Abstract
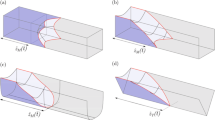
We present a device for passive unidirectional liquid transport. The capillary channels used are bioinspired by the shape of the spermathecae (receptaculum seminis) of rabbit fleas (Spilopsyllus cuniculi) and rat fleas (Xenopsylla cheopis). The spermatheca is an organ of female fleas that stores sperm until suitable conditions to lay eggs are found. We translated and multiplied the natural form and function of a spermatheca to create a continuous capillary system from which we designed our microfluidic device based directly on the model from nature. Applying the Young-Laplace equation, we derived a theoretical description of local liquid transport, which enables model-guided design. We arranged the bioinspired capillaries in parallel and engraved them in poly(methyl methacrylate) (PMMA) plates by CO2 laser ablation. The fabricated structures transport soapy water passively (i.e., without external energy input) in the forward direction at velocities of about 1 mm·s−1 while halting the liquid fronts completely in the backward direction. The bioinspired capillary channels are capable of unidirectional liquid transport against gravity. Distance and velocity measurements prove the feasibility of the concept. Unidirectional passive liquid transport might be advantageous in technical surfaces for liquid management, for instance, in biomedical microfluidics, lab-on-chip, lubrication, electronics cooling and in micro-analysis devices.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Change history
20 March 2018
The article <Emphasis Type="Bold">“Fluidic Diode” for Passive Unidirectional Liquid Transport Bioinspired by the Spermathecae of Fleas</Emphasis>, written by <Emphasis Type="Bold">Gerda Buchberger, Alexander Kogler, Agnes Weth, Richard Baumgartner, Philipp Comanns, Siegfried Bauer, Werner Baumgartner</Emphasis>, was originally published electronically on the publisher’s internet portal (currently SpringerLink) on January 20th 2018 without open access. With the author(s)’ decision to opt for Open Choice the copyright of the article changed in March 2018 to © The Author(s) 2018 and the article is forthwith distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
20 March 2018
The article ?Fluidic Diode? for Passive Unidirectional Liquid Transport Bioinspired by the Spermathecae of Fleas, written by Gerda Buchberger, Alexander Kogler, Agnes Weth, Richard Baumgartner, Philipp Comanns, Siegfried Bauer, Werner Baumgartner, was originally published electronically on the publisher?s internet portal (currently SpringerLink) on January 20th 2018 without open access. With the author(s)? decision to opt for Open Choice the copyright of the article changed in March 2018 to ? The Author(s) 2018 and the article is forthwith distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
Woias P. Micropumps - Past, progress and future prospects. Sensors and Actuators, B: Chemical, 2005, 105, 28–38.
Laser D J, Santiago J G. A review of micropumps. Journal of Micromechanics and Microengineering, 2004, 14, R35–R64.
Buguin A, Talini L, Silberzan P. Ratchet-like topological structures for the control of microdrops. Applied Physics A: Materials Science and Processing, 2002, 75, 207–212.
Rothschild M L. Arrangement of sperm within the spermatheca of fleas with remarks on sperm displacement. Biological Journal of the Linnean Society, 1991, 43, 313–323.
Traub R, Rothschild M L, Haddow J F. The Rothschild Collection of Fleas. The Ceratophyllidae: Key to the Genera and Host Relationships, Cambridge, University Press, UK, 1983.
Mead-Briggs A R. The structure of the reproductive organs of the European rabbit-flea, Spilopsyllus Cuniculi (dale) (Siphonaptera). Proceedings of the Royal Entomological Society of London, Series A, General Entomology, 1962, 37, 79–88.
Holland G P. Primary and secondary sexual characteristics of some Ceratophyllinae, with notes on the mechanism of copulation (Siphonaptera). Transactions of the Royal Entomological Society of London, 2009, 107, 233–248.
Hsu M H, Wu W J. Off-host observations of mating and postmating behaviors in the cat flea (Siphonaptera: Pulicidae). Journal of Medical Entomology, 2001, 38, 352–360.
Mitzmain M B. Some new facts on the bionomics of the California rodent fleas. Annals of the Entomological Society of America, 1910, 3, 61–82.
Comanns P, Winands K, Arntz K, Klocke F, Baumgartner W. Laser-based biomimetic functionalization of surfaces: From moisture harvesting lizards to specific fluid transport systems. International Journal of Design and Nature and Ecodynamics, 2014, 9, 206–215.
Comanns P, Buchberger G, Buchsbaum A, Baumgartner R, Kogler A, Bauer S, Baumgartner W. Directional, passive liquid transport: The texas horned lizard as a model for a biomimetic ‘liquid diode’. Journal of the Royal Society Interface, 2015, 12, 20150415.
Buchberger G, Hischen F, Comanns P, Baumgartner R, Kogler A, Buchsbaum A, Bauer S, Baumgartner W. Bio-inspired microfluidic devices for passive, directional liquid transport: Model-based adaption for different materials. Procedia Engineering, 2015, 120, 106–111.
Comanns P, Winands K, Pothen M, Bott R A, Wagner H, Baumgartner W. The Texas horned lizard as model for robust capillary structures for passive directional transport of cooling lubricants. Proceedings of SPIE - The International Society for Optical Engineering, 2016, 9797, 979711.
Kühn P T, de Miranda B S, van Rijn P. Directed autonomic flow: Functional motility fluidics. Advanced Materials, 2015, 27, 7401–7406.
Chaudhury M K, Whitesides G M. How to make water run uphill. Science, 1992, 256, 1539–1541.
Zheng Y, Bai H, Huang Z, Tian X, Nie F Q, Zhao Y, Zhai J, Jiang L. Directional water collection on wetted spider silk. Nature, 2010, 463, 640–643.
Weislogel M M. Steady spontaneous capillary flow in partially coated tubes. Aiche Journal, 1997, 43, 645–654.
Extrand C W. Retention forces of a liquid slug in a rough capillary tube with symmetric or asymmetric features. Langmuir, 2007, 23, 1867–1871.
Zimmermann M, Schmid H, Hunziker P, Delamarche E. Capillary pumps for autonomous capillary systems. Lab on a Chip, 2007, 7, 119–125.
Feng J, Rothstein J P. One-way wicking in open micro- channels controlled by channel topography. Journal of Colloid and Interface Science, 2013, 404, 169–178.
Malvadkar N A, Hancock M J, Sekeroglu K, Dressick W J, Demirel M C. An engineered anisotropic nanofilm with unidirectional wetting properties. Nature Materials, 2010, 9, 1023–1028.
He J, Mao M, Li D, Liu Y, Jin Z. Characterization of leaf-inspired microfluidic chips for pumpless fluid transport. Journal of Bionic Engineering, 2014, 11, 109–114.
Wang Q, Hong J, Yan Y. Biomimetic capillary inspired heat pipe wicks. Journal of Bionic Engineering, 2014, 11, 469–480.
Mates J E, Schutzius T M, Qin J, Waldroup D E, Megaridis C M. The fluid diode: Tunable unidirectional flow through porous substrates. ACS Applied Materials and Interfaces, 2014, 6, 12837–12843.
Adams M L, Johnston M L, Scherer A, Quake S R. Polydimethylsiloxane based microfluidic diode. Journal of Micromechanics and Microengineering, 2005, 15, 1517–1521.
Sochol R D, Deeble C J, Shen V, Nakamura M, Hightower B J, Brubaker T A, Lee K Y, Gao S, Kim M, Wolf K T, Iwai K, Glick C C, Lee L P, Lin L. Single-layer microfluidic “disc” diodes via optofluidic lithography for ultra-low Reynolds number applications. The 17th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers and Eurosensors XXVII), 2013, 2201–2204.
Plamadeala C, Hischen F, Friesenecker R, Wollhofen R, Jacak J, Buchberger G, Heiss E, Klar T A, Baumgartner W, Heitz J. Bioinspired polymer microstructures for directional transport of oily liquids. Royal Society Open Science, 2017, 4, 160849.
Herder V, Wohlsein P, Peters M, Hansmann F, Baumgartner W. Romeis–Mikroskopische Technik, Springer Berlin Heidelberg, Berlin, Heidelberg, Germany, 2015. (in German)
Washburn E W. The dynamics of capillary flow. Physical Review, 1921, 17, 273–283.
Berthier J, Silberzan P. Microfluidics in Biotechnology, 2nd ed., Artech House, Boston, USA, 2010.
Berthier J, Brakke K A, Berthier E. Open Microfluidics, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2016.
Zimmermann M, Hunziker P, Delamarche E. Valves for autonomous capillary systems. Microfluidics and Nanofluidics, 2008, 5, 395–402.
Lenormand R, Zarcone C. Role of roughness and edges during imbibition in square capillaries. SPE Annual Technical Conference and Exhibition, 1984, SPE-13264.
Dong M, Chatzis I. The imbibition and flow of a wetting liquid along the corners of a square capillary tube. Journal of Colloid and Interface Science, 1995, 172, 278–288.
Delamarche E, Juncker D, Schmid H. Microfluidics for processing surfaces and miniaturizing biological assays. Advanced Materials, 2005, 17, 2911–2933.
Safavieh R, Juncker D. Capillarics: Pre-programmed, self-powered microfluidic circuits built from capillary elements. Lab on a Chip, 2013, 13, 4180–4189.
Chu R C, Simons R E, Ellsworth M J, Schmidt R R, Cozzolino V. Review of cooling technologies for computer products. IEEE Transactions on Device and Materials Reliability, 2004, 4, 568–585.
Hancock M J, Sekeroglu K, Demirel M C. Bioinspired directional surfaces for adhesion, wetting and transport. Advanced Functional Materials, 2012, 22, 2223–2234.
Heitz J, Plamadeala C, Wiesbauer M, Freudenthaler P, Wollhofen R, Jacak J, Klar T A, Magnus B, Köstner D, Weth A, Baumgartner W, Marksteiner R. Bone-forming cells with pronounced spread into the third dimension in polymer scaffolds fabricated by two-photon polymerization. Journal of Biomedical Materials Research Part A, 2016, 105A, 891–899.
Coenjarts C A, Ober C K. Two-photon three-dimensional microfabrication of poly (dimethylsiloxane) elastomers. Chemistry of Materials, 2004, 16, 5556–5558.
Michel B, Bernard A, Bietsch A, Delamarche E, Geissler M, Juncker D, Kind H, Renault J P, Rothuizen H, Schmid H, Schmidt-Winkel P, Stutz R, Wolf H. Printing meets lithography: Soft approaches to high-resolution patterning. IBM Journal of Research & Development, 2002, 56, 527–542.
Mujahid A, Iqbal N, Afzal A. Bioimprinting strategies: From soft lithography to biomimetic sensors and beyond. Biotechnology Advances, 2013, 31, 1435–1447.
Kim P, Kwon K W, Park M C, Lee S H, Kim S M. Soft lithography for microfluidics: A Review. Biochip Journal, 2008, 2, 1–11.
Becker H, Heim U. Hot embossing as a method for the fabrication of polymer high aspect ratio structures. Sensors and Actuators, A: Physical, 2000, 83, 130–135.
Taniguchi J, Yoshikawa H, Tazaki G, Zento T. High-density pattern transfer via roll-to-roll ultraviolet nanoimprint lithography using replica mold. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures, 2012, 30, 06FB07.
Li Y, Ng H W, Gates B D, Menon C. Material versatility using replica molding for large-scale fabrication of high aspect-ratio, high density arrays of nano-pillars. Nanotechnology, 2014, 25, 285303.
Micheal I J, Vidyasagar A J, Bokara K K, Mekala N K, Asthana A, Rao C M. Foil assisted replica molding for fabrication of microfluidic devices and their application in vitro. Lab on a chip, 2014, 14, 3695–3699.
Acknowledgment
We thank the Institute of Polymer Science at Johannes Kepler University Linz for providing the setup for surface tension and contact angle measurement, and the Biologiezentrum Linz for lending us the sample of the flea. We are grateful to Anna Stadler for her help with Fig. 1. Furthermore, we thank Thomas Fritz, Kurt Thaller B.Sc. and Dr. Andreas Buchsbaum from the company RECENDT GmbH for technical assistance. We acknowledge financial support from Kimberly-Clark Corporation and from the European Research Council within the Advanced Investigators Grant SoftMap (Soft Matter Physics Team). Financial support from the European Commission is acknowledged within the “LiNaBioFluid” project within the scope of H2020-FETOPEN-2014-2015-RIA. This research was further supported by the Austrian Research Promotion Agency (FFG) under contract number FFGP13830002/MicroNeedle.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised due to a retrospective Open Access order.
Electronic supplementary material
Supplementary material, approximately 9.28 MB.
Supplementary material, approximately 5.64 MB.
Supplementary material, approximately 7.97 MB.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
This article is published with open access at Springerlink.com, corrected publication 03/2018
The original article has been corrected.
About this article
Cite this article
Buchberger, G., Kogler, A., Weth, A. et al. “Fluidic diode” for passive unidirectional liquid transport bioinspired by the spermathecae of fleas. J Bionic Eng 15, 42–56 (2018). https://doi.org/10.1007/s42235-017-0003-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-017-0003-7