Abstract
Mesoporous silica nanoparticles (MSNs) can promote the solubility and absorption of pesticides by plants and are widely used as a delivery system to improve the efficacy of pesticide applications. In this study, MSNs with 20 nm particle size were produced. Additionally, a water-soluble chitosan (CS) derivative, here called N-(2-Hydroxyl) propyl-3- tri-methyl-ammonium CS chloride (HTCC) was produced and used to cap the outer surface of the MSNs preloaded with the pesticide fludioxonil (Flu). The HTCC coating layers resulted in a pesticide loading efficiency of 84% on the MSNs in comparison to a loading efficiency of 20% of uncoated particles. A comparative in vitro analysis indicated that Flu@MSNs20nm-HTCC loaded with a 0.05 mg/L dose of fungicide had significant higher fungicidal activity than the same fungicide at 1 mg/L dose against F. oxysporum f. sp. radicis-lycopersici (FORL). Moreover, after an initial burst, MSNs20nm-HTCC kept releasing Flu for 21 d, compared to an activity of 7 d associated with the direct release of Flu. Greenhouse data showed that 0.1 mg/L Flu applied through MSNs20nm-HTCC is sufficient to reduce Fusarium crown and root rot disease severity to a value of less than 6% in tomato plants, without any noticeable phytotoxicity after 70 d. In comparison, 1.56 mL/L of the fungicide are required to reach a 27% disease severity level. Thus, we suggest that HTCC-decorated MSNs20nm has a great potential as a nanodelivery systems for agrochemical applications. We also suggest that this work contributes to the notion that agro-nanotechnology is a powerful, environmentally-safe and cost-effective approach for a sustainable and long term protection of plants from disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant diseases cause significant crop losses damage, with global losses estimated to be between 20 and 40% per year (Savary et al. 2012). The pathogenic Fusarium oxysporum is a serious soil-borne fungal species with over 150 formae specials that harm/infect a wide range of key agricultural crops across the world, resulting in significant crop output losses (Pareek and Rajam 2017). Fusarium crown and root rot is caused by a highly virulent strain, F. oxysporum f. sp. radicis-lycopersici Jarvis and Shomaker (FORL) and affecting the majority of the tomato-growing areas worldwide (Debbi et al. 2018).
Pesticides including insecticides, fungicides, and herbicides are heavily used in current pest management. Despite its numerous advantages, such as high availability, fast action, and reliability, chemical pesticides have passive effects on the ecological balance of the microbial ecosystems, the resurgence of the pest population, and the development of resistance (Ghormade et al. 2011). Additionally, only around 0.1 percent of synthetic chemical pesticides reach their intended target for pest management, while the remaining 99.9% disperse into the environment and are lost during or after application (Ghormade et al. 2011; Sinha et al. 2017; Zhao et al. 2018; Kumar et al. 2019; Hashim et al. 2019). Thus, there is increasing desire to develop cost-effective, high-performance, and environmentally friendly pesticides.
Nanotechnology offers great promises for efficient and safe delivery of biomolecules and agrochemicals including pesticides (Mosa and Youssef 2021), in order to reduce or minimize the indiscriminate use of conventional chemical pesticides because more than 90% of used pesticides usually fail to reach their targets into plants for many reasons including leaching (El Bahri and Taverdet 2007), evaporation (Nuruzzaman et al. 2016), deposition (Yin et al. 2018), hydrolysis (Nuruzzaman et al. 2016), aerobic and photo degradation (Ravier et al. 2005).
Many researches have been undertaken in this area since the discovery of Mobil Crystalline Material 41 (MCM-41) to fully exploit the benefits of mesoporous silica nanoparticles (MSNs). Because of its high dispersion, uniformly adjustable porous structure, and colloidal chemical characteristics, MSNs have been researched and widely used in various disciplines, including medicine (Farokhzad and Langer 2009; Shi et al. 2016), however, only in the past few years the focus on MSNs research and their applications in different fields has been shifted to agriculture and pest disease management (Popat et al. 2012; Wang et al. 2014). With the use of MSNs as delivery systems for biomolecules such as drugs (Huang et al. 2015; Zhang et al. 2015), urea, (Wanyika et al. 2012), nucleic acids, (Keasberry et al. 2017), and protein (Guo et al. 2012), MSNs can be translocated into different cells. As mesoporous silica nanoparticles are characterized by some unique properties including large surface area of their pores, tunable size and biocompatibility, the mesoporous silica nanoparticles can be easily encapsulated with guest molecules in order to control the pesticide release with high loading capacity and more economically. Thus, MSNs acting as pesticide delivery systems have a significant advantages in agriculture and plant disease management, as they could help in increase the utilization efficiency of pesticides and consequently reduce the pesticide dosage (Popat et al. 2012; Wang et al. 2014).
Polymers are well-known for being excellent natural shell materials for encapsulating any active molecules. Their unique properties, including such excellent biocompatibility, low toxicity, and multi-physiochemical properties, gave it the superior in biological processes. Chitosan (CS), a biopolymer compound that have been widely used in sustainable agriculture and commonly produced through de-acetylation process of chitin (Kashyap et al. 2015). Quaternized chitosan, especially N-(2-Hydroxyl) propyl-3- tri-methyl-ammonium CS chloride (HTCC) is considered one of the most prevalent chitosan derivatives that has attracted much attention in research. This is supported by HTCC’s physiochemical properties, which cationic charge retention at neutral pH, highly water solubility, permeability-enhancing properties, muco adhesiveness, and antimicrobial activity (Rwei et al. 2014). Hence, the positively charged HTCC layer is coated over the negatively charged MSNs by electrostatic attraction, without any complicated chemical grafting, which will be effective for MSNs as effective pesticide carriers the ideal strategy for surface modification.
Fludioxonil is a non-systemic broad-spectrum fungicide which is firstly introduced in 1993 by Syngenta Co., and commonly used to control a range of fungal diseases on fruit and vegetables against many phyto-pathogenic fungi including Fusarium, with high-efficiency and low-toxicity (Lewis et al. 2016). The mode of fludioxonil action is the inhibition of transport-associated phosphorylation of glucose as well as preventing glycerol synthesis of the fungal pathogen. fludioxonil was selected in this work as a model pesticide in order to investigate the possibility of MSNs capped HTCC as controlled release nanocarrier.
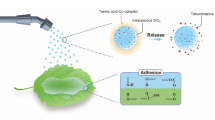
In the introduced work, a smart nano-delivery system using MSNs as the porous support and HTCC as the capping/coating agent was designed to deliver fludioxonil fungicide as a model of Celest pesticide (FS, 10% fludioxonil) for management of Fusarium crown and root rot disease (designated as Flu-MSNs20nm-HTCC) (Fig. 1). Moreover, a comparison between Fungicide-loaded MSNs and the chemical fungicide with respect to fungicidal activity under in vitro and in vivo conditions was designed. The loading capacity, controlled release activity, and pesticide final residues in tomatoes were also conducted. Finally, the present work aims to contribute to the concept of smart agro-nanotechnology in plant disease management and using very low amounts of pesticides showing the properties of a unique economic nanopesticide for sustainable plant protection.
Materials and methods
Materials
Cetyl-tri-methyl-ammonium bromide (CTAB) and sodium hydroxide (NaOH) were purchased from Sigma, St. Louis, MO, USA, tetra-ethyl-ortho-silicate (TEOS, 99%) were provided by Aladdin Industrial corporation (Shanghai, China), N-[3-(Tri-methoxy-silyl) propyl] ethylene-diamine (TSD) was purchased from Balinway Technology Co., Ltd. (Beijing, China). Also, Tianhua Biological Agents Ltd., Dongying, China, provided HTCC. The model fludioxonil (Flu) and the pesticide, Celest (FS 10%) was supplied by Syngenta (Switzerland). Dialysis membrane (molecular weight cutoff: 3 K Da) was provided by Thermo Fisher Scientific Co. USA. A Millipore Milli-Q water purification system (Burlington, MA, USA) was used in order to obtain deionized water.. All the other utilized chemicals are commercially available and were used without any further processing.
Fungal strain and molecular identification
A pathogenic F. oxysporum f. sp. radicis-lycopersici (FORL) isolated from tomato plants showing Fusarium crown and root rot symptoms was obtained from Mycology and Disease survey Research department, Plant pathology Research Institute (PPATHRI), Agricultural Research Center (ARC), Egypt, and deposited in the gene bank (NCBI) under the accession number (MW871619) ( El-Abeid et al. 2020).
Synthesis and characterization of MSNs
Synthesis process
Mesoporous silica nanoparticles were synthesized with three different sizes (20, 50, and 90) under alkaline conditions using Cetyl-tri-methyl-ammonium bromide (CTAB) acting as the structure-directing agent, and also TEOS solution as the precursor of silica following Radu et al. with some modification (Radu et al. 2004). In brief, CTAB (2.75 g) was firstly dissolved slowly in a mixture solution of 520 mL of deionized water (dH2O), and 3.5 mL of 2.0 M sodium hydroxide (NaOH) under 500 rpm magnetic stirring at room temperature. The temperature of mixture solution was then raised to 80 °C in an oil bath, then about (5 mL) of TEOS solution was wisely added at a rate of 10 drops/min followed by 0.85 mL TSD were later added dropwise. At 80 °C, the mixed solution was vigorously agitated for almost 3 h. Three times with ethyl alcohol followed by water, the white precipitate that formed throughout the operation was rinsed. Finally, it was vacuum-freeze-dried. The produced powdered mesoporous silica nanoparticles were then calcined at 550 °C for 7 h to entirely eliminate the surfactant.
Characterization
Electron microscopy studies were conducted using a TECNAI 10 transmission electron microscope (TEM, Philips, Amsterdam, the Netherlands) in order to investigate the morphological characteristics of the formed Flu@MSNs20nm-HTCC and MSNs. The Dynamic light scattering analysis was also done in order to measure the particles size of the formed MSNs by using a ZetaSizer Nano ZS Analyzer (Malvern Z90; Malvern Instruments, Malvern, UK). An FTIR spectrometer (Avatar-300, Nicolet, Green Bay, WI, USA) was used to record the Fourier transform infrared (FT-IR) spectra of the samples with potassium bromide pellet. At a spectral resolution of 4 cm−1, spectra were obtained in 400 to 4000 cm−1 range.
To investigate their pore properties, a surface area and pore size analyzer (TriStar II 3020; Micrometrics Instruments Corporation, USA) was used to investigate the nitrogen adsorption of both the produced MSNs alone and the Flu@MSNs20nm-HTCC at 196 °C. Before analysis, the samples were adequately degassed at 80 °C for around 12 h. The BET and BJH techniques were applied to the adsorption branches of the isotherms to examine the properties of the formed silica nanoparticles mesoporous structure.
Pesticide loading and its release (kinetics)
Fludioxonil loading into/onto MSNs20nm-HTCC and its efficiency
After preparation MSNs with different sizes (20, 50, and 90 nm), the better MSNs size will be selected to continue the work and loading the pesticide. The following was a common technique for the loading of fludioxonil into the formed MSNs20nm capped HTCC MSNs which was as follows: 20 mg of naked MSNs were disseminated in the fludioxonil -methanol solution (0.1 mg/mL, 1.0 mL). Then, the suspension was sonicated for 30 min at low power. After that, about 2.4 mL of HTCC aqueous solution (20 mg/mL) was added to the suspension in dropwise while it was sonified. Within 10 min of more sonification, the Fludioxonil-MSNs20nm capped HTCC were collected by centrifugation at 14,000 rpm for 5 min, the resulting precipitate was washed three times with deionized H2O, and allowed to dry at room temperature and then freeze dried. The fludioxonil-MSNs20nm capped HTCC were denoted as Flu@MSNs20nm -HTCC. The fludioxonil -loaded nacked MSNs were denoted as Flu@MSNs. GC–MS analysis was used to determine the concentration of the unloaded Celest in the supernatant by (Agilent 5890 Series II GC with 5972 MSD detector; PerkinElmer Autosystem GC with FID detector). The loading efficiency content (LC) of the fludioxonil was calculated according to equation:
Fludioxonil release
In order to evaluate fludioxonil release from the prepared Flu@MSNs20nm and Flu@MSNs20nm–HTCC compared to the conventional pesticide alone in the treated plants, twenty milligrams of Flu@MSNs20nm-HTCC were dispersed in 250 mL of phosphate buffer with 0.1% Tween-80 emulsifier, which was used as the release medium and had the same pH as those of tomato fruit (6.22), roots (6.96) and leaves (8.19). This mixture was then magnetically stirred at a speed of 300 rpm. The release test was then performed with a D-800LS dissolution tester (Tianjin University, Tianjin, China) at a stirring speed of 120 rpm. Flu@MSNs20nm -HTCC was held in the releasing medium using a dialysis membrane (PBS). About 800 µL of PBS was then taken for HPLC analysis during a given time interval, and 800 µL of fresh medium was carefully supplied to furnish the whole volume of the releasing medium. The release tests were carried out twice more. The rate of fludioxonil accumulative release was calculated following the below the equation:
Where Ep: the accumulative release (%) of Fludioxonil from the Flu@MSNs20nm -HTCC; Ve: 800 μL, the volume of the PBS (the release medium) taken in a time interval; Ci: the Fludioxonil concentration in the PBS at the sampling time n; Mp (mg): the total amount of fungicide loaded into mesoporous silica nanoparticles (MSNs20nm).
Antifungal activity
Firstly, the antifungal activity of the prepared MSNs at different sizes (20, 50, and 90) nm against FORL was determined following the growth rate method in order to select the more effective size of MSNs to be loaded further with the pesticide. Then, Flu@MSNs20nm-HTCC formulation containing different concentrations (0.05, 0.1, 0.2, 0.4, 0.8, 1) mg/L of fludioxonil and after selecting the MSNs size with better antifungal activity was evaluated similarly. Briefly, FORL mycelial discs (7 mm in diameter) developed on potato dextrose agar (PDA) plates were cut from the colony’s edges and deposited on the synthetic media plates supplemented with the above concentrations of Flu@MSNs20nm-HTCC. The inoculated plates were then incubated at 25 °C ± 1 for 4, 6, and 8 d. Mycelial fungal radial growth was monitored after incubation in all plates, and data was reported as the percentage of inhibition. Finally, the antifungal impact of all treatments was assessed by measuring the fungal hyphae growing radially in each inoculated plate (Çolak and Bicici 2013):
Where A represents the FORL mycelial radial growth on the control (untreated) plate and a represents the FORL mycelial radial growth on the plate treated with different concentrations of Flu@MSNs20nm-HTCC. Positive controls included the commercial fludioxonil fungicide (Syngenta, Switzerland), HTCC, and blank carrier MSNs-HTCC. All laboratory experiments were done three times under controlled conditions.
In vivo experiments
Inoculum preparation for in vivo experiments
For a week, FORL isolate was cultivated on PDA plates. Sorghum meals were used to make the fungal inoculum. In one-quart Mason jars, 200 g of sorghum (Sorghum bicolor (L.) Moench) seeds were steeped overnight in water and autoclaved twice. After cooling, 6–8 mycelial plugs containing Fusarium conidial spores were inoculated into the autoclaved sorghum seeds. The inoculated fungal plates were then incubated and allowed to develop on sorghum for 3.5 weeks; before being harvested and air dried. One part of the Fusarium-infested sorghum was then properly mixed with 100 parts of sterile 1:2 (soil: sand mixture) in order to prepare the inoculum for the tomato root infection assay.
Disease assessment
Flu@MSNs20nm-HTCC aqueous solution was firstly prepared at 0.1 and 1 mg/L concentrations. Thirty-day-old tomato (cv. TH99806) seedlings were slowly transferred from their wetted soil into 100 mL of each Flu@MSNs20nm-HTCC concentration (0.1 and 1 mg/L) for 5 min. Nine replicates for each Flu@MSNs20nm-HTCC concentration, as well as control conditions, were used. After incubation, tomato seedlings were slowly re-transplanted in the infested soil. The experiment was done in triplicates, each replicate have three pots and each pot has 4 tomato seedlings. Seedlings sprayed with a chemical fungicide (Celest FS 10% Fludioxonil, Syngenta, Egypt, at 1.56 mL/L) served as positive and negative controls in the studies, and mock-treated with H2O, respectively were also included. Plants were cultivated in a greenhouse. Starting on the tenth day post-inoculation (dpi), the number of tomato plants showing crown and root rot symptoms were recorded.
Three rating levels were followed to evaluate the percentage of disease severity in plant parts based on discoloration which scored according to (Huang et al. 2011) Finally, disease severity (DS) were calculated based on the following formulas: DSI (%) = [sum ( frequency class × the score of rating class)]/[(the total number of tomato plants) × ( the maximum level of disease index)] × 100. In order to figure out the residue behavior of Celest in tomato fruits, the concentration level of Fludioxonil as the active ingredient of Celest pesticide were determined during the fruiting stage (over a period of 60 d) using high performance liquid chromatography tandem mass spectrometry (HPLC–MS/MS).
Soil pot experiments
Two clean standard burettes (Borosil) of 50 mL capacity were prepared as soil columns. In this experiment, around 50 g of soil samples were taken from the depth of 2 feet to prepare the column. The soil was held in the column to 1/3 of the column length and a suitable a patch of cotton of cotton used as blocker in the lower end of the column, hence the soil could be retained in the column. Then, a known volume and concentration of celest pesticide solution was filled in the upper portion of the column and slow release of celest was noticed periodically over a course of time (21 d).
In parallel, and in a another set of the experiment, 2.5 g of Fludioxonil loaded MSNs20nm-HTCC was properly mixed under room temperature with 50 g of soil after stirring for 5 min at 200 rpm, and completed the second column in the same manner as the first (see Fig. 2). The columns were then drained of a known volume of water at a fixed flow rate, and the Celest fungicide released amounts were eventually collected at definite time intervals and determined analytically. The schematic experimental setup for soil pot experiment is shown in Fig. 2.
Statistical analysis
Statistical analysis was performed by using R software packages (version 4.0.5). Origin pro 9.1 software was also used for graphing. Differences between treatments with one-way analysis of variance (ANOVA) followed by Duncan’s new multiple range test were evaluated to confirm the statistical differences between treatments in some way. All experiments were done three times, and the findings were provided as mean ± standard deviation (SD). The results were considered statistically significant, when the p-value < 0.05.
Results
Preparation and characterization of Fludioxonil capped @MSNs20nm-HTCC
The construction process of fludioxonil -loaded MSNs capped HTCC was simply illustrated in Fig. 1. In the present study, different sizes of MSNs (20 nm, 50 nm, and 90 nm) (Supplementary material S1) were chemically produced following the liquid crystal templating mechanism using CTAB and TEOS solutions as the surfactant and silica precursor respectively. The different sizes of the produced MSNs were obtained by varying the proportion of sodium hydroxide and water, i.e., the pH of the solution, as shown in Table 1. It was found that the particle size increased when the pH of the solution increased, which was consistent with Lu’s study (Popat et al. 2012).
As shown in Fig. 3, the formed MSNs–HTCC possessed a porous structure and were regularly spherical with a relatively smooth surface (Fig. 3A); the particle size was around 20 nm (Fig. 3C). The particles were still spherical or sub-spherical, and the particle size was slightly larger around 25 nm (Fig. 3B, D). Among the three formed sizes of MSNs, MSNs-HTCC with the smaller size (20 nm) were selected to continue the next work and were loaded with Fludioxonil (the pesticide model) by immersion the MSNs20nm-HTCC nanoparticles in a methanol solution of Fludioxonil with high concentration.
The Fourier transform infrared (FTIR) spectroscopy analyses for each Flu, MSNs, HTCC and the Flu@MSNs20nm-HTCC are presented in Fig. 4. The analyses of Brunauer–Emmett–Teller (BET) surface area and Barrett–Joyner–Halenda (BJH) pore size and volume were applied in this work in a way to investigate the structure of the formed MSNs. Figure 5 showed the obtained data for the BET specific surface area (SBET), the BJH pore diameter (DBJH), and the total pore volume (Vt).
Optimization of fludioxonil loading
For reliable practical use in plant protection, loading the pesticides more efficiently with high contents and in sustainable manner is a critical important issue. The uptake and release behaviors of guest molecules were mostly determined by the pore size, surface area, and mesoporous structure of silica nanoparticles, when bare mesoporous silica nanoparticles without surface functionalization were exploited for biocide loading. HTCC coating had an effect on pesticide loading in this investigation. Table 2 summarizes the fludioxonil loading optimization results. With the larger mass ratio of Celest to MSNs, the loading content was raised when the amounts of MSNs and HTCC were fixed (see 1–4 & 5–6 entries, in Table 1). Without HTCC, the loading content of Fludioxonil was only 20%, demonstrating that coating with HTCC layers can significantly boost pesticide loading (see Table 2).
Fludioxonil release
The in vitro release behavior of fludioxonil from the produced MSNs and also MSNs-HTCC are presented in Fig. 6. In the obtained results, it was noted that the amount of Fludioxonil in the PBS over 12 h (as the sampling time) accumulated to no higher than 35%. In contrast to fludioxonil loaded in MSNs20nm and MSNs20nm–HTCC was rapidly released, which may be attributed to the mesoporous nature of the silica nanoparticles which modifying the crystalline nature of the loaded pesticide model to a non-crystalline form, that is commonly known to promote the rate of pesticide dissolution (Zhang et al. 2010).
All fludioxonil -loaded in MSNs exhibited the same releasing profiles of the pesticide, which is started with a high first burst release continued by a relatively slow and sustained release manner. In the PBS solution, fludioxonil molecules adsorbed on the surfaces of the HTCC could release more rapidly; However, HTCC layers were swelled to open the MSNs pores and might be partially dissolved giving a good chance to depart the MSNs, consequently faster release was noticed particularly within the short initial sampling time.
On the other hand, in a way to determine the reliable pH condition for fludioxonil release, the pH values of tomato (leaves, roots and fruits) showed 7.89, 6.94 and 6.21, respectively; phosphate puffer solution was used as the release medium with the same pH values like different tomato plant parts. Figure 6B shows the release curves of Flu@MSNs20nm-HTCC in the release medium with different pH values. The obtained data showed that the whole release process was slow and that MSNs20nm-HTCC had good controlled release ability. It was noticed that in the initial release, the three release curves were relatively similar. Subsequently, the rate of the accumulative release in the basic medium was noticeably faster than those that appeared in the neutral and acidic medium. Seventy-two hr. later the accumulate release rate reached 33.33, 36.2 and 44.0% at pH 6.21, 6.94 and 7.89, respectively.
Activity of Flu@MSNs20nm-HTCC against FORL
Firstly, the antifungal activity of the prepared MSNs at different sizes (20, 50, and 90) nm against FORL were evaluated. Also, the fungicidal activity of fludioxonil molecules, HTCC, and MSNs-HTCC were also tested as controls.
Based on this results, and as it was mentioned before, we selected the 20-nm MSNs to produce the formula of fludioxonil loaded MSNs capped HTCC which designed as (Flu@MSNs20nm-HTCC) for further work in the present study. The percentage of inhibition in the fungal mycelial growth rate for FORL after the different treatments with Flu@MSNs20nm-HTCC at different concentrations (0.05, 0.1, 0.4 and 1) mg/L in compared to fludioxonilalone at the same concentrations on 4, 6, and 8 d was demonstrated in Fig. 7A, B. The obtained results showed that, the inhibition percentages were 90.23 ± 1.35, 100, and 100% under the concentrations of 0.05, 0.1, and 0.2 mg/L, on the 8th d for Flu@MSNs20nm-HTCC in compared to inhibition rate with 20.12 ± 0.3, 32.19 ± 2.5 and 42.89 ± 2.5 in case of the fludioxonil alone at the same concentrations (Supplementary Material S2).
The fungicidal activity of Flu@ MSNs20nm-HTCC: A Mycelial growth inhibition of FORL on PDA plates supplemented with different concentrations (0.05, 0.1, 0.4, and 1.0) of Flu@ MSNs20nm-HTCC and after 8 d of incubation. B Mycelial growth inhibition curve of FORL on PDA plates with different concentration of Flu@ MSNs20nm -HTCC, fludioxonil alone, MSNs20nm–HTCC, HTCC, and MSNs20nm after 8 d of incubation
Greenhouse experiments
Flu@MSNs20nm-HTCC effectiveness against Fusarium crown and root rot
Based on in vitro results, two different effective fungicides doses (0.1 or 1 mg/L) of Flu@MSNs20nm-HTCC were selected to set experiments on tomato plants in greenhouse. Disease severity of Fusarium crown and root rot was monitored for about 70 dpi on tomato plants after their treatment with two selected doses of Flu@MSNs20nm-HTCC. The experiment also included plants treated with the conventional fungicide (Celest FS contains 10% fludioxonil).
In mock-treated tomato plants, crown and root rot disease symptoms were clearly noticed in 15 dpi, in contrast in tomato treated plants, the disease symptoms were significantly delayed and largely reduced to the lowest level (Fig. 8). Importantly, the conventional chemical fungicide Celest also scored a significant delay and reduction in disease symptoms in case of control treatments, but at a lesser extent than Flu@MSNs20nm-HTCC treatments (Fig. 8). These results demonstrated that the severity of Crown and root rot disease was minimized to 94 and 98% in the treated plants with 0.1 and 1 mg/L Flu@MSNs20nm HTCC, respectively (Fig. 8A).
In vivo treatments of tomato plants with Flu@MSN20nm-HTCC: A Disease severity index in % of tomato plants non-treated, treated with Flu@MSN20nm-HTCC or the conventional fungicide fludioxonil and then, challenged with FORL. Average and standard error of nine biological replicates are shown. B In vivo treatments of tomato plants with Flu@MSN20nm-HTCC in compared to the conventional fungicide. (1) Plants non-treated, (2) infected control, (3) treated with 0.1 mg/L Flu@MSN20nm-HTCC, and (4) treated with fludioxonil fungicide (1.56 mL/L). Pictures were taken at 70 dpi
On the other hand, it was also observed that no substantial differences were noticed in plants treated with 0.1 or 1 mg/L of Flu@MSNs20nm-HTCC (Fig. 8A). The obtained data also showed tomato plants treated with 0.1 or 1 mg/L of Flu@MSNs20nm-HTCC were more healthy than either the mock-treated or tomato plants treated with the chemical fungicide at high dosage (1.56 mL/L) at 70 dpi (Fig. 8B). Importantly, Flu@MSNs20nm-HTCC at either 0.1 or 1 mg/L did not show any degree of phyto-toxicity. Compared with commercial Celest pesticide, the obtained results indicated that Celest as a pesticide, can exhibit better antifungal activity at the very low concentration of 0.1 mg/L, than its routinely used high doses, if it was delivered on a suitable nano-delivery system with sustained release manner.
Fludioxonil controlled release activity
Data presented in Fig. 9 clearly indicated that the release of fludioxonil was relatively more rapid when it was directly applied, in contrast to its behavior when applied through MSNs20nm-HTCC as a nanocarrier system results in a noticeable slower release rate. Most importantly, the obtained results also indicated that in case of direct release of fludioxonil from the solution, the release noticeably continues up to a week (7 d), however, in case of fludioxonil loaded MSNs20nm-HTCC nanocarrier, the release process continues for a longer time reaching 21 d (Fig. 9A).
Release activity of fludioxonil fungicide. A Accumulation release activity of fludioxonil pesticide over time; B Photograph comparing between the effect of fludioxonil release through (1) controlled Release manner “Flu@MSN20nm-HTCC” a prolonged effect of Flu with very low conc. (0.1 mg/L) when loaded on@MSN20nm-HTCC”, (2) Direct Release manner “fludioxonil only” with its routinely used high doses “ 1.56 mL/L)
fludioxonil accumulation in tomatoes
The obtained data indicated that the final concentration of fludioxonil residue in tomatoes were less than 0.001 mg per kg. It is well known that the maximum residual limit (MRL) for fludioxonil in tomatoes established by Codex for fludioxonil in tomato is 0.3 mg/kg.
Discussion
The formed MSNs20nm had negative charges on their surfaces in the aqueous solution while HTCC layers were positive in their charges, as evidenced by a zeta potential of -20 mV with a Zeta Sizer Nano ZS Analyzer (Malvern Instruments Ltd, England). Those layers were effectively wrapped onto the delivered MSNs’s surface by the driving forces of both the electrostatic and hydrogen bonding interactions. Interestingly and after pesticide loading, it was noticed that Flu@MSNs20nm-HTCC had a relatively similar structure, size and dispersion (Fig. 3B, D). Herein, the pesticide loading process did not largely change the size of nanoparticles, since most pesticide molecules entered the mesoporous structure of silica nanoparticles which is suggested to be entrapped into the nanoparticle’s pores.
As shown in Fig. 4, the IR spectrum of fludioxonil molecules presents a noticeable strong absorption band centered at the wavelength 1400 cm−1and 920 cm−1, which attributes to the C = O functional group and also the skeleton vibration of the benzene ring founded in pesticide model molecules (fludioxonil). Also, the absorption band centered at 1075 cm−1was attributed to siloxane linkage (Si–O–Si) stretching vibrations. The absorption peak centered at 1450 cm−1 wavelength for the HTCC layers spectra may be attributed to the aliphatic C–H bending vibration of –trimethylamine (N(CH3)3) + , which denotes the presence of a quaternized amine group in the chitosan structure (Lim and Hudson 2004). Almost there is no noticeable C–H aliphatic stretch peaks found in the prepared MSNs, meaning that the CTAB as a surfactant was totally extracted (Kankala et al. 2015). The characteristic absorption bands of fludioxonil, MSNs and HTCC can be present in the formula Flu@MSNs20nm-HTCC, which indicated the successful coatings of MSNs20nm with the HTCC molecules and consequently loading of Fludioxonil.
Moreover, the type IV isotherm curve with an obvious step in the area in the range of 0.3—0.4 of P/P0 values, and the curve of pore size distribution (Fig. 5A) indicates that the formed mesoporous silica nanoparticles have a well-defined mesoporous structure. Most importantly, it was observed that a significant decrease was noticed on the nitrogen adsorption amount after coating the MSNs with HTCC, which may be backed to the sealing/coating effect of the outside layers of the HTCC (Fig. 5B). Furthermore, the pore volume was significantly reduced to 0.22 cm3/g, which introduces a more confirmation evidence of HTCC coating on MSNs.
It was thought that the molecules of fludioxonil in solution outside of the formed MSNs might be trapped in the layers of the produced HTCC through the coating process onto the outer MSNs20nm surface. Also, the concentration effect of the HTCC layers on loading content was also studied. When the MSN quantity was constant, more HTCC did not appear to improve LC, implying a saturation coating of HTCC. The pesticide-loaded nanoparticles' zeta potential is almost constant, which supports this notion. On the other hand, the positive zeta potential and its negative counterpart without decoration confirmed, HTCC coating success.
To continue the research, an equivalent mass ratio of MSNs, Fludioxonil, and HTCC was chosen based on the optimization. As it was noted in Fig. 6. the amount of fludioxonil in the PBS over 12 h accumulated to no higher than 35%. In contrast to fludioxonil loaded in MSNs20nm and MSNs20nm–HTCC was rapidly released, which may be attributed to the mesoporous nature of the silica nanoparticles which modifying the crystalline nature of the loaded pesticide model to a non-crystalline form, that is commonly known to promote the rate of pesticide dissolution (Zhang et al. 2010). The fludioxonil first burst release may be backed to the presence of small amounts from the fludioxonil adsorbed on the outer surface of MSNs-HTCC surface, which may give an advantage for the effectiveness of this system due to the need for immediate treatment against the target pathogen after direct application. The biocompatibility and high safety profile of polymer-coated MSNs received a continued great research interest not only for regulating the delivery of many essential molecules including chemical pesticides but also their release in a sustained manner.
As indicated in Fig. 6A, MSNs-HTCC released higher amounts (80%) of fludioxonil as the active ingredient of fludioxonil than bare MSNs (45%) within 180 min, this may be attributed to the fludioxonil molecules that were encapsulated inside the pores of the mesoporous silica nanoparticles and also absorbed by the outside layers of HTCC molecules.
In 2008, Lee and his co-workers reported a simple procedure for MSNs synthesis, SNs having variable densities of positive surface charges and a significant electrostatic repulsion force that triggers the controlled release of anionic drug molecules (Lee et al. 2008). Their results indicated that the physiochemical properties of the formed MSNs play a critical role in the cargo release behavior. In this regard, although HTCC layers are positive in charges, however the loaded molecules of fludioxonil is neutral. Meanwhile, we suggest that the H- bonding interaction between the fludioxonil molecules as the pesticide model and the MSNs-HTCC surfaces might be the main responsible that governs the fludioxonil release behavior and its level. Those results supposed that fludioxonil from Flu@MSNs20nm-HTCC may be release the fastest in tomato leaves and the slowest in both root and fruit tissues.
The antifungal activity of the formed MSNs was noticed increased with decreasing in their size. Which may be attributed to the high surface area of the formed MSNs particularly in case of more small sizes when contrasted with their larger counterparts, which may induce more interaction with the outer surface molecules of the target fungal cells as well as more loading of the pesticide and consequently its faster release (Dreyer et al. 2010; Goyal et al. 2018). Most importantly, the data presented in Supplementary Material: S2, clearly indicates that fludioxonil -loaded on MSNs20nm-HTCC demonstrated a more higher antifungal activity in compared to fludioxonil alone by about 16 times even under reducing the dose of fludioxonilfungicide, which not only reduced the pesticide applied dosages more greatly but also enhanced their utilization efficiency. It is worth noting that the MSNs20nm-HTCCs as a blank carrier also showed a degree of activity against FORL as the target fungal pathogen, which is an interesting observation that needs to be studied in the further.
The activity of Flu@MSNs20nm-HTCC against the causal fungal pathogen was then explored under in vivo experiments in a way to control the causal agent of Fusarium crown and root rot disease affecting tomatoes. The in vivo results demonstrated that the severity of crown and root rot disease was minimized to 94 and 98% in the treated plants with 0.1 and 1 mg/L Flu@MSNs20nm-HTCC, respectively. Meaning that Flu@MSNs20nm-HTCC provided longer protection than commercial Celest pesticide alone. (Fig. 8A). The significant effectiveness of Flu@MSNs20nm-HTCC could be backed to the MSNs20nm-HTCC carrier which could protect the activity of the active ingredients of Celest from degradation at premature stage and also improve/ induce smart release of them under alkaline conditions or reductive conditions which caused by fungal colonization.
On the contrary, naked commercial Celest certainly would not provide the same long protection for crops even under higher concentration and the same environmental conditions. Those finding are in agreement with previous reports, in which Gao et al. reported that using disulfide-bridged mesoporous organo-silica nanoparticles (MONs) was designed to deliver prochloraz (PRO) pesticide for controlling the fungal Sclerotinia disease (Gao et al. 2020). This quality is, thereby expected to effectively improve the pesticides effectiveness and its usage with a significant low pesticide application dosage. Moreover, their results study clearly showed a significant and promising approach for delivering pesticides for sustainable plant disease management and precision farming with lesser quantities and high efficiency.
In addition to the unique hydrophilic reactive oxygen functional groups founded on the MSNs surface together with the HTCC activity, MSNs easily disperses in water (Dreyer et al. 2010). One possible interpretation for the long lasting merits of Flu @MSNs20nm-HTCC could be that Fusarium root infection happens on newly seedling tomato plants in the begging of the season (Arias et al. 2013; Elmer and White 2016), demonstrating the significance of a disease/treatment window. In this regard, if the root cells of tomatoes have a sufficient amounts of Flu@MSNs20nm-HTCC with sustained release manner, host defense may significantly inhibit or minimize the Fusarium fungal infection as well as delay the onset of the disease symptoms to the point where that disease does not take hold or have a negative impact.
Another mechanism to consider is that the formula of Flu @MSNs20nm-HTCC may need to regulate tomato host defense related genes, which would help tomato plants to overcome the disease infection. Although our findings are important and promising, they do not completely clarify the exact Flu@MSNs20nm-HTCC mechanism of action that enables them to control the Fusarium crown and root rot disease. MSNs, on the other hand have been hypothesized to be able to penetrate the root tissue cells and also translocate into different other plant parts of the tomato treated plants conveying the loaded active molecules (Elmer and White 2016), thus inducing systemic resistance.
Soil pot experiments were carried out in a way to evaluate the benefits of fludioxonil (as a model of Celest pesticide) sustained release behavior from MSNs20nm-HTCC which acting as a delivery system. Meanwhile, the sustained release formulations of pesticides appear to serve a prolonged effect against the phytopathogens that kills the pests and destroy field crops. This was clearly indicated in Fig. 9B showing no noticeable Fusarium effect on treated plants with fludioxonil with controlled release manner using MSNs20nm-HTCC, in compared to plants treated with direct application of fludioxonil. Also, based on the obtained data of final residues of fludioxonil in tomatoes, i it can be concluded that the level of fludioxonil concentration in tomato fruit was lower than the permitted international MRL value. Meanwhile, fludioxonil delivery into tomatoes using mesoporous silica nanoparticles has a very low risk of fludioxonil accumulation inside plant tissues (particularly tomatoes and their edible parts).
Conclusions
The current study provides a simple and cost-effective method for extending pesticide release into tomato plants by trapping it in biopolymer-based nanocarriers. Fludioxonil, a Celest pesticide model, was effectively loaded into mesoporous silica nanoparticles, which may be easily synthesized using a simple immersion method to produce HTCC-coated silica nanoparticles using HTCC as a across linking agent. Both the hydrogen bond and the electrostatic interaction forces were considered as critical driving forces in the creation of MSNs. According to the findings, HTCC layers played an important part in the efficient pesticide loading process by introducing MSNs with a high positive charge value. The formed MSNs-coated HTCC showed a better performance more than the nacked MSNs for guest pesticide loading efficiency. The celest-loaded nanoparticles burst at first, but then continued to discharge for a long time. Also, it was indicated that the MSNs-coated with layers of HTCC released pesticide more rapid than the naked MSNs in the primary stage.
Taking into account the benefits of MSNs, such as their unique level of biocompatibility and wide dwelling space for guest molecules such as insecticides, mesoporous silica nanoparticles coated with HTCC layers demonstrated excellent effectiveness against the pathogenic FORL isolate. Interestingly, it was also observed that even under low doses of Celest pesticide, Flu@MSNs20nm-HTCC introduced a better and efficient fungicidal activity against the target pathogen either in vitro or in vivo, which obviously reduced the pesticide amounts, applied and essentially’ introduce a new strategy in controlling fungal pathogens for sustainable plant disease management and consequently precision farming.
We believe that with the further progress of cost-effective and environmentally acceptable MSNs synthesis processes, as well as the notion of smart delivery, functionalized mesoporous silica nanoparticles will be viable for large-scale and commercial application. Most importantly, although the reported results in our study bring to light the possibility of using engineered mesoporous silica nanoparticles to deliver very low amounts of pesticide into tomato plants more safely and efficiently in compared to the conventional pesticide. However, further research is still in need to secure the most suitable application protocols (foliar versus soil) applications for real field agricultural practices. Furthermore, those experiments should be applied on a wider range of crops at different stages of plant development in order to acquire a comprehensive view of plant responses to treatments using this novel delivery mechanism.
References
Arias MMD, Leandro LF, Munkvold GP (2013) Aggressiveness of Fusarium species and impact of root infection on growth and yield of soybeans. Phytopathology 103:822–832. https://doi.org/10.1094/phyto-08-12-0207-r
Çolak A, Bicici M (2013) PCR detection of Fusarium oxysporum f. sp. radicis-lycopersici and races of F. oxysporum f. sp. lycopersici of tomato in protected tomato-growing areas of the eastern Mediterranean region of Turkey. Turk J Agric for 37:457–467. https://doi.org/10.3906/tar-1203-71
Debbi A, Boureghda H, Monte E, Hermosa R (2018) Distribution and genetic variability of Fusarium oxysporum associated with tomato diseases in Algeria and a biocontrol strategy with indigenous Trichoderma spp. Front Microbiol 9:282. https://doi.org/10.3389/fmicb.2018.00282
Dreyer DR, Park S, Bielawski CW, Ruo RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240. https://doi.org/10.1039/B917103G
El-Abeid SE, Ahmed Y, Daròs JA, Mohamed MA (2020) Reduced graphene oxide nanosheet-decorated copper oxide nanoparticles: a potent antifungal nanocomposite against fusarium root rot and wilt diseases of tomato and pepper plants. Nanomaterials 10:1001. https://doi.org/10.3390/nano10051001
El Bahri Z, Taverdet JL (2007) Elaboration and characterisation of microparticles loaded by pesticide model. Powder Technol 172:30–40. https://doi.org/10.1016/j.powtec.2006.10.036
Elmer WH, White JC (2016) The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environ Sci Nano 3:1072–1079. https://doi.org/10.1039/C6EN00146G
Farokhzad OC, Langer R (2009) Impact of nanotechnology on drug delivery. ACS Nano 3:16–20. https://doi.org/10.1021/nn900002m
Gao Y, Xiao Y, Mao K, Qin X, Zhang Y, Li D, He S (2020) Thermoresponsive polymer-encapsulated hollow mesoporous silica nanoparticles and their application in insecticide delivery. Chem Eng Sci 383:123169. https://doi.org/10.1016/j.cej.2019.123169
Ghormade V, Deshpande MV, Paknikar KM (2011) Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv 29:792–803. https://doi.org/10.1016/j.biotechadv.2011.06.007
Goyal P, Chakraborty S, Misra SK (2018) Multifunctional Fe3O4-ZnO nanocomposites for environmental remediation applications. Environ Nanotechnol Monit Manag 10:28–35. https://doi.org/10.1016/j.enmm.2018.03.003
Guo HC, Feng XM, Sun SQ, Wei YQ, Sun DH, Liu XT, Yin H (2012) Immunization of mice by hollow mesoporous silica nanoparticles as carriers of porcine circovirus type 2 ORF2 protein. Virol J 9:1–10. https://doi.org/10.1186/1743-422X-9-108
Hashim AF, Youssef K, Abd-Elsalam KA (2019) Ecofriendly nanomaterials for controlling gray mold of table grapes and maintaining postharvest quality. Eur J Plant Pathol 154:377–388. https://doi.org/10.1007/s10658-018-01662-2
Huang CH, Roberts PD, Datnoff LE (2011) Silicon suppresses Fusarium crown and root rot of tomato. J Phytopathol 159:546–554. https://doi.org/10.1111/j.1439-0434.2011.01803
Huang X, Zhang T, Goswami A, Luo F, Asefa T (2015) Glutathione-triggered release of model drug molecules from mesoporous silica nanoparticles via a non-redox process. RSC Adv 5:28836–28839. https://doi.org/10.1039/C4RA08570A
Kankala RK, Kuthati Y, Liu CL, Mou CY, Lee CH (2015) Killing cancer cells by delivering a nanoreactor for inhibition of catalase and catalytically enhancing intracellular levels of ROS. RSC Adv 5:86072–86081. https://doi.org/10.1039/C5RA16023E
Kashyap PL, Xiang X, Heiden P (2015) Chitosan nanoparticle based delivery systems for sustainable agriculture. Int J Biol Macromol 77:36–51. https://doi.org/10.1016/j.ijbiomac.2015.02.039
Keasberry NA, Yapp CW, Idris A (2017) Mesoporous silica nanoparticles as a carrier platform for intracellular delivery of nucleic acids. Biochem (mosc) 82:655–662. https://doi.org/10.1134/S0006297917060025
Kumar S, Nehra M, Dilbaghi N, Marrazza G, Hassan AA, Kim K-H (2019) Nanobased smart pesticide formulations: emerging opportunities for agriculture. J Control Release 294:131–153. https://doi.org/10.1016/j.jconrel.2018.12.012
Lee CH, Lo LW, Mou CY, Yang CS (2008) Synthesis and characterization of positive-charge functionalized mesoporous silica nanoparticles for oral drug delivery of an anti-inflammatory drug. Adv Funct Mater 18:3283–3292. https://doi.org/10.1002/adfm.200800521
Lewis KA, Tzilivakis J, Warner D, Green A (2016) An international database for pesticide risk assessments and management. Hum Ecol Risk Assess 22:1050–1064. https://doi.org/10.1080/10807039.2015.1133242
Lim SH, Hudson SM (2004) Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr Res 339:313–319. https://doi.org/10.1016/j.carres.2003.10.024
Mosa MA, Youssef K (2021) Topical delivery of host induced RNAi silencing by layered double hydroxide nanosheets: An efficient tool to decipher pathogenicity gene function of Fusarium crown and root rot in tomato. Physiol Mol Plant Pathol 115:101684. https://doi.org/10.1016/j.pmpp.2021.101684
Nuruzzaman MD, Rahman MM, Liu Y, Naidu R (2016) Nanoencapsulation, nano-guard for pesticides: a new window for safe application. J Agric Food Chem 64:1447–1483. https://doi.org/10.1021/acs.jafc.5b05214
Pareek M, Rajam MV (2017) RNAi-mediated silencing of MAP kinase signalling genes (Fmk1, Hog1, and Pbs2) in Fusarium oxysporum reduces pathogenesis on tomato plants. Fungal Biol 121:775–784. https://doi.org/10.1016/j.funbio.2017.05.005
Popat A, Liu J, Hu Q, Kennedy M, Peters B, Lu GQM, Qiao SZ (2012) Adsorption and release of biocides with mesoporous silica nanoparticles. Nanoscale 4:970–975. https://doi.org/10.1039/C2NR11691J
Radu DR, Lai CY, Jeftinija K, Rowe EW, Jeftinija S, Lin VSY (2004) A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent. J Am Chem Soc 126:13216–13217. https://doi.org/10.1021/ja046275m
Ravier I, Haouisee E, Clément M, Seux R, Briand O (2005) Field experiments for the evaluation of pesticide spray-drift on arable crops. Pest Manage Sci 61:728–736. https://doi.org/10.1002/ps.1049
Rwei SP, Chen YM, Lin WY, Chiang WY (2014) Synthesis and rheological characterization of water-soluble glycidyltrimethylammonium-chitosan. Mar Drugs 12:5547–5562. https://doi.org/10.3390/md12115547
Savary S, Andrea F, Jean-Noël A, Clayton H (2012) Crop losses due to diseases and their implications for global food production losses and food security. Food Sec 4:519–537. https://doi.org/10.1007/s12571-012-0200-5
Shi Y, Miller ML, Di Pasqua AJ (2016) Biocompatibility of mesoporous silica nanoparticles? Comment Inorg Chem 36:61–80. https://doi.org/10.1080/02603594.2015.1088439
Sinha K, Ghosh J, Sil PC (2017) New pesticides: a cutting-edge view of contributions from nanotechnology for the development of sustainable agricultural pest control. In (ed) New Pesticides and Soil Sensors. Academic Press, London, pp. 47–79. https://doi.org/10.1016/B978-0-12-804299-1.00003-5
Wang Y, Cui H, Sun C, Zhao X, Cui B (2014) Construction and evaluation of controlled-release delivery system of Abamectin using porous silica nanoparticles as carriers. Nanoscale Res Lett 9:1–6. https://doi.org/10.1186/1556-276X-9-655
Wanyika H, Gatebe E, Kioni P, Tang Z, Gao Y (2012) Mesoporous silica nanoparticles carrier for urea: potential applications in agrochemical delivery systems. J Nanosci Nanotechnol 12:2221–2228. https://doi.org/10.1166/jnn.2012.5801
Yin J, Wang Y, Gilbertson LM (2018) Opportunities to advance sustainable design of nano-enabled agriculture identified through a literature review. Environ Sci Nano 5:11–26. https://doi.org/10.1039/C7EN00766C
Zhang Y, Ang CY, Li M, Tan SY, Qu Q, Luo Z, Zhao Y (2015) Polymer-coated hollow mesoporous silica nanoparticles for triple-responsive drug delivery. ACS Appl Mater Interfaces 7:18179–18187. https://doi.org/10.1021/acsami.5b05893
Zhang Y, Zhi Z, Jiang T, Zhang J, Wang Z, Wang S (2010) Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J Control Release 145:257–263. https://doi.org/10.1016/j.jconrel.2010.04.029
Zhao P, Cao L, Ma D, Zhou Z, Huang Q, Pan C (2018) Translocation, distribution and degradation of prochloraz-loaded mesoporous silica nanoparticles in cucumber plants. Nanoscale 10:1798–1806. https://doi.org/10.1039/C7NR08107C
Acknowledgements
Mohamed A. Mosa thanks Dr. Abd El-Moez A. Mohamed, School of Metallurgy and Materials, University of Birmingham, United Kingdom, for his valuable efforts in the structural analyses.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors agreed to participate.
Consent to publication
All authors agreed for publication.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mosa, M.A., El-Abeid, S.E., Khalifa, M.M.A. et al. Smart pH responsive system based on hybrid mesoporous silica nanoparticles for delivery of fungicide to control Fusarium crown and root rot in tomato. J Plant Pathol 104, 979–992 (2022). https://doi.org/10.1007/s42161-022-01122-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-022-01122-1