrell MJ, Kirby ML (2001) Cell biology of cardiac development. Int Rev Cytol 202(202):99–158
Severs NJ (2000) The cardiac muscle cell. BioEssays 22(2):188–199
Gelb BD (2013) Recent advances in understanding the genetics of congenital heart defects. Curr Opin Pediatr 25(5):561–566
Silvestri A, Boffito M, Sartori S, Ciardelli G (2013) Biomimetic materials and scaffolds for myocardial tissue regeneration. Macromol Biosci 13(8):984–1019
Mironov V, Reis N, Derby B (2006) Review: bioprinting: a beginning. Tissue Eng 12(4):631–634
Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, Sluijter JP (2012) Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 33(6):1782–1790
Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A (2016) Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 110:45–59
Duan B, Hockaday LA, Kang KH, Butcher JT (2013) 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A 101(5):1255–1264
Gaebel R, Ma N, Liu J, Guan J, Koch L, Klopsch C, Gruene M, Toelk A, Wang W, Mark P, Wang F, Chichkov B, Li W, Steinhoff G (2011) Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 32(35):9218–9230
Filová E, Straka F, Mirejovský T, Masín J, Bacáková L (2009) Tissue-engineered heart valves. Physiol Res 58 Suppl 2(6):S141–158
Ji B, Fisher J, Nyberg SL (2011) Liver regeneration and tissue engineering. In: Bernstein HS (ed) Tissue engineering in regenerative medicine. Humana Press, USA
Song ZW, Gupta K, Ng IC, Xing JW, Yang YA, Yu H (2018) Mechanosensing in liver regeneration. Semin Cell Dev Biol 71:153–167
Michalopoulos GK, DeFrances MC (1997) Liver regeneration. Science 276(5309):60–66
Bedossa P, Paradis V (2003) Liver extracellular matrix in health and disease. J Pathol 200(4):504–515
Natarajan V, Harris EN (2017) Kidambi S (2017) SECs (sinusoidal endothelial cells), liver microenvironment, and fibrosis. Biomed Res Int 1:4097205
Chang R, Emami K, Wu HL, Sun W (2010) Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2(4):045004
Bhise NS, Manoharan V, Massa S, Tamayol A, Ghaderi M, Miscuglio M, Lang Q, Zhang YS, Shin SR, Calzone G, Annabi N, Shupe TD, Bishop CE, Atala A, Dokmeci MR, Khademhosseini A (2016) A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 8(1):014101
Matsusaki M, Sakaue K, Kadowaki K, Akashi M (2013) Three-dimensional human tissue chips fabricated by rapid and automatic inkjet cell printing. Adv Healthc Mater 2(4):534–539
Ma XY, Qu X, Zhu W, Li YS, Yuan SL, Zhang H, Liu J, Wang PR, Lai CSE, Zanella F, Feng GS, Sheikh F, Chien S, Chen SC (2016) Deterministically patterned biomimetic human ipsc-derived hepatic model via rapid 3D bioprinting. Proc Nat Acad Sci USA 113(8):2206–2211
Nguyen DG, Funk J, Robbins JB, Crogan-Grundy C, Presnell SC, Singer T, Roth AB (2016) Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS ONE 11(7):e0158674
Snyder JE, Hamid Q, Wang C, Chang R, Emami K, Wu H, Sun W (2011) Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication 3(3):034112
Wang XH, Yan YN, Pan YQ, Xiong Z, Liu HX, Cheng B, Liu F, Lin F, Wu RD, Zhang RJ, Lu QP (2006) Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng 12(1):83–90
Zhu W, Castro NJ, Zhang LG (2015) Chapter 14-nanotechnology and 3D bioprinting for neural tissue regeneration. In: Geraghty F (ed) 3D bioprinting and nanotechnology in tissue engineering and regenerative medicine. Elsevier Inc, USA
Owens C, Marga F, Forgacs G (2015) Chapter 23-bioprinting of nerve. In: Haley M (ed) Essentials of 3D biofabrication and translation. Elsevier Inc, USA
Moneim M, Omer G (1998) Clinical Outcome Following Acute Nerve Repair. Management of Peripheral Nerve Problems. 1998:414–419
Cunha C, Panseri S, Antonini S (2011) Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomed Nanotechnol Biol Med 7(1):50–59
Subramanian A, Krishnan UM, Sethuraman S (2009) Development of biomaterial scaffold for nerve tissue engineering: biomaterial mediated neural regeneration. J Biomed Sci 16(1):108
Chen JL, Yin Z, Shen WLA, Chen XA, Heng BC, Zou XAH, Ouyang HW (2010) Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials 31(36):9438–9451
Ladak A, Olson J, Tredget EE, Gordon T (2011) Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp Neurol 228(2):242–252
Lee Y-B, Polio S, Lee W, Dai G, Menon L, Carroll RS, Yoo S-S (2010) Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp Neurol 223(2):645–652
Ferris CJ, Gilmore KJ, Beirne S, McCallum D, Wallace GG, Panhuis MIH (2013) Bio-ink for on-demand printing of living cells. Biomater Sci 1(2):224–230
Gu Q, Tomaskoviccrook E, Lozano R, Chen Y, Kapsa RM, Zhou Q, Wallace GG, Crook JM (2016) Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv Healthc Mater 5(12):1429–1438
Owens CM, Marga F, Forgacs G, Heesch CM (2013) Biofabrication and testing of a fully cellular nerve graft. Biofabrication 5(4):045007
Lee W, Pinckney J, Lee V, Lee JH, Fischer K, Polio S, Park JK, Yoo SS (2009) Three-dimensional bioprinting of rat embryonic neural cells. NeuroReport 20(8):798–803
Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, Boland T (2006) Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 27(19):3580–3588
Hopp B, Smausz T, Kresz N, Barna N, Bor Z, Kolozsvari L, Chrisey DB, Szabo A, Nogradi A (2005) Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng 11(11–12):1817–1823
Hsieh FY, Hsu SH (2015) 3D bioprinting: a new insight into the therapeutic strategy of neural tissue regeneration. Organogenesis 11(4):153–158
Du X, Zhou J, Shi J, Xu B (2015) Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem Rev 115(24):13165
Dou XQ, Feng CL (2017) Amino acids and peptide-based supramolecular hydrogels for three-dimensional cell culture. Adv Mater 29(16):1604062
Fleming S, Ulijn RV (2014) Design of nanostructures based on aromatic peptide amphiphiles. Chem Soc Rev 43(23):8150
Miller JS (2014) The billion cell construct: will three-dimensional printing get us there? PLoS Biol 12(6):e1001882
Niklason LE, Langer RS (1997) Advances in tissue engineering of blood vessels and other tissues. Transpl Immunol 5(4):303–306
Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro E, Chen AA, Galie PA, Yu X (2012) Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 11(9):768–774
Novosel EC, Kleinhans C, Kluger PJ (2011) Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev 63(4–5):300–311
Jeyaraj R, Natasha G, Kirby G, Rajadas J, Mosahebi A, Seifalian AM, Tan A (2015) Vascularisation in regenerative therapeutics and surgery. Mater Sci Eng C Mater Biol Appl 54:225–238
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (Project No. 21703253, 21774132, 21644007) and the Talent Fund of the Recruitment Program of Global Youth Experts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jian, H., Wang, M., Wang, S. et al. 3D bioprinting for cell culture and tissue fabrication. Bio-des. Manuf. 1, 45–61 (2018). https://doi.org/10.1007/s42242-018-0006-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-018-0006-1