Abstract
Fire blight is the most important bacterial disease in apple (Malus × domestica). Owing to the severity of the caused damages, fire blight resistance is an important breeding objective. In the past, various phenotypic screening methods and strategies have been used to identify new sources of fire blight resistance for breeding. In this study, breeding material, modern cultivars and heirloom accessions were phenotyped by artificial inoculation of shoots under greenhouse (n = 273) and flowers under field conditions (n = 20) and genotyped for known resistance genes and quantitative trait loci by using molecular markers. A comparison between the two phenotyping methods was made in relation to the two control varieties ‘Gala Galaxy’ and ‘Enterprise’. The results obtained for the resistance sources FB_MR5, Fb_E and FB_F7 are consistent with previously published data, showing a large effect of the two major resistance genes FB_MR5 (‘Malus × robusta 5’) and Fb_E (‘Evereste’). Genotypes carrying FB_F7 showed greater variation in their resistance levels, but were on average less susceptible than ‘Gala Galaxy’ and genotypes with no known resistance gene or quantitative trait locus (QTLs) in both tests. No correlation was found between the results of 18 genotypes phenotyped with both inoculation methods. The ranking of genotypes according to their flower and shoot fire blight resistance varied between the two methods. However, 11 of the 18 tested genotypes showed no significant difference between the results of the two methods. Additionally, it was found that flower shedding appears to be an important triggered mechanism for flower resistance to fire blight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fire blight, caused by the bacterium Erwinia amylovora, is a devastating disease for several genera of the Rosaceae family (Vanneste 2000). It is the most important bacterial disease in apple fruit production. The bacteria infect the host plant via flowers, twigs and suckers and overwinter in cankers on woody organs and infected plant material. During flowering, E. amylovora is actively spread by pollinating insects under optimal climatic conditions. After rapid multiplication on the flower organs (stigma and nectarthodes) (Spinelli et al. 2005), the bacteria invade the entire flower cluster and move into the shoot, further through the branch into the trunk and finally to the roots (Bogs et al. 1998).
In Switzerland, the first cases of fire blight infections were observed in 1989 (Duffy et al. 2005) and the first major disease outbreak occurred in 2007 (Holliger 2008). In 2016, the Swiss government completely banned the hitherto strictly regulated use of antibiotics for fire blight control. Currently, only products with a partial efficacy are available in Switzerland (e.g., LMA® [80% potassium aluminum sulfate], Myco-sin [65% sulfuric acid clay, plus silicates, yeast components and horsetail extract], BlossomProtect™ [5 × 109 cfu/g Aureobasidium pullulans], Bion® [50% acibenzolar-S-methyl], Regalis® Plus [10% prohexadione-calcium], Vacciplant® [45 g/L laminarin]; Reininger et al. 2021; Perren et al. 2023). The use of fire blight resistant cultivars in combination with other control strategies such as orchard phytosanitary management, targeted application of plant protection products and the use of resistant rootstocks are key factors in fire blight control. In fact, Reininger et al. (2021) showed that fewer fire blight infections occurred and plant protection products showed a higher efficacy against fire blight when applied to the fire blight robust cultivar ‘Ladina’ compared with the fire blight susceptible cultivar ‘Gala Galaxy’. However, only a few partially resistant cultivars have been released to date, and most modern cultivars are more or less susceptible to fire blight. For this reason, fire blight resistance is an important breeding goal in many apple breeding programs around the world.
Known sources of fire blight resistance (e.g. Fb_E (Durel et al. 2009), FB_MR5 (Peil et al. 2007) and FB_F7 (Calenge et al. 2005; Khan et al. 2006; Baumgartner et al. 2015) currently used in breeding were found by performing artificial shoot inoculation according to the method of Khan et al. (2006). Whether the resistances found with this method also provide flower resistance, i.e. resistance when the pathogen enters through the flowers and not through a wound in the tree, has not yet been tested for all resistances. Since the main entry of E. amylovora into a tree is through the flowers, it is mandatory for the application of these resistances in breeding that genotypes that show good shoot resistance to fire blight also show flower resistance. However, evaluating shoot resistance is much easier and less time-consuming than evaluating flower resistance.
This paper presents the results of the comparison between artificial inoculation of shoots and flowers used to study fire blight resistance within the Agroscope apple breeding material, as well as heirloom accessions and modern cultivars used in breeding. In addition, molecular markers were used to characterize and select for qualitative and quantitative fire blight resistance.
Materials and methods
Plant material
Five heirloom accessions from the Swiss apple collection of genetic resources, the two fire blight resistance donors ‘Evereste’ (Fb_E resistance; Durel et al. 2009) and ‘Malus × robusta 5’ (FB_MR5 resistance; Peil et al. 2007), and 264 genotypes from the Agroscope apple breeding program were tested in this study. ‘Gala Galaxy’ and ‘Enterprise’ were used as susceptible and resistant control genotype, respectively. For the artificial fire blight shoot inoculation test, the scions of each genotype were grafted onto the rootstock M9vf T337 and potted in rose pots (Stuewe & Sons, Inc., Tangent, OR, U.S.; 35.5 cm pot height, 7 cm diameter). For the artificial fire blight flower inoculation test, two- to three-year-old trees on rootstock M9vf T337 were potted in 10-L pots approximately one month before the inoculation.
Artificial fire blight shoot inoculation test
All 273 genotypes were inoculated by artificial shoot inoculation with E. amylovora in the biosafety quarantine greenhouse at Agroscope in Waedenswil, Switzerland, from 2012 to 2022 in a total of 16 independent trials (Supplementary Table S1). ‘Gala Galaxy’ and ‘Enterprise’ were included in each trial as susceptible and resistant control genotype, respectively. Per genotype, twelve grafted plants were grown during five to six weeks in a regular greenhouse (temperature: 16–24 °C, humidity: 65%). Actively growing, healthy plants with a preferred minimum shoot length of 13.5 cm were then transferred to the quarantine greenhouse (temperature: 16–24 °C, humidity: 65%) and inoculated the next day with E. amylovora Swiss strain Ea ACW 610 Rif (suspension at approx. 1 × 109 cfu/ml), which is a natural Rif mutant of the strain used for the flower inoculation, into the shoot tip using a syringe according to Khan et al. (2006). In each independent screening trial, actively growing and healthy shoot tips of five to twelve replicated plants per genotype were inoculated. Maximum shoot length and length of the visually fire blight free shoot part was measured per week (7, 14, 21 days after inoculation (DAI). Lesion length at 21 DAI was calculated by subtracting the measured length of the visually fire blight free shoot part 21 DAI from the maximum shoot length reached within 21 days.
Artificial fire blight flower inoculation test under field conditions
During the years 2013 to 2015, 2018, 2019 and 2021, 18 advanced breeding selections were tested plus the two control genotypes ‘Gala Galaxy’ and ‘Enterprise’ in each year; eight breeding selections carried the quantitative trait locus (QTL) FB_F7 (Calenge et al. 2005; Khan et al. 2006; Baumgartner et al. 2015), one genotype carried the resistance gene FB_MR5, and nine genotypes without known fire blight resistance genes or QTLs (Supplementary Table S2). All 18 genotypes had shown, on average, a lower shoot susceptibility than ‘Gala Galaxy’ in previously shoot inoculation trials and were additionally positively evaluated in the selection process for their fruit quality. The trials were conducted in spring at the Agroscope Stone Fruit Center Breitenhof in Wintersingen, Switzerland, in a completely netted plot with black insect-proof mesh (1 × 2 mm) on the side and a hail net (approx. 3 × 8 mm) on the top. ‘Gala Galaxy’ and ‘Enterprise’ were included in each trial as susceptible and resistant control genotype, respectively. Potted trees were placed in rows by using a randomized complete block design (three replicated blocks with four to five experimental trees per block, spaced 1 × 3 m) in the netted plot prior to bud break (approx. one month before inoculation). Trees were drip-irrigated as needed without fertilizer, and only vigorous and healthy trees were included in the evaluation. Bumblebees (Bombus terrestris; Andermatt Biocontrol Suisse AG, Grossdietwil, Switzerland) were released in the completely netted plot to promote pollination of the flowers. Around full bloom (BBCH stage 65; Meier et al. 1994) approximately 10 flower clusters per tree were labeled and inoculated with a hand spray bottle (500 ml) containing the E. amylovora bacterial suspension (Swiss strain Ea L 610/03/2013, adjusted to approx. 1.5 × 109 cfu/ml) prepared according to Reininger et al. (2021). Flowers in balloon stage (BBCH stage 59; Meier et al. 1994) were inoculated after manual opening. Fire blight symptoms on artificially inoculated flower clusters were scored approximately 28 DAI by using classes 0 to 5 of the evaluation scale explained in Table 1.
In 2022, six breeding selections (‘ACW 22800’, ‘ACW 18313’, ‘ACW 18252’, ‘ACW 22750’, ‘ACW 18769’, ‘ACW 21303’) and the susceptible control genotype ‘Gala Galaxy’ were inoculated and tested as previously described, assessing the symptoms at 14, 21 and 28 DAI (Supplementary Table S3). In addition, the number of flower clusters with flowers shed and the number of developing fruits per flower cluster were counted 14 and 21 DAI.
Molecular marker analysis
The 273 phenotyped genotypes were additionally genotyped with specific molecular markers for the fire blight resistance genes Fb_E (Durel et al. 2009), FB_MR5 (Peil et al. 2007) and the QTL FB_F7 (Calenge et al. 2005; Khan et al. 2006; Baumgartner et al. 2015) based on the presence or absence of the resistance source in their pedigree. Dried (LGC Genomics Ltd., Teddington, UK) or frozen (Ecogenics GmbH, Balgach, Switzerland) leaf samples were prepared for shipment according to the company’s requirements. Genotyping with simple-sequence repeat (SSR) markers and sequence characterized amplified region (SCAR) markers was performed between 2012 and 2019 at Ecogenics GmbH (www.ecogenics.ch) using multiplex PCR assays with fluorescently labeled primers. Genotyping with single nucleotide polymorphism (SNP) markers was performed between 2020 and 2022 at LGC Genomics Ltd. (www.lgcgroup.com) using the KASP™ PCR assays developed for Fb_E, FB_MR5 and FB_F7 (Table 2).
Statistical analysis
Statistical analysis was performed in the R software environment (v 4.2.2; R Core Team 2023). The tidyverse packages magrittr (v 2.0.3), dplyr (v 1.0.9), tidyr (v 1.2.0) and ggplot2 (v 3.4.0) were used for data preparation and visualization (Wickham et al. 2019). To compare the two phenotypic susceptibility tests with each other and over the years, the data were normalized using the two control genotypes ‘Gala Galaxy’ and ‘Enterprise’ to account for environmental and trial effects. Equation (1) and Eq. (2) were used to normalize the data of each repetition of the shoot and flower inoculation, respectively. The used normalization puts the data in relation to the control genotypes ‘Gala Galaxy’ and ‘Enterprise’. For this reason, the term relative susceptibility is used in the following. A relative susceptibility greater than 100 indicates that a genotype is more susceptible than ‘Gala Galaxy’. A relative susceptibility of zero indicates that a genotype is as resistant as ‘Enterprise’, whereas a genotype that is more resistant than ‘Enterprise’ has a negative relative susceptibility. For the comparison of the resistance genes in the shoot inoculation test, the term relative susceptibility corresponds to the mean value of the scaled relative lesion lengths (rLLsc) of all shoots per genotype across trials. When comparing shoot and flower tests, relative susceptibility is the scaled relative lesion length (rLLsc) per shoot or the mean of the scaled scoring classes (Csc) per tree, respectively.
where:
-
\({rLL}_{sc}\) = scaled relative lesion length
-
rLL = relative lesion length (lesion length / shoot length).
-
\(\stackrel{-}{r{LL}_{E}}\) = mean of the rLL from ‘Enterprise’ in the same trial.
-
\(\stackrel{-}{{rLL}_{G}}\) = mean of the rLL from ‘Gala Galaxy’ in the same trial.
where:
-
\({C}_{sc}\) = scaled scoring class
-
C = scoring class.
-
\(\stackrel{-}{{C}_{E}}\) = mean of the classes of all flowers of ‘Enterprise’ in the same trial
-
\(\stackrel{-}{{C}_{G}}\) = mean of the classes of all flowers of ‘Gala Galaxy’ in the same trial
Results
Effect of fire blight resistance Fb_E, FB_MR 5 and FB_F7 on shoot susceptibility
The relative susceptibility of the 271 genotypes were grouped according to the presence or absence of the fire blight resistances predicted by the molecular markers associated with Fb_E, FB_MR5 and FB_F7 (Fig. 1). Significant differences (post hoc Tukey test, p < 0.05) between groups of genotypes were found. As expected, the group of genotypes not carrying Fb_E, FB_MR5 and FB_F7 were on average the most susceptible. These genotypes were in general less susceptible than the susceptible control genotype ‘Gala Galaxy’ (relative susceptibility of ‘Gala Galaxy’ equal to 100). The group of genotypes carrying FB_F7 were on average only slightly (but not significantly) less susceptible than the previous group (“no resistance”). By contrast, all four groups of genotypes carrying either Fb_E or FB_MR5 resulted in a mean relative susceptibility of about zero and thus showed the same level of resistance as the resistant control genotype ‘Enterprise’. There was no significant difference between the four groups carrying either Fb_E or FB_MR5, regardless of whether or not FB_F7 was additionally present. To assess the repeatability and reliability of the obtained results, a Pearson correlation coefficient was calculated using the mean relative susceptibility of 48 genotypes tested in two independent trials. A positive correlation was found between two measurements of the same genotype (r[47] = 0.67; p = 1.35 × 10− 7; Supplementary Figure S1).
Comparison of the relative susceptibility among six groups of genotypes with different genetic backgrounds 21 days after artificial shoot inoculation. Relative susceptibility is a normalized value that sets the susceptible control genotype ‘Gala Galaxy’ to 100 and the resistant control genotype ‘Enterprise’ to 0 (upper and lower red dotted line, respectively). The numbers under the boxes indicate the number of evaluated genotypes per group. Each data point contains the mean of 5 to 34 inoculated shoots per genotype. The red dots represent the relative susceptibility of the resistance donors ‘Malus × robusta 5’ (FB_MR5) and ‘Evereste’ (Fb_E) tested in two different years. Letters indicate significant differences according to a post hoc Tukey test (p < 0.05)
Flower fire blight resistance
All 18 genotypes tested showed a significant difference in flower resistance level compared with the susceptible control ‘Gala Galaxy’ (Supplementary Figure S2). ‘ACW 18346’, without any known source of fire blight resistance, was significantly more susceptible than ‘Gala Galaxy’ in the artificial flower inoculation test. All the other 17 genotypes were significantly less susceptible than ‘Gala Galaxy’. Comparing the relative susceptibility of the 18 genotypes with the resistant control ‘Enterprise’, six genotypes showed no significant differences (Fig. 2). The genotypes were: ‘ACW 22800’ (without known resistance), ‘ACW 23794’, ‘ACW 13490’, ‘ACW 12556’, ‘ACW 19256’ (all four carrying FB_F7) and ‘1124_26’ (carrying FB_MR5). In addition, a very low level of flower susceptibility was observed in the two genotypes ‘ACW 20280’ and ‘ACW 21274’ (without known resistance) as well as in ‘ACW 14992’ and ‘ACW 16426’ (both carrying FB_F7).
Comparison of fire blight resistance between artificial shoot and flower inoculation
The Pearson correlation coefficient between the mean values of all 18 genotypes of both tests revealed no significant correlation between the two phenotyping methods (r[16] = − 0.014; p = 0.9562; Supplementary Figure S3). In fact, the ranking of genotypes according to their flower and shoot fire blight resistance varied between the two inoculation methods (Fig. 2). For instance, ‘ACW 18346’, ‘ACW 22744’ and ‘ACW 22984’ showed large and significant differences between the two tests (Wilcoxon rank sum test, p < 0.05). These three genotypes proved to have a high level of shoot fire blight resistance not significantly different from ‘Enterprise’, whereas they were highly susceptible in the flower inoculation test, with ‘ACW 18346’ being even more susceptible than ‘Gala Galaxy’. Also, ‘ACW 16756’, ‘ACW 12556’, ‘ACW 19256’ and ‘1124_26’ showed significant differences between the two inoculation methods. The flower fire blight resistance of ‘ACW 19256’ and ‘ACW 12556’ was higher than their level of shoot resistance, whereas the opposite was true for ‘ACW 16756’ and ‘1124_26’. However, the differences between the level of flower and shoot resistance for ‘ACW 12556’ and ‘1124_26’ were not large. All other genotypes did not show significant differences between the two inoculation methods. On average, genotypes with FB_F7 were classified as more resistant than genotypes without the QTL in both tests. In fact, the mean relative susceptibility in both tests was lower for genotypes carrying FB_F7. ‘1124_26’ is the first offspring of the third pseudo backcross generation of ‘Malus × robusta 5’, which was tested in both inoculation trials and carries the resistance gene FB_MR5. It proved to be at least as resistant as ‘Enterprise’ in both tests.
Comparison of fire blight resistance assessed by artificial shoot (21 days after inoculation) and flower (approx. 28 days after inoculation) inoculation of 18 genotypes. The data were normalized against the susceptible control ‘Gala Galaxy’ and the resistant control ‘Enterprise’ so that in both tests a relative susceptibility of 100 corresponds to the susceptibility of ‘Gala Galaxy’ and a relative susceptibility of 0 to the resistance of ‘Enterprise’. A single data point in the flower test corresponds to the average value of all flowers per tree, whereas in the shoot test a single data point corresponds to one value per shoot. The numbers in parentheses below the genotype name indicate the number of trees in the artificial fire blight flower inoculation test and the number of shoots in the artificial fire blight shoot inoculation test, respectively. In each group, the genotypes were ordered according to their median performance in the flower test. The dashed horizontal lines show the mean relative susceptibility for both tests in the two groups (‘no resistance’ and ‘FB_F7’). Asterisks indicate significant differences between the results of flower and shoot test for each genotype. Equal signs (“=”) mean no significant difference between the respective genotype and ‘Enterprise’. All other genotypes were significantly different from ‘Enterprise’ (“<” indicates significantly better performance than ‘Enterprise’). Compared with the susceptible control ‘Gala Galaxy’, all genotypes showed a significant difference, with only one genotype being significantly more susceptible. Significant differences were determined using Wilcoxon rank sum tests (p < 0.05)
Development of fire blight symptoms over time upon artificial flower inoculation under field conditions
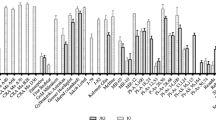
Clear differences between the tested genotypes were already visible after 14 DAI (Fig. 3). Three genotypes, ‘ACW 22800’ (without known resistance), ‘ACW 18313’ and ‘ACW 18252’ (both carrying FB_F7), showed the highest level of resistance and had more than 90% of “class 0” flower clusters and no symptoms were observed on flowers, peduncles, leaves and shoots. At 28 DAI, the same three genotypes were still the most resistant, and symptoms had worsened only slightly over time. The majority of flower clusters were still assigned to “class 0”, and infection of the young shoot (class 3) was observed in only about 10% of the flower clusters of ‘ACW 18313’ and ‘ACW 18252’. The remaining three genotypes (‘ACW 22750’, ‘ACW 18769’ and ‘ACW 21303’) and the susceptible control genotype ‘Gala Galaxy’ showed a different picture. Most of the young shoots were infected (class 3) already 14 DAI, and the symptoms worsened gradually over time. At 28 DAI, necrosis (class ≥ 3) was present in the wood of more than 75% of the infected flower clusters. Another difference between the two groups of genotypes concerned flower shedding. For the three genotypes showing a higher level of resistance (‘ACW 22800’, ‘ACW 18313’ and ‘ACW 18252’), it was observed that up to 50% of the inoculated flower clusters dropped flowers before 21 DAI. This behavior was observed for the more susceptible genotypes (‘ACW 22750’, ‘ACW 18769’ and ‘ACW 21303’) only for a few flower clusters, whereas no flower shedding was observed for ‘Gala Galaxy’. A similar behavior was observed for fruit development, i.e., the three most resistant genotypes developed fruits despite the artificial inoculation, whereas the more susceptible genotypes and ‘Gala Galaxy’ showed only few or no flower clusters with fruits.
Development of fire blight symptoms over time after artificial flower inoculation (14, 21 and 28 days after inoculation [DAI]) for six breeding selections and the susceptible control genotype ‘Gala Galaxy’. The left part of each panel shows the proportion of flower clusters in each class 14, 21 and 28 DAI (See Table 1 for description of the classes). The two bars on the right side of each panel show the proportion of flower clusters that have shed flowers or have developed fruit as a percentage of all inoculated flower clusters, from the time of inoculation to 21 DAI
Discussion
The fire blight resistances Fb_E (Durel et al. 2009), FB_MR5 (Peil et al. 2007) and FB_F7 (Calenge et al. 2005; Khan et al. 2006; Baumgartner et al. 2015), which explain between 37.5% (FB_F7), 50–70% (Fb_E), and up to 80% (FB_MR5) of the phenotypic variation of shoot resistance, respectively, were evaluated for their level of shoot and/or flower resistance to fire blight in selected breeding material, modern cultivars and heirloom accessions. Genotypes from the four groups carrying one of the major resistance genes (Fb_E or FB_MR5) were significantly less susceptible to fire blight in the artificial shoot inoculation test than genotypes without known resistance or with the QTL FB_F7 only (Fig. 1). All genotypes carrying the resistance gene from ‘Evereste’ (Fb_E) or ‘Malus × robusta 5’ (FB_MR5) were substantially less susceptible than ‘Gala Galaxy’. By contrast, the variation of the level of shoot fire blight resistance among genotypes carrying the QTL FB_F7 was much greater, and the average group level was not significantly different from ‘Gala Galaxy’ (Fig. 1). Some genotypes carrying FB_F7 were as susceptible as or even more susceptible than ‘Gala Galaxy’. Van de Weg et al. (2018) found epistatic interactions between FB_F7 (also present in ‘Enterprise’) and two other loci on linkage group (LG) 8 and 13. Their presence or absence in the tested genotypes could partially explain the large difference in resistance levels within the group of genotypes carrying FB_F7. Peil et al. (2021) reported that elite apple cultivars, such as ‘Fiesta’, ‘Cox’s orange Pippin’, ‘Enterprise’, ‘Delicious’, ‘Nova Easygro’ or ‘Florina’, are moderately resistant to fire blight. As many of these cultivars are present in the pedigree of the genotypes included in this study (data not shown), the inheritance of these (partially unknown) additional resistance QTLs could explain their unexpected, relatively high level of fire blight resistance (e.g., accessions ‘ACW 21274’ or ‘ACW 20280’ that both have ‘Florina’ in their pedigree).
Artificial shoot inoculations with a syringe or scissors in the greenhouse are the most used methods for assessing the shoot fire blight resistance level of an apple genotype (Peil et al. 2021). The results provided by this test mimics fire blight infections that in nature may occur when trees are injured (e.g., during a storm, a hail event or mechanical and manual pruning). Genotypes possessing a good level of shoot fire blight resistance are more easily sanitized in case of infection (e.g., by pruning diseased twigs and branches in summer or winter; Norelli et al. 2003). In such genotypes the pathogen is likely to spread less rapidly than in very susceptible genotypes. Nevertheless, the most important and major entry point of E. amylovora into the tree remains through the flowers (van der Zwet and Keil 1979). Testing the level of flower fire blight resistance is more costly and time consuming than the assessment of shoot resistance. For the artificial fire blight flower inoculation test, two- to three-year-old trees must first be produced, and depending on the country (e.g., Switzerland) elaborate biosafety measures may have to be fulfilled in order to perform inoculation with E. amylovora in field trials. If a good correlation between the level of shoot and flower fire blight resistance existed, the costly and time-consuming flower inoculation test could be avoided. For this reason, the results obtained by artificial shoot and flower inoculation tests were compared in this study and in the past. Horner et al. (2014) studied the correlation between shoot and flower fire blight resistance of individual progeny plants of a ‘Royal Gala’ × ‘Malus × robusta 5’ cross and found no correlations. Peil et al. (2019) performed a QTL study for flower fire blight resistance in a cross between ‘Idared’ and ‘Malus × robusta 5’ and found a major QTL on LG3. The same major QTL (FB_MR5) had previously been found by Peil et al. (2007) using the same mapping population for fire blight shoot resistance. Broggini et al. (2014) cloned FB_MR5 and used this gene to generate the first cisgenic fire blight resistant ‘Gala Galaxy’ line (‘C44.4.146’; Kost et al. 2015). Recently, Schlathölter et al. (2023) demonstrated that ‘C44.4.146’ showed high levels of shoot and flower fire blight resistance under greenhouse conditions. In our study, ‘1124_26’, an offspring of the first pseudo backcross generation (pBC’1) of ‘Malus × robusta 5’ carrying FB_MR5, also showed high levels of flower and shoot fire blight resistance (Fig. 2). Therefore, it can be concluded that FB_MR5 confers both types of fire blight resistance. It is reasonable to assume that the lack of correlation found by Horner et al. (2014) may be due to slightly different inoculation protocols or too much variability in the shoot inoculation test within a year, resulting in a weak correlation (0.474) of the test results between two different years. In this context, our normalization approach, using a resistant and a susceptible control cultivar can account for environmental and trial effects and allows for easier comparability between the two test methods and across years. Nevertheless, our normalization approach also has a limitation, as it assumes that the genotypes tested respond in a similar way to environmental changes, i.e. different weather conditions between years, as the control genotypes used to perform the normalization. With this study, we aimed to verify whether FB_F7, a QTL widely used in the Agroscope apple breeding program, is sufficient to provide resistance to fire blight in flowers and whether the level of resistance in flower and shoot is comparable. Six out of eight genotypes carrying QTL FB_F7 showed good and similar levels of resistance in both tests (exceptions: ‘ACW 16756’, ‘ACW 22984’; Fig. 2). However, for half of the eight FB_F7 breeding selections, a significant difference was found between the levels of flower and shoot resistance. The small number of genotypes tested does not allow definitive conclusions to be drawn. According to the genotypes tested, the presence of QTL FB_F7 seems to be a good prerequisite for a higher level of flower fire blight resistance (Supplementary Figure S2). However, this result still needs to be verified experimentally. Finally, the fire blight resistance of genotypes not carrying a known resistance gene or QTL was examined and it was additionally tested whether in this case there was a significant difference between the shoot and flower resistance levels. The largest differences between the two types of resistance and inoculation methods were observed in this group of genotypes, but a significant difference was only found for two out of nine genotypes included in this study. Therefore, also in this case, the level of shoot resistance seems to be a good indicator for the selection of genotypes to be further tested for their level of flower fire blight resistance under field conditions. For a more accurate assessment of the comparability of the two test methods, a larger group of unselected genotypes without resistance and with a broad resistance spectrum should be phenotyped with both methods. In our study, a single strain of E. amylovora (‘Ea ACW 610 Rif’, a natural Rif mutant of ‘Ea L 610/03/2013’) was used for both artificial shoot and flower inoculation tests. It was shown that a gene-for-gene relationship between FB_MR5 and E. amylovora exist (Vogt et al. 2013). Strains capable of overcoming this resistance gene were reported and that a single point mutation in the bacterial genome can lead to resistance breakdown. Therefore, the results presented in our study may only be valid for the strain used. Consequently, the use of a mixture of different E. amylovora strains for artificial inoculation should be considered in the future.
Based on observations from previous years, we decided in spring 2022 to additionally systematically record the number of flower clusters with shed flowers and the number of fruits developed despite artificial inoculation of the flower clusters. For the three most resistant genotypes (‘ACW 22800’, ‘ACW 18313’ and ‘ACW 18252’), flower shedding was observed in 25–50% of inoculated flower clusters. Therefore, flower shedding could be part of the resistance response. A repetition of this experiment including non-inoculated trees will allow to clarify the role of flower shedding in fire blight resistance. Flower shedding has previously been reported to occur after natural infection of the flower peduncle with apple scab (Venturia inaequalis) (Aćimović et al. 2019). In terms of fruit development, the three previously mentioned genotypes showed great differences. Whereas genotype ‘ACW 22800’ developed only very few fruits, 25% of the flower clusters of ‘ACW 18252’ developed fruits despite infection. To our knowledge, this behavior has not been described before and should be considered when phenotyping fire blight resistance of flowers in the future.
Field trials remain crucial for the evaluation of fire blight resistance of new breeding material under more natural conditions. In the near future, additional genotypes with resistances based on major genes, i.e., Fb_E (Durel et al. 2009; Parravicini et al. 2011) or FB_Mfu10 (Emeriewen et al. 2014), will become available (Bühlmann-Schütz et al., unpublished). Likewise, for these resistances, it is important to verify their level of flower resistance under field conditions in addition to their high level of shoot resistance in the greenhouse. The availability of advanced breeding selections carrying these major resistance genes will further allow their stacking to develop new cultivars with a high level of durable fire blight resistance.
References
Aćimović SG, Martin DKH, Turcotte RM et al (2019) Choosing an adequate pesticide delivery system for managing pathogens with difficult biologies: Case studies on Diplodia Corticola, Venturia Inaequalis and Erwinia amylovora. https://doi.org/10.5772/intechopen.87956
Baumgartner IO, Patocchi A, Frey JE et al (2015) Breeding Elite lines of Apple carrying Pyramided homozygous resistance genes against Apple Scab and Resistance Against Powdery Mildew and Fire Blight. Plant Mol Biol Report 33:1573–1583. https://doi.org/10.1007/s11105-015-0858-x
Bogs J, Bruchmüller I, Erbar C, Geider K (1998) Colonization of Host Plants by the Fire Blight Pathogen Erwinia amylovora marked with genes for Bioluminescence and Fluorescence. Phytopathology® 88:416–421. https://doi.org/10.1094/PHYTO.1998.88.5.416
Broggini GAL, Wöhner T, Fahrentrapp J et al (2014) Engineering Fire blight resistance into the apple cultivar ‘Gala’ using the FB_MR5 CC-NBS-LRR resistance gene of Malus × robusta 5. Plant Biotechnol J 12:728–733. https://doi.org/10.1111/pbi.12177
Calenge F, Drouet D, Denancé C et al (2005) Identification of a major QTL together with several minor additive or epistatic QTLs for resistance to Fire blight in apple in two related progenies. Theor Appl Genet 111:128–135. https://doi.org/10.1007/s00122-005-2002-z
Duffy B, Schärer H-J, Bünter M et al (2005) Regulatory measures against Erwinia amylovora in Switzerland*. EPPO Bull 35:239–244. https://doi.org/10.1111/j.1365-2338.2005.00820.x
Durel C-E, Denancé C, Brisset M-N (2009) Two distinct major QTL for resistance to Fire blight co-localize on linkage group 12 in apple genotypes ‘Evereste’ and Malus floribunda clone 821. Genome 52:139–147. https://doi.org/10.1139/G08-111
Emeriewen O, Richter K, Kilian A et al (2014) Identification of a major quantitative trait locus for resistance to Fire blight in the wild apple species Malusfusca. Mol Breed 34:407–419. https://doi.org/10.1007/s11032-014-0043-1
Fahrentrapp J, Broggini GAL, Kellerhals M et al (2013) A candidate gene for Fire blight resistance in Malus ×robusta 5 is coding for a CC–NBS–LRR. Tree Genet Genomes 9:237–251. https://doi.org/10.1007/s11295-012-0550-3
Holliger E (2008) Bekämpfung des Feuerbrands in der Schweiz: traditionelle Lösung oder Gentechnik? In: Kohler S, Maranta A, Sautter C (eds) Bekämpfung des Feuerbrands in der Schweiz—Traditionelle Lösung oder Gentechnik? ETH, Plant Science Center, pp 15–21
Horner MB, Hough EG, Hedderley DI et al (2014) Comparison of Fire blight resistance screening methodologies. N Z Plant Prot 67:145–150. https://doi.org/10.30843/nzpp.2014.67.5745
Jänsch M, Broggini GAL, Weger J et al (2015) Identification of SNPs linked to eight apple Disease resistance loci. Mol Breed 35:45. https://doi.org/10.1007/s11032-015-0242-4
Khan MA, Duffy B, Gessler C, Patocchi A (2006) QTL mapping of Fire blight resistance in apple. Mol Breed 17:299–306. https://doi.org/10.1007/s11032-006-9000-y
Khan MA, Durel C-E, Duffy B et al (2007) Development of molecular markers linked to the ‘Fiesta’ linkage group 7 major QTL for Fire blight resistance and their application for marker-assisted selection. Genome 50:568–577. https://doi.org/10.1139/G07-033
Kost TD, Gessler C, Jänsch M et al (2015) Development of the First Cisgenic Apple with increased resistance to Fire Blight. PLoS ONE 10:e0143980. https://doi.org/10.1371/journal.pone.0143980
Meier U, Graf H, Hack H et al (1994) Phänologische Entwicklungsstadien Des Kernobstes (Malus domestica Borkh. Und Pyrus communis L.), des steinobstes (Prunus-Arten), Der Johannisbeere (Ribes-Arten) Und Der Erdbeere (Fragaria x ananassa Duch). Nachrichtenblat Dtsch Pflanzenschutzd 46:141–153
Norelli JL, Jones AL, Aldwinckle HS (2003) Fire Blight Management in the twenty-first century: using New technologies that Enhance Host Resistance in Apple. Plant Dis 87:756–765. https://doi.org/10.1094/PDIS.2003.87.7.756
Parravicini G, Gessler C, Denancé C et al (2011) Identification of serine/threonine kinase and nucleotide-binding site–leucine-rich repeat (NBS-LRR) genes in the Fire blight resistance quantitative trait locus of apple cultivar ‘Evereste’. Mol Plant Pathol 12:493–505. https://doi.org/10.1111/j.1364-3703.2010.00690.x
Peil A, Garcia-Libreros T, Richter K et al (2007) Strong evidence for a Fire blight resistance gene of Malus robusta located on linkage group 3. Plant Breed 126:470–475. https://doi.org/10.1111/j.1439-0523.2007.01408.x
Peil A, Hübert C, Wensing A et al (2019) Mapping of Fire blight resistance in Malus ×robusta 5 flowers following artificial inoculation. BMC Plant Biol 19:532. https://doi.org/10.1186/s12870-019-2154-7
Peil A, Emeriewen OF, Khan A et al (2021) Status of Fire blight resistance breeding in Malus. J Plant Pathol 103:3–12. https://doi.org/10.1007/s42161-020-00581-8
Perren S, Egger B, Kuster T et al (2023) Empfohlene Pflanzenschutzmittel für den Erwerbsobstbau 2023. Agroscope Transf 461:1–23
R Core Team (2023) R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Reininger V, Schöneberg A, Holliger E (2021) Fire blight plant protection efficacy trial with resistant apple cultivar ‘Ladina’. J Plant Pathol 103:143–149. https://doi.org/10.1007/s42161-021-00741-4
Schlathölter I, Broggini GAL, Streb S et al (2023) Field study of the fire-blight-resistant cisgenic apple line C44.4.146. Plant J 113:1160–1175. https://doi.org/10.1111/tpj.16083
Spinelli F, Ciampolini F, Cresti M et al (2005) Influence of stigmatic morphology on flower colonization by Erwinia amylovora and Pantoea agglomerans. Eur J Plant Pathol 113:395–405. https://doi.org/10.1007/s10658-005-4511-7
van de Weg E, Di Guardo M, Jänsch M et al (2018) Epistatic Fire blight resistance QTL alleles in the apple cultivar ‘Enterprise’ and selection X-6398 discovered and characterized through pedigree-informed analysis. Mol Breed 38:5. https://doi.org/10.1007/s11032-017-0755-0
van der Zwet T, Keil HL (1979) Fire blight: a bacterial Disease of Rosaceous plants. U.S. Department of Agriculture
Vanneste JL (2000) Fire blight: the Disease and its causative agent, Erwinia amylovora. Cabi Publishing
Vogt I, Wöhner T, Richter K et al (2013) Gene-for-gene relationship in the host–pathogen system Malus × robusta 5–Erwinia amylovora. New Phytol 197:1262–1275. https://doi.org/10.1111/nph.12094
Wickham H, Averick M, Bryan J et al (2019) Welcome to the tidyverse. J Open Source Softw 4:1686. https://doi.org/10.21105/joss.01686
Acknowledgments
Funding was provided by the Federal Office for Agriculture (FOAG) and VariCom GmbH for the projects: ZUEFOS, ZUEFOS II (breeding of fire blight resistant fruit cultivars), GgFB (together against fire blight) and resistance breeding in apple at Agroscope. Our thanks go to all project partners, as well as to Jürgen Krauss, Mathias Schmid and Thomas Schweizer and their team for their technical support.
Funding
Agroscope internal funds. Open access funding provided by Agroscope.
Open access funding provided by Agroscope
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table S1
: Data from genotypes tested for fire blight resistance by artificial inoculation of shoots.
Supplementary Table S2
: Data from genotypes tested for fire blight resistance in artificial inoculation of shoots and flowers.
Supplementary Table S3
: List of genotypes tested for fire blight resistance in artificial flower inoculation in 2022.

Supplementary Fig. S1: Comparison of relative susceptibilities in repeated measurements of genotypes in the Fire blight shoot inoculation test
. Relative susceptibility is a normalized value that sets the susceptible control genotype ‘Gala Galaxy’ to 100 and the resistant control genotype ‘Enterprise’ to 0. In total 49 genotypes were measured twice. Each dot displays the mean of 5 to 12 inoculated shoots per genotype and trial. The different colors indicate the known resistance genes in the respective genotypes. The identity line is represented by a dashed line.

Supplementary Fig. S2: Relative susceptibility of 20 genotypes in the flower inoculation test
. Relative susceptibility is a normalized value that sets the susceptible control genotype ‘Gala Galaxy’ to 100 and the resistant control genotype ‘Enterprise’ to 0. A single data point corresponds to the average value of all flowers per tree. In each group, the genotypes were ordered according to their median relative susceptibility. The dashed horizontal lines show the mean relative susceptibility in the two groups (‘no resistance’ and ‘FB_F7’). Green asterisks indicate significant differences between the respective genotype and ‘Gala Galaxy’ and red asterisks display a significant difference between the genotype and ‘Enterprise’ according to a Wilcoxon rank sum test (p < 0.05).

Supplementary Fig. S6: Comparison of the relative susceptibilities measured in shoot and flower inoculation tests
. Relative susceptibility is a normalized value that sets the susceptible control genotype ‘Gala Galaxy’ to 100 and the resistant control genotype ‘Enterprise’ to 0. The dots indicate the mean values per genotype per test. The dashed lines indicate the 95% confidence interval of the mean per genotype and test (\(\bar x \pm 1.96 \times SE\)). The red dot and lines display the values for the control varieties ‘Enterprise’ and ‘Gala Galaxy’. They were not included in the calculation of the Pearson correlation coefficient shown in the plot.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bühlmann-Schütz, S., Hodel, M., Dorfmann, E. et al. Comparison between artificial fire blight shoot and flower inoculations in apple. J Plant Pathol (2023). https://doi.org/10.1007/s42161-023-01550-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-023-01550-7