Abstract
Haptic feedback is typically missing during telemanipulation of surgical robots in minimally invasive surgeries, i.e., surgeons cannot feel the interaction forces between the instruments and tissues. Instead, surgeons have to solely rely on visual feedback, which increases complexity of guiding the instruments and poses the safety threat of unperceivable contacts outside the field of view. We propose a novel series elastic actuation design for articulated robotic endoscopes to overcome these limitations and evaluate an according device with one joint. Similar to the actuation of human fingers, the joint is driven by antagonistic tendons. Springs are integrated in the transmission between the tendons and the motors outside of the endoscope shaft. We estimated the joint angle and thereby the endoscope shape, measured spring deflection, estimated tendon forces from that deflection, and implemented force control for the endoscope joint. Zero torque control and impedance control were evaluated under application of both a continuous force and an impact force to the endoscope tip. The springs reduced impact forces at the tip of the endoscope through their inherent compliance. At the same time, feeding back the estimated force resulted in a stable tendon force control and a tunable endoscope joint control: Zero torque control effectively reduced the external forces, while the endoscope joint showed the expected stiffness in impedance control. These results show that antagonistic series elastic actuation is a promising concept for endoscope joint actuation and that it can lead towards safer robot–tissue interactions in surgical robotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The concept of minimally invasive surgery aims at reducing the size and number of incisions that surgeons perform during an intervention. Depending on the intervention, benefits of minimally invasive surgery include less blood loss [1,2,3,4], shorter recovery times [1], and shorter hospital stays [4, 5]. However, minimally invasive surgery is associated with a considerable learning curve [6,7,8], since it is difficult for the surgeon to manually manipulate the surgical instruments through small holes [9]. Robots can assist with manipulating these instruments and allow the surgeon to focus more on the actual surgical task, such as inspecting the site, taking biopsies, and treating pathologies. A typical setup for robot-assisted surgeries features the robot at the operating table and the surgeon controlling it with a telemanipulation console. By eliminating the direct mechanical connection between surgeon and surgical instruments, robotic control can improve task performance. For example, filtering hand tremor [10] and motion scaling [11] can improve accuracy. Eliminating the fulcrum effect (i.e., the mirroring motion about the incision point) can make instrument manipulation more intuitive [12].
To realize robot-assisted minimally invasive surgery, the movable part that enters the human body must be small. Therefore, current flexible robotic endoscopes often have a remote actuation, i.e., the source of mechanical energy is placed outside the endoscope and forces are transmitted to the movable parts through a mechanical transmission. Most commonly, a transmission via cables, often referred to as tendons, is used. These tendons are placed under high pre-tension to reduce the effects of friction and backlash [13]. Even though this improves the performance for accurate motion control, a high pre-tension also renders the actuation stiff. Therefore, collisions of the endoscope with the surrounding tissue result in high interaction forces, which can damage the tissue and may lead to complications [14].
Eliminating the mechanical connection between surgeon and surgical instruments introduces another problem: The surgeon solely relies on visual feedback through the endoscopic camera because haptic feedback is missing. If the surgeon cannot perceive collisions of the endoscope with the tissue, e.g., when these collisions occur outside the camera’s visual field, these collisions are especially dangerous. In conventional non-robotic interventions, the surgeon could eventually perceive such collisions through direct haptic feedback when handling the endoscope. The lack of haptic feedback in robot-assisted interventions has been identified as one of the main drawbacks of current robotic minimally invasive surgical systems [9, 12, 15]. To provide haptic feedback, interaction forces must be measured. However, available force sensors are too bulky and often not sterilizable [16]. Furthermore, haptic perception involves the exchange of mechanical energy in two directions (unlike vision or hearing, which are both unidirectional). A bidirectional teleoperation system should be transparent, i.e., the surgeon console and the patient-side robot should follow each other without noticeable disturbances (e.g., from friction, inertia, delay, or gravity). To achieve acceptable transparency, the control gains need to be high. From a control systems perspective, this is challenging, since delays and disturbances cause instabilities in a feedback loop with high control gains [15, 17].
Instead of reducing the interaction forces between the endoscope and its environment with virtual compliance through digital control only, the endoscope can be made inherently compliant. A popular approach for compliant endoscopes is to use an elastic backbone, as seen in catheters and hand-controlled flexible endoscopes. Similarly, robots with elastic backbones, so-called continuum robots, have been developed for surgeries [18]. However, robots with elastic backbones cause challenges in shape estimation, which is required for precise motion control. They can bend anywhere along their flexible structure, making it complicated to reliably measure the device's shape without detailed knowledge of the environment the endoscope is in contact with [19]. Furthermore, when interaction forces are in fact needed to complete a surgical task (e.g., perforation, grasping, and cutting), continuum robots are limited by the stiffness of their backbone [20].
Inherently elastic robots that enable a more reliable shape reconstruction can be realized by integrating the elastic elements in the actuation outside of the endoscope rather than in the endoscope structure itself [21]. For example, the endoscope can be designed as an articulated structure with discrete joints, where the joint angles can easily be determined by measuring the tendon displacement or by directly measuring the joint angle with a sensor. Embedding an elastic element in the transmission between the actuator and the load is called Series Elastic Actuation (SEA) [22]. SEA is the opposite of traditional actuation and transmission systems, which are designed as stiff as possible. The spring reduces peak forces in the transmission, thus improves the shock tolerance, and acts as a low-pass filter between the motor and the load, but it also limits the achievable bandwidth of the output force [22]. Therefore, SEA realizes a steerable trade-off in the system design. Active research on SEA is done with applications in legged robotics [23], exoskeletons [24], and rehabilitation robotics [25], but to our best knowledge it has not been applied to tendon-driven endoscopes. However, using SEA for surgical robots could be beneficial in several ways. First, surgical robotics correspond to the typical application area of SEA—where robots must physically interact with an unknown and dynamic environment [22, 26]. Second, surgical tasks do usually not involve highly dynamic movements (i.e., the frequency of the required surgical movements is significantly lower than the modal frequency of the system due to the low mass of the movable parts [18]). Thus, the limited force control bandwidth may be acceptable. Third, the spring deflections and tendon lengths can be measured and used to estimate the endoscope’s shape and the external force with the spring’s stress–strain curve.
In this paper, we propose an actuation design for robotic endoscope joints similar to the actuation of human fingers. Antagonistic pairs of muscles in the forearm (the flexor and extensor muscles) initiate the movement, which is then transmitted to the finger via tendons. In the human motor apparatus, the stress–strain behavior of the elasticity is nonlinear, which leads to a variable stiffness of the joint [27]. Such antagonistic arrangements have been developed and studied for larger robot actuators [26, 28], but are also promising for tendon-driven surgical robots. With this paper, we aim to demonstrate the benefits of antagonistic series elastic actuation for robotic endoscopes by evaluating the control of a prototype.
2 Materials and Methods
We designed and manufactured a first prototype to investigate the concept of using SEA for one endoscope joint that was driven by a pair of antagonistic tendons.
2.1 Apparatus
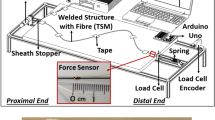
The mechanical system consisted of two parts: the endoscope and the drive units (Fig. 1). The endoscope part is supposed to be inserted into the patient in future applications; the drive units would remain outside. Thus, the endoscope can be controlled remotely without inserting sensors and actuators into the body. The endoscope consisted of two rigid links connected by a rotational joint with joint angle \(\varphi\). The second link, referred to as the endoscope tip, could move about the joint, actuated by two antagonistic tendons (Berkley NanoFil, Pure Fishing, Inc., Columbia, SC, USA). Applying more pulling force on tendon 1 moved the tip in negative \(\varphi\)-direction; applying more pulling force on tendon 2 moved the tip in positive \(\varphi\)-direction. The attachment of the tendons at the endoscope tip was designed to provide a constant lever arm regardless of the joint angle. This was achieved by winding the tendon around the joint by one complete turn before attaching it to the tip. The pulling force on the tendons originated from two independent drive units, each powered by an electric motor (RE25 DC motor, Maxon Motor AG, Sachseln, Switzerland). The drive unit housing was 3D-printed in ABS thermoplastic (Fortus 250mc 3D-printer, Stratasys, Ltd., Rehovot, Israel). At the output of these drive units, the tendon was wound on a winch. The transmission from the motor to the winch was not rigid but designed to include elasticity in the form of a torsional spring with spring stiffness \(k_{s} = 9 \times 10^{ - 4}\;\text{ Nm/}^\circ\) (Durovis AG, Perlen, Switzerland). We chose springs with a linear spring stiffness for the herein presented experiments, but the spring mounting was modular, i.e., the springs could be exchanged in future to get a different stress–strain behavior. The deflection of the spring was measured with two absolute rotational encoders (AMT23, CUI Devices, Tualatin, OR, USA): one that measured the angular position \(q_{m}\) of the shaft on the motor side (i.e., before the spring), and one that measured the angular position \(q_{l}\) of the output shaft on the load side (i.e., after the spring). To provide the necessary torque, the motor was connected to the input shaft via a gearbox with a mechanical advantage of 12:1. The current applied to the motors was controlled with a proportional-integral controller in torque control mode, which was provided by the manufacturer’s motor drives (MAXPOS 50/5, Maxon Motor AG, Sachseln, Switzerland) running at \(100\; {\text{kHz}}\). The target motor torque sent to the drives over EtherCAT was computed with a TwinCAT3 real-time control system (Beckhoff Automation GmbH & Co. KG, Verl, Germany) at a cycle rate of \(1 \;{\text{kHz}}\).
Overview of the proposed design for an antagonistic series elastic actuation of an endoscope joint. The schematic shows the functioning principle of the prototype: Two antagonistic tendons were pulled by two identical drive units, both including a spring between the motor and the output. Two encoders per drive unit were used to measure the deflection of the spring. The computer-aided design (CAD) rendering and the prototype show the technical realization
2.2 Control Algorithm
The control algorithm (Fig. 2) had a cascaded structure: the inner loop controlled the force on each tendon, \(F_{1}\) and \(F_{2}\), while the outer loop controlled the endoscope joint torque \(\tau_{\varphi }\). For the inner loop, a proportional-derivative control \(C_{{{\text{force}}}}\) was used to minimize the error between desired tendon force and the tendon force estimated by the spring deflection:
where \(r_{w}\) is the winch radius, \(K_{pF}\) and \(K_{dF}\) are the proportional and derivative gains, respectively, \(T_{s}\) is the sample time, \(\frac{z - 1}{z}\) is the time derivative in the discrete, complex frequency domain \(z\), \(F_{{i,{\text{des}}}}\) is the desired force on tendon \(i\) computed in the outer control loop, and \(\hat{F}_{i}\) is the estimated force on tendon \(i\). The tendon force was estimated using the spring deflection and assuming a linear stiffness of the torsional spring:
where \(k_{s}\) is the spring stiffness, \(q_{i,m}\) and \(q_{i,l}\) are the rotational position of the shaft before and after the spring, respectively, and \(r_{w}\) is the winch radius. The spring stiffness parameter was estimated for both drive units by attaching different weights to the tendon and by measuring the resulting spring deflection. A linear least-squares fit was used to determine the spring stiffness parameter \(k_{s}\). An initial estimate for the controller gains was obtained according to the Ziegler-Nichols method [29]. Fine-tuning was achieved by reducing the rise time of a closed-loop step response (step of \(F_{{i,{\text{des}}}}\) from \(4\) to \(5\;\text{N}\)), while keeping the overshoot within stability boundaries and thus ensuring a stable control loop. The used controller gains were \(K_{pF} = 22\) and \(K_{dF} = 0.25\).
An overview of the control system illustrates that the target torque to the motors (\({{\varvec{\tau}}}_{{\varvec{m}}}\)) is computed in a cascaded control scheme. The inner loop controls the force on the individual tendons by using the force estimated through spring deflection (\(\hat{F}_{i}\)) as feedback. The outer loop controls the endoscope joint position by using as feedback the estimated joint position (\(\hat{\varphi}\)). The output of the position controller is a joint torque command, which is then mapped to the desired tendon forces. User inputs are the desired tendon pre-tension (\(F_{\text{pret}}\)) and the desired endoscope joint angle (\({\varphi}_{\text{des}}\))
For the outer control loop, the desired force \(F_{{i, {\text{des}}}}\) on tendon \(i\) was computed as:
where \(F_{{{\text{pret}}}}\) is the desired pre-tension on the tendons, \(\tau_{{\varphi ,{\text{cmd}}}}\) is the output of the joint position controller \(C_{{{\text{pos}}}}\) for the respective tendon, and \(r_{\varphi }\) is the lever arm of the tendon at the endoscope joint. Depending on the sign of the controller output \(\tau_{{{\text{cmd}}}}\), it contributed to the desired force of either tendon 1 or 2. In other words, a position controller output corresponding to a positive direction in tip angle \(\varphi\) was added to \(F_{{2, {\text{des}}}}\), and vice versa.
The position controller \(C_{{{\text{pos}}}}\) was the high-level control that determined how the endoscope interacted with the environment. For our experiments, it was set up as a proportional controller of the form
where \(K_{pP}\) is the proportional gain, \(\varphi_{{{\text{des}}}}\) is the desired tip angle, and \(\hat{\varphi }\) is the estimated tip angle. This estimation was performed using a model of the forward kinematics:
where \(q_{1,l}\) and \(q_{2,l}\) are the encoder positions on the load side, \(r_{w}\) is the winch radius, and \(r_{\varphi }\) is the lever arm of the tendon attachment at the endoscope tip. For our experiments, two control modes were implemented:
-
Zero torque control, where the desired tendon force was always equal to the desired pre-tension, \(F_{{1,{\text{ des}}}} = F_{{2,{\text{ des}}}} = F_{{{\text{pret}}}}\), independent of the tip angle \(\varphi\). This means that the output of the position controller was not mapped to the desired tendon force. In practice, this was achieved by setting \(K_{pP} = 0\).
-
Impedance control, where the position controller output increased linearly with the tip angle error. Therefore, \(K_{pP} = 0.2\) corresponded to the largest possible value that did not lead to system instability for a reference step input of \(\varphi_{{{\text{des}}}} = 5^\circ .\) Additionally, the output torque of the position controller was limited to \(\pm 12.5 \times 10^{ - 3} {\text{Nm}}\).
2.3 Evaluation of Tendon Force Estimation
First, the tendon force estimation was evaluated in an experiment individually for each tendon. Hereby, the tendon was not yet fixed to the endoscope tip but instead attached to a commercial force sensor (Nano17 F/T sensor, ATI Industrial Automation, Inc., Apex, NC, USA). The zero positions of the encoders were set when the springs were in a relaxed state. To lock the position of the spring on the motor side, the motor drives were set to position control mode with a constant position command. The force sensor was moved back and forth by hand on a linear rail. During this experiment, the tendon force estimated through spring deflection and the force measured by the force sensor were recorded simultaneously over \(35\; {\text{s}}\) for later comparison.
2.4 Evaluation of Endoscope Joint Control
Both the zero torque control and the impedance control were evaluated for two cases of applied external forces: one where a continuous force was applied to the tip of the endoscope and one where the endoscope tip was subjected to an impact force. In both cases, the force was applied to the endoscope tip through a cable. During the experiments, the following variables were recorded: the endoscope tip angle \(\hat{\varphi }\), which was estimated with the forward kinematics from the encoder measurements, the endoscope tip angle \(\varphi_{ot}\), which was measured with an optical tracking system, and the endoscope joint torque \(\hat{\tau }_{\varphi }\), which was estimated using the spring deflection. When applying a continuous force, the cable from the endoscope tip was attached to the Nano17 force sensor and the magnitude of the measured force \(\varvec{F_{\text{fs}}}\) was also recorded. Before the measurements, the zero position of the encoders were set when the endoscope tip was straight (i.e., \(\varphi = 0^\circ\)) and the springs were in a relaxed state.
For the experiments with a continuous force applied, the force sensor was mounted on a linear rail and it was moved by hand (Fig. 3). The linear rail was mounted orthogonal to the longitudinal axis of the endoscope. Thus, for a tip angle \(\varphi = 0^\circ\), the entire external force was acting on the endoscope joint torque \(\tau_{\varphi }\). However, if the tip was tilted to an angle \(\varphi \ne 0^\circ\), only a portion of the external force (\(F_{{{\text{fs}} \bot }}\)) affected the joint torque, the other portion (\(F_{{{\text{fs}}\parallel }}\)) acted on the joint bearing (Fig. 4). To compensate for that, the position of the force sensor was tracked with the optical tracking system and the force measured by the sensor \(\varvec{F_{\text{fs}}}\) was projected onto the direction orthogonal to the endoscope tip:
The different control modes were tested with both a continuous and an impact force applied. A force sensor was used to measure the external forces in case of continuous forces applied. For the case of an impact force, a weight was dropped from a height of \(5\;\text{cm}\), which resulted in impact force \(F_{\text{imp}}\) on the tendon connected to the endoscope tip. Both the endoscope (\(\varvec{\text{e}}_{1..4}\)) and the force sensor (\(\varvec{\text{fs}}\)) were equipped with markers. Therefore, the endoscope angle \(\boldsymbol{\varphi }\) and the projection of the external force to the orthogonal direction contributing to the endoscope joint torque \(F_{{\text{fs}}\perp }\) could be computed
For the impact force experiments, the cable from the endoscope tip was attached to a weight \(m = 29\;\text{g}\) via a pulley. The weight was lifted from the initial position by \(5\;\text{cm}\) before dropping it, resulting in a linear momentum at the point of impact of \(p = m v = m \sqrt {g h} = 0.02\;\text{Ns}\). The theoretical impact force that results from such a free fall depends on the time \(t_{d}\) it takes for the weight to decelerate to zero velocity. For the theoretical force, we assumed that the cable was attached to a fixed object instead of the endoscope tip. Preliminary tests showed that the duration of the impact \(t_{d}\) was in the order of \(10^{ - 3} {\text{s}}\). The range of the theoretical impact force was therefore estimated to be \(F = \frac{p}{{t_{d} }} \approx \left( {2 {\text{N }},20 {\text{N}}} \right)\). To confirm this theoretical value, the cable was attached to the fixed Nano17 force sensor while dropping the weight four times. The observed maximum forces were in the range of \(2.2\)–\(8.4\;\text{N}\).
For both experiments, the endoscope tip was tracked with an optical tracking system (Qualisys AB, Göteborg, Sweden) as a baseline for the forward kinematic model. Three infrared cameras (Miqus M5 cameras by Qualisys) were used to measure the location of optical markers placed on the endoscope: Two markers were placed per endoscope link (Fig. 4). The data were acquired from the cameras with a motion capture software at a sampling rate of \(100\;\text{Hz}\) (QTM version 2020.2 by Qualisys), then transmitted to the real-time system via UDP. The endoscope joint angle was then computed as the angle between the two vectors along the links:
where \(\varvec{\text{e}}_{i} = \left( {\begin{array}{*{20}c} {{\text{e}}_{ix} } & {{\text{e}}_{iy} } & 0 \\ \end{array} } \right)^{{\text{T}}} , i \in 1,..,4\) is the position of marker \(i\), assuming all markers lie in the plane \(z = 0\).
3 Results
The maximum force registered by the force sensor was \(11.6 \;{\text{N}}\) and \(7.88 \;{\text{N}}\) for tendon 1 and 2, respectively (Fig. 5). The mean absolute error between measured and estimated tendon force was \(0.92\;{\text{N}}\) (tendon 1, corresponding to \(7.9 \%\) of \(11.6\;{\text{N}}\), the maximum force registered on tendon 1) / \(0.26\;{\text{N}}\) (tendon 2, corresponding to \(3.3 \%\) of \(7.88\;{\text{N}}\), the maximum force registered on tendon 2), the root mean square error was \(1.16\;{\text{N}}\) (tendon 1, \(10 \%\)) / \(0.36 \; {\text{N}}\) (tendon 2, \(4.6 \%\)), and the maximum absolute error was \(2.89\; {\text{N}}\) (tendon 1, \(24.9 \%\)) / \(0.94\; {\text{N}}\) (tendon 2, \(11.9 \%\)).
When applying external forces in zero torque control, the endoscope tip settled to the mechanical joint limit of about \(50^\circ\) and stayed there when the force was released (Fig. 6). In impedance control, the tip moved back towards the desired tip angle of \(0^\circ\) once the external force was released. When applying a continuous force, the maximum joint torque that occurred during zero torque control was \(0.48 \times 10^{ - 3} \;{\text{Nm}}\), compared to \(12.5 \times 10^{ - 3} \; {\text{Nm}}\) during impedance control. Compared to the joint angle measured with the optical tracking system, the endoscope tip angle estimated by the encoders had a maximum error of 4.7° (corresponding to \(4.7 \%\) of the total joint range of \(\pm 50^\circ\)) for a continuous force applied, and \(19.8^\circ\) (\(19.8 \%\)) during impact. The root mean square error of the endoscope tip angle estimation was \(2.5^\circ\) (continuous force, \(2.5 \%\)) and \(4.3^\circ\) (impact force, \(4.3 \%\)).
The evaluation of endoscope joint control is shown for all four cases: when a continuous force was applied versus when an impact force was applied; for zero torque control mode versus impedance control mode. Shown in blue are the joint angle estimated by the encoders (\(\hat{\varphi}\)) and as reference the joint angle measured with the optical tracking system (\(\varphi_\text{ot}\)). The joint torque estimated by spring deflection (\(\hat{\tau}_{\varphi}\)) and the magnitude of the sensor force (\(F_\text{fs}\)) are shown in red and yellow, respectively. The Y-axis of the joint torque and the external force is scaled by the lever arm of the endoscope tip such that the magnitude of the variables are comparable. The graph shows the measured variables over a time of 10 s (continuous force) and 1 s (impact force)
The torque–displacement behavior of the endoscope joint for the four test cases (Fig. 7) illustrates the differences between the control modes. In zero torque control, the joint torque remained close to zero for all angles. In impedance control, the torque increased linearly with the tip angle up to the safety limit of \(12.5 \times 10^{ - 3} \;{\text{Nm}}\).
The joint torque is shown over the joint angle for the different cases: zero torque control (ZT) and impedance control (IC) for both a continuous and impact force applied. In impedance control, the safety limit of the commanded joint torque \(\tau_{\varphi,\text{cmd}}\) was set to \(12.5\;{\text{mNm}}\). As a reference, the theoretical stiffness of the spring (converted through forward kinematics) is shown. The temporal direction of the data points is indicated with an arrow
4 Discussion
Measuring the deflection of a remotely placed spring resulted in reliable estimates of endoscope tendon forces, which is an important prerequisite for estimating endoscope joint torques. The tendon force estimation in drive unit 1 showed greater errors than the tendon force estimation in drive unit 2. This difference is likely due to misalignment and poor tolerances in the 3D-printed parts, causing friction in the system. Thus, these errors could be partly eliminated with a more precise manufacturing. Compared to our previous proof-of-concept prototype [21], drive unit 2 of the proposed prototype showed a lower mean absolute error in tendon force estimation, and the related maximum error could be improved from 19 to 12% of the measured range. Even though errors in the order of 10% might not seem like an impressive result for tendon force measurements, it is important to note that the force was measured remotely after the tendon transmission, and thus the error includes mechanical losses such as friction. When estimating the endoscope joint torques from remote sensor measurements, these losses will be present regardless of the sensor modality. However, our proposed design has advantages when detecting impact forces. For stiff force sensors, the sampling rate of the analog-to-digital signal conversion needs to be fast enough to detect a short force spike. Integrating a spring changes the impulse response of the sensor and the impact force can be detected even with a slower sampling rate. The impact force measurement clearly demonstrated the advantage of SEA over other actuation, since reactions of a compliant mechanical system are faster than a system that purely relies on sensory feedback. Accordingly, the SEA endoscope reacted faster to external impacts than the optical tracking system even provided the measurement results. Furthermore, the estimation of the joint angle from the encoder signals ran at a faster rate (\(1 \;{\text{kHz}}\)) than the optical tracking system (\(100\; {\text{Hz}}\)). Therefore, the error in the joint angle between the optical tracking system and the robotic endoscope during fast movements (i.e., after external impact) was likely caused by the low sampling rate and delays of the optical tracking system. During slower movements, however, the comparison of the estimated and the optically tracked endoscope joint angles revealed acceptably small errors of below 5°. Consequently, our method of estimating the endoscope joint angle enabled a reliable endoscope shape estimation and could even be superior to marker-based optical tracking techniques in situations with fast movements. This also has to be seen in the context of the mechanical measurement accuracy that can be achieved with 3D-printed structures in combination with optical tracking markers that are placed on a rather short endoscope tip. Similarly, when trying to avoid high impact forces, systems with stiff force sensors can only react to the forces once the impact has already happened. The elasticity of SEA, on the other hand, inherently reduces these peak forces, which was shown in the evaluation of the system under external impact forces. While theoretical impact forces and preliminary measurements with a stiff force sensor indicated peak forces in the range of \(2\)–\(20 \;{\text{N}}\), the forces measured with our system were below \(1.5 \;{\text{N}}\). When the applied external forces were continuous, the differences between the control modes were more clearly visible. The zero torque control effectively reduced the contact forces. This control mode could be used in situations where the endoscope must yield to a fragile environment. During impedance control, the endoscope joint exhibited the expected stiffness. This could be useful in situations where endoscope motion needs to be precise despite minor external forces. For larger external forces, springs with a higher stiffness need to be used, since the achievable endoscope joint stiffness under system stability is limited by the stiffness of the springs. Using nonlinear springs would enable to change the passive stiffness of the endoscope joint by adjusting the pre-tension on the tendons [26], which might also be interesting for certain surgical applications [20]. While we demonstrated the feasibility of SEA for a single endoscope joint, certain surgical applications might require multiple joints in series to achieve more degrees of freedom and a greater maneuverability at the endoscope tip. A challenge that arises for multiple tendon-driven joints in series is the cross-coupling between consecutive joints (i.e., movement of a distal joint will cause movement in a proximal joint). This challenge also exists for non-SEA articulated endoscopes and has been addressed by routing the tendons over pulleys [30]. These pulley mechanisms increase the complexity and size of the endoscope design. Furthermore, routing the tendons over pulleys will introduce friction in the transmission, which affects the sensitivity of the joint torque estimation and thus impairs the torque control performance. Therefore, choosing the number of joints in a future SEA endoscope design will be a trade-off between torque control performance and size on one side, and maneuverability on the other side.
5 Conclusion
In this paper, we proposed a novel actuation design based on the concept of series elastic actuation to control an articulated endoscope with one joint driven by antagonistic tendons. The conducted experiments showed that the use of SEA is a promising method to measure external forces in minimally invasive surgery. At the same time, it enables stable control of the torque and position of an endoscopic joint (i.e., the endoscope shape for articulated endoscopes). The required stress–strain behavior of the springs has yet to be derived from surgical requirements, but could lead towards safer robot–tissue interactions in surgical robotics since the smart mechanical structure can react faster to external forces than any closed-loop controlled robotic system.
References
de Rooij, T., van Hilst, J., van Santvoort, H., Boerma, D., van den Boezem, P., Daams, F., van Dam, R., Dejong, C., van Duyn, E., Dijkgraaf, M., van Eijck, C., Festen, S., Gerhards, M., Koerkamp, B. G., de Hingh, I., Kazemier, G., Klaase, J., de Kleine, R., van Laarhoven, C., Luyer, M., Patijn, G., Steenvoorde, P., Suker, M., Abu Hilal, M., Busch, O., Besselink, M. (2019). Minimally invasive versus open distal pancreatectomy (LEOPARD). Annals of Surgery, 269(1), 2–9. https://doi.org/10.1097/sla.0000000000002979
Chimento, G. F., Pavone, V., Sharrock, N., Kahn, B., Cahill, J., & Sculco, T. P. (2005). Minimally invasive total hip arthroplasty. The Journal of Arthroplasty, 20(2), 139–144. https://doi.org/10.1016/j.arth.2004.09.061
Patel, A. A., Zfass-Mendez, M., Lebwohl, N. H., Wang, M. Y., Green, B. A., Levi, A. D., Vanni, S., & Williams, S. K. (2015). Minimally invasive versus open lumbar fusion: A comparison of blood loss, surgical complications, and hospital course. The Iowa Orthopaedic Journal, 35, 130–134.
van Hilst, J., de Rooij, T., Klompmaker, S., Rawashdeh, M., Aleotti, F., Al-Sarireh, B., Alseidi, A., Ateeb, Z., Balzano, G., Berrevoet, F., Björnsson, B., Boggi, U., Busch, O. R., Butturini, G., Casadei, R., Chiaro, M. D., Chikhladze, S., Cipriani, F., van Dam, R., Damoli, I., van Dieren, S., Dokmak, S., Edwin, B., van Eijck, C., Fabre, J.M., Falconi, M., Farges, O., Fernández-Cruz, L., Forgione, A., Frigerio, I., Fuks, D., Gavazzi, F., Gayet, B., Giardino, A., Groot Koerkamp, B., Hackert, T., Hassenpflug, M., Kabir, I., Keck, T., Khatkov, I., Kusar, M., Lombardo, C., Marchegiani, G., Marshall, R., Menon, K.V., Montorsi, M., Orville, M., de Pasteno, M., Pietrabissa, A., Poves, I., Primrose, J., Pugliese, R., Ricci, C., Roberts, K., Røsok, B., San Sánchez-Cabús, S., Sandström, P., Scovel, L., Solaini, L., Soonawalla, Z., Souche, F.R., Sutcliffe, R.P., Tiberio, G.A., Tomazic, A., Troisi, R., Wellner, U., White, S., Wittel, U., Zerbi, A., Bassi, C., Besselink, M.G., Abu Hilal, M. (2019). Minimally invasive versus open distal pancreatectomy for ductal adenocarcinoma (DIPLOMA). Annals of Surgery, 269(1), 10–17. https://doi.org/10.1097/sla.0000000000002561
Harrington, J., & French, P. (2008). Open versus minimally invasive lumbar microdiscectomy: Comparison of operative times, length of hospital stay, narcotic use and complications. Minimally Invasive Neurosurgery, 51(1), 30–35. https://doi.org/10.1055/s-2007-1004543
King, J., Stamper, D. L., Schaad, D. C., & Leopold, S. S. (2007). Minimally invasive total knee arthroplasty compared with traditional total knee arthroplasty. The Journal of Bone & Joint Surgery, 89(7), 1497–1503. https://doi.org/10.2106/jbjs.f.00867
Sclafani, J. A., & Kim, C. W. (2014). Complications associated with the initial learning curve of minimally invasive spine surgery: A systematic review. Clinical Orthopaedics and Related Research, 472(6), 1711–1717. https://doi.org/10.1007/s11999-014-3495-z
van Workum, F., Stenstra, M. H. B. C., Berkelmans, G. H. K., Slaman, A. E., van Berge Henegouwen, M. I., Gisbertz, S. S., van den Wildenberg, F. J. H., Polat, F., Irino, T., Nilsson, M., Nieuwenhuijzen, G. A. P., Luyer, M. D., Adang, E. M., Hannink, G., Rovers, M. M., & Rosman, C. (2019). Learning curve and associated morbidity of minimally invasive esophagectomy. Annals of Surgery, 269(1), 88–94. https://doi.org/10.1097/sla.0000000000002469
Simaan, N., Yasin, R. M., & Wang, L. (2018). Medical technologies and challenges of robot-assisted minimally invasive intervention and diagnostics. Annual Review of Control, Robotics, and Autonomous Systems, 1(1), 465–490. https://doi.org/10.1146/annurev-control-060117-104956
Veluvolu, K. C., & Ang, W. T. (2010). Estimation and filtering of physiological tremor for real-time compensation in surgical robotics applications. The International Journal of Medical Robotics and Computer Assisted Surgery, 6(3), 334–342. https://doi.org/10.1002/rcs.340
Prasad, S. M., Prasad, S. M., Maniar, H. S., Chu, C., Schuessler, R. B., & Damiano, R. J. (2004). Surgical robotics: Impact of motion scaling on task performance. Journal of the American College of Surgeons, 199(6), 863–868. https://doi.org/10.1016/j.jamcollsurg.2004.08.027
Vitiello, V., Lee, S.-L., Cundy, T. P., & Yang, G.-Z. (2013). Emerging robotic platforms for minimally invasive surgery. IEEE Reviews in Biomedical Engineering, 6, 111–126. https://doi.org/10.1109/rbme.2012.2236311
Kosari, S. N., Ramadurai, S., Chizeck, H. J., & Hannaford, B. (2013). Control and tension estimation of a cable driven mechanism under different tensions. Volume 6A: 37th Mechanisms and Robotics Conference, Portland, OR, USA. https://doi.org/10.1115/detc2013-13548
Bouras, T., & Sgouros, S. (2013). Complications of endoscopic third ventriculostomy. World Neurosurgery, 79(2), S2.e29-S22.e12. https://doi.org/10.1016/j.wneu.2012.02.014
Enayati, N., Momi, E. D., & Ferrigno, G. (2016). Haptics in robot-assisted surgery: Challenges and benefits. IEEE Reviews in Biomedical Engineering, 9, 49–65. https://doi.org/10.1109/rbme.2016.2538080
Trejos, A. L., Patel, R. V., & Naish, M. D. (2010). Force sensing and its application in minimally invasive surgery and therapy: A survey. Proceedings of the Institution of Mechanical Engineers, Part C: Journal of Mechanical Engineering Science, 224(7), 1435–1454. https://doi.org/10.1243/09544062jmes1917
Okamura, A. M. (2018). Haptics in robot-assisted minimally invasive surgery. The encyclopedia of medical robotics (pp. 317–339). World Scientific.
Burgner-Kahrs, J., Rucker, D. C., & Choset, H. (2015). Continuum robots for medical applications: A survey. IEEE Transactions on Robotics, 31(6), 1261–1280. https://doi.org/10.1109/tro.2015.2489500
Shi, C. Y., Luo, X. B., Qi, P., Li, T. L., Song, S., Najdovski, Z., Fukuda, T., & Ren, H. L. (2017). Shape sensing techniques for continuum robots in minimally invasive surgery: A survey. IEEE Transactions on Biomedical Engineering, 64(8), 1665–1678. https://doi.org/10.1109/tbme.2016.2622361
Loeve, A., Breedveld, P., & Dankelman, J. (2010). Scopes too flexible….and too stiff. IEEE Pulse, 1(3), 26–41. https://doi.org/10.1109/mpul.2010.939176
Fasel, L., Gerig, N., Cattin, P. C., & Rauter, G. (2020). Tendon force control evaluation for an endoscope with series elastic actuation. Proceedings of MESROB 2020: New Trends in Medical and Service Robotics, Basel, Switzerland, 118–126. https://doi.org/10.1007/978-3-030-58104-6_14
Pratt, G. A. & Williamson, M. M. (1995). Series elastic actuators. Proceedings 1995 IEEE/RSJ International Conference on Intelligent Robots and Systems: Human Robot Interaction and Cooperative Robots, Pittsburgh, PA, USA. https://doi.org/10.1109/iros.1995.525827
Zhou, X. D., & Bi, S. S. (2012). A survey of bio-inspired compliant legged robot designs. Bioinspiration & Biomimetics, 7(4), 041001. https://doi.org/10.1088/1748-3182/7/4/041001
Veneman, J. F., Ekkelenkamp, R., Kruidhof, R., van der Helm, F. C. T., & van der Kooij, H. (2006). A series elastic- and bowden-cable-based actuation system for use as torque actuator in exoskeleton-type robots. The International Journal of Robotics Research, 25(3), 261–281. https://doi.org/10.1177/0278364906063829
Yu, H., Huang, S., Chen, G., Pan, Y., & Guo, Z. (2015). Human–robot interaction control of rehabilitation robots with series elastic actuators. IEEE Transactions on Robotics, 31(5), 1089–1100. https://doi.org/10.1109/tro.2015.2457314
Vanderborght, B., Albu-Schaeffer, A., Bicchi, A., Burdet, E., Caldwell, D. G., Carloni, R., Catalano, M., Eiberger, O., Friedl, W., Ganesh, G., Garabini, M., Grebenstein, M., Grioli, G., Haddadin, S., Hoppner, H., Jafari, A., Laffranchi, M., Lefeber, D., Petit, F., Stramigiolic, S., Tsagarakise, N., Van Dammef, M., Van Hamf, R., Visser, L.C., Wolf, S. (2013). Variable impedance actuators: A review. Robotics and Autonomous Systems, 61(12), 1601–1614. https://doi.org/10.1016/j.robot.2013.06.009
Hogan, N. (1984). Adaptive control of mechanical impedance by coactivation of antagonist muscles. IEEE Transactions on Automatic Control, 29(8), 681–690. https://doi.org/10.1109/tac.1984.1103644
Petit, F., Chalon, M., Friedl, W., Grebenstein, M., Albu-Schäffer, A., & Hirzinger, G. (2010). Bidirectional antagonistic variable stiffness actuation: analysis, design & implementation. Proceedings of 2010 IEEE International Conference on Robotics and Automation (ICRA), Anchorage, AK, USA. https://doi.org/10.1109/robot.2010.5509267
Ziegler, J. G., & Nichols, N. B. (1942). Optimum settings for automatic controllers. ASME Transactions, 64, 759–768.
Madhani, A. J. & Salisbury, J. K. (1998). Articulated surgical instrument for performing minimally invasive surgery with enhanced dexterity and sensitivity. U.S. Patent No. 5,792,135
Acknowledgements
The work in this paper was conducted as part of the MIRACLE project, and we are grateful for the generous funding by the Werner Siemens Foundation.
Funding
Open access funding provided by University of Basel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fasel, L., Gerig, N., Cattin, P.C. et al. Control Evaluation of Antagonistic Series Elastic Actuation for a Robotic Endoscope Joint. J Bionic Eng 19, 965–974 (2022). https://doi.org/10.1007/s42235-022-00180-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-022-00180-6